COPPER BINDING ABILITY OF SUWANNEE RIVER HUMIC ACID IN SEAWATER
by
Megan Brook Kogut
ENO
B.S. Chemistry University of Washington
(1995)
Submitted to the Department of Civil and Environmental Engineering in Partial Fulfillment of the Requirements for the Degree of
MASTER OF SCIENCE
In Civil and Environmental Engineering at the
Massachusetts Institute of Technology MASSACHUSETTS INSTITUTE
J OF TECHNOLOGY
June 2000
I
O
@Massachusetts Institute of Technology LI BRAR ES All rights reserved.
Signature of Author_____________
Department of Civil and Environmental Engineering 5 May 2000 Certified by
Bettina Voelker Professor, Civil and Environmental Engineering Thesis Supervisor Accepted by_
Daniele Veneziano Chairman, Departmental Committee on Graduate Students
COPPER BINDING ABILITY OF SUWANNEE RIVER HUMIC ACID IN SEAWATER by
Megan Brook Kogut
Submitted to the Department of Civil and Environmental Engineering on 5 May 2000 in partial fulfillment of the requirements for the Degree of Master of Science in Civil and Environmental Engineering
ABSTRACT
Elevated total copper concentrations ([CulT) due to industrial and municipal pollution are toxic to microorganisms in coastal areas. Copper is up to 99.99% complexed by strong ligands in the water column and is not immediately bioavailable, so that the toxicity of copper is frequently
correlated to the free copper concentration ([Cu2+]) and not [Cu]T. However, [Cu2+] is too small to measure directly with current methods. The copper binding ability, or the total effect of
copper ligands, of these coastal areas is therefore as important an indicator of copper toxicity of the waters as [Cu]r. However, binding ability is more difficult to measure and predict than [Cu]T and therefore is the limiting factor in our ability to monitor and regulate copper as a toxin.
Several sources of copper ligands have been proposed as major sources of strong copper ligands to coastal areas. Strong copper ligands (KcuL = 101-10214) are produced by phytoplankton, perhaps as a defense mechanism against copper toxicity. However, planktonic ligands are created at some metabolic cost, possibly affecting phytoplankton viability. Recent field research has shown that rivers, sewage outfalls, and sediment porewaters also contribute strong copper ligands to coastal areas. The strong ligands in these samples are difficult to characterize, so suspected ligands must be investigated separately.
Terrestrial humic substances are well recognized as weak ligands in sediments, rivers, and sewage. They are ubiquitous because they have plentiful sources (plants, soils, sediments) and are
recalcitrant to degradation. However, they are usually not considered to be strong copper ligands because there are no published studies of low concentration, high strength binding sites in humic substances. For the first time, we have taken methods developed by oceanographers to study copper speciation at ambient copper levels in seawater and applied them to a humic acid extract, Suwannee River Humic Acid (SRHA). We show that SRHA contains strong copper ligands and propose that other sources of humic acids may play a previously unrecognized role in buffering copper toxicity in coastal areas.
Thesis Supervisor: Dr. Bettina Voelker
Acknowledgements
Deepest thanks to Tina Voelker for providing tireless, instructive feedback on thesis drafts and a healthy balance of academic freedom and guidance. The "we" in this paper is far from the royal "we"! This research was supported by a Parsons Fellowship and by her Doherty Professorship for Young Investigators.
Endless thanks to my parents for freedom and guidance and support in all aspects of life. I am very grateful to Glen T. Shen for letting me loose in his lab and then pointing eastward.
TABLE OF CONTENTS ABSTRACT 3 ACKNOWLEDGEMENTS 5 LIST OF TABLES 8 LIST OF FIGURES 9 CHAPTER 1. INTRODUCTION 10
1.1.. Importance of copper speciation. 10
1.2. Copper ligand strength and influence on speciation. 13
1.3. Investigated sources of strong copper ligands. 14
1.4. Terrestrial humic acids and Suwannee River Humic Acid. 17
CHAPTER 2. INTRODUCTION TO METHODS 20
2.1. Competitive Ligand Exchange and Copper Titrations. 20
2.2. Interpretating and Modeling Copper Titration Data. 24
2.3. Adsorptive Cathodic Stripping Voltammetry. 29
2.4. Calibration and surfactant effect. 30
CHAPTER 3. PROCEDURE AND METHODS DEVELOPMENT 34
3.1. Sample preparation. 34
3.2. Titration set up and sample analysis. 37
3.3. Kinetics of humic acid and SA competition. 397
3.4. True sensitivity of ACSV with 1 mg/L SRHA. 41
3.5. Change in Cu(SA)x electrode sensitivity with SA speciation. 44 3.6. Surfactant effects of 1 mg/L SRHA on standard curves. 46
CHAPTER 4. RESULTS AND DISCUSSION. 57
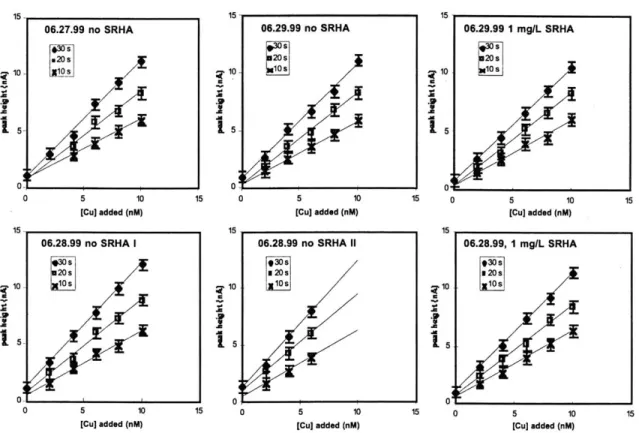
4.1. Titrations of 1 mg/L SRHA. 57
4.2. Modeling titrations of 1 mg/L SRHA. 61
4.3 Graphical presentation of the binding ability of 1 mg/L SRHA. 67 4.4. Comparison of SRHA titrations to estuary field sample titrations. 70
4.5. Suwannee River Humic Acid as Source of Copper Ligands 72
CHAPTER 5. SUMMARY AND FUTURE RESEARCH 73
5.1. Results of methods development. 73
5.2. Implications of binding ability of SRHA on coastal copper speciation. 74
5.3. Future Research and Directions. 75
APPENDIX. ERROR ANALYSIS. 78
LIST OF FIGURES
Figure 2.1. Modeled copper titrations, both with SRC(AL)= 103 ([AL] = 1 gM and log
KCu(AL)2 = 15, assuming a bis complex). 23
Figure 2.2. Diagram of E[CuLi] versus [Cu2+] modeled with MINEQL for several ligand
mixtures titrated with total copper from 2 to 100 nM in a seawater sample. 25 Figure 3.1. Molecular structures of (a) salicylaldoxime (SA) and (b) benzoylacetone (bzac).
36 Figure 3.2. Sample potential scans: solid lines are samples with 1 mg/L SRHA, and
dashed lines are for the samples run the same day without SRHA. 39 Figure 3.3. Signal, or peak height, versus equilibration time for 1 gM SA and 10 nM
copper without (E) and with (0) 1 mg/L SRHA. 41
Figure 3.4. MINEQL models of 1 mg/L SRHA and several concentrations of a) SA
and b) bzac. 43
Figure. 3.5. Relative sensitivity of Cu(SA)2 reduction with increasing [SA]. Inset is
the absolute sensitivity of the same samples. 47
Figure 3.6. Peak height versus deposition time for 5 uM SA and 10 nM copper. 50 Figure 3.7. Standard curves of UV-SW and overload titrations of 1 mg/L SRHA
with 25 [tM SA. 51
Figure 3.8. Standard curves corresponding to titrations presented. 55 Figure 4.1 (A-E). Sample titrations of Suwannee River Humic Acid. 58 Figure 4.2. Langmuir linearizations of all titrations presented. 63 Figure 4.3. Langmuir linearizations of all SA titrations presented. 64 Figure 4.4. Plot of I[CuLi] versus [Cu2
+] for all eight copper titrations of 1 mg/L SRHA,
plus ASV results of SRHA binding at high copper calculated from reported stability
constants and concentrations per mg/L SRHA. 68
Figure 4.5. Comparison of three copper titrations of 1 mg/L SRHA with multiple copper
LIST OF TABLES
Table 1.1. Copper toxicity thresholds of selected species of microorganisms. 12 Table 3.1. Side reaction coefficients used in modeling and titration calculations. 45
Table 3.2. Slopes of UW-SW standard curves and overload titrations of 1 mg/L SRHA
and 25 gM SA conducted over three days. 53
Table 4.1. Average conditional binding strengths and ligand concentrations of ligand
CHAPTER 1. INTRODUCTION 1.1. Importance of copper speciation.
