HAL Id: hal-01976054
https://hal.archives-ouvertes.fr/hal-01976054
Submitted on 9 Jan 2019
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Setting-up a billboard of marine invasive species in the
ESENIAS area: current situation and future
expectancies
Paraskevi Karachle, Maria Corsini Foka, Fabio Crocetta, Jakov Dulčić, Nina
Dzhembekova, Marika Galanidi, Petya Ivanova, Noa Shenkar, Marius Skolka,
Elitsa Stefanova, et al.
To cite this version:
Paraskevi Karachle, Maria Corsini Foka, Fabio Crocetta, Jakov Dulčić, Nina Dzhembekova, et al.. Setting-up a billboard of marine invasive species in the ESENIAS area: current situation and future expectancies. Acta Adriatica, Institute of Oceanography and Fisheries, Split, 2017, 58 (3), pp.429-458. �hal-01976054�
Setting-up a billboard of marine invasive species in the
ESENIAS area: current situation and future expectancies
Paraskevi K. KARACHLE
1,*, Maria CORSINI FOKA
2, Fabio CROCETTA
1,
Jakov DULČIĆ
3, Nina DZHEMBEKOVA
4, Marika GALANIDI
5, Petya IVANOVA
4,
Noa SHENKAR
6, Marius SKOLKA
7, Elitsa STEFANOVA
4, Kremena STEFANOVA
4,
Victor SURUGIU
8, Irfan UYSAL
9, Marc VERLAQUE
10and Argyro ZENETOS
1 1 Institute of Marine Biological Resources and Inland Waters, Hellenic Centre for MarineResearch, 46.7 km Athens Sounio ave., P.O. Box 712, 19013 Anavyssos Attiki, Greece
2 Institute of Oceanography, Hydrobiological Station of Rhodes,
Hellenic Center for Marine Research, Cos Street, 85100 Rhodes, Greece
3 Institute of Oceanography and Fisheries, Šetalište I. Meštrovića 63, 21000 Split, Croatia 4 Institute of Oceanology, Bulgarian Academy of Sciences,
First May Street 40, P.O.Box 152, Varna 9000, Bulgaria
5 Institute of Marine Sciences and Technology, Dokuz Eylül University,
Haydar Aliyev Bul., No:100, 35430, Inciraltı-Izmir, Turkey
6 School of Zoology, George S. Wise Faculty of Life Sciences, Tel-Aviv University,
Tel-Aviv 6997801, Israel
7 Ovidius University of Constanta, Bd. Mamaia 124 RO 900527, Constanta, Romania 8 Alexandru Ioan Cuza University of Iași, Faculty of Biology,
Bd. Carol I, No. 20A, 700507 Iași, Romania
9 Ministry of Forestry & Water Affairs, Marine Protected Areas Division,
Alparslan Turkes cad. No: 72 Bestepe, Ankara, Turkey
10 Aix-Marseille University, Mediterranean Institute of Oceanography (MIO), CNRS/INSU,
INTRODUCTION
Biological invasions, both in land and sea, have been worldwide acknowledged as a man-induced ecosystem pressure that should be monitored and managed (ROY et al., 2015, 2017).
The Mediterranean, has been characterized as a “sea under siege” (GALIL, 2000), due to the
large number of marine alien species recorded, which are introduced by a variety of pathways/ vectors (ZENETOS et al., 2010, 2012). In particular,
the Eastern Mediterranean, due to its proximity to the Suez Canal, has been susceptible to bio-logical invasions and hosts more than 775 alien and cryptogenic species (ZENETOS et al., 2012).
Similarly, the Black Sea, an enclosed marine system of particular physicochemical character-istics, low diversity and high marine traffic, is vulnerable to marine invasions (LEPPÄKOSKI &
MIHNEA, 1996). Of particular importance among
aliens are the invasive alien species (IAS), which are considered one of the greatest threats to biodiversity and on ecosystem services (KAT-SANEVAKIS et al., 2014).
