HAL Id: inserm-00484910
https://www.hal.inserm.fr/inserm-00484910
Submitted on 19 May 2010
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
High-frequency stimulation produces a transient
blockade of voltage-gated currents in subthalamic
neurons.
Corinne Beurrier, Bernard Bioulac, Jacques Audin, Constance Hammond
To cite this version:
Corinne Beurrier, Bernard Bioulac, Jacques Audin, Constance Hammond. High-frequency stimu-lation produces a transient blockade of voltage-gated currents in subthalamic neurons.. Journal of Neurophysiology, American Physiological Society, 2001, 85 (4), pp.1351-6. �inserm-00484910�
High-Frequency Stimulation Produces a Transient Blockade of
Voltage-Gated Currents in Subthalamic Neurons
CORINNE BEURRIER,1 BERNARD BIOULAC,1 JACQUES AUDIN,1 AND CONSTANCE HAMMOND2
1
Laboratoire de Neurophysiologie, Centre National de la Recherche Scientifique, Unite Mixte de Recherche 5543, Universite´ Bordeaux II, 33076 Bordeaux Cedex; and2Institut National de la Sante´ et de la Recherche Me´dicale U29, 13273 Marseille Cedex 09, France
Received 8 June 2000; accepted in final form 22 December 2000
Beurrier, Corinne, Bernard Bioulac, Jacques Audin, and Con-stance Hammond. High-frequency stimulation produces a transient
blockade of voltage-gated currents in subthalamic neurons. J Neuro-physiol 85: 1351–1356, 2001. The effect of high-frequency stimula-tion (HFS) of the subthalamic nucleus (STN) was analyzed with patch-clamp techniques (whole cell configuration, current- and volt-age-clamp modes) in rat STN slices in vitro. A brief tetanus, consist-ing of 100-s bipolar stimuli at a frequency of 100–250 Hz during 1 min, produced a full blockade of ongoing STN activity whether it was in the tonic or bursting mode. This HFS-induced silence lasted around 6 min after the end of stimulation, was frequency dependent, could be repeated without alteration, and was not synaptically induced as it was still observed in the presence of blockers of ionotropic GABA and glutamate receptors or in the presence of cobalt at a concentration (2 mM) that blocks voltage-gated Ca2⫹channels and synaptic
trans-mission. During HFS-induced silence, the following alterations were observed: the persistent Na⫹current (INaP) was totally blocked (by
99%), the Ca2⫹-mediated responses were strongly reduced including
the posthyperpolarization rebound (⫺62% in amplitude) and the pla-teau potential (⫺76% in duration), suggesting that T- and L-type Ca2⫹currents are transiently depressed by HFS, whereas the Cs⫹
-sensitive, hyperpolarization-activated cationic current (Ih) was little
affected. Thus a high-frequency tetanus produces a blockade of the spontaneous activities of STN neurons as a result of a strong depres-sion of intrinsic voltage-gated currents underlying single-spike and bursting modes of discharge. These effects of HFS, which are com-pletely independent of synaptic transmission, provide a mechanism for interrupting ongoing activities of STN neurons.
I N T R O D U C T I O N
The observation that deep brain stimulation applied at a high-frequency (HFS) in the subthalamic nucleus (STN) and its surgical destruction, both greatly ameliorate motor signs of Parkinson’s disease in patients, led to the hypothesis that HFS blocks, partly or completely, the activity of STN neurons. In keeping with this, HFS in the STN has been shown to signif-icantly decrease the frequency of extracellularly recorded STN neurons in rats in vivo (Benazzouz et al. 1997). As STN neurons are glutamatergic excitatory output neurons (Ham-mond et al. 1978; Robledo and Fe´ger 1990; Smith and Parent 1988), the immediate consequence of their reduction of activity could be the decrease of activity in target nuclei [substantia nigra pars reticulata (SNr) and entopeduncular nucleus/globus
pallidus internal part (EP/GPi)] as observed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys and naive rats (Benazzouz et al. 1995; Burbaud et al. 1994; Hayase et al. 1996). It has also been suggested that the consequence of clinical HFS will be to somehow counteract the abnormal bursting pattern recorded in the STN in animal models of Parkinson disease (Bergman et al. 1994; Hassani et al. 1996; Hollerman and Grace 1992; Vila et al. 2000).