Many highly populated coastal waters have dangerously elevated total copper concentrations due to industrial and municipal pollution. For example, copper is released from metals processing to coastal waters via wastewater effluents and runoff, and in recreational and shipping areas, copper leaches from antifouling paints on boat hulls. Total dissolved copper concentrations range from 2 nM in well -flushed coastal areas (Moffett 1997) to more than 100 nM in the most heavily impacted areas such as San Francisco Bay (Donat 1994). The US EPA national ambient marine water quality criterion for total dissolved copper is 2.9 gg/L, or 46 nM. This criterion, chosen to be below copper concentrations that cause acute toxicity to a species of crab, is a starting point for assessment of the effects of copper on aquatic ecological health. However, the fact that total copper concentrations are often greater than current EPA mandated levels suggests that copper pollution is a problem and that conflicts between regulators and polluters are likely.
Assessing the effects of copper on aquatic organisms is complicated by the fact that not all copper is immediately bioavailable. Complexation of copper by strong ligands reduces the concentration of free copper, [Cu2+]. Lower [Cu2
+] in the water column lead to decreased rates of
copper uptake by phytoplankton (Sunda 1976; Anderson 1978). Copper complexation also decreases copper toxicity to fish, in which copper transport seems to occur primarily through binding sites on gill tissue (Meyer 1999). Because the percentage of strongly complexed copper varies widely from 80 to over 99.99% in different coastal systems, [Cu2+] is in the range of low
September 1995 copper speciation data from Waquoit Bay, Massachusetts, show that [Cu]T is about 20 nM, but strong copper ligands decrease [Cu2+] to roughly 1 pM (Moffett 1997). In the
San Francisco Bay, California, [Cu]T is about 50 nM, while [Cu2+] is 5 to 10 nM (Donat 1994). San Francisco Bay has much higher [Cu2+] and theoretically higher copper toxicity than Waquoit Bay although the two samples have comparable [Cu]T. Because [Cu2+] is a better indicator of
copper toxicity than [Cu]T, many biologists and toxicologists now report the toxic effects of copper in relationship to measured [Cu2+]. Several species of microorganisms have copper toxicity thresholds within the range of [Cu2+] found in coastal areas (Table 1.1); therefore copper toxicity may affect ecosystems with dissolved copper levels currently in EPA compliance for ambient water quality.
Instead of measuring [Cu2+] of a sample, usually below detection limits in natural waters, many scientists measure the copper binding ability of that sample (the sum of the binding abilities of the copper ligands in the sample). They then calculate [Cu2+] of the sample as a function of the sample binding ability, (e.g. Moffett 1997; Sedlak 1997; van den Berg 1987). The speciation of copper between free ionic and complexed forms is determined by the relationship between [CuT] and the binding ability of the sample. The copper binding ability is much more difficult to measure and predict than [Cu]T and therefore is the limiting factor in our ability to monitor copper speciation and regulate copper as a toxin.
Table 1.1. Copper toxicity thresholds of selected species of microorganisms. Values of [Cu2
+] reported are the threshhold concentrations at which the indicated effects occur
significantly. *Free copper concentrations measured by electrochemical methods; otherwise, [Cu2+] held constant during experiment by complexation with excess concentrations of synthetic metal chelators (NTA or tris).
Organism Toxicity Indicator [Cu2
+] (nM) Reference
Eurytemora affinis 90% of control survival 2.0 (Hall 1997)*
(copepod) (8 days)
Acartia tonsa Decreased survival rate 0.5 to 0.05 (Sharp 1997)
(copepod) (48 hr)
Acartia tonsa Supressed grazing activity 0.1 (Sharp 1997)
(24 hr)
Acartia hudsonica Supressed grazing activity 0.01 (Sharp 1997)
(copepod) (24 hr)
Nannochloris atomus Inhibited growth rate 0.04 (Sunda 1976)
(alga)
Thalassiosira Inhibited growth rate 0.003 (Sunda 1976)
pseudonana (diatom)
synechococcus 50% of maximum 0.005 (Moffett 1997)*
1.2. Copper ligand strength and influence on speciation.
The binding strength of a copper ligand is represented by its conditional binding constant, KcuLi= [CuLi]/([Cu2
+][Li]), (1.1)
for which [CuLi] is the concentration of copper bound to ligands of class Li, [Cu2+] is the concentration of free copper, and [Li] is the concentration of ligands in that class not bound to copper. Copper ligands are polar or charged oxygen-, nitrogen-, and sulfur-containing sites on molecules that bind copper. The binding site strength depends partly on electron cloud
"softness" of the binding site, (both "hard" 0 binding sites and "soft" S and N groups complex "borderline soft" Cu2+), and number of chelators, or "claws", available to attach to the copper ion. Because other cations (e.g. H*, Ca2+, Mg2+) compete with copper for binding sites, the
conditional constant KCuL depends on the concentrations of these cations and their abilities to compete with copper. KCuL is also conditional because ionic strength effects may shield the electrostatic attraction of Cu2
+ to negatively charged binding sites. This decrease in ligand
strength due to competition and ionic strength effects is potentially important in estuaries, where cation concentrations and ionic strength increase by several orders of magnitude from freshwater to seawater.
The speciation of copper in natural waters is described by the equation
[Cu]T = [Cu2+] + I[CuLi], (1.2)
where the sum of concentrations of all copper species is the total copper concentration. Substituting Eq. 1.1 into Eq. 1.2 gives a speciation relationship that includes ligand binding strengths and concentrations,
[CU]T= [Cu2+] + X(KCuLi[Li][Cu2+]). (1.3)
Dividing all terms by [Cu2+] gives the relationship [Cu]T/[Cu2
+] = 1 + l(KCuLi[Li]), (1.4)
which relates the extent of copper complexation directly to ligand binding strengths and
concentrations. The quantity (KCuLi[Li]), also called the side reaction coefficient (SRC) of ligand class Li, is a useful value for comparing copper binding abilities of different ligand classes based on their average strengths and concentrations.
Oceanographers have classified copper binding ligands based on their conditional copper binding constants KCuLi. The strongest ligands found in coastal areas approaching seawater salinity have a conditional copper binding constant of 1012 and greater. This ligand class is dubbed "LI" and controls [Cu2
+] when [Cu]T is less than [L1]1T. At higher copper concentrations, the ligand class
"L2", with conditional binding constants in the range of 108 to 1011, controls copper speciation when the stronger L1 ligand class is copper saturated. The distinction between LI and L2 ligand classes is likely artificial in coastal waters, where relative contributions of different sources of different types of stronger and weaker ligands to the binding abilities of water samples are unknown. However, ligand class models are useful for quick numerical comparisons of copper binding in different waters.
1.3. Investigated sources of strong copper ligands.
experiments have repeatedly proven that strong organic copper ligands (KcL = 1012-1014) dominate copper speciation in the open ocean. The ligand concentration versus depth profiles suggest a plankton source of these ligands in the upper mixed layer of the water column (van den Berg 1984; Coale 1988; Moffett 1990). One ubiquitous species of marine blue-green algae,
Synechococcus sp., excretes copper-complexing ligands (Kce = 1013) when copper stressed in culture (Moffett 1996). Another culture study shows that Emiliania huxleyi, a marine
microalgae, excretes a compound with a copper binding constant of about 1012 (Leal 1999). Excretion of copper-binding compounds is suspected to be a strategy to protect against copper toxicity, since more copper ligands are produced in cultures with elevated copper concentrations in both studies. Planktonic ligands are created at some metabolic cost to the organisms that produce them, possibly compromising their viability as they compete for resources in the ecosystem.