Several international agreements such as the Convention on Biological Diversity (CBD), the EU Biodiversity Strategy (EU, 2011), and the
European Marine Strategy Framework Directive (EU, 2008), recognise the negative impacts of IAS and highlight the growing concerns of
pol-icy-makers, scientists, stakeholders and society. It is widely acknowledged among scientists that an early warning system should be elaborat-ed, and one of the best ways is through horizon scanning (ESSL et al., 2015; ROY et al., 2015, 2017;
and references therein). Horizon scanning is the
pro-cess of gathering, analysing and disseminating added-value information to support decision-making (ROY et al., 2014). However, for most
regional seas, lists of current or future biological invasions are not available to date. This is also the case for many countries in the Mediterranean region regarding invasive species in the marine environment. Moreover, it is essential to priori-tise IAS, their pathways and the areas of higher likelihood to appear, in order to manage and successfully encounter IAS issues (MCGEOCH et
al., 2016).
In the present study, effort has been made to compile a list of marine IAS in the network of the ESENIAS (East and South European Network for Invasive Alien Species) countries, including those species already present in this area and those with a high likelihood of appear-ance. The aim of this work was to create a catalogue (list of invasive / potential invasive species of ESENIAS concern) to serve as a basis for an early warning system, through a horizon scanning process (ROY et al., 2015). This would
allow ESENIAS countries to a) identify the most
Of the 160 species comprising the list, 149 were already present in the ESENIAS countries, while eleven were invasive species either present in the Mediterranean or in other European Seas, likely to be recorded in the ESENIAS countries. The majority of the species were of Red Sea/Indo-Pacific origin (97 species; 60.6%). Italy, Turkey and Greece were the countries with the highest representation of species (159, 152 and 139 species respectively), due to their extended coastline and the number of scholars working on marine invasive species. The highest number of established species was recorded in Turkey (116 species), whereas in Italy and Greece the most numerous species were the “expected” ones (85 and 48 species, respectively). The eastern Adriatic Sea countries (i.e. Albania, Croatia, Montenegro and Slovenia) had generally low numbers of species in this list, many of which are still “expected” to arrive from the neighbouring countries of Greece and Italy. Finally, the most frequently potential pathway was transfer stowaways (ship ballast water: 41 cases; ship hull fouling: 55), whereas unaided spread of Lessepsian immigrants followed (95 cases).
This list is intended to serve as an early warning system that through horizon scanning process would assist ESENIAS countries to prioritise invasive alien species, their pathways and the areas of higher likelihood to appear, in order to take management measures.
harmful species within the ESENIAS area; b) support the establishment of an effective early warning and rapid response mechanisms; c) support any eradication attempts of prioritised species at an early stage of invasion, and d) take management measures for IAS that are already widely spread, as requested by the EU Regu-lation No 1143/2014 (EU, 2014). Detailed fact
sheets of the ten most important species, based on their invasive potential, were also assembled.
MATERIAL AND METHODS
In the framework of ESENIAS-Tools pro-ject, a list of invasive/potential invasive species in the ESENIAS countries with marine borders (Albania, Bulgaria, Croatia, Greece, Italy, Mon-tenegro, Romania, Slovenia, and Turkey) was built up. The criteria used for this compilation were the following: a) invasive species already present in the area as reported in the national lists or other literature; b) invasive species in the Mediterranean/Black Sea that have not yet exhibited invasive behaviour in ESENIAS coun-tries and c) invasive species in European Seas, which are likely to invade ESENIAS countries. Cryptogenic species were not considered.
Consequently, based on the existing litera-ture, as well as on expert judgment, the species were classified for the ESENIAS area in the countries as established (species with an already established population in the area), casual (spe-cies with just one or few records in the country), invasive (species that are established/invading, with effects – positive and/or negative – on the ecosystem and its services), unknown (species whose presence/status in a country is not yet clarified), and expected (species likely to appear in a country, based on expert judgment and their presence in adjacent areas, and their ecological/ environmental requirements). This latter catego-ry was further expanded for Croatia and Italy, as expected in the southern and northern parts of the country. The list also includes the species’ origin and potential pathway/vector of introduc-tion (CBD, 2014).
