Development of Natural and Engineered
Bacteriophages as Antimicrobials
by
Robert James Citorik
BSc. in Microbiology, University of New Hampshire (2008)
Submitted to the Microbiology Graduate Program
in partial fulfillment of the requirements for the degree of
Doctor of Philosophy
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
June 2018
@
Massachusetts Institute of Technology 2018. All rights reserved.
Signature redacted
A uthor ...
Microbiology Graduate Program
Signature reaacted
May 25, 2018
C ertified by ...
...
Timothy K. Lu
Associate Professor of Biological Engineering
and Electrical Engineering and Computer Science
Thesis Supervisor
Signature redacted
A ccepted by ...
Kristala L. Jones Prather
MASSACHUSMlS INSTrTUTEOF TECHNOWGY
rthur D.
Little Professor of Chemical Engineering
Chair of Microbiology Program
JUL
09
2018
LIBRARIES
Development of Natural and Engineered Bacteriophages as
Antimicrobials
by
Robert James Citorik
Submitted to the Microbiology Graduate Program on May 25, 2018, in partial fulfillment of the
requirements for the degree of Doctor of Philosophy
Abstract
One of the major public health concerns of the modern day is the emergence and spread of extensively antibiotic-resistant pathogens. We have already seen the arrival of infections caused by bacteria resistant to all available antibiotics in the therapeutic arsenal. In addition, we have learned much of the incredible importance of the microbial communities that cohabit our bodies, and of how perturbations to these communities can lead to long-lasting health effects. Bacteriophages may pro-vide a solution for both of these problems, in that they are narrow-spectrum and can be used to specifically kill target microbes without disrupting whole commu-nity structure through off-target effects. Here, various approaches to creating phage-based therapeutics are explored, including the isolation and application of naturally occurring wild-type phages, the conversion of temperate phages to obligately lytic phages to permit their use as a resource in phage therapeutics, and the creation of programmable, sequence-specific antimicrobials through phage-mediated genetic pay-load delivery. These efforts are expected to contribute to the field by expanding the approaches available to develop next-generation, phage-based antimicrobials.
Thesis Supervisor: Timothy K. Lu
Title: Associate Professor of Biological Engineering and Electrical Engineering and Computer Science
Acknowledgments
I would like to take this opportunity to first express my sincere gratitude to all of
the teachers, friends, family, and other role models who have helped and inspired me to complete this leg of my journey. Without all of you, none of this would have been possible.
To Tim Lu, my supervisor and mentor for the past 7 years: I thank you for giving me the opportunity to explore exciting and creative science under your guidance. I recognize that being in this laboratory has been a unique experience, and that many are not given the same freedom and independence to pursue new ideas and explore interesting side projects.
To my thesis committee members, Jim Collins, Eric Alm, and Deb Hung: I am grateful for all of your guidance and expertise in pursuing, as well as finishing, my PhD research. None of you needed to say yes when I asked for your time in this endeavor, and for this I am very thankful. I hope that our conversations continue beyond my time at MIT.
To my program: I feel extremely lucky to have found the Microbiology Program at MIT, and I thank Alan Grossman for creating this opportunity for such interdisci-plinary research, as well as Bonnielee Whang for helping keep us in line and all those faculty who have helped to organize and lead the way. This unique program allowed me to venture beyond basic science research and enter into the exciting fields of bio-logical engineering and synthetic biology, where I have found an exciting interface in which to build upon my previous research in pathogenic microbiology.
To my wife: Jenna, I would never have survived this experience without your support and understanding. Your effort and commitment to giving your all to your students, as well as your husband, is truly inspiring. This degree belongs to the both of us. I love you with all of my heart, and being able to start forever with you has been an unanticipated highlight of my graduate school tenure. If we can survive being a PhD student at MIT and an elementary school teacher working tireless hours, we can survive anything. On to the next chapter!
about my education from the very first day of school. I know the days of getting help with homework are long gone, but those lessons in problem-solving, as well as how to look out for your children, will last a lifetime. I thank my brother and sister, as well as my brother- and sister-in-law, for always being there for me. They are the kind of support system crucial to success in any aspect of life. I also thank my grandparents and the others who have helped teach me lessons far beyond the realm of academia. To my friends and labmates: I thank you for your day-to-day support, from the little things to the major ones. From bringing joy to mundane tasks to having a drink after impressive failures. You are all the best. I cannot believe the number of lifelong friends I have made by spending too much time together in the lab. To Mark, I thank you for being a simultaneous peer, friend, and mentor. I hope that you have benefited even a fraction as much as I have from our lab bromance.
To Area Four: I thank you for your caffeine. I have seen many baristas come and go during my tenure, but the coffee has stood the test of time.
And to all those not specifically mentioned here: the people who have touched my life from my early years to today are countless, and the journey would never have been the same save for all of these interactions. So a final thank you goes out to the unnamed, whose influence neither of us may even remember, but who have helped me to be where I am today. And with these words, I will continue seeking to leave a positive mark on the world as I go forth and SCIENCE!
