Deciphering how the viscoelastic properties of
mussel-inspired metal-coordinate hydrogels dictate their
adhesive and interfacial mechanics
by
Erica L. Lai
S.B., Massachusetts Institute of Technology (2014)
Submitted to the Department of Materials Science and Engineering in the field of
Polymers and Soft Matter in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Materials Science and Engineering
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
February 2020
© Massachusetts Institute of Technology 2020. All rights reserved.
Author ………..
Department of Materials Science and Engineering
Program in Polymers and Soft Matter
January 9, 2020
Certified by ………..
Niels Holten-Andersen
Associate Professor
Thesis Supervisor
Accepted by ………..
Donald R. Sadoway
Chairman, Departmental Committee on Graduate Studies
Deciphering how the viscoelastic properties of mussel-inspired
metal-coordinate hydrogels dictate their adhesive and interfacial mechanics
by
Erica L. Lai
Submitted to the Department of Materials Science and Engineering
in the field of Polymers and Soft Matter on January 9, 2020,
in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Materials Science and Engineering
Abstract
In the world of adhesives, tunable viscoelasticity and adhesion to wet surfaces are two highly desirable properties. Mussels have already mastered both of these properties within the threads they create to anchor themselves in harsh intertidal conditions (collectively called the byssus). The key to both the mussel’s ability to stick to a wide variety of surfaces and the highly energy-dissipative viscoelastic behavior of its byssal threads is a type of reversible bonding called metal-ligand coordination, which is comprised of amino acid functional groups binding to metal ions. Recently, researchers have incorporated metal-coordinate cross-links into various types of polymeric networks to improve their mechanical properties, particularly toughness, self-healing, and adhesion. However, there is not as much fundamental understanding of how the linear viscoelastic properties of these networks dictate adhesive behavior, both cohesively and at an interface.
In this thesis, we use shear rheology, tack tests, and spherical probe indentation tests to explore correlations between linear viscoelastic properties (i.e., plateau modulus, 𝐺𝑝, and characteristic relaxation time, 𝜏𝑐) and adhesive behavior (e.g., peak stress, energy dissipation per volume or work of debonding per area) of transiently cross-linked hydrogels comprised of histidine-functionalized 4-arm PEG coordinated with Ni2+. It is important to note that this fully transient model system is technically a viscoelastic fluid even if it has gel-like behavior on the timescales studied. To control the viscoelastic properties of the transient networks, we varied the Ni2+-histidine ratio, the polymer
wt %, or the choice of buffer; in a case study, we also added Co2+ for a second relaxation timescale. The experimental conditions of pull rate and substrate choice were also varied.
From our tack results, a strong dependence of peak stress on 𝐺𝑝 and 𝜏𝑐 was observed, and this correlation between network dynamics and mechanics under tensile load is in good quantitative agreement with our theoretical framework for peak stress, which includes the linear viscoelastic properties as parameters. Energy dissipation per volume is also influenced by 𝐺𝑝 and 𝜏𝑐, with an additional dependence on the polymer wt % at higher strains when the network is remodeling. These findings are consistent with previously proposed molecular mechanics of reversible HisxNi2+ cross-links. From our ongoing spherical probe indentation tests, we have demonstrated
are starting to provide quantitative information about how that contribution is modulated by probe material choice and buffer-influenced timescales.
In addition to the adhesive studies, we also replicated the effect of the macroscopic byssal thread structure – a stiff metal-coordinate coating surrounding a compliant core – on its mechanical behavior. To do so, we mimicked the thread structure by coating PDMS fibers with dried 4-arm PEG that was end-functionalized with Dopa or nitroDopa and coordinated with Fe3+, and
performed tensile tests on these coated fibers. From these studies, we demonstrated that the coating allowed for improved toughness, with the magnitude dependent on the coating composition (i.e. pH and covalent cross-linking content). Collectively, these findings provide us with new insights into the correlations between bulk mechanics and adhesive dynamics of gels with transient metal-coordinate cross-links, as well as ways to tune the toughness of mussel-inspired materials during larger extensions under tensile load.
Thesis Supervisor: Niels Holten-Andersen Title: Associate Professor
Acknowledgements
First, I would like to acknowledge my committee, Professor Krystyn Van Vliet, Professor Brad Olsen, and my advisor Professor Niels Holten-Andersen. As my main mentor, Niels has been the biggest cheerleader for my research and a strong supporter of my endeavors outside the lab, allowing for a reasonably healthy work-life balance. He cares deeply about helping and mentoring others, and that is very admirable.Krystyn and Brad’s guidance helped make this thesis stronger, especially in areas where Niels and I were a bit out of our depth.
I would like to thank both former and current members of the Laboratory for Bio-inspired Interfaces. Despite my labmates having quite disparate projects, each of them contributed to my work in some way, and they are a great group of people to work with. I would also like to thank my collaborators. Dr. Bavand Keshavarz was integral in providing a complementary perspective to my data, thus making my analysis more nuanced. Without Dr. Joseph Sandt and Dr. Mathias Kolle, none of the early project ideas would have had testable samples, and the entire thesis may have gone in a very different direction. Daniel Darby and Dr. Jonathan Pham at the University of Kentucky have been the best teammates on our ongoing project and I look forward to seeing where Dan takes our joint project in his graduate studies.
I would like to acknowledge my family, both biological and chosen. I am eternally thankful for my support network, comprised of friends near and far (including those from Holmdel, the National Youth Science Camp, Next 5W, DMSE, PPSM, and WVC) and of my relatives, particularly the ones who live in the greater Boston area. I would especially like to thank my younger sister, Alicia, who has been an easily accessible shoulder to lean on; my dad, John, who always knows how to distract me from whatever I’m worrying about; and my boyfriend, Andrew, whose direct contributions to this thesis are (1) writing a code that saved me hours of tedious data processing, and (2) adopting Chessie (see Figure 0) – both of them never fail to brighten my day. Lastly, I would like to thank my mother, Anhuey. Without her determination to provide my sister and me with the best of every opportunity growing up, I doubt the two of us would be who and where we are today. At numerous points in the past seven years, I really wished I could ask for her wisdom or be given a pep talk, but in her absence I learned how to persevere. I am eternally grateful to be her daughter, and I dedicate this thesis to her and her legacy.
The work presented in this thesis was funded in part by the Anne M. Mayes Fellowship through the Department of Materials Science and Engineering at MIT, the National Science Foundation via the Graduate Research Fellowship Program under Grant No. 2388357, the Office of Naval Research (ONR) under the Young Investigators Program Grant ONR.N00014-15-1-2763, and the MRSEC Program of the National Science Foundation under Award DMR-1419807. Collaborative work with Dan and Jon was supported by the University of Kentucky start-up funds and the National Science Foundation under Grant No. 1832889.