Finally, fact sheets were compiled for ten species that were commonly agreed, among
experts, as of high importance. The decision was based on their expanding/invading char-acter according to biological traits and existing data on their invasion history, combined with the absence of published fact sheets at the time (January 2016), excepting Mya arenaria that has recently invaded the Adriatic Sea
(CROC-ETTA & TUROLLA, 2011). Each fact sheet includes
the following information: a) description and diagnostic features; b) biology and ecology; c) habitat and distribution (both native and in the ESENIAS area); d) pathway/vector of intro-duction; e) impacts on biodiversity, ecosystem services and human health; and f) risk assess-ment and manageassess-ment (when applicable). Maps presented here include information until August 2017. The full fact sheets are published at the ESENIAS Scientific Reports (TRICHKOVA et al.,
2017), whereas here only a brief account per
spe-cies is given.
RESULTS AND DISCUSSION
The list of invasive/potential invasive species
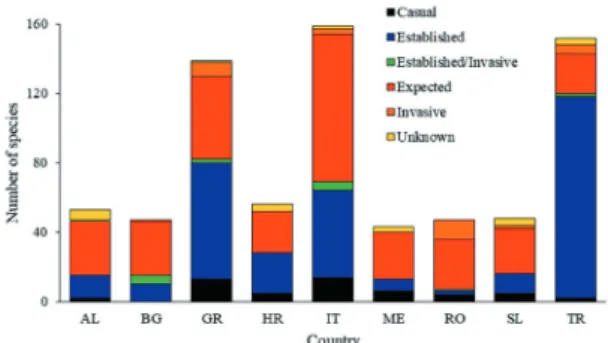
Overall, 160 species are given in the list, 149 of which are already present in the ESENIAS countries and eleven have not yet been recorded in the area. Approximately two thirds of the species (97 species; 60.6%) were of Red Sea/ Indo-Pacific origin, followed by those of Pacific origin (28 species; 17.5%) (Table A, on-line supplement). From the species included in this list, the countries with the highest representa-tion were Italy, Turkey and Greece (159, 152 and 139 species respectively; Table 1; Table A, on-line supplement; Fig. 1). A plausible expla-nation could be the fact that these countries have the longest coastlines among the ESENIAS ones, as well as the highest number of marine experts working on invasive species (KARACHLE
et al., 2017). Moreover, a recent work on marinas
across the Mediterranean has added consider-ably to the distribution of many invasive species in Turkey, Greece and Italy (ULMAN et al., 2017)
Turkey was the country with the highest number of established species (116 species), a fact that it can be attributed to its geographic
position and proximity to the Suez canal, and, along with Croatia, had the lowest number of expected ones (23 and 24 species, respec-tively; Table 1; Table A, on-line supplement; Fig. 1). On the other hand, countries with the most numerous expected species were Italy and Greece (85 and 48 species, respectively) fol-lowed by Albania, Bulgaria and Romania (31, 31 and 29 species, respectively). In the case of Italy, the high number could be attributed to three main reasons: a) this country is in the middle of the Mediterranean Sea, and hence has influxes of invasive species from both east (mainly Lessepsian immigrants) and west (Atlantic entries); b) intensive oyster activities, mainly in the northern Adriatic, led to accidental transfer of IAS through mariculture, and is thus vulnerable to similar introductions in the future; and c) the country holds major ports and is susceptible to ship transferred species. The last reason stands also for Bulgaria and Romania in the Black Sea. Concerning Greece, it is strongly affected by the Lessepsian invasion, and, due to the proximity to Turkey that holds a substantial number of established alien species, is likely to receive quite many additional IAS.
Finally, a small number of species rep-resented in the list characterizes the eastern Adriatic Sea countries, i.e. Albania, Croatia, Montenegro and Slovenia, in general. Yet, all the aforementioned counties display a high number of “expected” IAS, compared to their total IAS. This fact could be related to the low number
of experts working on alien species in these countries (KARACHLE et al., 2017). In addition,
the neighbouring Greece and Italy display high numbers of alien species (e.g. ZENETOS et al., 2015; MARCHINI et al., 2013; ZENETOS & KARACHLE,
2017), many of which have not been reported to
date in the East Adriatic countries (KATSANEVA-KIS et al., 2011).