Contents
1 Introduction
1.1 Introduction . . . .
1.2 An Overview of Phage Therapy . . . .
1.3 Engineered Phages as Therapeutics and Tools for Health .
1.4 New Phage Engineering Strategies . . . .
1.5 Bacteriophages and the Microbiome . . . .
1.6 Chapter Overviews . . . .
2 Phages from Without: Isolation and Characterization of Phag
from the Environment
2.1 Introduction . . . .
2.2 Isolation of Novel Bacteriophages . . . .
2.3 Characterization of Bacteriophages . . . .
2.4 Murine Gastrointestinal Colonization Model . . . .
2.5 D iscussion . . . .
2.6 Experimental Details . . . .
3 Phages from Within: Utilization of Prophages for Bacterial
Target-ing
3.1 Introduction . . . .
3.2 Discovery and Characterization of <}Kpn852 . . . .
3.3 Construction of Lytic Phage Derivatives . . . .
3.4 D iscussion . . . . 7 13 . . . . 13 . . . . 13 . . . . 16 . . . . 20 . . . . 22 . . . . 24 27 28 29 29 33 37 39 43 44 47 50 54 E~s
4 Phages born Anew: Using CRISPR Payloads to Create
Sequence-Specific Antimicrobials 59
4.1 Introduction ... ... 60
4.2 Transformation Assays for Validation ... 62
4.3 Cell-Based Delivery . . . . 64
4.4 Phage-Based Delivery . . . . 66
4.4.1 Toxin-Antitoxin Activation . . . . 69
4.5 Targeting Virulence Genes in Galleria mellonella Models of Infection 71 4.6 Population Sculpting . . . . 73
4.7 D iscussion . . . . 74
4.8 Experimental Details . . . . 78
5 Conclusion 87
List of Figures
2-1 Plaque assay on propagation hosts . . . . 31
2-2 Mutant derivative K6.2 shows probable capsule alteration . . . . 31
2-3 Double-agar spotting assay for determining host range of inhibitory activity . . . . 32
2-4 Visualization of selected bacteriophages by transmission electron mi-croscopy (TEM ) . . . . 34
2-5 Murine gastrointestinal colonization model . . . . 36
2-6 Phage therapy reduces K. pneumoniae levels in murine gut . . . . 36
2-7 Phage <DKPNIH1-1 is not maintained in the gut . . . . 37
3-1 Two disparate lifestyles for bacteriophages . . . . 44
3-2 Overview of yeast assembly for phage genome reconstruction . . . . . 46
3-3 K. pneumoniae KPNIH31 harbors a viable prophage . . . . 48
3-4 Polishing of the <DKpn852 genome . . . . 49
3-5 Phage <bKpn852 is a relative of E. coli phage N15 . . . . 50
3-6 TEM visualization of K. pneumoniae phage <DKpn852 and E. coli phage N 15 . . . . 51
3-7 Phage <bKpn852 genome rebooting is inefficient in E. coli . . . . 51
3-8 Overexpression of antirepressor antC in E. coli C-1 prevents lysoge-nization by phage N15 . . . . 52
3-9 Engineering an obligately lytic derivative of temperate phage N15 . . 53
4-1 RGN overview schematic . . . . 61
4-3 Characterization of escape mutants that tolerated transformation of a
cytotoxic RGN construct . . . . 64
4-4 Mobilizable RGNs can be conjugated into target bacteria for selective
removal of multidrug resistance . . . . 65
4-5 RGN constructs delivered via bacteriophage particles (DRGN) exhibit efficient and specific antimicrobial effects against strains harboring
plasmid or chromosomal target sequences . . . . 67
4-6 Characterization of 4RGN-mediated killing of antibiotic-resistant
bac-teria . . . . 68
4-7 Treatment of E. coli with 4RGNs induces DNA damage and an SOS
response in cells that possess a cognate target sequence . . . . 69
4-8 RGN-mediated targeting of toxin-antitoxin systems can lead to
cyto-toxicity . . . . 71
4-9 'IRGN particles elicit sequence-specific toxicity against
enterohemor-rhagic E. coli in vitro and in vivo . . . . 72
4-10 Minimum inhibitory concentrations (MICs) . . . . 73
4-11 Comparison of 4RGNeae to conventional antibiotic treatment of
EHEC-infected Galleria mellonella larvae . . . . 73
4-12 Programmable remodeling of a synthetic microbial consortium . . . . 75
4-13 Bacterial strains used in this study . . . . 79
10
List of Tables
2.1 Isolation of bacteriophages against K. pneumoniae . . . . 30
2.2 TEM morphological characterization . . . . 35
2.3 Bacterial strains for phage isolations . . . . 39
3.1 Infectivity of KPNIH strains by <DKpn852 . . . . 48
Chapter 1
Introduction
Portions of this chapter are adapted from reviews written or co-written by the
author [1-3].
1.1
Introduction
In an age when antibiotic-resistant bacterial infections are increasingly common and lead to more treatment failures, new solutions are sorely needed. One promising answer to these mounting concerns is a century-old solution forgotten by Western medicine that employs natural predators of bacteria: enter the bacteriophages.
1.2
An Overview of Phage Therapy
Like many antibiotics, bacteriophages (phages) are naturally occurring and have the capacity to kill bacteria. Unlike antibiotics, however, phages are viruses and
so can replicate and amplify within their target bacteria. These 'bacteria eaters'
are conceptually similar to human viruses, but can only infect bacterial cells. From a human perspective, phage therapy is a good example of the proverbial saying 'the enemy of my enemy is my friend'. Phages reproduce by infecting bacteria and turning them into phage manufacturing plants, which can prove lethal to the bacterial cell. Once the progeny have been assembled, the phage initiates enzyme-mediated lysis,
rupturing the host bacterium from the inside and releasing the next generation of infectious particles to repeat the cycle.
Phage therapy dates back to the early 20th century, shortly after the independent discovery of bacteriophages by bacteriologists Frederick Twort (1915) [4] and Felix d'Herelle (1917) [5]. It was found that sometimes a phenomenon occurred resulting in zones of clearing on bacteria growing in Petri dishes or a healthy, cloudy culture of bacteria to suddenly lyse and become clear. The lysed culture could be filtered and added to other cultures of the bacteria to cause a repeatable clearing effect. Phages able to lyse cultures of the intestinal pathogen Shigella were found alongside the bacteria in patients with dysentery, with an increase in phage concentration often corresponding to recovery, and so were thought to be part of the natural course of disease [6].
After some reported successes, research and commercial development of phage-based therapies expanded over the following decades, although there was still contro-versy surrounding the nature of the agent or agents causing the bactericidal effects. An early debate centered on whether phages were indeed of a viral nature or instead were autolytic enzymes produced by the bacteria themselves, and it was not until 1940 that electron micrographs were published visually depicting what was responsible [7]. In the beginning, scientists did not yet fully appreciate the diversity of phages and the specificity of each one to its target. Unlike broad-spectrum antibiotics, which kill bacteria fairly indiscriminately, phages are usually highly specific to a target bacterial species, and often limited to certain strains within a particular species. Early experiments and trials were likely impacted by these and other gaps in knowledge, and choosing the wrong phages or using suboptimal phage preparations to treat a bacterial infection may have led to inconsistencies in treatment outcomes. As a result, the initial excitement associated with phage therapy was short-lived. The appearance of antibiotics in the physician's toolbox around the mid-20th century coincided with a decline in phage therapy for various reasons, including the incredible efficacy of the new drugs, an insufficient understanding of phages and mixed reports of success, and even political motivations.
Bacteriophages for Human Health
Despite the complicated history leading to phage therapy falling out of favor in
the West [8], it has continued to some extent in other parts of the world,
primar-ily in Russia and Eastern Europe. The George Eliava Institute of Bacteriophages, Microbiology and Virology in Tbilisi, Georgia, is one of the most well-known of the clinics still specializing in this antibacterial therapy. As well as preparations designed for off-the-shelf use for particular disease indications, the institute has a large bac-teriophage collection that can be screened against bacterial pathogens from patient samples to create personalized treatments.
For phage therapy to become accepted in modern Western medicine, there are sev-eral challenges that must be addressed, the first of which may be to definitively prove safety and efficacy. Perhaps the first safety trials were performed by d'Herelle, who administered phage to himself as well as to family members, noting no adverse effects from the preparations [8]. As a result of the discontinuation of phage therapy in the first half of the 20th century, safety and efficacy testing did not evolve concomitantly with the more rigorous clinical testing standards, which now include blind or double-blind trials with appropriate controls and strict statistical analyses. Accordingly, the efficacy of phage therapy versus standards of care has never been convincingly demonstrated to Western standards, although renewed efforts are emerging [9, 10].