To understand the contribution of HFS in pathological con-ditions, it is clearly essential to determine whether a HFS of the STN could modify or block the intrinsic activities of STN neurons and to analyze the underlying mechanisms. This is best achieved in vitro, as slice preparations enable to better isolate the various effects of a tetanus on neuronal properties. In the present study, using patch-clamp recordings of rat STN neurons in slices, we report that HFS of the STN suppresses the spontaneous activity of both single-spike and bursting STN neurons. The effects of HFS are synaptic-independent and are mediated by a blockade of the voltage-gated currents and particularly the persistent Na⫹ (INaP) current and the L- and
T-type Ca2⫹currents (ICaLand ICaT) that are known to generate
the intrinsic spontaneous discharge modes of STN neurons (Beurrier et al. 1999, 2000; Bevan and Wilson 1999). M E T H O D S
Slice preparation
Experiments were performed on STN neurons in slices obtained from 20- to 28-day-old male Wistar rats. Rats were anesthetized with ether and decapitated. The brain was quickly removed, and a block of tissue containing the STN was isolated on ice in a 0 –5°C oxygenated solution containing (in mM) 1.15 NaH2PO4, 2 KCl, 26 NaHCO3, 7
MgCl2, 0.5 CaCl2, 11 glucose, and 250 saccharose, equilibrated with
95% O2-5% CO2(pH 7.4). This cold solution, with a low NaCl and
CaCl2content, improved tissue viability. In the same medium, 300- to
400-m-thick coronal slices were prepared using a vibratome (Camp-den Instruments, Loughborough, UK) and were then incubated at room temperature in a Krebs solution containing (in mM) 124 NaCl, 3.6 KCl, 1.25 N-[2-hydroxyethyl]piperazine-N⬘-[2-ethanesulfonic acid] (HEPES), 26 NaHCO3, 1.3 MgCl2, 2.4 CaCl2, and 10 glucose,
equilibrated with 95% O2-5% CO2 (pH 7.4). After a 2-h recovery
period, STN slices were transferred individually to an interface-type
Address for reprint requests: C. Hammond, INSERM U29, Route de Luminy, 13273 Marseille Cedex 09, France (E-mail: hammond@inmed.univ-mrs.fr).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
1351 0022-3077/01 $5.00 Copyright © 2001 The American Physiological Society
recording chamber, maintained at 30 ⫾ 2°C (mean ⫾ SD) and continuously superfused (1–1.5 ml/min) with the oxygenated Krebs solution.
STN stimulation
The stimulating electrode was positioned in the middle of the STN identified as an ovoid structure just lying at the border of the basal part of the cerebral peduncle. Two types of stimulating electrodes were tested: The bipolar concentric electrode measuring 0.5 mm in diameter (NEX-100, Rhodes Medical Instruments) used by Burbaud (Burbaud et al. 1994) and Benazzouz (Benazzouz et al. 1995) for the in vivo stimulation of the rat STN and a much thinner electrode (0.01 mm in diameter) that we designed to avoid any mechanical lesion of the STN.