The vast majority of the listed species (112 species) had only one potential pathway/vector of introduction, while the remaining ones had either two (42 species) or even three (6 species) pathways/vectors (Table A, on-line supplement). In 94 cases species were transferred as stowa-ways (ship ballast water: 39 cases; ship hull fouling: 55), whereas in 95 cases species were Lessepsian immigrants.
There were only 15 species transported as contaminants on animals (accidentally with aquaculture) and 10 species by other vectors (aquaria intentional releases, aquaculture escap-ees) (Table A, on-line supplement). It should be noted that invasive cold water species intro-duced as contaminants in Northern Europe are likely to be introduced to the Black Sea coun-tries and Northern Adriatic but not to the south-ern countries. In this context, tropical species that are already invasive in the Levantine are more likely to spread in the southern countries but not in the Black Sea.
Species’ factsheets: a brief account
Ten species were selected, based on their spreading and invasive character, namely
Alex-andrium monilatum (J.F.Howell), Bonnemaiso-nia hamifera Hariot, Streblospio gynobranchia-ta Rice & Levin, 1998, Cassiopea androm-eda (Forsskål, 1775) upside down jellyfish, Oithona davisae Ferrari & Orsi, 1984, Penaeus aztecus Ives, 1891 brown shrimp, Mya arenaria
Linnaeus, 1758 sand gaper, Diadema setosum (Leske, 1778) long-spined sea urchin,
Micro-cosmus exasperatus Heller, 1878 and Pterois miles (Bennett, 1828) Devil firefish/lionfish.
The major pathway/vector of introduction for the above mentioned species was transfer stowa-ways (ship ballast water: 9 cases; ship hull
foul-Fig. 1. Number of species per different category in the ESE-NIAS countries with marine borders (AL=Albania, BG=Bulgaria, GR=Greece, HR=Croatia, IT=Italy, ME=Montenegro, RO=Romania, SL=Slovenia, TR=Turkey)
Table 1. List of pr
esent (absent species ar
e indicated by an asterisk *), and expected
invasive species in the ESENIAS countries with marine bor
ders (AL=Albania, BG=Bulgaria,
GR=Gr eece, HR=Cr oatia, IT=Italy , ME=Montenegr o, RO=Romania, SL=Slovenia, TR=T urkey). Est=established, cas=casual, unk=unknown establishment status, inv=invasive, exp=expected (expS=expected south; expN=expected
north). Empty cells indicate
that the species has
not been r
eported yet or is expected
in the country
.
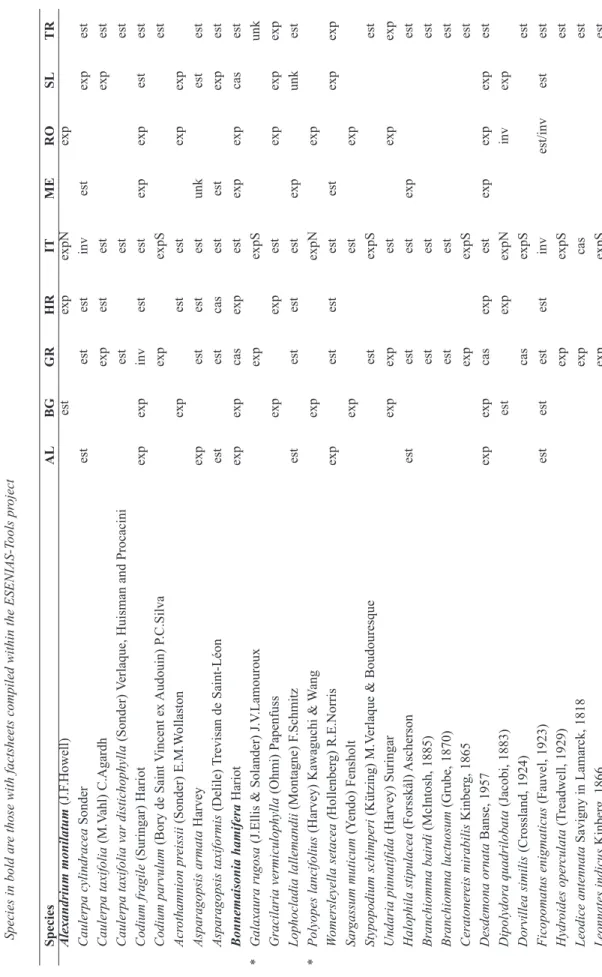
Species in bold ar
e those with factsheets
compiled within the ESENIAS -T ools pr oject Species AL BG GR HR IT ME RO SL TR Alexandrium monilatum (J.F .Howell) est exp expN exp Caulerpa cylindracea Sonder est est est inv est exp est Caulerpa taxifolia (M.V ahl) C.Agardh exp est est exp est Caulerpa taxifolia var distichophyll a (Sonder) Verlaque,
Huisman and Procacini