Contents
Chapter 1: Introduction ………. 11
1.1 The Importance of Viscoelasticity in Pressure Sensitive Adhesives (PSAs) ……….... 11
1.2 Transient Bonds to Improve Adhesive Toughness ……….... 11
1.3 Mussel Byssal Threads and Metal-Ligand Coordination: Inspiration for the design of better adaptable adhesives ……….. 12
1.4 Thesis Overview ……….... 12
Chapter 2: Background ……….. 15
2.1 Rheology: Mechanical Tests for Viscoelastic Materials ………... 15
2.1.1 Oscillatory Strain Test: Frequency Sweep ………... 15
2.1.2 Stress Relaxation and Creep Tests ……… 16
2.1.3 Constitutive Models: The Maxwell Model ………... 17
2.2 Supramolecular (Transient) Polymer Networks ……… 18
2.2.1 Tying Bond Dynamics to Mechanical Behavior in Transient Polymer Networks …... 19
2.3 Metal-ligand Coordination in Natural and Bio-inspired Materials ……… 20
2.3.1 Metal-ligand Coordination in the Mussel Byssal Threads ………... 20
2.3.2 The Tunability of Mussel-inspired Metal-ligand Coordination in Synthetic Materials ……….. 22
2.4 Adhesion, Adhesives, and Contact Mechanics ……….. 26
2.4.1 Adhesive Testing in a Confused Geometry: Probe Tack Tests ……… 27
2.4.2 Adhesive Contact Mechanics: Spherical Probe Indentation Tests ………... 28
Chapter 3: Methodology ………. 31
3.1 Synthesis of 4PEG-His ……….. 31
3.2 Synthesis of 4PEG-Dopa ………... 31
3.3 Synthesis of 4PEG-nitroDopa ………... 31
3.3.1 Conversion of Dopamine Hydrochloride to Nitrodopamine Sulfate ……… 31
3.3.2 Coupling of nitroDopa to 4PEG ………... 32
3.4 Synthesis of 4PEG-His + M2+ Hydrogel Samples ………. 32
3.4.1 Single-Ion Samples (Ni2+) for Tack Tests ……… 32
3.4.2 Double-Ion Samples (Ni2+ and Co2+) for Tack Tests ………... 32
3.4.3 Single-Ion Samples (Ni2+) for Spherical Probe Indentation Tests ……… 32
3.5 Synthesis of Byssal Thread Mimic Samples ………. 32
3.5.1 PDMS Fiber Extrusion ………. 32
3.5.2 Coating Process ……… 33
3.6 Small Angle Oscillatory Shear (SAOS) ……… 33
3.7 Tack Tests ……….. 33
3.8 Theoretical Prediction for the Temporal Evolution of the Tack Force ………. 34
3.9 Spherical Probe Indentation Tests ………. 35
3.9.1 Equipment Setup ……….. 35
3.9.2 Moving Profile – Software Setup ………. 36
3.9.3 Pull-Off Measurement ………. 36
3.9.4 Maintenance of Sample Composition and Shape During Tests ………... 38
3.10 Tensile Tests for Byssal Thread Mimic Samples ………. 38
3.10.3 Standard Tensile Test ………. 39
3.10.4 Cyclic Tensile Testing: Repeated Incremental Extension ……….. 39
Chapter 4: Deciphering How the Viscoelastic Properties of Mussel-inspired Metal -coordinate Transiently Cross-linked Gels Dictate Their Tack Behavior ……... 41
4.1 Results and Discussion ……….. 41
4.1.1 Linear Viscoelastic Properties (𝐺𝑝 and 𝜏𝑐) of Transiently Cross-linked 4PEG-His + Ni2+ Hydrogels ………. 41
4.1.2 Qualitative Categorization of the Tack Tests via Visual Documentation ……… 42
4.1.3 The Relationship between Peak Stress (𝜎max) and Linear Viscoelastic Properties ….. 44
4.1.4 The Relationship between Energy Dissipation and Linear Viscoelastic Properties …. 45 4.2 Conclusion ………. 47
Chapter 5: An Attempt to Optimize Adhesive Performance with Multiple Relaxation Modes ……… 49
5.1 Results and Discussion ……….. 49
5.1.1 Linear Viscoelastic Behavior of 4PEG-His + Ni2+ & Co2+ Hydrogels ……… 49
5.1.2 Tack Performance of 4PEG-His + Ni2+ & Co2+ Hydrogels ……….. 51
5.2 Conclusion ………. 53
Chapter 6: Probing Interfacial Adhesion of Mussel-inspired Metal-coordinate Transient Networks ………... 55
6.1 Preliminary Results and Discussion ……….. 55
6.1.1 Changing Interfacial Interactions by Changing Probe Material ………... 55
6.1.2 Changing Plateau Modulus by Changing Polymer wt% ……….. 55
6.1.3 Changing Relaxation Time by Changing Buffer Choice, in Relation to Dwell Time ………. 57
6.2 Conclusion ………. 61
Chapter 7: Exploring the Mechanical Properties of Bio-inspired Metal-coordinate Coatings on Soft Polymer Fibers ……… 63
7.1 Results and Discussion ……….. 63
7.1.1 Qualitative Coated Fiber Failure Behavior ………... 63
7.1.2 Tensile Testing ………. 64
7.1.3 Cyclic Tensile Testing: Repeated Incremental Extension ……… 65
7.2 Conclusion ………. 67
Chapter 8: Conclusion and Future Outlook ………. 69
8.1 Summary ………... 69
8.2 Improvements, Future Directions, and Open Questions ……… 69
8.2.1 Application of Knowledge from Fully Transient Network to Hybrid Network ……... 69
8.2.2 Optimization of Adhesive Performance via Multiple Strategic Relaxation Timescales ………... 70
8.2.3 Version 2.0 of Spherical Probe Indentation Experiments ……….... 70
8.2.4 The Effect of the Unbound Ligand Concentration on Interfacial Adhesion ………… 71
8.2.5 Ways to Improve the Byssal Thread Mimic Samples and Testing ………... 72
Appendix: Theoretical Prediction for the Temporal Evolution of the Tack Force – The Full Derivation ………. 73
List of Figures
Chapter 1: Introduction ………. 11
1.1 Mussel byssal threads, their tensile mechanical behavior, and their structure ……….. 13
Chapter 2: Background ……….. 15
2.1 Graphical relationship between 𝐺′, 𝐺′′, and phase lag (𝛿) ……… 15
2.2 Viscoelastic stress relaxation and creep ……… 16
2.3 Representative frequency sweep for 4PEG-His + Ni2+ hydrogels ………. 18
2.4 Maxwellian behavior modeled by springs and dashpots ………... 18
2.5 Byssal thread regions and their tensile mechanical behavior ……… 22
2.6 Types of bonds formed by Fe3+ and Dopa to create a hybrid network ……….. 22
2.7 4PEG-Dopa gels and pH-dependent Fe3+-Dopa complexes ……….. 23
2.8 HisxNi2+ complexes as a function of Ni2+-His ratio ………... 24
2.9 Dependence of 𝐺𝑝 and 𝜏𝑐 on polymer wt% or buffer choice ……… 24
2.10 Frequency sweeps for gels of four different Ni-Co compositions – reference ……….. 25
2.11 Contribution of LxMn+ complexes to gel cohesion and interfacial adhesion ………. 26
2.12 Stress-strain curve from probe tack test on a PSA ……… 28
Chapter 3: Methodology ………. 31
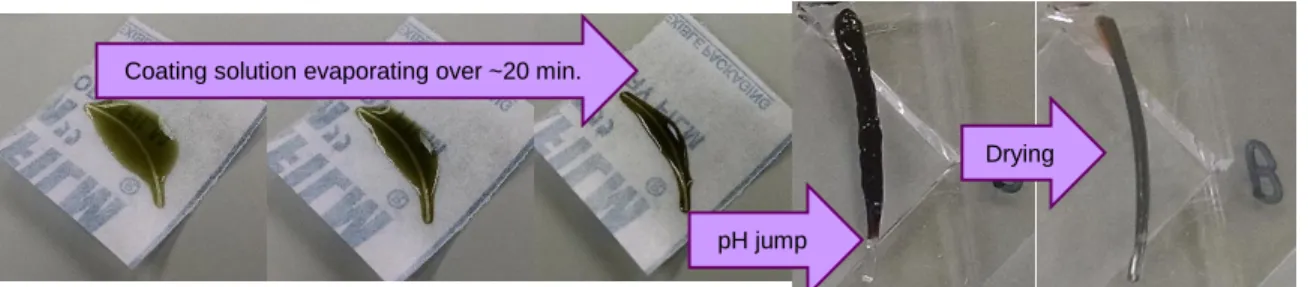
3.1 Process for coating PDMS fiber with dried metal-coordinate PEG hydrogel ………... 33
3.2 Constant strain rate tack test, with 𝜎max and EDV definitions ……….. 34
3.3 Equipment setup of spherical probe indentation tests ………... 35
3.4 Moving profile for spherical probe indentation tests ………. 36
3.5 Pull-off force-displacement curve, with 𝑥max, 𝐹max, and 𝑊DB definitions ……….. 37
3.6 Image estimation of contact area, 𝐴0 ………. 37
3.7 Change in sample shape and 𝐴0 over time ……… 38
3.8 Byssal thread mimic fiber sample testing setup ……… 39
Chapter 4: Deciphering How the Viscoelastic Properties of Mussel-inspired Metal -coordinate Transiently Cross-linked Gels Dictate Their Tack Behavior ……... 41
4.1 Average 𝐺𝑝 and 𝜏𝑐 for 4PEG-His + Ni2+ gels at different Ni2+-His ratios and wt% ………. 42
4.2 Qualitative categories of tack test sample behavior ……….. 43
4.3 Peak stress (𝜎max) vs. strain rate (𝜀̇) and cavitation threshold during tack tests …………... 43
4.4 𝜎max/𝐺𝑝 vs. Wi compared to theoretical prediction (Equation 3.1) during tack tests ……... 45
4.5 Energy dissipation per volume (EDV) vs. 𝜀̇ during tack tests ………... 46
4.6 EDV/𝐺𝑝 vs. Wi – pre-peak and post-peak – during tack tests ………... 46
Chapter 5: An Attempt to Optimize Adhesive Performance with Multiple Relaxation Modes ……… 49
5.1 Frequency sweeps for gels of four different Ni-Co compositions – experimental ………… 50
5.2 Estimation of concentration of HisxM2+, at different Ni-Co compositions ………... 50
5.3 𝜎max vs. 𝜀̇ for hydrogels of differing Ni-Co composition during tack tests ……….. 51
5.4 Total EDV vs. 𝜀̇ for hydrogels of differing Ni-Co composition during tack tests ………… 52
Chapter 6: Probing Interfacial Adhesion of Mussel-inspired Metal-coordinate Transient
Networks ………... 55
6.1 Apparent stress vs. displacement curves on three different probe materials ………. 56
6.2 Apparent stress vs. displacement curves for 10 and 15 wt% gels ………. 56
6.3 Force vs. time curves for gels buffered in PBS and MOPS ……….. 57
6.4 Evolution of contact area over dwell time ………. 58
6.5 Apparent stress vs. displacement curves for different buffers and dwell times ……… 59
6.6 Average 𝜎max/𝐺𝑝 for different buffers, probe materials, and dwell times ……… 60
Chapter 7: Exploring the Mechanical Properties of Bio-inspired Metal-coordinate Coatings on Soft Polymer Fibers ……… 63
7.1 Four main coated fiber failure mechanisms ………... 64
7.2 SEM image of byssal thread cuticle damage vs. mimic coating damage ……….. 64
7.3 Stress-strain curves of 4PEG-Dopa vs. 4PEG-nitroDopa coated fibers ……… 65
7.4 Stress-strain curves of 1:3, 1:2, vs. 1:1 Fe3+:Dopa coated fibers ………... 66
7.5 Images of corresponding fiber samples to Figure 7.4 prior to tensile testing ………... 66
7.6 Multiple cycles of tensile testing, with incrementally increased maximum strain ………… 67
7.7 Modulus & modulus loss vs. maximum engineering strain per cycle ………... 68
Chapter 8: Conclusion and Future Outlook ………. 69
Chapter 1: INTRODUCTION
1.1 The Importance of Viscoelasticity in Pressure Sensitive Adhesives (PSAs)
Adhesives, or substances that can stick or bond substrates together via surface attachment, are a prevalent and essential category of materials in our society; one would be hard-pressed to find a commercial product that does not contain an adhesive. One class commonly found in consumer applications is the pressure sensitive adhesive (PSA); as the name implies, PSAs are non-reactive and only require a bit of applied pressure to activate bonding capabilities. Common products that employ PSAs are Post-It Notes, Blu Tack, postage stamps, wound care dressings, and a wide variety of labels and tapes. In order to perform this type of adhesion well, PSAs need to be
viscoelastic, which means that they need to exhibit a balance of solid-like and liquid-like behavior
when deformed. The liquid-like (viscous) behavior allows the adhesive to flow and wet the substrate to create good contact, while the solid-like (elastic) behavior provides strength for the adhesive to resist debonding. The Dahlquist criterion is an important threshold to allow for good adhesive (tack) behavior; it states that the shear elastic (storage) modulus, 𝐺′, should be less than 0.3 MPa at room temperature. A viscoelastic material generally behaves more elastically when deformed at fast timescales or at low temperatures, and behaves more viscously when deformed at slow timescales or at high temperatures; a designer of a novel PSA, however, wants to more specifically control its bulk viscoelastic properties to optimize adhesive performance for both bonding (application) and debonding (removal).