Electrophysiological recordings
Slices were visualized using a dissecting microscope and the re-cording electrode was precisely positioned in the STN. Electrophys-iological recordings of STN neurons were performed in the current- or voltage-clamp mode using the blind patch-clamp technique in the whole cell configuration. Patch electrodes were pulled from fila-mented borosilicate thin-wall glass capillaries (GC150F-15, Clarck Electromedical Instruments, Pangbourne, UK) with a vertical puller (PP-830, Narishige, Japan) and had a resistance of 10 –12 M⍀ when filled with the following (in mM): 120 Kgluconate, 10 KCl, 10 NaCl, 10 ethylene glycol-bis(b-aminoethyl ether)-N,N,N⬘,N⬘-tetraacetic acid (EGTA), 10 HEPES, 1 CaCl2, 2 MgATP, and 0.5 NaGTP, pH 7.25. Reagents
Drugs were applied by bath. Reagents were procured from Sigma (St. Louis, MO), except 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX),
D-(⫺)-2-amino-5-phosphopentanoic acid (D-APV), and bicuculline, which were purchased from Tocris (Bristol, UK).
Data analysis
Membrane potential was recorded using Axoclamp 2A or Axopatch 1D amplifier (Axon Instruments, Foster City, CA), displayed simul-taneously on a storage oscilloscope and a four-channel chart recorder (Gould Instruments, Longjumeau, France), digitized (DR-890, Neuro-Data Instruments, New York), and stored on a videotape for subse-quent off-line analysis. During voltage-clamp recordings, membrane currents were fed into an A/D converter (Digidata 1200, Axon Instru-ments), stored, and analyzed on a PC using pCLAMP software (ver-sion 6.0.3, Axon Instruments). Corrections for the liquid junction potential were performed according to Neher (1992):⫺6 mV for the K-gluconate-based pipette solution as estimated with a 3 M KCl ground electrode.
R E S U L T S
HFS-induced arrest of single-spike or bursting activity STN activity was recorded in current-clamp mode (whole cell configuration) for at least 1 min before the HFS was applied. Using a bipolar concentric stimulating electrode sim-ilar to that used in rat in vivo (see METHODS), a brief (1 min)
HFS consisting of 100 s stimuli of 5–8 V amplitude, pro-duced a blockade of ongoing activity whether it was in single-spike (Fig. 1) or bursting (Fig. 2) mode. This effect was frequency dependent (Figs. 1A and 2) with an optimal fre-quency of 166 up to 250 Hz that produced a full blockade of the activity (n⫽ 17). The latency of the HFS-induced silence could not be determined in detail as during the 1-min
stimu-lation period, artifacts prevented analysis of the activity. Nev-ertheless as shown in Figs. 1B and 2, above a certain fre-quency, the onset of the blockade was immediately obvious by the end of the train. Interestingly, HFS blocked both single spike (Fig. 1) and burst firing (Fig. 2) modes, suggesting that its mechanisms do not involve a current(s) that is expressed only in one type of discharge.
The suppression of STN spontaneous activity was observed for 5.8⫾ 0.7 min (range: 1.1–18.0, n ⫽ 31) after HFS. At the end of the silence period, spontaneous activity slowly recov-ered in the same mode as before stimulation (Figs. 1B to 6). During cell silence, membrane potential remained stable at
⫺52.2 ⫾ 0.8 mV (range: ⫺40 to ⫺68, n ⫽ 45) for tonic cells
and at⫺56.2 ⫾ 1.4 mV (range: ⫺48 to ⫺61 mV, n ⫽ 8) for bursting cells. These membrane potentials were significantly more depolarized than the potentials at which cells were silent in control conditions: before HFS, cells tested in the tonic mode were silent at⫺60.2 ⫾ 0.6 mV (range: ⫺49 to ⫺68 mV, n⫽ 45, P ⬍ 0.001, paired t-test) and cells tested in the bursting mode were silent at⫺63.5 ⫾ 1.3 mV (range: ⫺56 to ⫺68 mV, n⫽ 8, P ⫽ 0.015, paired t-test). This suggested that HFS did not stop STN cell activity simply by transiently hyperpolariz-ing the membrane.