On this front, the large-scale Phagoburn trial has been funded by the European Union and sponsored by Pherecydes Pharma. Phagoburn, which focuses on phage treatment of infected burn wounds, was launched in 2013, with the first patients entering into the phase I/II clinical study in 2015. In order to test safety, efficacy and pharmacodynamics, cocktails of around a dozen different phages targeting either
Escherichia coli or Pseudomonas aeruginosa were designed to treat enrolled patients
identified as carrying these specific pathogens. Additionally, AmpliPhi Biosciences, working with the University of Adelaide and Flinders University, recently dosed their first patient with phages targeting Staphylococcus aureus in a phase I safety trial at the Queen Elizabeth Hospital in Adelaide, Australia. The design and implementation of these types of trials will undoubtedly prove critical to moving the technology into modern medicine.
Beyond safety and efficacy trials, potential phage therapies may need to overcome other hurdles, including the release of endotoxin or other toxic bacterial products when infected bacteria rupture. Other issues are the emergence of bacterial resistance to phages, bacterial strain coverage, pharmacokinetics and how to dose a self-replicating agent, as well as the patient immune responses, which may target the phage when administered into the body.
These challenges are already being addressed to some degree, with efforts demon-strating phages designed to kill bacteria with limited endotoxin release [11, 12] and the selection for phages with improved residence time in the body [13]. Bacterial strain coverage can be improved using phage cocktails, which have multiple phages able to target different receptors on a bacterium, which could also decrease the likeli-hood of resistance. Additionally, phages can be naturally evolved to subvert defensive tactics employed by bacteria that have become resistant to infection, meaning that components of a cocktail that are no longer effective could be re-derived instead of shelved alongside defunct antibiotics.
The field of phage therapy is poised very differently now than it was a century ago. The understanding of phage biology that we have today promises to help us to select bacteriophage cocktail components appropriately, produce and purify them according to suitable standards, and design and execute rigorous clinical trials.
1.3
Engineered Phages as Therapeutics and Tools
for Health
As an alternative approach to using only naturally derived phages, some engineer-ing efforts in synthetic biology have been made toward addengineer-ing functions or improvengineer-ing existing phages. For example, Lu and Collins [14] incorporated the gene encoding DspB, an enzyme that degrades a polysaccharide adhesin implicated in biofilm for-mation, into an engineered T7 phage. This modified phage effectively cleared F. coli biofilms through cycles of infection, phage-mediated lysis, and release of the
Bacteriophages for Human Health
nant dispersin enzyme to enzymatically degrade the biofilm material itself and expose protected cells. Additionally, the phage was modified to carry a gene from phage T3 in order to expand its host range and permit infection of the biofilm-forming strain used in the study. However, despite the promise of phage therapeutics, bacteria can dis-play resistance toward phages through innate means, such as restriction-modification systems [15, 16], as well as adaptive means, typified by clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (Cas) systems [17]. More-over, mechanisms may emerge in a bacterial population during the course of selective pressure by phages, including phenotypic [18] and genotypic [19] causes of decreased phage adsorption, among others [20]. These hurdles may be tackled through the use of phage cocktails [21], high-throughput phage evolution, or perhaps, given predictable evolutionary pathways, through the rational engineering of phages [22].
In contrast to taking advantage of a phage's natural ability to lyse a target cell, some studies have focused on using virus particles only for their capacity to deliver nucleic acids to target cells. Such an approach was taken by Westwater et al. [23], in which the group utilized the non-lytic, filamentous phage M13 to deliver special-ized phagemid DNA in place of the phage genome to target cells. The engineered phagemids (plasmids carrying signals to enable packaging into phage particles) were designed to encode the addiction toxins Gef and ChpBK to elicit destruction of tar-get cells. Hagens and Blisi [11] also applied this toxic payload concept using M13 to deliver genes encoding the restriction enzyme BglII or the A S holin to kill target E.
coli by the introduction of double-stranded breaks in the chromosome or the creation
of cytoplasmic membrane lesions, respectively. Subsequently, delivery of BglII was used to rescue mice infected with P. aeruginosa by adapting the system with an en-gineered derivative of the P. aeruginosa filamentous phage Pf3 [12]. These methods also resulted in a marked decrease in release of endotoxin, one of the major concerns with lytic phage therapy [24], as compared to killing via lysis by a lytic phage [11, 12]. More recently, M13-derived particles were used to express a lethal mutant of catabolite activator protein in E. coli 0157:H7, a food-borne pathogen that causes outbreaks of hemorrhagic colitis [25]. Biotechnology companies have also begun to
Chapter 1
make use of recombinant phage methods, such as virus-like particles that deliver genes encoding small, acid-soluble proteins to cause toxicity to target cells through
non-specific binding to DNA [26].
Rather than encoding killing functions directly within phage particles, Edgar et al. [27] used phage A as a chassis to generate antibiotic-resensitizing particles through the delivery of dominant wild-type copies of rpsL and gyrA. The transduction of these genes into target cells resistant to streptomycin and fluoroquinolones, conferred by mutations in rpsL and gyrA, respectively, resulted in the production of wild-type enzymes susceptible to the formerly ineffective drugs. In another demonstration, Lu and Collins [28] engineered M13 to carry genes encoding transcription factors that modify the native regulation of bacterial gene networks. Constructs encoding the LexA3 repressor or SoxR regulator were used to disable the SOS response and DNA repair or to modulate the response to oxidative stress in target cells, respectively, thus potentiating the toxic effects of antibiotic treatment and even resensitizing a resistant bacterial strain. A dual-function phage was also created and validated by using M13 harboring the global regulator csrA, to inhibit biofilm formation and the associated increase in antibiotic resistance, and the porin ompF, to improve drug
penetration. Examples such as these demonstrate the capacity for bacteriophages
to be engineered as gene delivery devices in order to perturb genetic networks in bacteria for both research and therapeutic applications. With this approach, one can alter a gene network at a particular node and observe the qualitative and quantitative effects in order to better characterize native regulatory systems. As models of the interactions in complex regulatory webs of pathogens grow increasingly robust, the ability to know which strands to tug to elicit desired effects may enable rationally designed novel therapeutics based on predictable behaviors.
The development and improvement of next-generation sequencing technology has enabled genomic and metagenomic analyses of phage populations [29, 30]. For ex-ample, sequencing of gut viral metagenomes has implicated phages as reservoirs of antibiotic-resistance genes [31] and their role in influencing the intestinal microbiota has been of recent interest [32]. Since bacteriophages must encode mechanisms to
IM1,11.1 I INIMPITI ""M
Bacteriophages for Human Health
control their host cells in order to infect, divert cellular resources to propagate, build progeny phages, and, in many cases, lyse their hosts to release new particles, phage genomes constitute a vast library of parts that can be used to manipulate bacteria for study or treatment. On the basis of this concept, Liu and colleagues [33] devel-oped a method for mining such tools to generate novel therapeutics against S. aureus.
Predicted phage open reading frames were cloned with inducible expression into the target strain and screened for growth-inhibitory properties. Identified phage proteins were used to pull bacterial targets out of cell lysates and a library of small molecules was screened to identify inhibitors of the protein-protein interaction, with the hy-pothesis that these molecules might demonstrate similar modulatory action on the host target. In this way, the authors identified novel compounds capable of inhibiting the initiation of bacterial DNA replication in analogy with the phage proteins. Since currently available drugs that target replication only act on topoisomerases, this work demonstrates that mining phage proteins long evolved to inhibit bacterial processes has the potential to expand the antibiotic repertoire by leading us to discover drugs against previously unused targets [33, 34].