In this thesis, we mainly seek to provide new insights into the correlation between bulk mechanics and adhesive dynamics. We focus on a model system of four-arm polyethylene glycol (4PEG) hydrogels cross-linked with bio-inspired metal-ligand coordination complexes; using previously established ways to tune the bulk viscoelastic behavior of these hydrogels in a controlled manner, we note the effects on adhesive behavior and correlate parameters that characterize the linear viscoelastic properties of an adhesive material to those that characterize its adhesive properties. The maximum 𝐺′, called the plateau modulus or 𝐺𝑝, of these hydrogel systems tested was less than 25 kPa, which is an order of magnitude lower than the Dahlquist criterion. While the model system as is should by no means be considered a viable PSA, knowledge obtained from these studies is still informative, and perspectives from the PSA community have been borrowed to assess the behavior of these materials.
1.2 Transient Bonds to Improve Adhesive Toughness
The base material for PSAs is typically an elastomer (“elastic polymer”) cross-linked by covalent bonds (or in the case of thermoplastic elastomers, by block copolymer phase separation). To make an elastomer more adhesive or “tacky”, a chemical compound called a tackifier can be blended in to increase viscoelasticity and the ability to bond to different substrates. Another way to increase viscoelasticity is to incorporate transient bonds into a covalent network. Transient bonds are reversible and thus allow for network structure remodeling to relax stress; remodeling is dependent on the bond dissociation rate, and thus transient networks are by nature viscoelastic and also energy dissipative. Network remodeling also allows a material to be more durable and tough because when it is deformed, the transient bonds are sacrificed to prevent irreversible damage to the covalent network, and so the material is able to be more extensible.1
Metal-ligand coordination is a type of transient cross-linking that can also contribute to interfacial adhesion due to ligand interaction at the adhesive-substrate interface. In this thesis, we
aim to determine how metal-ligand coordination contributes to both cohesive strength and interfacial adhesion, specifically when the network is fully transient. It is important to know that the model hydrogel system is technically a viscoelastic fluid even if it has gel-like behavior on the timescales studied.
1.3 Mussel Byssal Threads and Metal-Ligand Coordination: Inspiration for
the design of better adaptable adhesives
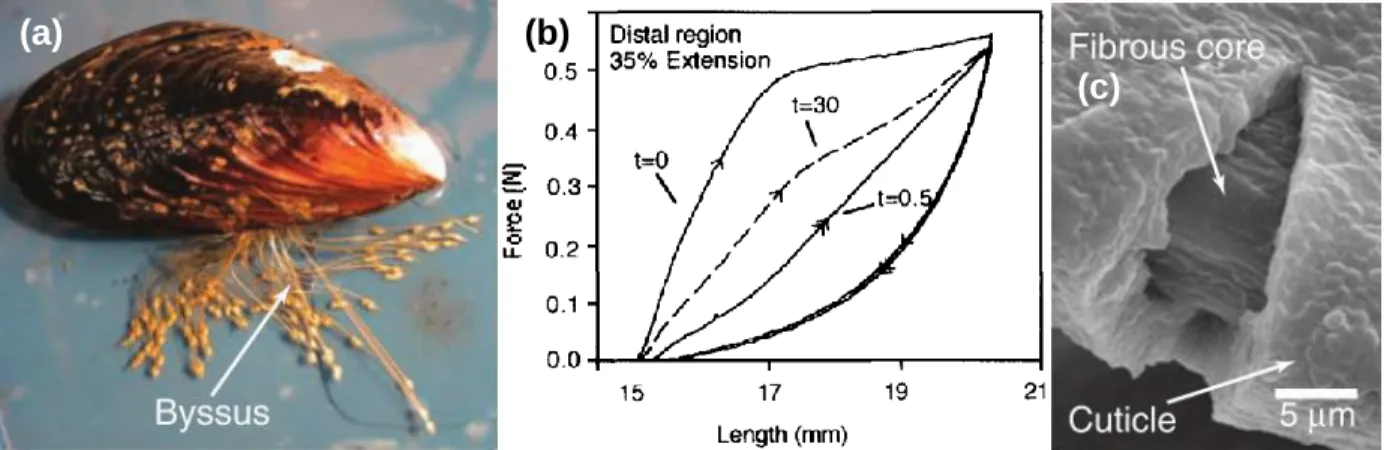
A mussel is an aquatic bivalve mollusk that can be found in intertidal zones. In order to stay tethered onto hard substrates like rocks and seabeds, the mussel secretes fibers, one at a time, from the ventral groove in its foot via a process similar to polymer injection molding (Figure 1.1a). The mussel takes 2-5 minutes to produce a single fiber, and they create a bundle of 20-60 fibers called a byssus.2 These hair-thin threads (~0.1 mm diameter) remain anchored in both wet and dry
environments, withstanding strong hydrodynamic forces. The structure of the byssal threads allows for high toughness and repeated hysteretic energy dissipation; after uniaxial tension past its yield point, the thread is able to recover after some time (Figure 1.1b).3 It is hypothesized that a stiff self-healing cuticle surrounding a more compliant core contributes to these mechanical properties (Figure 1.1c).2 The cuticle’s behavior is theorized to be due to sacrificial metal-ligand complexes formed by Fe3+ and 3,4-dihydroxyphenylalanine (Dopa), a post-translational modification of tyrosine and a catechol moiety.2,4,5 Dopa is also found in high concentration at the interface
between the substrate and the end of the byssal thread (called the adhesive plaque); here, it contributes strongly to interfacial adhesion.6,7 In addition, Dopa can interact with one another to create diDopa covalent cross-links and thus create a hybrid network of covalent and reversible bonds.8–10
Histidine (His) is another amino acid that can also coordinate with metal ions as a sacrificial bond. It has been demonstrated that histidine is concentrated within the core of the thread.8,11–13 Together, Dopa and histidine coordination complexes likely create the strong, sacrificial cross-links that allow for high stiffness, high extensibility, high hysteresis, and the self-healing behavior of the threads. These, combined with the oxidative cross-linking, contribute to cohesive strength within the byssal thread. Since histidine performs metal-ligand coordination like Dopa in mussels but is not sensitive to oxidation, it is an appropriate ligand to use in model transient hydrogels for adhesive studies.