Spikes could still be evoked during the silence period in all tested neurons (n⫽ 60). However, in half of the cells, spike threshold was significantly higher during the silence period
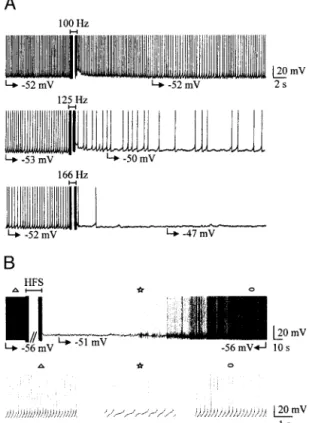
FIG. 1. Effect of high-frequency stimulation (HFS) on the spontaneous single-spike activity of 2 subthalamic nucleus (STN) neurons. A: frequency dependence of HFS. Applied at 100 Hz, HFS had nearly no posteffect whereas at 125 Hz it decreased the frequency of tonic activity and at 166 Hz stopped single-spike activity for 5 min. B: continuous chart recording (top) at slow time resolution of the activity of an other STN neuron. HFS (250 Hz) stopped single-spike activity at a potential of⫺51 mV for 1 min 12 s. Symbols indicate the parts of the top recording that are shown at an expanded time scale in
bottom traces.
(⫺39.3 ⫾ 1.6 mV, n ⫽ 30) compared with the control (⫺47.5 ⫾ 0.6 mV, n ⫽ 30, P ⬍ 0.001; Fig. 3). So was also input membrane resistance, which was significantly increased during HFS-induced silence, when tested at Vm⫽ ⫺65 mV by
applying hyperpolarizing current pulses of⫺100/-200 pA am-plitude (247.2⫾ 21.1 vs. 226.1 ⫾ 16.3 M⍀, P ⫽ 0.035, n ⫽ 20). A second tetanus, applied after the cell recovered from the first one, reversibly silenced the cell again (n⫽ 15). This could be repeated as long as patch recording could last. Therefore HFS does depress neuronal activity in slices, and this effect is short lasting and can be repeated. In subsequent experiments, we used a thinner electrode designed to avoid any mechanical lesion of the STN. We chose to use the same parameters of train duration (1 min) and of bipolar stimuli intensity (5– 8 V) and duration (100s) but to vary their frequency in the train (range 100 –500 Hz) to obtain a clear-cut suppression of ac-tivity during which a long-lasting analysis of currents or spe-cific responses could be performed.
HFS-induced suppression of activity is independent of synaptic activity
An important issue was to determine whether effects of the train were mediated by synaptic transmission. Bath
applica-tions of ionotropic glutamate and GABAA receptor
antago-nists, CNQX (20M), D-APV (40 M), and bicuculline (10 M) failed to prevent the effects of HFS (n ⫽ 6, Fig. 4).
Furthermore HFS still suppressed single-spike activity when synaptic transmission was blocked by 2 mM Co2⫹ (n ⫽ 16, Fig. 5A, top). Since the silencing effect of HFS did not require Ca2⫹-dependent transmitter release, we tested whether it was possible to mimic this effect with intracellular stimulation of the recorded cell. When comparing the two types of HFS (extracellular and intracellular) in the same tonically active STN neurons (n⫽ 8), it appeared that both HFS resulted in a silence of the cell. However, intracellular HFS had a different effect on membrane potential: there was a strong hyperpolar-ization of the membrane at the break of the intracellular pulses (to⫺63.2 ⫾ 3.1 mV) that declined in about 20 s to ⫺48.1 ⫾ 4.1 mV, a potential at which tonic activity recovered (n⫽ 8, data not shown). Such an after hyperpolarization and slow membrane repolarization were never observed after extracel-lular HFS where membrane potential remained stable during cell silence (Figs. 1– 6).