In addition to random-discovery screens, phage lysins have been specifically in-vestigated in recent years as potential antimicrobials. These enzymes are employed
by bacteriophages to degrade the bacterial cell wall and permit the release of progeny
phages [35]. In another functional metagenomic study, phage DNA was isolated from a mixture of feces from nine animal species, cloned into a shotgun library for
in-ducible expression in E. coli, and used in primary and secondary screens to detect
lysins from the phage DNA pool [36]. As a discovery tool, a specific lysin from a phage of Bacillus anthracis was used to develop a novel antimicrobial by identifying an enzyme involved in the production of the lysin target and designing a cognate chemical inhibitor [37]. Though lysins are considered useful antimicrobials for Gram-positive pathogens, Gram-negative bacteria possess an outer membrane that prevents access of these extracellular enzymes to the cell wall [38]. To overcome this barrier, a chimeric protein composed of the translocation domain of the Yersinia pestis bac-teriocin, pesticin, and the enzymatic domain of lysozyme from the E. coli phage T4
Chapter 1
was engineered. The hybrid bacteriocin was shown to be active against E. coli and
Y. pestis strains, including those expressing the cognate immunity protein conferring
resistance to unmodified pesticin [39, 40].
Bacteriophages have also been used to implement real-world applications of biosens-ing [41-45]. In areas from healthcare and hospital surfaces to food preparation and other industrial processes, methods for the rapid detection of pathogenic organisms are paramount in preventing disease and avoiding the public relations and finan-cial burdens of recalling contaminated products. The amount of time necessary for many conventional detection methods is long due to the requirement for bacterial enrichment before detection of the few bacteria present in complex samples in order to achieve sufficient assay sensitivity and specificity [46]. Engineered
bacteriophage-based detectors have the advantage of rapid read-outs, high sensitivity and specificity, and detection of live cells [47]. A common design strategy is the creation of reporter-based constructs packaged within phage or phage-like particles that infect target cells and ultimately result in the production of fluorescent, colorimetric, or luminescent signals. Furthermore, sensor designs can include genetically engineered phage that express a product causing ice nucleation [48] or that incorporate tags for linking to detectable elements such as quantum dots [49]. Though most of these examples of specifically modified phages have been enabled by advancements in engineering and synthetic biology to achieve real-world applicability, the concept of using natural phage as sensing tools is not a new one. Phage typing and other techniques have made use of the narrow host range of phage to identify species or strains of bacteria based on a target bacteria's ability to bind, propagate, or be lysed by non-engineered viruses [47].
1.4
New Phage Engineering Strategies
Historically, modifications to bacteriophage relied on random mutagenesis or ho-mologous recombination, both of which are inefficient and necessitate intensive screen-ing to identify mutants of interest. The relatively large size of most bacteriophage
20 Chapter 1 Citorik
Bacteriophages for Human Health
genomes and their inherent toxicity to bacterial hosts has confounded the use of
con-ventional molecular biology techniques for engineering. However, recent synthetic
biology tools have revitalized the ability to make rational additions or modifica-tions to phage genomes. Among such improvements, the phage defense function encoded by CRISPR-Cas systems, previously adapted for genome editing [50-55] and reviewed in [56], has been described for improving recombineering in bacteriophages
by counter-selecting unmodified phages with wild-type target sequences [57]. In vitro
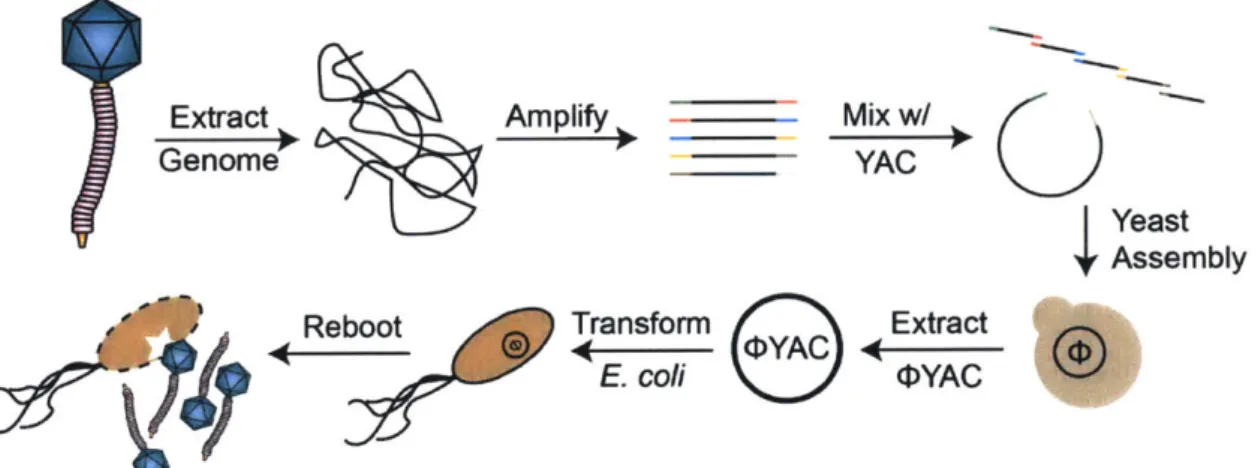
assembly of large constructs has also been made possible with techniques such as Gibson assembly [58], which enzymatically stitches together DNA fragments with overlapping homology, thus allowing for insertions of heterologous DNA and site-directed mutagenesis using PCR. Moreover, transformation of overlapping fragments into yeast in conjunction with a compatible yeast artificial chromosome leads to in
vivo recombination-based assembly of large constructs [59, 60]. Genomes can be
as-sembled with modifications or be modified post-assembly in yeast, where they are non-toxic to the host, and then purified and rebooted in bacteria to produce engi-neered phage progeny. Current DNA synthesis technology, in concert with in vivo and in vitro recombination, also permits de novo chemical synthesis of bacteriophage genomes. Smith et al. [61 utilized this approach to synthesize, clone, and produce infectious particles of the 5386 bp phage <DX174, and a similar scaled-up approach has created the first bacterial cell with a synthetic genome of 1.1 Mb [62]. These
in vitro approaches to creating engineered phage genomes have the potential to be
combined with newer methods in efficiently rebooting phages from genomic DNA, including efficient uptake of full genomes by cell wall deficient L-form bacteria [63] and completely cell-free synthesis of viable phages [64, 65]. By rendering bacterio-phages genetically accessible, synthetic biology can permit more precise studies of their underlying biology and inspire creation of novel therapeutic agents.