Therefore, although planktonic ligands are likely to be major contributors to strong copper ligand pools in coastal areas, we are also interested in nonplanktonic sources of copper ligands from an ecological standpoint. Nonplanktonic ligands could decrease the toxic effects of copper on coastal
ecosystems without metabolic cost to its organisms. Recent field research in riverine and coastal waters has shown that terrestrial, anthropogenic, and porewater ligand sources may contribute
significant amounts of ligands with binding strengths comparable to those of planktonic ligands produced in the water column. Stable copper sulfide complexes, which seem to resist oxidation by oxygen for several days and have large conditional stability constants (e.g., KCu(HS) = 1013,
several rivers in Connecticut (Rozan 1999). The sources of sulfide seem to be both anoxic wetland areas and waters that are anoxic due to nutrient overloading. Determination of copper speciation in several treated sewage effluents and creeks in South San Francisco Bay (Sedlak 1997) shows that effluent contains about 10 to 25 nM strong copper ligands (K > 10-'.) which are subsequently released to the bay. Assuming conservative mixing and no UV or biological destruction of these ligands, Sedlak et al. proposed that these sources could contribute enough strong ligands so that their average concentrations in the South Bay in winter and summer are as high as 7 nM and 3 nM, respectively. Finally, estuarine sediment porewaters in Chesapeake Bay contain high concentrations of ligands (around 10,000 nM) with binding strengths as high as K =
1015. If these ligands are stable in the oxic water column, they could contribute to the pool of strong Cu ligands in the whole estuary based on ligand flux measurements and a circulation model of Chesapeake Bay (Skrabal 1997).
All of the above authors suggest humic substances as a possible identity of weak (L2) copper binding ligands from these sources. Because humic acids have plentiful terrestrial sources (plants, soils, sediments), are ubiquitous in aquatic systems, and are relatively recalcitrant to microbial destruction, they are an interesting addition to the suite of ligand sources. Rozan et al. suggest based on comparisons of the binding abilities of whole river water samples and humic and fulvic acid standards that humic and fulvic acids contribute to the copper binding abilities of rivers. Sedlak et al. propose that humic acids contribute to the weaker ligand class associated with wastewater copper (average KCuL = 107, [L] = 200 to 500 nM), citing a study of fulvic acid
1996). Humic acids are well recognized as contributors to the weaker ligand class L2, e.g.
(Cabaniss 1988), but are usually not included in discussions of strong copper binding because there are no published studies of very low concentration, high strength binding sites in humic
substances.
The one study that has probed binding sites with conditional binding strengths greater than 108 in seawater is a study of humic acid isolated from swampwater. The binding ability of a 1 mg/L humic acid solution could be modeled with a three ligand model as follows: KCuLa > 1011, [La]= 50 nM; KCuLb = 9.2, [Lb] = 200 nM; KCuLc = 6.6; [L] =1,800 nM (Hering 1988), where the subscripts a, b, and c denote the strongest, weaker, and weakest ligand classes titrated with copper. This study proves that at low copper concentrations, humic acid ligands are detected that were copper saturated in other studies (and therefore not probed), and have a stronger binding ability than humic ligands at higher concentrations. The detection limits of anodic
stripping voltammetry in this study prevented probing of binding sites at concentrations lower than 50 nM, leaving open the possibility of stronger binding sites at lower concentrations which
could contribute to L1 in coastal waters. For the first time, we have taken the methods developed by oceanographers to study planktonic ligands and applied them to humic acid extracts in order to probe these stronger sites in saline waters.
1.4. Terrestrial humic acids and Suwannee River Humic Acid.
Terrestrial humic substances have long been suspected to bind metals because they contain a high concentration of polar and acidic functional groups. Humic substances are operationally defined
as the fraction of dissolved organic matter that can be collected on a hydrophobic resin (XAD-8) at pH = 2. Lowering the pH of the solution protonates acidic functional groups, neutralizing
their negative charge and causing the humic acids to become more hydrophobic and sorb to the resin. The column is then rinsed with a strongly basic solution, and the dissolved organic matter (DOM) released off the resin in this basic solution is defined as humic substances. In this way, humic substances are separated from hydrophobic DOM with less acidic character, which remains sorbed to the resin at higher pH. Subsequent reacidification of the collected humic substance solution causes a fraction of the humic substances to precipitate; centrifugation of the solution separates the precipitate, defined as humic acid, from the dissolved material, defined as fulvic acid.
In and near terrestrial systems, major components of humic substances collected from water samples are highly oxidized, aromatic, and stable degradation products, such as lignin, of trees, grasses, and other plant material (Aiken 1985). For example, DOM derived from the degradation of the salt marsh grass Spartina alterniflora is 34% humic substances collected with an XAD-8 column (Moran 1994). About 24% of these humic substances are degraded within 7 weeks, but the remainder of the humic substances are resistant to further degradation on the timescale of weeks to months. Sunlight increases the rate of degradation of humic substances by a factor of three (Moran 1999), and there is a wide range of degradation rates of different humic acids (Moran 1994). However, humic acids found in aquatic systems on average have a much greater halflife than the rest of the DOM in the water column.
Plentiful terrestrial sources and chemical recalcitrance of humic substances (e.g. Moran 1994) are consistent with findings of significant concentrations of lignin found in rivers and estuaries (Mantoura 1983). The terrestrial signal in DOM (assumed to be proportional to total lignin, phenolic components in the cell walls of vascular plants) can be traced from rivers and marshes along the coast of Georgia to the edge of the continental shelf (Moran 1991). Concentrations of humic acid in rivers and estuaries are consistently greater than 1 mg/L and can reach 5 and 10 mg/L in some systems, (e.g. Rozan 1999).
Of the previously isolated humic acids available from the International Humic Substance Society (IHSS), we use Suwannee River aquatic humic substance standards from the base of the
Okefenokee Swamp in Georgia, USA. Humic substances were collected in one large batch, separated into standard humic and fulvic acids, and freeze-dried. Initial studies on Suwannee River Humic Acid (SRHA) character and structure were published together in a U.S.G.S. report (Averett 1989). The aquatic humic acid is a mixture of grass, tree, and other degradation products has an average lifetime of three years and an average molecular weight of 1000 Daltons (Averett 1989). We chose these humic acid reference materials because they are well studied and because we can compare our results to those of the study of binding ability of SRHA at higher copper concentrations (Hering 1988).
CHAPTER 2. INTRODUCTION TO METHODS
Competitive Ligand Exchange Adsorptive Cathodic Stripping Voltammetry (CLE-ACSV) is the method used to measure the copper binding ability of water samples containing natural ligands. This method is a two step process: first, known concentrations of copper and a
well-characterized and purified synthetic ligand, AL, are added to the sample with the natural ligands, and the natural ligands and AL compete with each other for copper. When the sample has
reached equilibrium, the amount of copper complexed with AL is measured with ACSV. Because the concentrations of copper complexed with AL (nanomolar range) are orders of magnitude greater than those of free copper (usually in the picomolar to femtomolar range in ocean waters), competitive ligand exchange and measurement of copper complexed by AL greatly increases the sensitivity of ACSV. CLE-ACSV and copper titrations of water samples have been used to probe planktonic ligands (Moffett 1996) and natural ligands in marine systems (Donat 1994; Campos 1994). In this chapter, CLE theory and data modeling practices are discussed first. ACSV is introduced separately because related analytical issues are important to the presentation of methods development discussed in Chapter 3.