It may seem surprising to be using a model hydrogel system to study adhesive properties; moisture is typically a problem for adhesives because it is considered a surface contaminant or weak boundary layer. However, because ligands bind more strongly to metal ions or a substrate than to water,14 mussel-inspired adhesion is a good candidate for e.g. implementation in medical adhesives and sealants or the basis of antifouling coatings in underwater settings. In this thesis, we use a hydrophilic polymer (PEG) and typically keep the network well-hydrated so that we can focus on the behavior of the metal-ligand coordination cross-links.
1.4 Thesis Overview
In the next two chapters, we provide the background and methodology related to all projects presented in this thesis. In Chapter 2, we will go into more depth about mechanical tests for viscoelastic materials, transient polymer networks, metal-ligand coordination in both natural and bio-inspired materials, and adhesion tests. In Chapter 3, all the methodology, including sample synthesis, equipment setup, and testing protocol, are provided. Both of these chapters contextualize the research presented in the subsequent chapters.
Figure 1.1. (a) A mussel and its byssal threads.2 (b) Given time, a byssal thread is able to recover after
uniaxial tension.3 (c) The cuticle-core structure of the byssal thread.2
This thesis is comprised of three main projects, presented in four chapters. In Chapter 4, we present our first approach to determine how the adhesive behavior of metal-coordinate transiently cross-linked hydrogels are informed by their viscoelastic properties. We did so by performing tack tests (introduced in Section 2.4) on hydrogels with Ni2+-histidine complexes that differed in
polymer wt% and metal-ligand ratio. Our results were published in Langmuir in October 2019.15 Chapter 5 also involves tack tests, but on hydrogels with two metal-coordinate cross-links for more complex viscoelastic behavior. Unlike the more fundamental understanding study presented in Chapter 4, this shorter case study was done in an attempt to optimize adhesive performance.
Because what we learned in the project presented in Chapter 4 gave more information about the cohesive contributions to adhesive properties, in Chapter 6 we present an ongoing study that focuses more on the interfacial contributions to adhesive properties. In collaboration with Dr. Jonathan Pham and Mr. Daniel Darby at the University of Kentucky, we have been performing spherical probe indentation tests (a form of contact mechanics) on hydrogels with Ni2+-histidine complexes that thus far differ in polymer wt% and buffer choice to isolate effects on one viscoelastic parameter at a time. The results that are presented are preliminary but promising, and the project will hopefully continue with Dan and Jon.
In Chapter 7, we present a project that was a precursor to the ones detailed in Chapters 4-6. It was not a study of adhesive properties, but is related due to similar attention to interfaces and behavior of metal-coordinate materials at large extensions. We created a crude mussel byssal thread mimic by coating a polymeric fiber with dried hydrogel containing either a hybrid covalent-coordinate network or just metal-covalent-coordinate complexes alone. We studied the tensile mechanics of these coated fibers to determine if the structure of a stiff metal-coordinate coating surrounding a compliant core exhibited similar behavior as a byssal thread. This project was done with the assistance of Dr. Joseph Sandt and Dr. Mathias Kolle in the Department of Mechanical Engineering at MIT.
A summary of this thesis is presented in Chapter 8, as well as thoughts on future directions. We hope that this documentation allows you to learn how adding metal-ligand coordination to pressure sensitive adhesives could allow for tunable viscoelastic and thus adhesive behavior; perhaps one day, a baseline adhesive formation that contains metal-coordinate bonds could be designed to perform in a wide variety of applications, ranging from removable (e.g. resealable
(a) (b)
Chapter 2: BACKGROUND
2.1 Rheology: Mechanical Tests for Viscoelastic Materials
To measure the time-dependent mechanical properties of soft viscoelastic materials, typically an instrument called a rheometer is used. The three main shear geometry measurement techniques used are oscillatory strain, stress relaxation, and creep; each of these are briefly explained in the following text. The resulting data can be fit with models to quantify material behavior and make comparisons, as described in Section 2.1.3.
2.1.1 Oscillatory Strain Test: Frequency Sweep
A frequency sweep is a common technique which allows one to assess the mechanical response of a sample over a range of timescales. In this test, an oscillatory strain, defined by Equation 2.1, is applied to the sample, where 𝛾(𝑡) is the strain at time 𝑡, 𝛾0 is the strain amplitude, and 𝜔 is the angular frequency.
𝛾(𝑡) = 𝛾0sin(𝜔𝑡) (2.1) Equation 2.2 describes the resulting stress, where 𝜎(𝑡) is the stress at time 𝑡, 𝜎0 is the maximum stress, and 𝛿 is the phase lag between the stress and strain signals.
𝜎(𝑡) = 𝜎0sin(𝜔𝑡 + 𝛿) (2.2)
For a purely elastic material, 𝛿 = 0°, which means the stress and the strain are in phase. For a purely viscous material, the strain lags behind the stress with 𝛿 = 90°, or completely out of phase. For a viscoelastic material, the strain lags behind the stress with 0° < 𝛿 < 90°. With some mathematical tricks, one could define 𝜎(𝑡) in terms of its in phase and out of phase components, as shown in Equation 2.3.
𝜎(𝑡) = 𝜎0[cos(𝛿)sin(𝜔𝑡) + sin(𝛿)cos(𝜔𝑡)] (2.3) = 𝐺′sin(𝜔𝑡) + 𝐺"cos(𝜔𝑡)
𝐺′, defined as 𝜎0𝑐𝑜𝑠(𝛿), is the storage modulus, and 𝐺", defined as 𝜎0𝑠𝑖𝑛(𝛿), is the loss modulus. A helpful way to conceptualize the relationship between the storage modulus, loss modulus, and phase lag is to use Figure 2.1. The projection of the complex modulus, 𝐺∗, on the real x-axis is 𝐺′; the projection of 𝐺∗ on the imaginary y-axis is 𝐺".16
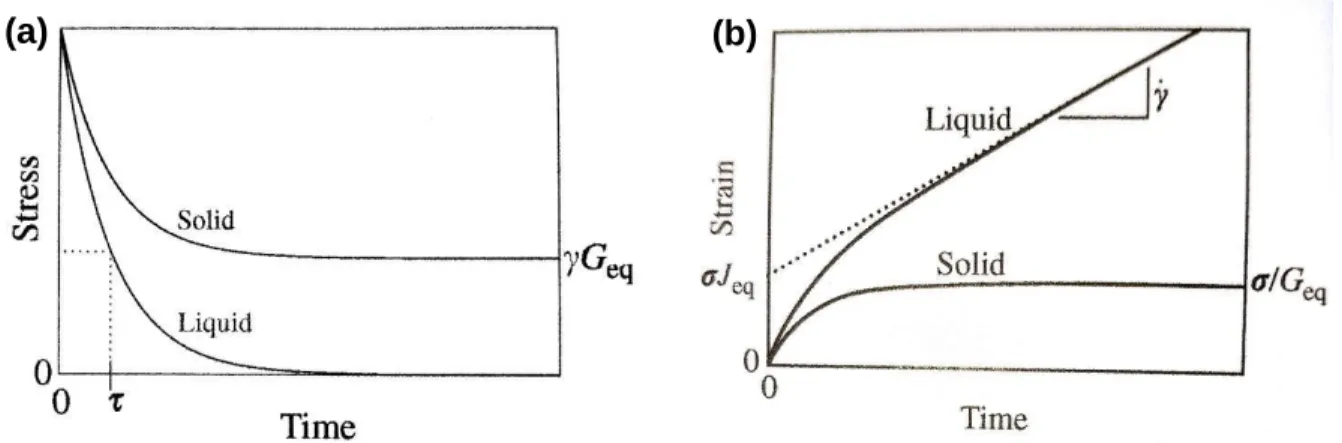
Figure 2.2. Schematics for a viscoelastic solid and viscoelastic liquid undergoing (a) stress relaxation after
the application of a step strain and (b) creep after the application of a constant stress.16
2.1.2 Stress Relaxation and Creep Tests
To perform a stress relaxation test, a step strain, 𝛾, is applied to an initially relaxed sample at time 𝑡 = 0, and the decrease in stress over time from its initial value, 𝜎0, is recorded. The stress relaxation modulus, 𝐺(𝑡), is defined in Equation 2.4, where 𝜏 is the relaxation time.
𝐺(𝑡) = 𝜎(𝑡) 𝛾⁄ ≈ 𝐺(𝜏)exp(− 𝑡 𝜏⁄ ) for 𝑡 > 𝜏 (2.4) For a viscoelastic liquid, 𝐺(𝑡) is a nearly exponential decay towards zero. For a viscoelastic solid, there would be a plateau at 𝛾𝐺eq, where 𝐺eq is the equilibrium shear modulus. 𝐺(𝑡) is independent of strain at small values of 𝛾.16
Generally, to perform a creep test, a constant stress 𝜎 is applied to an initially relaxed sample, and the strain is monitored. The shear creep compliance, 𝐽(𝑡), is defined in Equation 2.5.