Despite advances in rational engineering of bacteriophages, tampering with sys-tems finely tuned by evolution can lead to fitness defects [66]. For example, roughly
30% of the genome of the bacteriophage T7 was refactored, a process whereby genes
and their respective regulatory elements were separated into distinct modules to
mit systematic analysis and control [67]. The refactored genome produced viable bacteriophage, albeit with significantly reduced fitness. Multiple rounds of in vitro evolution restored wild-type viability at the expense of some of the design elements, implying that rational design can be coupled with evolution to ensure the creation of robust biological systems [68]. Similarly, the evolutionary stability of a T7 phage engi-neered to infect encapsulated E. coli by producing a capsule-degrading endosialidase as an exoenzyme was investigated in vitro [69]. While the engineered phage permitted replication in the encapsulated strain, the benefit conferred by endosialidase produc-tion was shared by wild-type, non-producing 'cheater' phages, which could quickly outcompete the engineered viruses due to their higher fitness. Although these studies point to the fragility of current synthetic biology efforts, bacteriophage-based systems can serve as an excellent platform to understand the constraints placed on synthetic genetic circuits by evolution and inform future designs.
1.5
Bacteriophages and the Microbiome
Subtractive therapies may employ chemicals, peptides, or even replicating entities to remove bacteria from the gut with varying degrees of specificity. In medicine, this is currently accomplished through the use of antibiotics, which tend to be broad-spectrum in nature, exhibiting activity towards many different bacteria. As a result, treatment of a patient aimed to remove an infectious pathogen also leads to the unintended reduction of other members of the microbiota. This community shifting may cause the patient to become susceptible to other temporary or chronic conditions to which they are normally protected, including antibiotic-associated infections with C. difficile. The development of targeted antimicrobials, such as bacteriocins and bacteriophages, could yield more effective subtractive therapies.
Bacteriophage therapy is a highly specific method of killing bacteria through the use of natural or engineered viral parasites. The application of phages as antimi-crobials has seen a renewed interest with the growing threat of antibiotic-resistant pathogens [9, 70]. Though early phage therapies targeted intestinal pathogens [71],
Bacteriophages for Human Health
clearance issues have recently been reported wherein bacterial and phage populations
stably coexist in the murine gut [72, 73]. Knowledge attained from research into
the ecology of phages in the gut may be pivotal in determining factors that lead to successful therapies in this complex ecosystem.
A newer focus of the field is to examine the natural role of these viruses in shaping
host-associated bacterial populations [32]. Metagenomic research of the human mi-crobiome has described the fecal virome of both healthy and diseased donors. These studies include measuring phage diversity, variability, and stability [74], and analyzing changes associated with diet [75] or inflammatory bowel disease (IBD) [76] in humans, or antibiotic treatment in mice [31]. For example, a study of the viromes of monozy-gotic twins and their mothers revealed high interpersonal variation of virome compo-sition, but low intrapersonal diversity that was dominated by temperate phages, or those that can exist in a silenced life cycle within bacteria [74]. Diet has been iden-tified as one factor that affects the viral community, as putting human subjects on a controlled diet altered community structure and resulted in a level of convergence for individuals on the same diet [75]. Interestingly, whereas IBD correlates with a reduced level of bacterial diversity, multiple cohorts have revealed a concomitant in-crease in bacteriophage richness, specifically those belonging to Caudovirales [76]. To explore phage-bacterial host dynamics, gnotobiotic mice were seeded with a defined, 15-member human commensal community and challenged orally with virus-like par-ticles (VLPs) purified from healthy human donors. In this study, the authors made several interesting observations, including an increase in specific phages correlating with a transient decrease in specific bacteria, different phages and bacteria showing non-simultaneous temporal population dynamics, and evidence of phage resistance likely due to ecological, rather than genetic, factors [77]. These types of compari-son and challenge studies should continue to yield important information useful for designing phage-based strategies for targeting pathogens or altering microbial com-munities in the gut, particularly in regards to determining the factors that lead to transient versus stable community rearrangements.
Engineered phages, or those modified to carry additional or alternative functions
Chapter 1
to those naturally occurring, may prove useful for therapeutics as design can be in-formed by new knowledge. Recently, it was found that certain phages possess Ig-like protein domains on their capsids that enhance association with mucus [78]. This or alternative localization domains may be useful for improving residence time in the gut or helping concentrate phages to relevant biogeographies. Phage engineering efforts have included altering host adsorption factors to change host range [79] or encoding a dispersal enzyme to help break up bacteria in protective biofilms [14]. Phages have also been used as DNA delivery agents to reverse antibiotic resistance [27, 28] or to exert broad-spectrum [12, 23, 80] or sequence-specific [81, 82] antimicrobial activity. Additionally, genome engineering and tools such as CRISPR-Cas [57] and
assem-bly methods including Gibson [58] and yeast [79] assemassem-bly should prove useful for
the development of new phages with augmented capabilities in modulating microbial communities. We believe that the use of natural or engineered phages as therapeutics for microbiota-related diseases has been understudied relative to the complementary modality of introducing natural or engineered microbes, and thus represents a fasci-nating area of investigation.
1.6
Chapter Overviews
This thesis is presented as a series of chapters focusing on different aspects of de-veloping bacteriophage therapeutics for targeting bacteria. In Chapter 2, the premise is to isolate novel phages by beginning with a panel of bacteria one wishes to tar-get. The focus of this chapter is on carbapenem-resistant K. pneumoniae and phages are collected from unprocessed sewage. These new isolates are characterized for host range on the panel of bacteria and morphology is assessed through visualization by transmission electron microscopy (TEM). One bacteriophage is selected for in vivo testing in a murine gastrointestinal colonization model and shown to elicit a significant decrease in bacterial load, though clearance was not achieved.
Whereas Chapter 2 focuses on the use of wild-type phage isolates, Chapter 3 introduces the concept of modifying phages for use in therapeutics, specifically
utiliz-24 Chapter 1 Citorik
Bacteriophages for Human Health
ing a phage engineering platform to create obligately lytic derivatives from wild-type temperate phages. Prophage prediction is used to mine a panel of K. pneumoniae strains for potential temperate phages residing within the bacterial genomes and phage 4Kpn852 is successfully isolated (formerly referred to as pKPN-852). This temperate phage, induced from host strain KPNIH31, is visualized by TEM, assayed for host range, and genomic DNA is extracted in order to polish the ends of the previously submitted sequence. Though initial efforts encountered roadblocks during the engineering pipeline, a highly related phage of E. coli, phage N15, was targeted for the same modifications planned for TKpn852. A null mutation introduced into the major repressor protein of N15 resulted in the generation of an obligately lytic derivative as evidenced by the clear plaque phenotype. Additionally, the platform was used to interrupt the repressor gene with a fluorescent reporter gene in a simultaneous knock-out/knock-in approach.
In Chapter 4, the creation of modified bacteriophages is shifted from modifica-tions of the phage genome to replacement with an alternative, engineered payload. In brief, phage M13 is loaded with a DNA construct encoding a CRISPR-Cas system programmed to recognize and cleave undesirable genetic signatures in recipient bacte-ria. These phages encoding RNA-guided nucleases (4RGNs) are able to elicit specific killing in bacterial strains harboring antibiotic resistance and virulence determinants, while having no ill effects toward similar or even otherwise isogenic strains lacking the determinants. The IRGNs are applied to demonstrate specific remodeling of a three-strain microbial consortium in vitro and shown to significantly improve survival of
Galleria mellonella larvae when challenged with enterohemorrhagic E. coli 0157:H7.