2.1. Competitive Ligand Exchange and Copper Titrations.
Competitive Ligand Exchange between added and natural ligands allows us to determine very low values of [Cu2+], but [Cu2+] and the concentration of copper complexes must be determined indirectly When equilibrium exists among copper, natural ligands and the added ligand,
where CuAL is the mono complex and CuAL2 is the bis complex of copper with the added ligand. Concentrations of AL are often high enough so that the concentrations of mono and bis copper complexes are comparable. The concentration of copper complexed with added ligand is therefore
I[Cu(AL),] = [CuAL] + [Cu(AL)2]. (2.2)
The value of I[CuLi] is obtained by rearranging Eq. 2.1 and omitting the negligible variable [Cu2+],
I[CuLi] = [Cu]T - I[Cu(AL)x]. (2.3)
The value of [Cu2+] is calculated from I[Cu(AL)], [AL], and the conditional complex formation constants of the mono and bis complexes, KCuAL and ICu(AL)2- Because the value determined with
ACSV is X[Cu(AL)] (Section 2.3), SRC(AL) is used, where
SRC(AL) = KCuAL[AL] + PCu(AL)2[AL]2, (2.4)
and [AL] is equal to [ALIT as long as [Cu]T is much less than [ALIT. Therefore,
[Cu2+] = Y[Cu(AL)x]/SRC(AL). (2.5)
The fraction of total copper complexed by inorganic hydroxide and carbonate species in seawater is usually a negligible fraction of X[CuLi] in samples that contain strong ligands. Even at the high concentrations of hydroxide and carbonate in seawater with a pH of 8.3, the sum of
concentrations of major inorganic species CuCO3* and ternary carbonate/hydroxide species is about 24 times greater than [Cu2+] (Byrne 1985) and usually at least two orders of magnitude
In order to determine the binding ability of a sample over a range of [Cu]T, CLE-ACSV is used to analyze a copper titration of a natural ligand sample. Analysis after each addition of copper determines the portion of total copper complexed by the added ligand. As [CulT is increased, stronger natural ligands are saturated with copper and weaker natural ligands cannot compete as well as the stronger ligands for additional copper. The fraction of each copper addition complexed by AL increases as [Cu]T increases, as shown with a sample modeled with MINEQL (Westall 1994) in Figure 2.1. Eventually, if all natural ligands are completely saturated with copper, an additional copper added to the sample ([CU]added) will be complexed by the added ligand, so that
the increase in I[Cu(AL)x] (1[Cu(AL)x]formed) is equal to [Cu]added (0, Fig. 2.1).
Finally, one copper titration with one SRC(AL) cannot probe the whole range of natural ligands found in coastal waters. The titration range of one sample is constrained by detection limits and error propogation during calculation of
X[CuLi]formed = [Cu]added -X[Cu(AL)x]formed (2.6)
for each copper addition. At the beginning of the titration, if X[Cu(AL)x]formed is much less than
[Cu]added because AL cannot effectively compete with natural ligands for copper, I[CuLi]formd is equal to [Cu]added. The only information that has been gained from competitive ligand exchange is that natural ligands with an SRC much greater than that of SRC(AL) are present. If
X[Cu(AL)x]fomed is very close to the value of [Cu]added then E[CuLi]ormed is the very small difference between two large numbers. The range of values of X[CuLi] defined by these two limiting conditions is the analytical window of the titration (Miller 1997).
5E-8 _
* LI and L2
- LI only a
an
4E-8 -no natural ligands 3
3E-8 C
6 2E-8 a
a" 1E-8
OE+O
OE+O IE-8 2E-8 3E-8 4E-8 5E-8
[Cu]T (M)
Figure 2.1. Modeled copper titrations, both with SRC(AL) =103 ([AL] =1 M and log KCu(AL)2 = 15, assuming a bis complex). 0 Sample with one strong ligand class (5 nM, log K = 13, SRC(L1)= 104.
*
Sample with strong ligand2.2. Interpretating and Modeling Copper Titration Data.
From Equations 2.3 and 2.5 and a copper titration where I[Cu(AL),] is obtained as a function of [Cu]T, a plot of [Cu2+] versus I[CuLi] can be calculated, showing the binding ability of the ligands over a range of free copper values (Fig. 2.2). For any sample where [Cu2+]/I[CuLi] < 0.01, which is usually true in the presence of natural ligands, I[CuLi] is approximately equal to [Cu]T*, the total copper that would be present in the water sample in the absence of AL but with identical [Cu2+]. From equation 2.3, [Cu]r* is therefore approximated by
[Cu]T*= [CulT -X[Cu(AL)x]. (2.7)
The values of [Cu]T* are lower than the corresponding values of [Cu]T for the titration with AL, so that, depending on the analytical window of the titration, ligands with concentration of less than [Cu]T of the original sample can be analyzed.
The concentration of [Cu2
+] corresponding to the concentration of copper originaly present in the
sample ([CuIT,o) can be read directly off this kind of plot ([Cu]T* = [Cu]T,o= I[CuLi]). This graphical representation of the sample's binding ability is also useful for predicting [Cu2+] of the natural ligands in the sample if it became more copper polluted, for example, by finding the value of [Cu2
+] which corresponds to a greater value of [Cu]T*. The visual representation of binding
ability also clearly shows the analytical window of the titration, so that a value of [Cu]T* can be chosen within the analytical window for an accurate prediction of [Cu2+]. Corresponding
1 E-8
1 E-9 'd'ILJ IIL" ""' u IIYUI AIU'' ^' JYIII IIU IVIIII '*
1 E-10
IE-11
1E-12
IE-13
IE-14
1 E-9 1 E-8 1 E-7
I[CuL ] (M)
Figure 2.2. Diagram of I[CuLJ] versus [Cu2
+ modeled with MINEQL for several ligand mixtures titrated with total copper from 2 to 100 nM in a seawater sample. LI has a conditional copper binding constant of KcuL1 = 10, while L2 has a
conditional copper binding constant of KcuL2 = 1011. When LI and L2 are saturated with copper, and if no other weaker ligands are present (true for these examples), the copper binding ability of the sample approaches that of carbonate and
hydroxide at salinity equal to 35%o. See text for additional discussion of the relative binding abilities of mixtures.
in these cases it is difficult to calculate accurately the binding ability of ligands at very low or high [Cu]T*.
Comparisons of the binding ability of different waters is theoretically easy with this graphical method; overlaying the curves shows relative binding ability, which is a function of both ligand strength and concentration, both at lower copper concentrations (stronger ligands) and at higher copper concentrations (weaker ligands). This is demonstrated by modeling combinations of ligands titrated from 2 to 100 nM total copper with MINEQL (Fig. 2.2). On a log-log scale, 20 nM strong ligands is offset at lower [Cu2+] than 10 nM strong ligands (* and 0), and the same magnitude of offset occurs for a factor of two difference in concentration of weaker ligands
(*
and ZS). In addition, weak ligands lower [Cu2+] slightly before the stronger ligand has been titrated completely at low [Cu]T*(*
and 8), and the strong ligand still influences [Cu2+] at high
[Cu]T* (* and 0). This overlap is evident for modeling two ligands with conditional binding constants separated by two orders of magnitude. The overlap is more important for modeling copper titrations of actual samples, which may contain ligands with a spectrum of copper
binding strengths, as combinations of ligand classes with average binding constants (Cernik 1995). However, in general, as the copper titration proceeds, less abundant stronger ligands become less important and the more abundant weaker ligands more important in the role of controlling [Cu2+].
When L 1 and L2 are saturated copper, and if no weaker ligands exist besides carbonate and hydroxide, the slope of the binding ability plot increases sharply until the line approaches and eventually overlaps the carbonate/hydroxide line (-).
It is common practice to model the ligands titrated in a sample as several classes, or bins, of ligands with average copper binding strengths. The main purpose of dividing ligands pools into bins with average binding strength is to compare concentrations of stronger and weaker ligands among samples. There are several ways to model copper titrations as one or several ligand classes with average conditional binding constants. The number of bins needed to model the natural ligands in a sample depends on the analytical window of the titration and the ligand pool of the sample. Two common techniques for determining average ligand binding strengths and
concentrations are the Langmuir linearization and the Scatchard plot, as discussed in Miller (1997). We use the Langmuir linearization here.