HFS-induced decrease of voltage-gated currents
We hypothesized that HFS induced a modification of volt-age-sensitive currents essential for the expression of tonic and burst-firing modes (Beurrier et al. 2000; Bevan and Wilson 1999). In the tonic mode, the silencing effect of HFS did not require Ca2⫹ influx since it was still observed in the presence of 2 mM Co2⫹nor increase of intracellular Ca2⫹concentration since it was present in BAPTA-loaded cells (n⫽ 4, data not shown). We therefore tested the effect of HFS on spontaneous tonic activity and INaPrecorded from the same STN neurons by
shifting from current- to voltage-clamp mode before, during, and after HFS-induced silence. In voltage-clamp mode, in response to a voltage ramp and in the continuous presence of Co2⫹, a TTX-sensitive inward current that had the character-istics of a persistent Na⫹current was recorded. It was strongly reduced during HFS-induced silence (Fig. 5). I-V relationships before and during HFS-induced silence showed that peak am-plitude of INaP was reduced by 99% during cell silence as
compared with the control (from⫺122.2 ⫾ 13.1 to ⫺1.1 ⫾ 1.1 pA, n⫽ 9; Fig. 5, B and C). This effect reversed to 78% of control (to⫺92.5 ⫾ 9.9 pA, n ⫽ 8) once cell activity
recov-FIG. 2. Frequency-dependent effect of HFS on spontaneous bursting
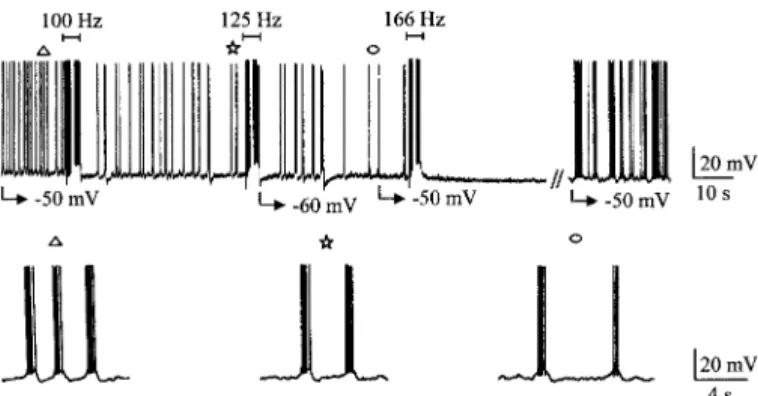
activ-ity of a STN neuron. Chart recording at slow time base of a bursting STN neuron (burst firing was evoked by continuous injection of⫺150 pA). At 100 and 125 Hz, HFS decreased burst frequency (bottom traces) whereas at 166 Hz it totally suppressed bursting activity for 1 min and 49 s. Activity then recovered in burst firing mode. Symbols indicate the parts of the top recording that are shown at an expanded time scale in bottom traces.
FIG. 3. Increase of threshold potential for Na⫹-dependent spikes during HFS-induced silence. Chart recording at low time base of a tonic STN neuron (top). HFS (250 Hz) stopped single-spike activity at a potential of⫺48 mV for 1 min 25 s. Bottom: Na⫹-dependent spikes evoked by a depolarizing pulse of 100 ms, before HFS (a, 80 pA), during HFS-induced silence (b, 100 pA), and after recovery of activity (c, 80 pA).
FIG. 4. HFS-induced silence is independent of ionotropic synaptic trans-mission. HFS (500 Hz) reversibly stopped single-spike activity of a STN neuron for 8 min at⫺45 mV (control). A 2nd HFS (500 Hz) was applied in the continuous presence ofD-APV, CNQX, and bicuculline (Bic). Single-spike activity was stopped at⫺45 mV for 9 min.
ered. When applied at the end of the experiment, TTX (1M) totally blocked this current, confirming that it was INaP
(Fig. 5A).
Spontaneous bursting mode and ICa were then analyzed.