These experiments show the potential of 4RGNs for development as anti-infectives and for manipulating complex microbial communities.
By exploring multiple pathways for the development of phage-based therapeutics,
this work seeks to improve the opportunities for its successful translation.
Citorik Chapter 1
25
26 Chapter 1 Citorik
Chapter 2
Phages from Without: Isolation
and Characterization of Phages
from the Environment
Abstract
Bacteriophages represent an alternative class of antimicrobials that may have spe-cific utility against pathogens for which antibiotics have become ineffective. In order to facilitate phage-based approaches to combat multidrug-resistant K. pneumoniae, bacteriophages were isolated from sewage against a small panel of highly resistant bacterial strains. Phage isolates were characterized for morphological traits and the host range of inhibitory effects, indicating a diversity of infectivity towards the dif-ferent strains. A selected phage against the carbapenem-resistant strain KPNIH1 was assayed in a murine gastrointestinal colonization model and demonstrated a sig-nificant reduction in K. pneumoniae load, though total bacterial clearance was not observed.
2.1
Introduction
The Gram-negative bacterium Klebsiella pneumoniae is a silent colonizer of the mammalian gut that, under some conditions, has the potential to escape to other body sites and cause infectious pathologies [83]. The emergence of extensively drug-resistant strains, including at least one clinical case in the U.S. in which the isolate was resistant to all available antibiotic treatments [84], has placed this microbe as an urgent public health concern [85]. Particularly for cases where the current an-timicrobial arsenal is dry, research and development of bacteriophage therapies is an important area of exploration for alternative or next-generation therapeutics.
The genomic bases of antibiotic resistance and virulence determinants that allow
K. pneumoniae to be a successful pathogen have been studied in detail [85]. One
especially important factor is the polysaccharide capsule that the organism produces to protect itself from immune and other challenges, which has been intimately tied with virulence [86]. Isolates of K. pneumoniae are notorious for variability in capsule composition and synthesis [87, 88], which poses an issue for developing natural immu-nity, vaccination, and even phage therapies, which are often highly specific to capsule type. Efforts to isolate phages against this organism often identify new capsule types to which these phages are specific [89-91]. In light of this, phage collections capable of targeting as many strains as possible will prove crucial toward the development of phage therapies against K. pneumoniae.
In 2011, an outbreak of carbapenem-resistant K. pneumoniae occurred when an index patient at the National Institutes of Health (NIH) Clinical Center led to the colonization of 18 patients and 6 deaths from bloodstream infections [92]. A collec-tion of these clinical isolates was subjected to whole genome sequencing [93], and a panel of these KPNIH strains was acquired for the development of bacteriophage-based therapeutics. Here, a collection of novel bacteriophages was isolated against these outbreak strains in order to further efforts toward translational phage therapy. While this chapter focuses on isolating wild-type phages, the subsequent chapter will introduce engineering efforts for K. pneumoniae phage therapeutics. Not covered
Bacteriophages for Human Health
in this work is the potential of capsule-degrading enzymes that can be mined from
phages and used as standalone treatments [941, though this represents a potential
future direction for developing agents based on the newly isolated phages.
2.2
Isolation of Novel Bacteriophages
Since K. pneumoniae is often found residing within the gastrointestinal tract, sewage was chosen as a potential source for isolating bacteriophages against the or-ganism. In brief, raw sewage from the City of Cambridge was collected and filtered to remove any cells or larger debris. To acquire phages against a particular bacterial strain, filtered source material was first enriched in liquid culture with the target strain to amplify any infectious phages present, then individual phages were detected
by spotting samples onto lawns of the enrichment strain and assessing for formation
of visible plaques (Fig. 2.1). Phage isolates were serially passaged in order to en-sure populations contained single phages. This process was repeated as necessary to attempt to improve strain coverage (Table 2.1).
After serial purification, bacteriophage isolates were spotted onto their respective indicator host strains in order to observe plaque morphology and confirm homogeneity prior to further characterization (Fig. 2-1). Plaque morphology can be an indicator of particular characteristics of a phage, where traits such as larger plaques may indicate a larger burst size (number of phages released from a single cell) and halos of lighter growth around plaques may be evidence of polysaccharide-degrading enzymes capable of altering host outer membrane structures. All phage preparations formed plaques on their respective propagation strains and no contaminants were observed.
2.3
Characterization of Bacteriophages
The majority of bacteriophages tend to be very specialized for the particular hosts with which they have evolved. This is believed to be a major advantage of phage therapy, in that there should be minimal off-target effects when attempting
Chapter 2
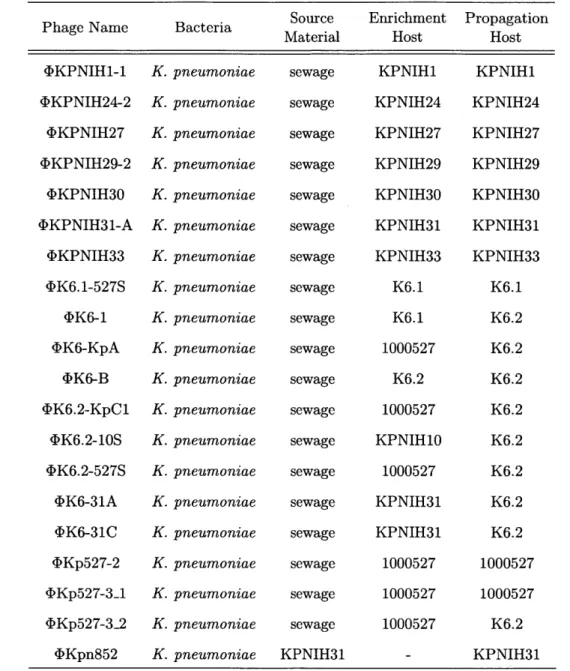
Phage Name Bacteria Source Enrichment Propagation
Material Host Host
<IKPNIH1-1 <kKPNIH24-2 <}KPNIH27 <bKPNIH29-2 <bKPNIH30 <DKPNIH31-A <bKPNIH33 <DK6.1-527S <DK6-1 <>K6-KpA <DK6-B <DK6.2-KpC1 <DK6.2-10S <bK6.2-527S <bK6-31A <bK6-31C <bKp527-2 4<Kp527-3_1 <IKp527-3-2 <bKpn852 K. K. K. K. K. K. K. K. K. K. K. K. K. K. K. K. K. K. K. K. pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae pneumoniae
Table 2.1: Isolation of bacteriophages against K. pneumoniae: A panel of bacterial strains was used as bait to collect new bacteriophages from source material. Filtered sewage was enriched using the indicated enrichment hosts and phages were isolated and purified by serial plaque passaging. In some cases, a strain different from the enrichment strain was found to serve as a better host for the isolate phage and was thus used for continuing the passaging and for propagation, where relevant.