To obtain the strength and concentration of the natural ligands with the Langmuir linearization, titration data is plotted as [Cu2+]/1[CuLi] versus [Cu2
+] so that
[Cu2+]/X[CuLi] = [Cu2+]/[Li]T + 1/(KcuLi*[Li]T). (2.8)
Therefore, the inverse of the intercept of this plot, i, is KcuLi*[Li]T and the inverse of the slope, s, is [Li]T. If the linearization shows a curved line that is best fit by more than one straight line, the
titration is best fit more than one ligand bin. The straight line corresponding to the stronger ligand class La is fit as in the one ligand case, with [La] = 1/si and KCuLa = 1*/(i*[La]). For the line at higher [Cu2
+] that represents the weaker ligand class Lb, the intercept, i2, is
i2= [Lb]/KCuLa*([La] +[Lb]) 2}. (2.9)
The slope s2 is
where subscripts 1 and 2 refer to the lines at lower and higher [Cu2
1], and the subscripts a and b
refer to the stronger and weaker ligand classes in the analytical window of that titration. Finally, iteration should be used to optimize the accuracy of a two ligand fit (Miller 1997). But, if the fit of the two lines of a Langmuir linearization produce two ligand classes with conditional binding constants no more than an order of magnitude apart, then iteration could fail to separate the contributions of La to the Lb portion of the linearization and vice versa. Therefore modeling Lb is possible only when there is enough curvature in a Langmuir linearization to produce two discreet ligand classes.
The distinction between La and LI (and Lb and L2) is subtle because with CLE-ACSV analytical windows, SRC(La) is in the range of SRC(L1) found in coastal samples. SRC(La) is often centered near SRC(AL) of any titration, suggesting that SRC(La) is not a value corresponding to a
"real" concentration of LI and a single fixed conditional binding constant (van den Berg 1990). In addition, linearization methods underestimate the concentration and overestimate the binding strength of the weaker ligand class if the weaker ligand class is not fully titrated (Miller 1997). It is impossible to fully titrate the spectrum of binding sites in humic acid, so for any titration of humic acid, Lb will depend on the range of [Cu]T* obtained. However, La and Lb classes reasonably represent titration data, and these ligand classes could be used to roughly compare strong and weak ligand concentrations per mg/L SRHA to LI and L2 classes reported in coastal copper speciation literature.
2.3. Adsorptive Cathodic Stripping Voltammetry.
Adsorptive Cathodic Stripping Voltammetry is used to measure I[Cu(AL).] in each equilibrated sample. After the competitive ligand exchange step, the sample is transferred to a Teflon@ cup and the cup attached to a hanging mercury drop electrode (HMDE). The sample is purged of oxygen (because oxygen causes signal interferences when it is reduced), a fresh mercury drop is produced, and added ligand copper complexes in the sample are adsorbed to the mercury drop for a fixed amount of time called the deposition time.
The deposition step concentrates species on the drop as a function of their charge and
hydrophobicity. For example, for the added ligand salicylaldoxime (SA), with a net ligand charge of 0 at pH = 8.3, both the Cu(SA) and the Cu(SA), complexes have a net positive charge of +2. Experiments of deposition potential versus peak height show that more negative deposition potential (and therefore more negative charge on the mercury drop) is correlated to increased peak height, consistent with the expected increase in electrostatic attraction of positively charged SA
species [Campos, 1994 #64].
During the deposition step, a certain amount of Cu(AL)2 is collected on the drop, [Cu(AL)2
]drop,-After the deposition step, scanning the electrical potential on the mercury drop in the negative direction creates a more reducing environment on the drop. A potential negative enough to cause the reduction of Cu(II) creates a flow of electrons measured as current. When current is plotted as a function of potential, a peak centered on the reducing potential of the Cu(II) species appears. All Cu(II) sorbed to the drop is reduced, and the potentials at which different Cu(II) species are
reduced (Cu2+, Cu(AL), Cu(AL)2) are more negative for more stable copper complexes, so that different peaks corresponding to the reduction of each copper species are obtained. The peak caused by the reduction of Cu(AL)2 on the drop dominates the potential scan for the added ligands that we used. The height of the peak is proportional to [Cu(AL)2]1rop, which in turn is proportional to the deposition time, to the deposition potential, and to [Cu(AL)x] in the solution. The sensitivity, or the relationship between peak height and [Cu(AL)x] is determined by
calibration.
2.4. Calibration and surfactant effect.
The sensitivity of ACSV is determined by calibration by standard additions of copper in a sample to generate a linear plot of peak height (corresponding to the reduction of Cu(AL)2) versus [Cu]T where no strong ligands are present and the SRC of carbonate/hydroxide copper complexation is negligible compared to AL), AL complexes almost all the copper, and [Cu2
+] is
negligible compared to the concentrations of the complexes. In this case,
[CU]T = I[Cu(AL)x] = [CuAL] + [Cu(AL)2], (2.12)
where [AL] is equal to [AL]T if [AL]T is much greater than [Cu]T. Because [AL]T is usually in the micromolar range and [Cu]T is usually in the nanomolar range, the assumption that [AL]T
is much greater than [Cu]T is usually correct.
The sensitivity, S,
S = I/[Cur. (2.14) Therefore, although we are measuring the height of the peak corresponding to the reduction of Cu(AL)2, for example, the peak height is proportional to X[Cu(AL)]. Although in principle we could instead define S as the sensitivity of [Cu(AL)2] and add a calculated [CuAL] to both
standard curve and titration, it is easier to calibrate both the standard curve and the corresponding titration as a function of I[Cu(AL)x] for each [AL]. Since [Cu(AL)]dop: [Cu(AL)2]drop is not
necessarily equal to [Cu(AL)]:[Cu(AL)2], calibration of the standard curve in terms of
1[Cu(AL)]drop insures that one calibration step accounts for any unpredictable mercury drop surface chemistry among AL, CuAL, and Cu(AL)2. In this way, we determine an empirical relationship between sensitivity and I[Cu(AL)x] in the sample.
Determining the sensitivity of copper titrations of samples with high DOM content is difficult because hydrophobic portions of DOM may sorb to the mercury drop surface and decrease the sensitivity by hindering the simultaneous sorption of Cu(AL)x. This phenomenon is called the surfactant effect. An external calibration using a standard curve in a DOM-free sample will overestimate the sensitivity of samples with a significant surfactant effect.
To avoid the surfactant effect, internal calibrations are often used to determine the sensitivity of sample. For this method, one assumes that at the end of the titration, all strong natural ligands that can compete successfully with AL for copper are already saturated with copper. Therefore, at higher [CU]T, one assumes a situation analogous to the UV-oxidized standard curve, where
[CUladded is equal to X[Cu(AL)x]ormed after each copper addition. This assumption is based on the fact that a titration with competitive ligand exchange between AL and one ligand of
comparable strength and concentration is parallel to the UV-oxidized standard curve when all the sample ligands have been filled with copper (0, Fig. 2.1). An internal calibration assumes that the straight portion of the titration is the true sensitivity of the ACSV in that water sample, and thatonly surfactants cause any decrease in sensitivity.
However, if weaker ligands are able to compete with AL for copper, then the slope will be underestimated by an internal calibration. This is demonstrated in Fig. 2.1 (*), where a weaker ligand of high concentration continues to complex some of the added copper because it is not saturated with copper. Therefore, although the titration appears linear at greater [Cu]T, the slope remains less than that of the UV-oxidized (no natural ligands) standard curve. Therefore, the decrease in signal in an internal calibration could be due to complexation of some added copper by weaker natural ligands as well as to surfactant effects. Comparison of titrations of the same sample at different detection windows is the only way to tell the difference between natural ligand binding of copper and surfactant effects as causes of decreased slope during internal calibrations.