est est est Codium fragile (Suringar) Hariot exp exp inv est est exp exp est est Codium parvulum (Bory de Saint V incent ex Audouin) P .C.Silva exp expS est Acr othamnion pr eissii (Sonder) E.M.W ollaston exp est est exp exp Asparagopsis armata Harvey exp est est est unk est est Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon est est cas est est exp est Bonnemaisonia hamifera Hariot exp exp cas exp est exp exp cas est * Galaxaura rugosa
(J.Ellis & Solander) J.V
.Lamouroux exp expS unk Gracilaria vermiculophylla (Ohmi) Papenfuss exp exp est exp exp exp Lophocladia lallemandii (Montagne) F .Schmitz est est est est exp unk est * Polyopes lancifolius
(Harvey) Kawaguchi &
W ang exp expN exp W omersleyella setacea (Hollenber g) R.E.Norris exp est est est est exp exp Sar gassum muticum (Y endo) Fensholt exp est exp Stypopodium schimperi (Kützing) M.V erlaque & Boudouresque est expS est Undaria pinnatifida (Harvey) Suringar exp exp est exp exp Halophila stipulacea (Forsskål) Ascherson est est est exp est Branchiomma bair di (McIntosh, 1885) est est est Branchiomma luctuosum (Grube, 1870) est est est Ceratoner eis mirabilis Kinber g, 1865 exp expS est Desdemona ornata Banse, 1957 exp exp cas exp est exp exp exp est Dipolydora quadrilobata (Jacobi, 1883) est exp expN inv exp Dorvillea similis (Crossland, 1924) cas expS est Ficopomatus enigmaticus (Fauvel, 1923) est est est est inv est/inv est est Hydr oides oper culata (T readwell, 1929) exp expS est Leodice antennata Savigny in Lamarck, 1818 exp cas est Leonnates indicus Kinber g, 1866 exp expS est
Leonnates persicus W esenber g-Lund, 1949 cas expS est Notomastus mossambicus (Thomassin, 1970) exp exp est Polydora cornuta Bosc, 1802 exp est cas exp est exp inv exp est Prionospio krusadensis Fauvel, 1929 exp expS est Prionospio saccifera
Mackie & Hartley
, 1990 unk expS est Pseudoner eis anomala Gravier , 1900 exp est est est Pseudopolydora paucibranchiata (Okuda, 1937) exp exp est exp est exp exp exp est/inv Spir obranchus kraussii (Baird, 1865) exp expS est Streblospio gynobranchiata Rice & Levin, 1998 exp expN inv est Amathia verticillata (delle Chiaje, 1822) exp exp est/inv est est exp exp unk unk Diadumene lineata (V errill, 1869) est exp est exp exp unk est Clytia hummelincki (Leloup, 1935) exp exp unk est exp exp exp Clytia linearis (Thorneley , 1900) exp est exp est exp exp est Garveia franciscana (T orrey , 1902) exp est exp exp est exp exp exp exp Macr or hynchia philippina Kirchenpauer , 1872 exp expS est Cassiopea andromeda (Forsskål, 1775) exp est expS cas exp est Phyllor hiza punctata Lendenfeld, 1884 est est est Rhopilema nomadica Galil,
Spanier & Fer
guson, 1990 cas cas est Grandidier ella japonica Stephensen, 1938 exp exp est exp exp exp Megabalanus tintinnabulum (Linnaeus, 1758) exp exp est unk est exp exp unk exp * Heter osaccus dollfusi Boschma, 1960 exp expS est * Mytilicola orientalis Mori, 1935 exp expN exp Oithona davisae
Ferrari & Orsi, 1984