However, since the recording of Ca2⫹ currents requires the presence of K⫹ channel blockers, a procedure incompatible with the recording of burst firing in current-clamp mode, the amplitude of Ca2⫹currents was therefore evaluated from the evoked potentials they underlie: the rebound depolarization, also called low-threshold Ca2⫹spike (LTS), that results from the activation of a T-type Ca2⫹current and the plateau poten-tial that results from the combined action of the nifedipine-sensitive L-type Ca2⫹ current and a Ca2⫹-activated inward current (Beurrier et al. 1999). Following HFS, during minutes of silence, plateau duration was reduced by 62% (from
1119.4⫾ 150.6 to 425.6 ⫾ 111.4 ms, n ⫽ 32) sometimes with a total suppression of the after spike depolarization (Fig. 6, A, top and middle, and B, left). Concomitantly, the amplitude of the rebound potential was reduced by 75.9% (from 8.8⫾ 0.4 to 2.1⫾ 0.5 mV, n ⫽ 23; Fig. 6, A, top and bottom, and B, right). Once cell activity recovered, the effects on plateau potential duration and on the amplitude of rebound potential reversed to 66% of control (to 739.4⫾ 217.9 ms, n ⫽ 18) and to 39% of control (to 3.4⫾ 0.8 mV, n ⫽ 14), respectively.
In contrast, the Cs⫹-sensitive, hyperpolarization-activated cation current (Ih) was not affected by HFS at potentials
normally traversed by the membrane during tonic firing. It was
FIG. 6. Effect of HFS on Ca2⫹-mediated responses. A, top: HFS (500 Hz)
stopped the activity of a burst-firing STN neuron (at⫺63 mV). Middle: plateau potential triggered by a depolarizing current pulse at Vm⫽ ⫺65 mV (⫹80 pA,
100 ms), lasted 760 ms before HFS (a), lasted 150 ms during cell silence (b, 70 s after HFS), and lasted 520 ms during recovery of bursting activity (c, 17 min after HFS).2 indicates the after spike depolarization (ADP) present in a and c and absent in b. Bottom: rebound potential (2) recorded at the break of a hyperpolarizing pulse (⫺80 pA, 500 ms) had an amplitude of 10 mV before HFS (a), of 2.5 mV during silence (b, 8 min 30 s after HFS), and of 5 mV after recovery of bursting activity (c, 34 min after HFS). In the same traces, note the absence of modification of the depolarizing sag that developed during the current pulse. All recordings were obtained from the same STN neuron. B, left: histogram of plateau potential duration before (control), during (HFS), and after (recovery) HFS-induced silence. Right: histogram of rebound potential amplitude before, during, and after HFS-induced silence. *, comparison with preceding column;E, comparison with control. *P⬍ 0.05; ** orE E: P⬍
0.001; *** orE E E: P⬍ 0.0001. FIG. 5. Effect of HFS on the persistent Na⫹ current in the absence of
synaptic transmission. A: HFS (500 Hz) applied in the continuous presence of Co2⫹(2 mM) stopped the activity of a tonically firing STN neuron (for 18 min
at⫺60 mV; top). In response to a 5-mV/s depolarizing ramp from ⫺80 to ⫺10 mV, a slow inward current was recorded before HFS (a), 110 s after HFS (b, during HFS-induced silence), and 36 min after HFS (c, after recovery of activity). TTX (1M) applied at the end of the experiment totally abolished the slow inward current as well as the fast ones (c, bottom). B: I-V relationships of the persistent Na⫹current obtained from the same cell in A. The curve HFS-TTX represents the subtraction of traces Ab–Ac. The curve control-TTX represents the subtraction of traces Aa–Ac. C: histogram of INaPpeak
ampli-tude before (control), during (HFS), and after (recovery) HFS-induced silence. *, comparison with preceding column;E, comparison with control. * orE:
P⬍ 0.05; **: P ⬍ 0.001; ***: P ⬍ 0.0001.
reduced between⫺80 and ⫺110 mV (by 26.5% at ⫺90 mV, n ⫽ 5, Fig. 7). Consistent with these findings on Ih, the
amplitude of the depolarizing sag observed during a hyperpo-larizing current pulse was not significantly affected (it was reduced by 4.8%, from 5.21⫾ 0.82 to 4.99 ⫾ 0.80 mV, P ⫽ 0.69, n⫽ 12; Fig. 6A, bottom).