𝐽(𝑡) = 𝛾(𝑡)/𝜎 (2.5) For a viscoelastic solid, the strain eventually plateaus at 𝜎/𝐺eq. For a viscoelastic liquid, the slope
of 𝛾(𝑡) at long times is the steady shear rate, 𝛾̇. The shear rate can be used to calculate viscosity, 𝜂. The long-time creep compliance can be extrapolated to 𝑡 = 0, and this intercept is 𝜎𝐽eq, where 𝐽eq is the steady state creep compliance, or a measure of the stored elastic energy in flow. Using
this information, we can write Equation 2.6.
𝐽(𝑡) = 𝐽eq+ 𝑡/𝜂 for 𝑡 ≫ 𝜏 (2.6) By using the Boltzmann superposition principle, the relation of the steady state creep compliance to the stress relaxation modulus can defined as shown in Equation 2.7.
𝐽eq ≈ 1/𝐺(𝜏) ≈ 𝜏/𝜂 (2.7)
Equations 2.6 and 2.7 combined to define the long-time behavior of a liquid in creep (Equation 2.8).
𝐽(𝑡) ≈ (𝑡 + 𝜏)/𝜂 for 𝑡 ≫ 𝜏 (2.8)
(b) (a)
Figures 2.2a and 2.2b provide graphical schematics for the output of a stress relaxation and a creep
experiment, respectively, to help visualize all the parameters.16
2.1.3 Constitutive Models: The Maxwell Model
Constitutive models relate the stress, strain, and the time derivatives of stress or strain of a material; they can be used to fit experimental data from mechanical testing of materials. Perfectly elastic solids can be modeled as springs; according to Hooke’s law of elasticity, the shear stress, 𝜎, is linearly proportional to shear strain, 𝛾, via the shear modulus, 𝐺, i.e. 𝜎 = 𝐺𝛾. Perfect liquids can be modeled as dashpots; according to Newton’s law of viscosity, 𝜎 is linearly proportional to the shear rate, 𝛾̇, via the shear viscosity, 𝜂, i.e. 𝜎 = 𝜂𝛾̇. Because viscoelastic materials have both viscous and elastic behavior, the simplest way to model them is the Maxwell model, or a spring and a dashpot in series, as written in Equation 2.9.16
𝜎 + 𝜎̇𝜂 𝐺⁄ = 𝜂𝛾̇ (2.9) The frequency sweep for a fully transient 4PEG-His + M2+ metal-coordinate hydrogel fits a single-mode Maxwell model, as shown in Figure 2.3. This model suggests that at high frequencies, 𝐺′ ∝ 𝜔0 and 𝐺” ∝ 𝜔−1; at low frequencies, 𝐺′ ∝ 𝜔2 and 𝐺” ∝ 𝜔1. The plateau modulus, 𝐺
𝑝, is
defined as the limit of the storage modulus at high frequency, and it is directly proportional to the cross-link density in the material. The characteristic relaxation frequency, 𝜔𝑐, or relaxation time, 𝜏𝑐 = 1/𝜔𝑐, is defined as the crossover point where 𝐺′ and 𝐺” are equal and tan𝛿 = 1, and is related to the dominant relaxation mode of the material. More generally, 𝐺’ and 𝐺" for a single-mode Maxwellian viscoelastic material are defined as
𝐺
′(𝜔) =
𝐺𝑝𝜔2𝜏𝑐2 1+𝜔2𝜏 𝑐2 (2.10)𝐺"(𝜔) =
𝐺𝑝𝜔𝜏𝑐 1+𝜔2𝜏 𝑐2 (2.11)Multiple Maxwell elements in parallel can be summed together discretely (Equations 2.12 and 2.13) or continuously (Equations 2.14 and 2.15), where 𝐻(𝜏) is defined as the relaxation spectrum; the analogous spring-dashpot models are shown in Figure 2.4.17,18
𝐺
′(𝜔) = ∑
𝐺𝑖𝜔2𝜏𝑖2 1+𝜔2𝜏 𝑖2 𝑁 𝑖 (2.12)𝐺"(𝜔) = ∑
𝐺𝑖𝜔𝜏𝑖 1+𝜔2𝜏 𝑖2 𝑁 𝑖 (2.13)𝐺
′(𝜔) = ∫
𝐻(𝜏)
𝜔2𝜏2 1+𝜔2𝜏2𝑑ln𝜏
∞ −∞ (2.14)𝐺"(𝜔) = ∫
∞𝐻(𝜏)
𝜔𝜏𝑑ln𝜏
(2.15)Figure 2.3. Representative frequency sweep for one of the 4PEG-His + Ni2+ hydrogels studied; the points
are experimental data, while the solid line is the single-mode Maxwellian fit. From the fit, the plateau modulus (Gp) and the characteristic relaxation time (𝜏𝑐) were obtained for each hydrogel sample.
Figure 2.4. A schematic demonstrating (from left to right) how Maxwellian behavior can be modeled by
springs and dashpots. From left to right: single-mode, multiple discrete modes, and a continuous spectrum.18
2.2 Supramolecular (Transient) Polymer Networks
A supramolecular polymer network is one that has transient, reversible cross-links. (“Supramolecular”, “transient”, “reversible”, “dynamic”, and “physical” will be used interchangeably in this thesis to describe our model system.) These types of networks exist as gels (i.e. swollen with solvent) or melts (without solvent), and examples of cross-links include hydrogen bonds, ionic bonds, hydrophobic interactions, and metal-ligand coordination. Supramolecular networks in which metal-ligand coordination is used to control mechanical properties are dubbed “metallopolymers” or “metallo-supramolecular networks”.
1/𝜏
𝑐G
pω (rad/s)
G’, G” (Pa)
Supramolecular networks are best utilized in energy dissipative applications; as hydrogels on their own, applications have included super-absorbent materials, drug delivery, and scaffolds.19 These networks have also been included in tough double-network hydrogels.20–23 In
supramolecular networks, energy dissipation or stress relaxation occurs via bond dissociation and successful re-association with a different binding partner, i.e. network remodeling; this is further discussed in Section 2.2.1.
2.2.1 Tying Bond Dynamics to Mechanical Behavior in Transient Polymer Networks
Understanding of transient polymer networks at the microscopic level, i.e. bond dynamics, can provide insights into the origin of the behavior observed at the macroscopic level. According to Leibler, Rubinstein, and Colby, at timescales longer than the lifetime of a reversible cross-link, the successive breaking of only a few cross-links allows a linear chain to self-diffuse via sticky reptation. This motion is determined by the concentration and lifetime of the cross-links. Four time regimes qualitatively characterize the stress relaxation behavior, 𝐺(𝑡), of physical gels. First, at times shorter than the Rouse time of an entanglement strand (𝜏𝑒), the gels act the same way as those without reversible cross-links. Second, at times between 𝜏𝑒 and the lifetime of a cross-link
(𝜏), there is a rubbery plateau with contributions to modulus from both cross-links and entanglements, i.e. elastically active chains. Third, at times greater than τ, cross-links dissociate and relieve stress, resulting in a second plateau modulus only dependent on the entanglements. Finally, the stress relaxation modulus drops off after reaching the terminal relaxation time, as derived from the self-diffusion coefficient.24
Long et al. provide further insight by modelling the time-dependent mechanical behavior of a dual cross-link self-healing gel under moderate strain and connecting it to the kinetics of association and dissociation of reversible cross-links. In this model, it is assumed that there are no entanglements, which is true for gels of a low volume fraction. It is also assumed that when a polymer chain detaches from a reversible cross-link, the strain energy in that chain is relaxed; when the relaxed chain reattaches to a reversible cross-link, it deforms and contributes to the macroscopic stress starting from a state of zero strain energy (a Gaussian chain). Therefore, the kinetics of chain detachment and reattachment need to be tracked, and the stress needs to take both time and deformation history into account. The rates of chain detachment and reattachment are independent of the strain in the sample, and the stress at a given time has contributions from permanent chains, temporary chains that have survived up until that time, and reattached chains. The parameters of Long et al.’s model are fitted experimentally using stress relaxation and constant strain rate uniaxial tensile tests. The stress relaxation test provides the parameters related to detachment, while the constant strain rate test provides the parameters related to reattachment, or healing. They discover that reattached temporary chains decay faster than the surviving temporary chains, and that the characteristic detachment time is much longer than the characteristic reattachment time. Thus, not all the reversible bonds in a material have the same stability.25
Tensile tests and adhesive tests put a material through high deformation, which results in non-linear behavior that is not yet well understood. Supramolecular polymer networks have been known to demonstrate either shear-thinning or shear-thickening behavior depending on testing conditions, with the mechanism behind that behavior still being investigated.19 Shear thickening
is when viscosity increases with shear strain rate. Xu and Craig found that in their metallo-supramolecular polymer networks, shear-thickening occurred if network chain relaxation is faster than re-association of cross-link, while shear-thinning occurred in the inverse case.26
A study of the relationships between structure, mechanics, and dynamics for Ni2+-histidine coordination bonds within a transient polymer gel network has been done by Tang and Olsen, with a polymer that has the ligands as side groups on a linear poly(N,N-dimethylacrylamide) backbone (instead of the ligands at the ends of a four-arm polymer). They make the point that “materials mechanics is affected by not only the sticker bond chemistry but also the sticker position [end groups vs. pendant side groups], the polymer structure, and the physical environment of the associating polymer such as concentration and solvent quality.”27 They also note that the
characteristic relaxation time (taken from the crossover of G’ and G” in frequency sweeps) can be interpreted as the time for sticker exchange between junctions, 𝜏ex, as this exchange reduces the number of elastically active chains and thus induces network relaxation. Meanwhile, the sticker dissociation time constant, 𝜏off, can be interpreted as an intrinsic sticker exchange time constant,
or the fastest network relaxation time; it is related to dissociation rate constant, 𝑘𝑑. 𝜏off is smaller
than 𝜏ex, meaning that stickers dissociate frequently but don’t necessarily exchange to contribute to chain relaxation.27 𝜏ex is also called the “renormalized bond lifetime”.18 The probability for a
successful exchange decreases with increased gel concentration, even while the total concentration of unbound stickers also increases. Hindered chain relaxation, i.e. the decrease in the effective diffusivity of stickers, requires renormalization of the bond lifetime. In summary, 𝜏off governs the
relaxation dynamics on timescales near the bond lifetime, while hindered self-diffusion is at play at longer timescales.27
As mentioned in Section 2.1.3, our fully transient metal-coordinate hydrogel systems exhibit single-mode Maxwellian behavior. This is because the short PEG arms of equal length cannot entangle and the bond coordination kinetics dictate the mechanical behavior as the rate-limiting step. However, the fit is not perfect. The discrepancy at high frequencies, where 𝐺" flattens just like 𝐺′, is due to all the cross-links behaving elastically at that fast timescale. Even when that is taken into account, our 4PEG-His metal-coordinate systems only form quasi-ideal reversible networks because loops and local defects (i.e. dangling chains, three arms coordinated with one ion) can occur. Parada created a similar system without these defects by having the ends of the 4PEG functionalized with either all “group A” or all “group B”; A and B were complementary and thus could only form reversible cross-links with each other. This ideal model system allowed him to more clearly relate bond dynamics to mechanical behavior.28
While none of the model systems described in these five selected studies directly correspond to the transient polymer network system studied in this thesis, insights from all five can help inform how to interpret the non-linear viscoelastic mechanical behaviors witnessed in coordinately cross-linked four-arm polymer adhesive supramolecular hydrogels.