to treat a specific infection. On the other hand, however, phages are restricted to infecting only specific isolates of any given target, meaning a single phage is unable to provide comprehensive coverage for all strains of a particular bacterial species. It
Chapter 2 sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage sewage KPNIH31 30 Citorik KPNIH1 KPNIH24 KPNIH27 KPNIH29 KPNIH30 KPNIH31 KPNIH33 K6.1 K6.1 1000527 K6.2 1000527 KPNIH10 1000527 KPNIH31 KPNIH31 1000527 1000527 1000527 KPNIH1 KPNIH24 KPNIH27 KPNIH29 KPNIH30 KPNIH31 KPNIH33 K6.1 K6.2 K6.2 K6.2 K6.2 K6.2 K6.2 K6.2 K6.2 1000527 1000527 K6.2 KPNIH31
Bacteriophages for Human Health 100 10-1 102 10- 10-4 10- 10- 10-7 100 10-1 102 4 10-OKPNIH1-1 4K6-1 POW _ Q)KPNIH24-29 0KPNIH27 4'KPNIH29-2 DKPNIH30 OKPNIH31-A
@ @ *
OKPNIH33 ISIS OKp527-3_1I
1:
0Kp527-2* 9*
4 4K6.1-527S.
10-7 OK6-KpA Ww
WI
K6-BI
K6.2-KpC1I
K6.2-10S 0K6.2-527S 4VK6-31A 4)K6-31C cOKp527-3_2 4,Kpn852*Figure 2-1: Plaque assay on propagation hosts: Bacteriophages isolated from sewage on strains of K. pneumoniae were serially passaged at least 6 times to ensure homogeneity of the population. Lysates generated from purified phage isolates were spotted onto double-agar plates of the strains used for propagation in order to check for contaminants and observe plaque morphology.
is, therefore, imperative to know the host range of a bacteriophage to understand its effectiveness toward the various strains that may be relevant for a given pathogen.
Kp K6.1 Kp K6.2
Figure 2-2: Mutant derivative K6.2 shows probable capsule alteration: An
isolation streak of K. pneumoniae resulted in the appearance of a colony morphotype with a reduction of mucoid appearance versus the parental colonies. The opaque (K6.1) and translucent (K6.2) colonies were restreaked for isolation and used to com-pare the infectivity of various phage isolates.Chapter 2 31
Bacteriophages for Human Health K. pneumoniae Strains 4KPNIH1-1 ciKPNIH24-2 PKPNIH27 4KPNIH29-2 4'KPNIH30 DKPNIH31-A* 4KPNIH33 (DK6.1-527S OK6-1 DK6-KpA 0K6-B 0K6.2-KpC1 0K6.2-10S 4K6.2-527S 4K6-31A 4K6-31 C cIKp527-3_1 4Kp527-3_2 4IKp527-2 4tKpn852 o) m r- M~ o C' r- ( LO r. UN C) r' o a~o~ n O~0 O 0C ) O r-
-El
".EE*
EL.
a. a.5-a-a.aEpEaE8C
- K1, -"
UM EMEE.
',t'**UEES*EE
MM
.UEB
r
.__ME.
Figure 2-3: Double-agar spotting assay for determining host range of
in-hibitory activity: Newly isolated bacteriophages were assayed to determine their
range of infectivity beyond the initial enrichment and propagation hosts. Double-agar plates were prepared with all bacterial hosts and phage lysates containing around 10 or 10" PFU/mL were spotted on all strains. Spots shown here are representative of the qualitative results of three independent assays. *Phage <DKPNIH31-A is thought to only have infected strain KPNIH31 with any efficiency, while the inhibition ob-served from this sample on other bacterial strains is thought to have been due to contamination of KPNIH31-propagated phage with the temperate phage <DKpn852, which lysogenizes KPNIH31.
To analyze the host range of the bacteriophages isolated above, a spotting assay was performed for each phage across the panel of bacterial isolates. Results indicated
U) '5 0 U) U) 0) 'U 0. 0 0 U EU Citorik Chapter 2 32
Bacteriophages for Human Health
that the isolates possessed uniqueness in terms of the breadth of strains on which
they were capable of forming zones of inhibition or plaques (Fig. 2-3).
Of particular interest, the bacterial strain K. pneumoniae K6.2 was isolated from
K6 as a colony appearing less opaque than the parental (K6.1) strain (Fig. 2-2).
These strains showed a marked difference in both host range and apparent efficiency
of bacterial killing as evidence by phage host range assays (Fig. 2-3). Strain K6.2
is anticipated to have a mutation resulting in a reduction or change in capsule
pro-duction, which seems to render it much more susceptible to infection by all phages
that infected both strains, with the exception of the temperate phage <bKpn852
(iso-lated and characterized in Chapter 3), which only efficiently infected K6.1 and did
not form detectable plaques on K6.2. Future genomic studies, including the
sequenc-ing of both bacterial isolates, are expected to reveal the nature of this relationship.
Though capsules can render bacteria less susceptible to insults including infection by
some phages, other phages are known to require specific capsule structures in order
to facilitate binding and infection.
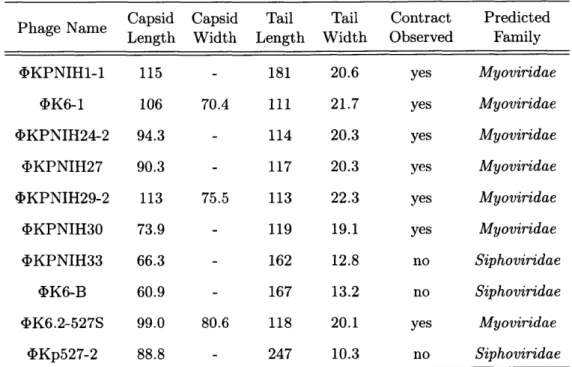
The structure and morphology of bacteriophages can be visualized using
trans-mission electron microscopy (TEM), during which an electron beam is used to probe
a sample negatively stained with an electron dense solution of a metal salt, such as
uranyl acetate [95]. A selection of isolated phages was subsequently concentrated
and visualized by TEM to determine morphological characteristics of phage
parti-cles (Fig. 2-4). Family level classification of the isolates was called using previously
determined characteristics, including tail length, if present, and the observation of
contracted particles [96, 97]. Measurements were taken and averaged as indicators of
approximate capsid and tail dimensions (Table 2.2).
2.4
Murine Gastrointestinal Colonization Model
In collaboration with the Segre Lab at the National Human Genome Research
Institute (NHGRI), selected bacteriophages were subjected to initial testing in an
established mouse model of gastrointestinal colonization by K. pneumoniae MKP103,
Chapter 2
Normal
Iz
a-0 CO) CO C6Triggered
29
Figure 2-4: Visualization of selected bacteriophages by transmission elec-tron microscopy (TEM): Phage samples were loaded onto FCF200-Ni grids and stained with 2% uranyl acetate. Grids were visualized on a Tecnai Spirit Transmission Electron Microscope (FEI) operating at 80 kV.