We developed a third method of calibration to avoid the uncertainty between depression of signal due to surfactant effects or copper complexation by natural ligands. This external calibration method accounts for the surfactant effect by using a great enough concentration of added ligand to
titration is designed so that SRC(AL) is much larger than the SRC of any of the natural ligands. The successful overload titration would be a straight line whose slope would be either equal to or less than the slope of the UV-oxidized standard curve, depending on the magnitude of the
surfactant effect. The success of the overload titration could be tested in lab by titrating natural ligands at several different overload values of [AL]. Consistency among observed slopes of these overload titrations would suggest that the natural ligands do not complex any added copper, and that the constant slope is the true sensitivity. The "overload" titration does not produce copper speciation data, so unlike for internal calibrations, additional titrations at lower SRC(AL) must be performed on separate aliquots of the same sample.
CHAPTER 3. PROCEDURE AND METHODS DEVELOPMENT 3.1. Sample preparation.
Only Teflon@ bottles were used to minimize adsorption of copper and ligands to bottle surfaces and leaching of phthalate plasticizers into the samples. The Teflon@ bottles were washed in detergent, soaked in 10% HCl (Aldrich, Analytical Grade) for two days, and rinsed six times with distilled deionized water (DDW).
The Suwannee River Humic Acid (SRHA) collection was conducted as follows (Averett 1989): river water was passed through a 0.45 gm filter, acidified to pH < 2, and collected on a nonpolar resin (XAD-8) column. The column was then rinsed with 0.1 M NaOH and about 95% of the DOM on the column was released into solution and collected. This solution was reacidifed to pH = 1, and the precipitate that subsequently formed was separated by centrifugation and
collected as humic acid and freeze dried.
Stock solutions of 100 mg/L SRHA were made fresh every month in our lab from freeze-dried SRHA and DDW. SRHA dissolved quickly in water without any addition of base. Humic acid stock solutions were stored in the refrigerator until use.
The sample matrix was buffered, UV oxidized Sargasso seawater (UV-SW) collected with trace metal clean methods. Organic matter in Sargasso seawater was destroyed by ultraviolet light oxidation with a mercury lamp (Ace Glass, 1000 W) for at least 8 hours. One mL of
UV-oxidized 1 M boric acid (EM Science, Suprapur@), adjusted to pH of 8.2 with 0.35 M ammonia, was added to one liter of UV-SW for a final pH buffer concentration of 1 mM.
Primary copper standards were made fresh every day from a 1 mM acidified copper stock solution diluted with DDW from an Aldrich atomic absorption standard solution. The primary copper standard was diluted with seawater for the secondary copper stock solution of 1 gM Cu and a pH of about 7.
The added ligand was either benzoylacetone (bzac), developed as a competing ligand for CLE-CSV by Moffett (1995), or salicylaldoxime (SA), developed by Campos and van den Berg (1994) (Figure 3.1). Both were recrystallized from 10-3 M aqueous ethylenediamine-tetraacetic acid (EDTA) in DDW, rinsed with cold DDW, and filtered. After recrystallization, they were redissolved, recrystallized, and filtered twice in DDW to rinse the added ligand of EDTA. Dissolution was increased by gentle heating below 650 C for bzac, and below 400 C for SA because SA decomposes at higher temperature. Bzac was dissolved in methanol (J. T. Baker, HPLC Solvent) for a final stock solution concentration of 10-2 M. SA was dissolved in DDW for a final stock solution concentration of 10-4 M. SA and bzac stock solutions were replaced every three months and refrigerated when not in use.
a. Saliyhldoxirne (SA)
OH
b. Benoyhcetan (bzac)
Figure 3.1. Molecular structures of (a) salicylaldoxime (SA) and (b) benzoylacetone (bzac).
OH
CH
3.2. Titration set up and sample analysis.
At the start of the experiment, one half of buffered, UV-irradiated seawater solution (500 mL) was separated for UV-SW standard curves and determination of total copper of UV-SW, and 0.5
mL of the humic acid stock solution was added to the other 500 mL for a humic acid
concentration of 1.0 mg/L. The 1.0 mg/L humic acid solution was equilibrated for at least several hours at room temperature in the dark.
For competitive ligand exchange with SA, copper titrations were set up as one sample to which aliquots of copper were sequentially added. The sample was a 10.0 mL aliquot of the 1.0 mg/L humic acid solution pipetted directly into the electrode sample cup. SA was also added to the sample at this time. Copper from the secondary standard was added to the sample after each titration point analysis for concentrations of total added copper from 0 to 30 nM for 3 pM SA titrations and from 10 to 150 nM for 1 pM SA titrations. Solutions were equilibrated for 5 minutes after each copper addition and before analysis of the corresponding titration point. Longer equilibration times did not results in a change in [Cu(SA),] measure (see section 3.3). The
sensitivity remained constant with ten consecutive measurements of the same sample.
In contrast, sensitivity dropped significantly between repeated measurements of the same sample with bzac as the competing ligand. Bzac is much less water soluble than SA and likely sorbs rapidly to the discarded mercury drop at the bottom of the cup, significantly reducing its concentration in solution. Because repeated measurements were not practical, bzac titrations were conducted with individual titration point samples. Ten to twelve 10.0 mL aliquots of the
1.0 mg/L humic acid solution were pipetted into individual 30 mL Teflon@ bottles. Copper from the secondary standard was added to each sample to make concentrations of total added copper varying from 10 to 150 nM. The samples were equilibrated for two to three hours, and then bzac from the 1 0- M stock solution was added to each sample and allowed to equilibrate with copper
and humic acid ligands for an additional one or two hours before analysis. Overnight equilibration periods with bzac titrations also caused a loss of apparent sensitivity, perhaps due to slow but significant sorption of bzac copper complexes to the walls of the Teflon@ sample bottles.
The samples were analyzed using differential pulse adsorptive cathodic stripping voltammetry (DP ACSV) with a PAR 303A static mercury drop electrode and a EG&G PAR 394 analyzer. Instrument settings for both SA and bzac titrations were as follows: deposition potential,
-0.08 V (versus Ag/AgCl electrode); scan range, -8 to -600 mV; scan rate, 10 mV/s; drop time, 0.2 s; pulse height, 25 mV. This instrument setting protocol was adapted from that used for bzac (Moffett 1995), and was also found to provide the best sensitivity for SA, so was used for SA although another protocol has been suggested (Campos 1994). Stir bar speed setting was usually slow to avoid stir bar knocking, and the mercury drop size setting was large to increase
sensitivity at short deposition times. The purge time was 5 minutes. Deposition times in general were 10, 20, and 30 seconds. The rest period between deposition time and initialization of scan was five seconds.
i I -40.00 -35.00 -30.00 -25.00 > -20.00 -15.00 -500.0 I -400.0 -300.0 E I mV -200.0 -100.0
El mV
-32.00 -28.00 -24.00 -20.00 >-16.00 -12.00 -8.00 -4.00 -500.0 -400.0 -300.0 -200.0 -100.0E/ mV
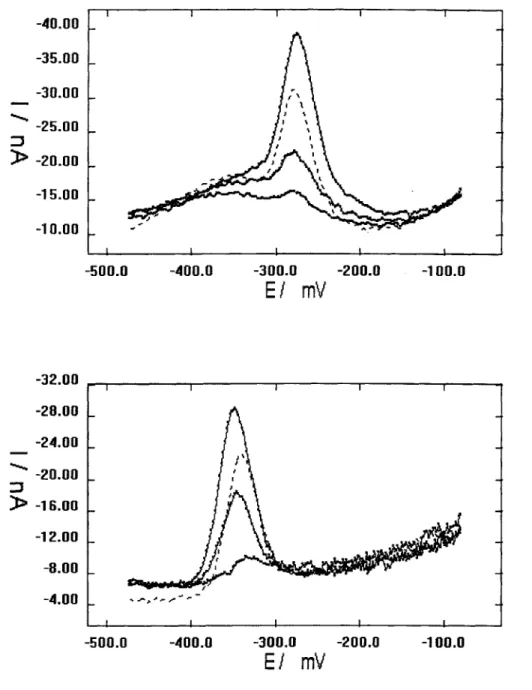
Figure 3.2. Sample potential scans: solid lines are samples with 1 mg/L SRHA, and dashed lines are for the samples run the same day without SRHA. A) 200 pM bzac, SRHA, and 40, 60, and 120 nM copper. UV-SW sample is with 60 nM copper. B) 1 pM SA, SRHA, and 2, 20, and 40 nM copper. UV-SW sample is with 20 nM copper.
defined and occurred at -290 mV for bzac copper complexes and -330 to -400 mV for SA copper complexes. The average background noise was 0.5 nA; therefore, peaks below 1 nA in height were discarded. At the shorter deposition times used (less than 30 s), there was no signal caused by the reduction of adsorbed humic complexes in samples with or without added ligand near the area of the measured peak (Fig. 3.1).