2.3 Metal-ligand Coordination in Natural and Bio-inspired Materials
2.3.1 Metal-ligand Coordination in the Mussel Byssal Threads
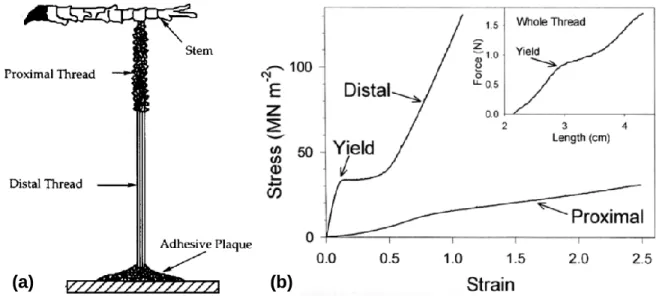
Researchers seeking to design bio-inspired adhesives for underwater or medical device applications have increasingly drawn inspiration from proteins within the mussel byssal threads.7,14,29 As previously mentioned in the Introduction, a mussel secretes an anchoring bundle of threads called a byssus to withstand strong hydrodynamic forces in intertidal zones. Each byssal thread is comprised of four regions along its length: (1) the stem, which attaches the thread to the tissue of the mussel; (2) the proximal region, which is about one-third of the total fiber; (3) the distal region, which is about two-thirds of the fiber; and (4) the adhesive plaque, which is in contact with the substrate (Figure 2.5a).3 The proximal region is like a fiber-reinforced composite; it has a corrugated coating surrounding a core of short, loosely arranged, coiled fibrils, and it
predominantly contains preCol-P, a protein with collagen and elastic domains arranged like a block copolymer. The distal region has a smoother sheath around bundles of densely packed filaments, which are held together by intermolecular cross-links. It contains preCol-D, a protein with collagen and silk-like domains. The adhesive plaque has a coat of collagen-like fibers over a spongy matrix. The distribution of preCols likely plays a major role in the gradient of mechanical properties along the thread, especially because preCols make up almost all of the protein component of the distal region. Because all fiber components are acellular, the fiber’s mechanical properties are intrinsic to the materials system.8,11,30–32
One of the more interesting components of the byssal thread is the viscoelastic distal region, as its purpose is to yield and undergo a large extension before failure. Representative tensile tests of the distal and proximal regions of a byssal thread, compared to that of the whole thread, can be found in Figure 2.5b.30,31 The structure of mainly the distal region of the thread allows for increased toughness and repeated hysteretic energy dissipation that is demonstrated in Figure 1.1b. For example, a byssal thread of the Mytilus californianus stretched to 35% strain can recover 25% of its lost modulus and strain energy after ten minutes of self-healing. Strain softening occurs when the thread is extended past yield, at around 16% strain; this is reversible up until 40% strain.8
As stated previously, it is hypothesized that a stiff cuticle containing sacrificial Fe3+-Dopa coordination complexes surrounds a more compliant core to allow for toughness and self-healing. The theory is supported by the higher concentration of metal-ligand complexes in the distal region, particularly in the cuticle, as shown in Raman imaging.2,4,5 In this Chapter 7 of this thesis, we mimic the structure of the byssal thread with Fe3+-(nitro)Dopa in the “cuticle” of a coated polymer fiber to see if we can replicate the macroscopic mechanical behavior.
Dopa is also found in high concentration within mfp-3 and mfp-5 at the plaque/substrate interface, where it contributes strongly to interfacial adhesion via mechanisms like bidentate coordination bonds, hydrogen bonds, and intermolecular interactions.6,7 In addition to participating in the metal-ligand complexes and adhering to surfaces, Dopa can also interact with one another to create diDopa covalent cross-links. The covalent diDopa linkages dominate in acidic conditions, while Fe3+-Dopa coordination dominates in basic conditions. Without the presence of Fe3+, Dopa would auto-oxidize in basic conditions.9 Thus, by starting at an acidic pH with metal ion present and increasing the pH, a hybrid network of covalent and reversible bonds can be created, as summarized in Figure 2.6.10 The byssal thread likely possesses this hybrid network, as its strong and highly dissipative behavior suggests that there are elastomeric components in parallel with the metal-ligand domains.6,33,34 This would allow for sacrificial metal-ligand bonds to reassemble.8
We have already established that histidine can also coordinate with metal ions as a sacrificial bond, in addition to cation-π, π-π stacking, and hydrogen-π interactions. The theory that histidine is concentrated in the core of the thread is supported by the presence of a high concentration of divalent transition metals (e.g. Co2+, Ni2+, Cu2+, Zn2+).8,11–13 In addition, His has a strong presence in mfp-4, which is found at the junction between collagen microfibrils and the foam-like adhesive plaques of the byssal threads.35 M2+-His interactions serve as load-bearing bonds in other natural materials as well, including the mandibles of insects and spider fangs; however in those materials they are used for stiffening or hardening instead of energy dissipation or self-healing.36 Through spatio-temporal hierarchical coordination, together Dopa and histidine coordination complexes are hypothesized to contribute to the energy dissipative and self-healing behavior of mussel adhesive threads.8,11–13,35
Figure 2.5. (a) The four regions of the byssal thread.3 (b) Tensile tests of the distal and proximal regions of
a byssal thread compared to the entire thread.31
Figure 2.6. The types of bonds formed by Fe3+ and Dopa to create a hybrid network.10
2.3.2 The Tunability of Mussel-inspired Metal-ligand Coordination in Synthetic Materials
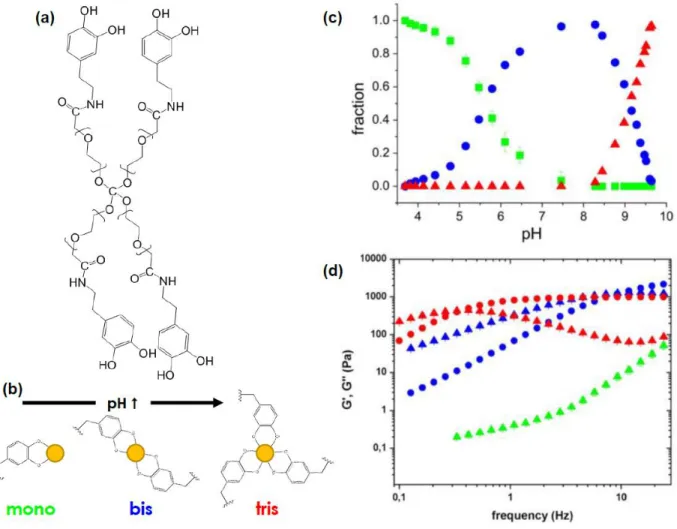
The Laboratory for Bio-inspired Interfaces (LBI) has its roots in Professor Holten-Andersen’s post-doctorate work at the University of Chicago, particularly in his 2011 PNAS paper, in which it was demonstrated how four-arm Dopa-modified polyethylene glycol (4PEG-Dopa) can form metal-ligand coordination bonds with Fe3+ to produce pH-dependent, colorful physical gels (Figure 2.7a). If Fe3+ is first bound to Dopa at an acidic pH to create mono-complexes, ferric precipitation is prevented during the pH increase. By setting the pH and thus affecting the deprotonation of the Dopa hydroxyls, the stoichiometry of the Fe3+-Dopa complexes can be