34 Chapter 2 Citorik
Normal
Triggered
04 (%4 CL) CO) I C\1 CJp
Chapter 2 Citorik 34Bacteriophages for Human Health
Capsid Capsid Tail Tail Contract Predicted Phage Name Length Width Length Width Observed Family
,DKPNIH1-1 115 - 181 20.6 yes Myoviridae
4)K6-1 106 70.4 111 21.7 yes Myoviridae
4KPNIH24-2 94.3 - 114 20.3 yes Myoviridae
bKPNIH27 90.3 - 117 20.3 yes Myoviridae
4KPNIH29-2 113 75.5 113 22.3 yes Myoviridae
4KPNIH30 73.9 - 119 19.1 yes Myoviridae
DKPNIH33 66.3 - 162 12.8 no Siphoviridae
4K6-B 60.9 - 167 13.2 no Siphoviridae
<DK6.2-527S 99.0 80.6 118 20.1 yes Myoviridae
4Kp527-2 88.8 - 247 10.3 no Siphoviridae
Table 2.2: TEM morphological characterization: Ten bacteriophage isolates were subjected to visualization via transmission electron microscopy (TEM) to deter-mine morphological characteristics. Measurements were taken during image acquisi-tion using AMT Capture Engine (AMT, Woburn, MA). Values are given in nm and represent the means of at least ten measurements.
a derivative of strain KPNIH1 with blaKPC-3 deleted from plasmid pKpQIL [98]. In this model, mice are first subjected to antibiotic treatment to disrupt the normal microbiota and improve colonization, then the bacteria are introduced via gavage and remain detectable for at least 7 days.
Since 4KPNIH1-1 was previously isolated and shown to efficiently infect the parental strain K. pneumoniae KPNIH1, this phage was selected for testing efficacy
in vivo. To assay for reduction of colonization by Kp, bacteriophages were delivered
by gavage 2 h prior to bacterial administration and again two days later, and bacterial
and phage titers were monitored by performing viable counts on fecal samples taken 1, 2, 4, and 7 days post-infection (Fig. 2-5).
When administered by gavage, 4KPNIH1-1 treatment was found to significantly decrease the levels of K. pneumoniae MKP103 detectable by viable cell counting at
2 (p<0.002), 4 (p<0.0
2), and 7 (p<0.01) days post-infection (Fig. 2-6).
Though a significant decrease was observed in bacterial counts with phage
admin-Chapter 2
35
I
I Antibiotics Cocktail&I
it
-i4,
A1
Figure
2-5:
Murine gastrointestinal colonization model: A model for
coloniza-tion of C57BL/6J mice by K. pneumoniae has been previously established. Mice are given an antibiotics cocktail for 7 days prior to oral gavage of bacteria to facilitate colonization of the gut. For phage therapy experiments, the bacteriophage prepara-tion ("<D") is gavaged 2 h prior to bacterial administration ("Kp"). Fecal samples are collected at 1, 2, 4, andviral titer.
Day I
0 0*
7 days post-infection and used to assay bacterial load and
Day 2 S S 00 Day 4 *
o
0 00e .0 Day 7 0 00 0 0 0e 0 Control * OKPNIHI-1Figure 2-6: Phage therapy reduces K. pneumoniae levels in murine gut: Bacteriophage <kKPNIH1-1 was selected to test against K. pneumoniae MKP103 in a murine gut colonization model. Phages or buffer were gavaged 2 h prior to bacterial gavage, and again at two days post-infection. Fecal samples were collected on days 1, 2, 4, and 7 and plated under antibiotic selection to quantify the load of viable K.
pneumoniae. Data are presented as mean values for 6 mice per treatment condition.
Statistical significance was determined using Welch's t-test (* indicates p<0.05 and
** indicates p<0.01).
istration, complete decolonization was not observed. To assess whether 4DKPNIH1-1 was able to stably exist in the gut in the context of colonization, fecal samples as used above were assayed for viable phage. Despite including a phage boost by additional
1010. -o 109 -1 07 -(0 1 06 . 105. S104- 103-U__ I Chapter 2 Citorik 36
Bacteriophages for Human Health
gavage at 2 days post-infection, it was observed that levels were mostly undetectable
by Day 7 (Fig. 2-7). Future efforts should be aimed at determining the cause of this
low residence time in order to improve treatment and decolonization. 109 -0 108 IL. 107-10 c 105 104 0 101 eg sos 10 P' s9: * Q lime Post-Inoculation
Figure 2-7: Phage <bKPNIH1-1 is not maintained in the gut: Bacteriophage levels for the murine decolonization model were determined by plating supernatants from homogenized fecal samples onto double-agar lawns of K. pneumoniae and enu-merating plaque forming units (PFU). Data are presented as mean values for 6 mice. When phages were undetectable in this assay, values were set to the lower limit of
detection (103 PFU/g).
2.5
Discussion
Bacteriophages are a resource that could prove invaluable as therapeutics against bacteria for which treatment options are rapidly dwindling. Since they are dependent on their bacterial host(s) for providing the means for propagation, they are highly evolved to efficiently deliver DNA into host cells. Unlike antibiotics, which require extensive screening of microbes and compound libraries to uncover even candidate molecules, bacteriophages can readily be isolated from an appropriate source material where they would be expected to coexist with their hosts. As such, there is no shortage of phages, since they are continually evolving to successfully infect and propagate on continually evolving hosts.
Here, a collection of bacteriophages against carbapenem-resistant K. pneumoniae was isolated and shown to elicit inhibitory effects on various combinations of the
Chapter 2 37
bacterial panel. After characterization of these new isolates, one phage was selected for initial experimental treatments aimed at reducing K. pneumoniae colonization of the gut in a murine model. Though a significant reduction was observed, the results seem to imply that further optimization of the phage would be useful.
A potential major limitation of translating phage therapies from the lab into the
clinic is the differences that can be observed in infectivity of the phage toward its bacterial host in the lab and in an animal. For example, bacteria in the gut will be in different growth conditions resulting in potentially altered receptor expression or a growth state not permissive to phage infection. One study showed that E. coli phages T4 and T7 differed in their ability to infect and propagate in vivo, despite both being extremely efficient bacteria killers in vitro [72]. Additionally, studies have shown the potential for stable coexistence of phage and their bacterial hosts, which contrasts with the desired mutual exclusivity for using a phage therapeutic [73]. It has been suggested that some bacteria may be protected from potential phage predators by localization within mucus [99]. The ability to engineer phages, which is the focus of the next two chapters, may permit the optimization of wild-type phage isolates, through methods such as appending mucus localization domains as has been found in the proteins of some phage capsids [78]. Optimization for gut residence time may also be possible through serial evolution of potential phage candidates, as has been performed previously to improve phage residence time in the blood through repeated passaging [13]. Resistance of bacteria to phages will not be discussed in detail here, but phage evolution and cocktail approaches are commonly accepted approaches to decrease the rate of occurrence. The ability to isolate, engineer, and evolve phages is a great benefit of their biological nature as compared to traditional chemotherapeutic antimicrobial approaches.
38 Chapter 2
Citorik