3.3. Kinetics of humic acid and SA competition.
To determine how quickly copper, strong SRHA binding sites, and SA equilibrate after mixing, we conducted a series of measurements of a single sample of 1 gM SA and 10 nM copper before and after the addition of 1 mg/L SRHA. The Cu(SA)2 signal remains constant for addition of SA to 10 nM copper after repeated measurements (0, Fig. 3.2). After addition of SRHA, the analytical signal will decrease with time due to competitive uptake of copper by SRHA, if
equilibration time between species is on the order of minutes. After addition of 1 mg/L SRHA to this sample, the signal decreased within 2.5 minutes to a value approximately 35% of the original signal, and then did not change significantly within a time period between 5 minutes and 24 hours later (M, Fig. 3.3). The quick decrease in signal after addition of SRHA is possibly the result of the surfactant effect as well as quick exchange (< 2 minutes) of copper from SA to SRHA. However, supporting results show that the surfactant effect is negligible with 30 second deposition times (Section 3.6). Therefore, this experiment shows that equilibration between copper, SA, and SRHA is quick.
O no SRHA - no SRHA average m 1 mg/L SRHA 1 mg/L SRHA average : LI 0 5 10 15 Equilibration 20 Time (minutes) 25 30
Figure 3.3. Signal, or peak height, versus equilibration time for 1 pM SA and 10 nM copper without (0) and with (0) 1 mg/L SRHA. The reduction in peak height after addition of SRHA is mostly if not completely due to competitive complexation of copper by SRHA (see discussion in text). The final two points for the SRHA times series were taken 24 hours after addition of SRHA to SA and copper. Because no change in peak height occurs between time 2.5 mintes and 24 hours after addition of SRHA, we assume that SRHA equilibrates within 2.5 minutes. The lines represent the average value for all points for each sample.
10 8 6 signal (nA) 4 2 0 .L 2
For bzac titrations, the experimental protocol was a three hour equilibration of humic acid with added copper and a two to three hour equilibration of these samples with bzac before analysis (Moffett 1997). Much longer equilibration times were not possible because bzac seems to sorb to the surfaces of the Teflon@ bottles, resulting in noticeably lowered sensitivity after six to eight hours.
3.4. True sensitivity of ACSV with 1 mg/L SRHA.
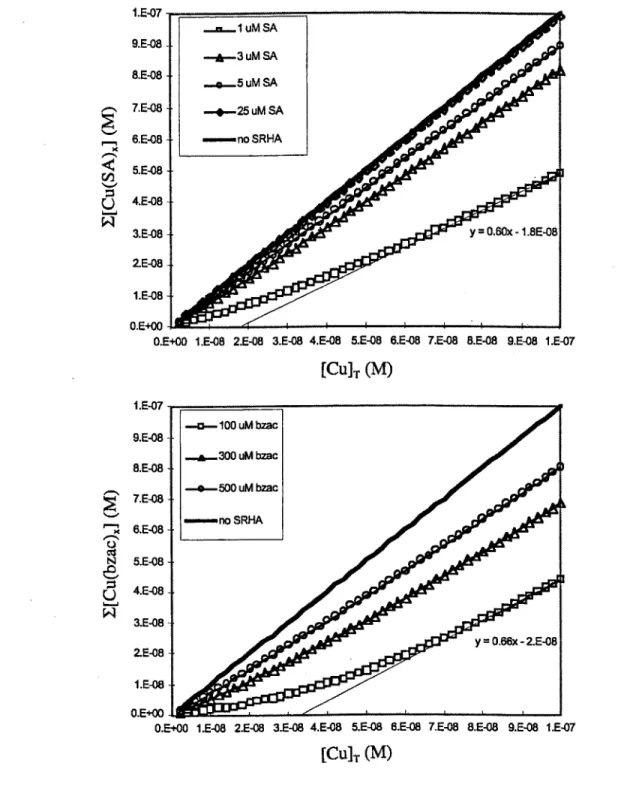
Our calculations using previous results of revious copper titrations of the weaker ligands in SRHA (Hering 1988) show that the large concentration of weaker ligands in 1 mg/L SRHA may interfere with obtaining the true sensitivity of titrations when using internal calibrations. With MINEQL, we modeled the reported set of copper ligand bins over a range of values of [Cu]T and SRC(AL). We compared the behavior of weaker ligands at higher [Cu]T with values of SRC(AL) near that of the L1 class (10,000) and with SRC(AL) corresponding to the highest concentrations of SA and bzac already calibrated in the literature (Campos 1994; Moffett 1995). Although the relationship among titration points appears linear at [CulT = 100 nM and greater, this linear relationship does not represent the true sensitivity of the titration because weaker ligands are still competing with AL for copper. Using the slope of the higher [Cu]T of the titration as the
sensitivity underestimates the sensitivity by as much as 40% with these detection windows (0, Fig 3.4.b).
1.E-07 ..=... uM SA 9.E-08 -1-3 uM SA 8.E-08 ....o-5 uM SA _ 7.E-08 -. *-25 uM SA 6.E-08 --.. no SRHA 5.E-08
0
4.E-08 3.E-08 y 0.60x - 1.8E-08 ZE-08 1.E-08 O.E+00O.E+00 1.E-08 2.E-08 3.E-08 4.E-08 5.E-08 6.E-08 7.E-08 8.E-08 9.E-08 1.E-07
[CulT (M) 1.E-07 -o- 100 uM bzac 9.E-08 .-..-. 300 uM bzac 8.E-08 -o-500 uM bzac 7.E-08 x 6.E-08 - SRHA 5.E-08 u 4.E-08 3.E-08 y 0.66x -2.E-08 2.E-08 1.E-08 0.E+00
0.E+00 1.E-08 2.E-08 3.E-08 4.E-08 5.E-08 6.E-08 7.E-08 8.E-08 9.E-08 1.E-07
[CUlT (M)
Figure 3.4. MINEQL models of 1 mg/L SRHA and several concentrations of a) SA and b) bzac. The models show that the slope of the titration does not return to unity at higher [CU]T The line fits are best fits through the linear portion of the titrations; the slope of each line should be unity if it represented the sensitivity of the
titrations. Here, weak humic binding decreases the slope below unity, showing that internal calibrations would underestimate the sensitivity except for very high SCR(AL) (25 pM SA), in which case SA outcompetes all humic ligands for copper.