controlled (Figure 2.7b). Mono-coordination dominates below pH 5.6, bis-coordination between pH 5.6 and 9.1, and tris coordination above pH 9.1 (Figure 2.7c). These crossover points are related to the pKa values of the Dopa hydroxyls. Mono-complexes generate a green-blue viscous fluid, while bis- and tris-complexes generate a purple and red physical gel, respectively (Figure 2.7d). The average relaxation times for bis- and tris-Fe3+-Dopa gels are 0.070 and 2.56 seconds, respectively.37
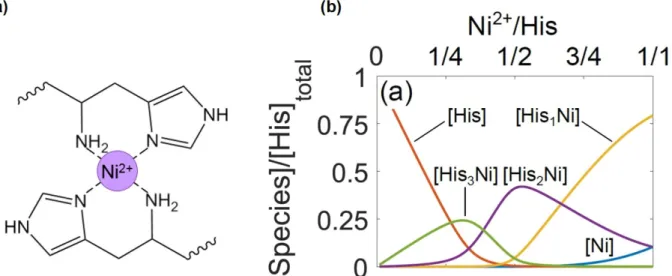
Based on this work, similar transient gel systems have been studied both within LBI and the greater mussel-inspired community.15,17,44–48,18,36,38–43 Attention will be focused on the works of Grindy et al., which used histidine ligands, as we also chose to focus exclusively on histidine as the coordinating ligand because of its insensitivity to auto-oxidation. Histidine can participate in mono-, bis-, and tris-complexes with divalent transition metal ions. An example of a bis-Ni2+-His complex is shown in Figure 2.8a. One of the ways to influence the amount of each type of complex present in a gel is to shift the metal-ligand ratio. Using the equilibrium constants of Ni2+-histamine
complexes (since the constants for histidine do not exist yet), the concentrations of HisxNi2+
species can be predicted, as shown in Figure 2.8b. These predictions inform the trends seen in plateau modulus, 𝐺𝑝, and characteristic relaxation time, 𝜏𝑐, with changes in the metal-ligand ratio.39
Figure 2.8. (a) A bis-Ni2+-histidine complex. (b) HisxNi2+ species present as a function of Ni2+-His ratio.
Ligand protonation/deprotonation is neglected and buffer is not included.39
Figure 2.9. A comparison of plateau modulus, 𝐺𝑝, and characteristic relaxation time, 𝜏𝑐, when either the
polymer wt% or buffer is changed. These were for 4PEG-His + Ni2+ hydrogels with a Ni2+-His ratio of 0.33,
using 0.2M buffers at pH 7.4. Increasing from 10 to 15 wt% increased 𝐺𝑝 with negligible change to 𝜏𝑐, while
changing from phosphate buffer solution (PBS) to MOPS buffer increased 𝜏𝑐 with a slight increase in 𝐺𝑝.
If one wanted to just affect the elasticity or 𝐺𝑝 of a fully transient Ni2+-His hydrogel system with negligible effect on the 𝜏𝑐, they could alter the polymer concentration (wt%); increasing the wt% increases the cross-link density and does not influence bond kinetics significantly.39 If one wanted to instead drastically change 𝜏𝑐with less of an effect on 𝐺𝑝, they could consider changing the buffer or pH control mechanism to change the chemical environment (i.e. competing ligands).18 For example, phosphate buffer solution (PBS) contains the weakly coordinating PO43− group but
Yet another way to control relaxation times is to exploit the redox chemistry of metal-coordinate complexes by creating hydrogels in which the oxidation state of the transition metal ion can be altered with a UV-generated radical. Grindy and Holten-Andersen demonstrated that with the radiation of a photoinitiator, free radicals could be produced to oxidize or reduce the metal ions. When Co2+, for instance, is oxidized to Co3+, the results are a 100-1000x increase in storage modulus across all measured frequencies and accordingly a material that behaves more like a solid with effectively permanent cross-links instead of a more fluid material.40
Finally, by tuning the relative concentration of two types of metal-ligand cross-links in a gel, spatial structure and mechanical performance can be decoupled.17,18 Because cross-link ligand exchange kinetics primarily dictate the behavior of these systems, and each type of metal-ligand cross-link exhibits distinct single-mode Maxwellian behavior, a system containing two of these types of cross-links can be modeled as two parallel Maxwell elements. For simplicity, let the two types of metal-ligand cross-links be designated as a slow-relaxing and fast-relaxing mode. At short timescales, i.e. 𝜏 < 𝜏𝑐,fast < 𝜏𝑐,slow, both modes actively contribute to more elastic behavior. At long timescales, i.e. 𝜏𝑐,fast < 𝜏𝑐,slow< 𝜏, both modes are no longer active and the material flows.
At a mid-range timescale, i.e. 𝜏𝑐,fast < 𝜏 < 𝜏𝑐,slow, the fast-relaxing mode will be inactive but the slow-relaxing mode will still be contributing to network behavior. These timescales are important to think about in the context of remodeling during tack tests.
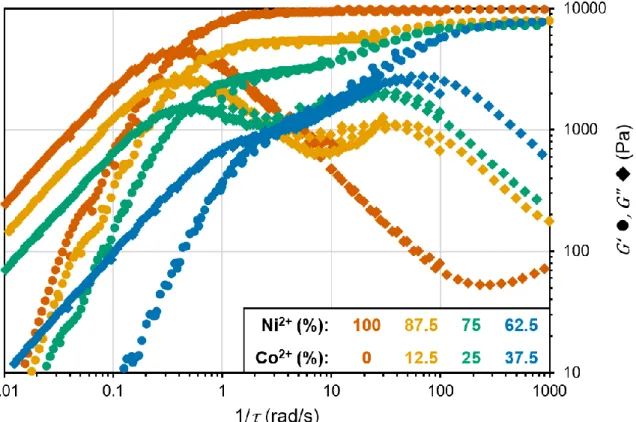
In an oscillatory frequency sweep for a dual-ion metal-coordinate hydrogel, each Maxwell element has a corresponding peak in 𝐺” and possibly a plateau in 𝐺′. These are exhibited in Figure
2.10, which is of a 4PEG-His + Ni2+ & Co2+ system at a total metal-ligand ratio of 0.33 with the
Figure 2.10. Oscillatory frequency sweeps for gels of four different Ni-Co compositions (62.5-100% Ni2+),
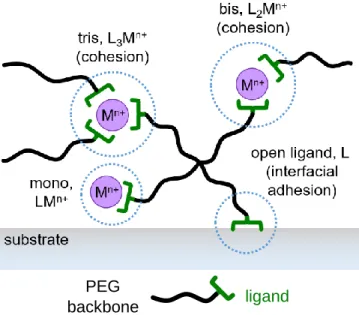
Figure 2.11. A schematic of LxMn+ complexes in a 4PEG-ligand (L) + metal ion (Mn+) transient hydrogel and
their proposed contributions to gel cohesion and interfacial adhesion.
percent of Ni2+ varied (62.5-100%). In these conditions, HisxNi2+ complexes have a slower 𝜏𝑐 than
HisxCo2+ complexes. From this data, Grindy noticed that the 𝐺” peaks shift along the frequency
axis relative to their position in the corresponding single-ion frequency sweep; the slow-relaxing mode becomes faster, and the fast-relaxing mode become slower in the dual-ion case.18 Thus, the 𝜏𝑐 speeds up with decreasing Ni2+ content as the 𝐺” peak for Ni2+ shifts to higher frequencies.
With a judicious choice of the transition metal ion(s) used to cross-link four-arm histidine-modified PEG (4PEG-His) networks, energy dissipation timescales can be designed in both the pre-UV and post-UV state. This, combined with choice of other parameters such as polymer wt%, metal-ligand ratio, buffer, and pH, allow for a wide range of tunable metal-coordinate network behavior. Studying the adhesive properties of these systems and relating them to their linear viscoelastic properties are the focus of Chapters 4 and 5.