D I S C U S S I O N
Our results show that HFS blocks the spontaneous activity of tonic and bursting STN neurons with a mechanism that does not require Ca2⫹-dependent transmitter release. The silencing effect of HFS has a short latency, is brief, reversible, can be repeated several times with little change, and is frequency dependent. It is mediated by a dramatic reduction of Na⫹and Ca2⫹ voltage-gated currents leading to an interruption of the spontaneous activities of the neurons. In fact, in single-spike activity, a TTX-sensitive, persistent Na⫹current (INaP),
under-lies the slow pacemaker depolarization that spontaneously de-polarizes the membrane from the peak of the after spike hy-perpolarization to the threshold potential for spike initiation (Beurrier et al. 2000; Bevan and Wilson 1999). In contrast, in burst-firing mode, the interplay between a T-type Ca2⫹current (ICaT), an L-type Ca
2⫹ current (I
CaL), and a Ca
2⫹-activated
inward current, all insensitive to TTX, underlie recurrent mem-brane oscillations (Beurrier et al. 1999). The blockade of these subliminal currents can also explain the increase of membrane resistance observed during HFS-induced silence.
The silencing effect of HFS does not result from the activa-tion of a local network and is not mediated by the stimulaactiva-tion of afferents to the STN, since it was still observed in the presence of blockers of glutamatergic and GABAergic iono-tropic synaptic transmission and in the presence of cobalt at a concentration that totally blocked synaptic transmission in the STN. It was in fact reproduced by direct stimulation of the recorded STN cell as previously tested by Borde et al. (2000) in hippocampal CA1 pyramidal neurons. In this preparation, a low-frequency intracellular stimulation induced a depression of activity that developed rapidly, was reversible, persisted up to
3 min and was still observed when synaptic transmission was strongly reduced by the P-type Ca2⫹ channel blocker -aga-toxin IVA or enhanced by 4-aminopyridine. The insensitivity of depression to synaptic blockade indicates little if any in-volvement of synaptic mechanisms and implies that postsyn-aptic mechanisms are key factors as observed in the present study with extracellular HFS. However, mechanisms underly-ing intracellular stimulation may be different from those un-derlying extracellular HFS. The silencing effect of intracellular stimulation is Ca2⫹-dependent since it requires Ca2⫹influx and intracellular Ca2⫹increase in the stimulated cell (Borde et al. 2000), whereas that of extracellular HFS is Ca2⫹-independent (the present study).
As the pattern of discharge of STN neurons may play an important role in the physio-pathology of parkinsonism (Berg-man et al. 1994; Holler(Berg-man and Grace 1992), it is tempting to correlate the present effects of in vitro HFS on the spontaneous STN activity, to the beneficial effects of high-frequency deep brain stimulation in the STN of MPTP-treated monkeys (Benazzouz et al. 1992; Hayase et al. 1996) or parkinsonian patients (Benabid et al. 1994; Limousin et al. 1998). However, such a direct correlation needs further experiments. First, clin-ical HFS is performed in vivo where it could affect the whole basal ganglia network, at least at the onset of stimulation. Second, clinical HFS is efficient at lower frequencies (125–185 Hz) than sometimes in vitro HFS does. This could be explained by the differences in the characteristics of the stimulating electrode. Finally, beneficial clinical effects are observed dur-ing the continuous application of the stimulation and only for a short while after the stimulation, whereas in the present study, only events that followed the stimulation have been studied. Nevertheless, the present results give some insights in the way intrinsic activity of STN neurons can be depressed.
Present address of C. Beurrier: Dept. of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, 1201 Welch Rd., Palo Alto, CA 94304-5485.
REFERENCES
BENABIDAL, POLLAKP, GROSSC, HOFFMANND, BENAZZOUZA, GAODM, LAURENT A, GENTIL M,AND PERRET J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotac Funct
Neurosurg 62: 76 – 84, 1994.