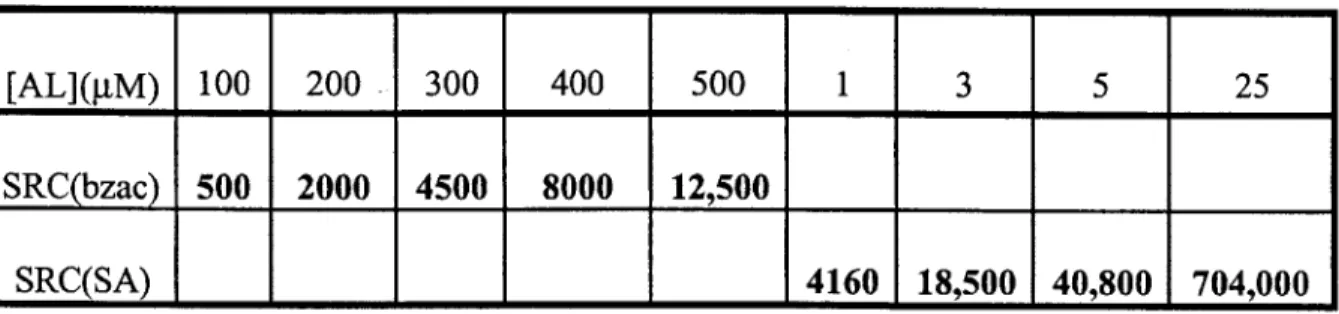
Because very high concentrations of SA (25 piM) can outcompete all ligands (Fig. 3.4.a), we used overload titrations with 25 gM SA to determine the true sensitivity of humic acid titrations with SA as the added ligand. We could not use overload titrations with bzac as the added ligand because 500 gM bzac does not provide a strong enough detection window to outcompete all humic ligands, and bzac is not completely soluble at concentrations much higher than 500 gM (Table 3.1).
3.5. Change in Cu(SA), electrode sensitivity with SA speciation.
The overload titration as external calibration has a complication: the speciation of CuAL and Cu(AL)2 will change in the range of [AL] used for titration and standard curve. For example, at 1 gM SA, [CuSA] is slightly greater than [Cu(SA)2], but at 25 gM SA, [Cu(SA)2] is much greater than [CuSA]. This change in AL speciation will affect the sensitivity because only Cu(SA)2 is reduced at the mercury drop during the ACSV step, and the relationship between [Cu(SA)2]drop
and I[Cu(SA)] in the sample will change with a change in [SA]. Campos et al. (1994) found that the sensitivity of Cu(SA)x increased significantly with increased [SA] up to 25 pM SA. They found that sensitivity was roughly correlated to the ratio of [Cu(SA)2] oto I[Cu(SA)x], which increases until Cu(SA)2 approximately equal to I[Cu(SA),] at [SA]= 25 gM. Correlation between sensitivity and [Cu(SA)2]/I[Cu(SA)] led Campos et al. to suggest that SA speciation at
[AL](gM) 100 200 300 400 500 1 3 5 25 SRC(bzac) 500 2000 4500 8000 12,500
SRC(SA) 4160 18,500 40,800 704,000
Table 3.1. Side reaction coefficients used in modeling and titration calculations. Bzac calibration data taken from Moffett, 1995, where Kcu(bzac) is neglected and pCu(bzac)2 -1010'7. SA calibration data taken from Campos and van den Berg, 1994, where Kcu(sA)= 109-7 and $Cu(SA)2 = 1015.
We too compared the slopes of standard curves obtained with 1 -5 gM and 25 gM SA in UV-SW to determine the sensitivity of the electrode at each SA concentration, and we obtained a different relationship between sensitivity and [SA] (Fig. 3.5). We used a different deposition potential than Campos et al. (-0.08 versus -1.
lV),
and Campos et al. observed that the sensitivity increased dramatically with deposition potential especially above -0.8V. There is no guarantee that the ratios of [Cu(SA)], [Cu(SA)2], and [SA] adsorbed to the drop are linearly related to their ratios in the sample solution because the physical conditions at the drop (charge,hydrophobicity) may affect the rate of adsorption of each of the SA species differently.
Therefore the deposition potential may effect SA speciation on the drop in some unpredictable but consistent fashion. Because we observed a reproducible relationship between sensitivity and [SA] for different deposition times and different experiments, we used our analytical results instead of those of Campos et al.
Comparisons of 25 pM SA sensitivities to 1 and 3 gM SA sensitivies give correction factors of 55% and 78%, respectively. These correction factors are used for all SA titrations presented. The percent standard deviation for each value averaged over the three deposition times is
approximately 5%.
3.6. Surfactant effects of 1 mg/L SRHA on standard curves.
As discussed in Section 2.4, we have two external calibration methods to obtain the true
1.20 1.00 1.5 0.80 081.0 0.60 o0 A A 0 0. 5 04) A 0.40 I ,0.0 ---30s 0 10 2 30 0.20 20 s 10 s [SA] (gM) 0.00 0 10 20 30 [SA] (jIM)
Figure. 3.5. Relative sensitivity of Cu(SA)2 reduction with increasing [SAJ. Inset is the absolute sensitivity of the same samples, at deposition times of 10, 20, and 30 seconds.
-analytical sensitivity. If we do not know the magnitude of the surfactant effect, we must add an excess ("overload") concentration of AL to complex all added copper so that the slope of the line of peak height versus [Cu]added = I[Cu(AL)x]ormed is the true sensitivity of the sample.
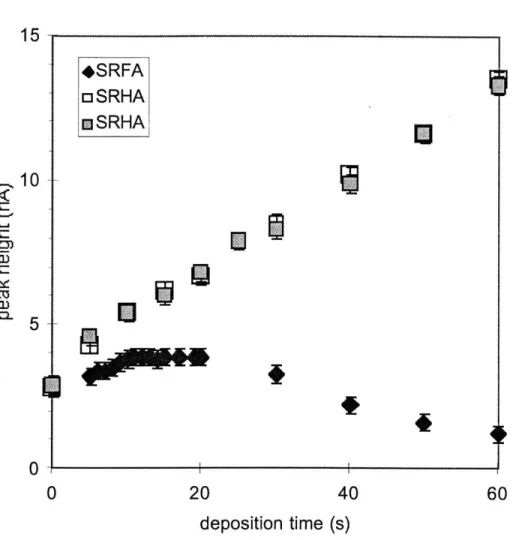
Do humic substances at concentrations typically found in natural systems significantly interfere with the adsorption and reduction of Cu(AL)2 during ACSV? To answer this question we determined the magnitude of the surfactant effect of 1 mg/L SRHA in our samples. Standard curves at 25 pM SA were conducted in UV-SW with and without 1 mg/L SRHA. Because 25 gM SA outcompetes all SRHA binding sites for added copper, any decrease in sensitivity
between samples with and without humic substances is due to humic interference at the electrode drop. The deposition time should be as long as possible while avoiding large surfactant effects. We observed very large and troublesome surfactant effects at one and two minute deposition times with 1 mg/L SRHA; the surfactant effect caused a net loss in sensitivity (data not shown). Because the surfactant effect is more pronounced at longer deposition times, a quick qualitative check for its interference can be obtained from repeated analysis of the same sample with several different deposition times to verify that a linear relationship between peak height and deposition time exists. Because this method is quick, it can be used as a rough check for surfactant effects before a sample titration. For example, the relationship between sensitivity and deposition time was linear for deposition times of at least one minute for samples of 5 RM SA and 1 and 2 mg/L humic acid (Fig. 3.6). The peak heights are the same for both concentrations of humic acid because 5 gM SA has complexed most of the copper. It is worth noting for didactic and warning
![Table 1.1. Copper toxicity thresholds of selected species of microorganisms. Values of [Cu 2 +] reported are the threshhold concentrations at which the indicated effects occur significantly](https://thumb-eu.123doks.com/thumbv2/123doknet/14493664.526383/12.918.123.815.415.708/toxicity-thresholds-selected-microorganisms-threshhold-concentrations-indicated-significantly.webp)
![Figure 2.1. Modeled copper titrations, both with SRC(AL) =103 ([AL] =1 M and log KCu(AL)2 = 15, assuming a bis complex)](https://thumb-eu.123doks.com/thumbv2/123doknet/14493664.526383/23.918.158.660.100.731/figure-modeled-copper-titrations-src-kcu-assuming-complex.webp)
![Figure 2.2. Diagram of I[CuLJ] versus [Cu 2 + modeled with MINEQL for several ligand mixtures titrated with total copper from 2 to 100 nM in a seawater sample.](https://thumb-eu.123doks.com/thumbv2/123doknet/14493664.526383/25.918.85.756.91.875/figure-diagram-versus-modeled-mineql-mixtures-titrated-seawater.webp)