2.4 Adhesion, Adhesives, and Contact Mechanics
A good adhesive requires a balance between cohesive and interfacial strength, i.e. between bulk dissipation and surface energy, between bulk and interfacial viscoelasticity. It also needs to have the ability to wet surfaces rapidly and to resist debonding. To achieve this, a number of variables need to be considered. Among them are link density; if there is too little cross-linking, cohesive failure would result, and if there is too much, there will be adhesive failure at an interface. Cavitation (i.e. the formation of air bubbles within the material) and fibrillation (i.e. the extension of the material to create fibers) reduce an adhesive’s ability to withstand high loads, but fibrillation simultaneously increases energy dissipation. Therefore, a material that can endure high loads and dissipate a lot of energy before debonding is a good adhesive.49
Pressure-sensitive adhesives (PSAs) tend to be in a confined geometry, i.e. thin films, where the radius of the sample contact area is much larger than its thickness. In the adhesive industry, these products are usually tested in three main ways: shear, tack (tensile), and peel. On the other hand, contact mechanics studies prefer surfaces to be “infinitely thick” to ignore confinement
ligand
PEG backbone
issues; this way, small-scale interactions can be tested in an easier fashion. In the following two subsections, we will focus on probe tack tests and adhesive contact mechanics with a spherical probe, which are the two ways we studied the adhesive properties of our mussel-inspired fully transient metal-coordinate hydrogels. We know that the ligands (Dopa, histidine) play an important role in surface adhesion and bulk cohesion in the mussel adhesive plaque; in our synthetic 4PEG system, we investigate these roles as well, as schematically shown in Figure 2.11.
2.4.1 Adhesive Testing in a Confined Geometry: Probe Tack Tests
The probe tack test is a method to assess instant adhesion under light pressure, i.e. for PSAs. From these tests, information on the mechanisms of debonding can be obtained. The applied compressive force, the contact time, the debonding rate, and the temperature are all parameters that can be independently controlled. The only other parameters that would influence the results would be the rheological properties of the adhesive and the interfacial interactions with the surfaces of the substrate and the probe. Probe geometry varies, but the standard in industry is the flat-end. This geometry gives a more uniform stress field and strain rate under the probe surface, and is more suitable for very soft adhesives, which undergo both cavitation and fibrillation under tensile stress.50
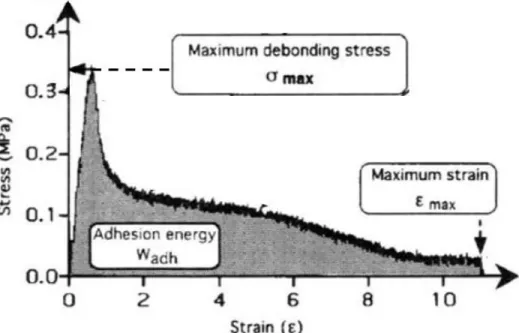
A stress-strain curve from a probe tack test for a typical PSA is shown in Figure 2.12. This evaluation can be coupled with video observation of the bonding and debonding process to understand the underlying mechanisms. A viscoelastic adhesive material first undergoes homogenous void-free deformation in tension, corresponding to the initial increase in tensile stress. Nucleation and rapid growth of cavities (and for some materials, the growth of Saffman-Taylor instabilities from the edges of the sample) correspond to the peak of the curve. The positive skew of the curve is a result of further cavitation, further fingering, and the onset of fibrillation as the cavities laterally grow and walls between them thin. These fibrils eventually either debond from the probe (interfacial failure) or break (cohesive failure), and the stress decreases slowly to zero. The type of debonding failure can be dependent on compressive force or contact time, due to viscoelasticity. For example, a material may demonstrate interfacial failure after a short contact time because the diffusion of adhesive components within the material may be too slow to reach the surface.50 Therefore, it is important to have consistent tack testing protocols to make valid
comparisons.
From the stress-strain curves of PSA materials, the maximum debonding (peak) stress, 𝜎max,
and the adhesion energy, 𝑊adh, are obtained, as marked in Figure 2.12. According to Lakrout et
al., 𝜎max is directly related to the onset of cavitation and shows a good correlation with the linear
shear modulus of the adhesive (i.e. proportional to 𝐺′ at a given frequency), while 𝑊adh is mainly
related to the non-linear elongational properties of the adhesive. In general, both 𝜎max and 𝑊adh should increase with increasing debonding rate due to viscoelastic losses, and both are affected by the nature of the probe surface.50 For samples that undergo extensive fibrillary debonding, 𝑊
adh
is a better way to characterize adhesion; this is because a low plateau modulus, coupled with slight branching and cross-linking, allow for stable fibrils.50
Again, it is important to note that our model system is not elastomeric like a typical PSA; instead, it is fully transient and technically a viscoelastic fluid even if it has gel-like behavior on the timescales studied. Thus, we not only can gain insight from tack tests on PSAs, but also on fluids.51–55 Tirumkudulu et al., for example, saw cavitation when the separation rate of the probe
from the sample was large, and that resulted in forces lower than predicted for their system.51 All of these studies can inform what we observe when testing our systems.
Figure 2.12. A schematic of the resulting stress-strain curve from a probe tack test on a PSA. The maximum
debonding stress (𝜎max) and the adhesion energy (𝑊adh) can be obtained from the curve. Adapted from
Lakrout et al.50
2.4.2 Adhesive Contact Mechanics: Spherical Probe Indentation Tests
Contact mechanics, the study of the deformation of two touching solid materials, has evolved over many years to encapsulate increasingly diverse scenarios. It started with Hertz in 1882, who determined how two nonadhering elastic spheres deformed.56 Adhesion, or surface energy, was added to the Hertz theory by Johnson, Kendall and Roberts (JKR) in 1971, but only within the area of contact.56,57 In 1975, Derjaguin, Muller, and Toporov (DMT) presented an alternate way to include adhesion in the Hertz model of elastic contact by assuming the adhesive forces came from outside the contact area.56,58 Both of these models have their limitations; JKR theory was found to
be more appropriate for soft materials with large surface energies and large contact radii, and DMT theory more appropriate for small rigid spheres with low surface energies.56 In 1992, Maugis, based on the work of Dugdale, was able to merge the JKR and DMT theories into one model by connecting the fields of contact mechanics and linear elastic fracture mechanics.56,59 This also addressed mechanical equilibrium of the system, not just the thermodynamic one.60 However, because these clastic models assume one is starting with two purely elastic curved surfaces and that the system is in equilibrium, none of them are able to capture the dynamic behavior of viscoelastic materials, with contact areas that grow over time.
A common class of viscoelastic materials to study via adhesive contact mechanics are elastomers. Because it is viscoelastic, the pull-off rate affects the energy dissipation rate, which is related to the relaxation times of the elastomer.60 There is a critical pull-off rate at which behavior of the elastomer transitions from behaving as a viscous liquid to an elastic solid, changing the energy dissipation mechanism from viscous losses to small internal losses.61 The effect of dwell
time on interfacial adhesion is of particular interest as it affects adhesive strength in elastomers along with other interfacial properties such as surface energy.62 It is hypothesized that generally, adhesion strength increases with dwell time because molecular rearrangements (i.e. polymer chain relaxation) slowly occur at the interface that increase bond strength.61,62 Changes in contact area,
chain interpenetration across the interface, and surface roughness also contribute to increased adhesion strength with contact time because both rearrangement and relaxation allow for a more perfect contact with the probe surface.62 Increasing the temperature of an elastomer while it is in
contact with a substrate increases the rate of molecular rearrangement but not the rate of bonding reactions at that interface.61 For particularly soft elastomers (i.e. with high liquid content), Pham et al. found that the JKR theory could be modified by accounting for surface tension of the liquid component, in addition to elasticity and adhesion.63
Many on these adhesive contact mechanics experiments use a spherical probe or flat cylindrical punch and have the adhesive material of interest as a flat slab. The choice of what probe geometry to use depends on the measurement scale; a spherical probe eliminates experimental alignment issues, while a flat punch allows for better control over stress distributions because of its contact area. The ratio of the contact radius to the sample thickness, 𝑎/𝑡, is of great importance to interpreting what occurs during these tests, as a small change to that ratio can drastically affect measured results. This is especially true when 𝑎/𝑡 > 1.64 In this thesis, we will be using a spherical
probe for our adhesive contact mechanics experiments, and call our overall procedure “spherical probe indentation tests”.
All of these prior works set the groundwork for how to approach studying the adhesion of metal-coordinate transient hydrogel networks with spherical probe indentation testing. The added complexities of this material system are the fully transient nature of the cross-links; the material may be viscoelastic like an elastomer, but it will eventually flow and relax stress relatively quickly. In addition, the ligands that are a critical component of metal-coordinate cross-links can also interact with the interface with the probe and thus contribute to adhesive behavior. To our knowledge, a system like this has not been studied in this manner before; this is the focus of Chapter 6.