BENAZZOUZA, GAODM, PIALLATB, BRESSANDK,ANDBENABIDAL. Inhibi-tory response of substantia nigra reticulata neurons to high frequency stimulation of the subthalamic nucleus is independent of globus pallidus activation. Abstr Soc Neurosci 83: 3, 1997.
BENAZZOUZA, GROSSC, F´EGERJ, BORAUDT, ANDBIOULACB. Reversal of rigidity and improvement in motor performance by subthalamic high frequency stimulation in MPTP-treated monkeys. Eur J Neurosci 5: 382–389, 1992. BENAZZOUZA, PIALLATB, POLLAKP,ANDBENABIDAL. Responses of
sub-stantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data.
Neurosci Lett 189: 77– 80, 1995.
BERGMAN H, WICHMANN T, KARMON B, AND DELONG MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkin-sonism. J Neurophysiol 72: 507–520, 1994.
BEURRIERC, BIOULACB,ANDHAMMONDC. Slowly inactivating sodium current (INaP) underlies single-spike activity in rat subthalamic neurons. J
Neuro-physiol 83: 1951–1957, 2000.
BEURRIERC, CONGARP, BIOULACB,ANDHAMMONDC. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci 19: 599 – 609, 1999.
FIG. 7. Effect of HFS on the hyperpolarization-activated cation current Ih.
Left: from a holding potential of⫺50 mV, a family of currents was evoked in
response to 1,500-ms hyperpolarizing steps from⫺60 to ⫺110 mV (10-mV increment) before HFS (control) and during HFS-induced silence (HFS).
Right: I-V relationship of Ih before (control), during HFS-induced silence
(HFS), and in the presence of 1–3 mM cesium in the bath (VH⫽ ⫺50 mV).
Values of I were obtained by subtracting the value of the current at the beginning of the hyperpolarizing pulse from that at the end of the pulse. Currents were normalized (I/Imax) to the maximal current (Imax) recorded at ⫺110 mV.
BEVANMDANDWILSONCJ. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci 19: 7617–7628, 1999.
BORDEM, CAZALETSJR,ANDBUNOW. Activity-dependent response depres-sion in rat hippocampal CA1 pyramidal neurons in vitro. J Neurophysiol 74: 1714 –1729, 2000.
BURBAUDP, GROSSC,ANDBIOULACB. Effect of subthalamic high frequency stimulation in substantia nigra pars reticulata and globus pallidus neurons in normal rats. J Physiol (Paris) 88: 359 –361, 1994.
HAMMONDC, DENIAUJM, RIZKA,ANDFEGERJ. Electrophysiological dem-onstration of a excitatory subthalamonigral pathway in the rat. Brain Res 151: 235–244, 1978.
HASSANI OK, MOUROUX M, AND FEGER J. Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience 72: 105–115, 1996.
HAYASEN, FILIIONM, RICHARDH,ANDBORAUDT. Electrical stimulation of the subthalamic nucleus in fully parkinsonian (MPTP) monkeys. In: The
Basal Ganglia V, edited by Ohye C. New York: Plenum, 1996, p.
241–248.
HOLLERMAN JR AND GRACE AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain Res 590: 291–299, 1992.
LIMOUSINP, KRACKP, POLLAKP, BENAZZOUZA, ARDOUINC, HOFFMANND,
AND BENABID AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 339: 1105–1111, 1998. NEHERE. Correction for liquid junction potentials in patch clamp experiments.
Methods Enzymol 207: 123–131, 1992.
ROBLEDOPANDFEGERJ. Excitatory influence of rat subthalamic nucleus to substantia nigra and the pallidal complex: electrophysiological data. Brain
Res 518: 47–54, 1990.
SMITHYAND PARENT A. Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res 453: 353– 356, 1988.
VILAM, PERIERC, FEGERJ, YELNIKJ, FAUCHEUXB, RUBERGM, RAISMAN -VOZARI R, AGID Y,AND HIRSCHEC. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed with metabolic and electrophysiological measure-ments. Eur J Neurosci 12: 337–344, 2000.