est exp est est Pseudodiaptomus marinus Sato, 1913 exp exp est exp est Ater gatis r oseus (Rüppell, 1830) est expS est Callinectes sapidus Rathbun, 1896 est est est est est est est exp est Charybdis japonica (A. Milne-Edwards, 1861) exp exp exp exp cas exp exp exp exp Charybdis longicollis Leene, 1938 est expS est Eriocheir sinensis H. Milne Edwards, 1853 exp exp cas cas exp exp Hemigrapsus sanguineus (De Haan, 1835) exp cas exp cas exp * Hemigrapsus takanoi Asakura & W atanabe, 2005 exp exp expN exp exp exp Ixa monodi
Holthuis & Gottlieb,
1956 est expS est Leptochela pugnax de Man, 1916 exp expS est Species AL BG GR HR IT ME RO SL TR
Matuta victor (Fabricius, 1781) exp expS est Metapenaeopsis aegyptia Galil & Golani, 1990 est expS est Metapenaeopsis mogiensis consobrina (Nobili, 1904) est expS est Metapenaeus monocer os (Fabricius, 1798) exp expS est Metapenaeus stebbingi Nobili, 1904 exp expS est Myra subgranulata Kossmann, 1877 est expS est Ogyrides mjoeber gi (Balss, 1921) exp expS est Penaeus aztecus Ives, 1891 inv est cas inv Penaeus japonicus Spence Bate, 1888 unk exp cas exp unk Penaeus pulchricaudatus Stebbing, 1914 est expS est Penaeus semisulcatus
De Haan, 1844 [in De Haan, 1833-1850]
exp exp est Per cnon gibbesi (H. Milne Edwards, 1853) est inv est est exp exp est Portunus segnis (Forskål, 1775) exp est est est Rhithr opanopeus harrisii (Gould, 1841) est/inv est inv exp Trachysalambria palaestinensis (Steinitz, 1932) est cas est Erugosquilla massavensis (Kossmann, 1880) est cas est Ber oe ovata Bruguière, 1789 est/inv cas unk inv cas est Mnemiopsis leidyi A. Agassiz, 1865 exp est/inv est est est/inv exp inv est est Anadara kagoshimensis (T okunaga, 1906) est est/inv exp est est/inv exp inv est est Anadara transversa (Say , 1822) exp est est est/inv exp est est Ar cuatula senhousia (Benson, 1842) unk exp est est/inv unk cas inv est Brachidontes pharaonis (P . Fischer , 1870) est est unk est cas est Chama pacifica Broderip, 1835 est expS est Clementia papyracea (Gmelin, 1791) cas expS est Dendostr ea cf. folium (Linnaeus, 1758) unk est expS est Fulvia fragilis (Forsskål in Niebuhr , 1775) est est est est Gafrarium savignyi (Jonas, 1846) exp expS est Goniobranchus annulatus (Eliot, 1904) est expS est Gouldiopa consternans
(Oliver & Zuschin, 2001)
exp expS cas Magallana/Crassostr ea sp./spp. unk exp est est est/inv unk cas inv est Malleus r egula (Forsskål in Niebuhr , 1775) est expS est Mya arenaria Linnaeus, 1758 est/inv est exp est inv exp est Paratapes textilis (Gmelin, 1791) exp expS cas Species AL BG GR HR IT ME RO SL TR
Pinctada imbricata radiata (Leach, 1814) est inv est est est exp est Ruditapes philippinarum
(Adams & Reeve,
1850) est exp exp est inv cas exp est est Spondylus spinosus Schreibers, 1793 cas expS est Xenostr obus securis (Lamarck, 1819) exp est exp exp Bulla arabica Malaquias & Reid, 2008 est expS est Bursatella leachii Blainville, 1817 est est est est est est est Conomur ex persicus (Swainson, 1821) unk est expS exp est
Corambe (Doridella) obscura
(A. E. Verrill, 1870) est expN inv Diodora ruppellii (G. B. Sowerby I, 1835) exp expS est Finella pupoides A. Adams, 1860 exp expS est Melibe viridis (Kelaart, 1858) exp est est est est cas est * Mur ex forskoehlii Röding, 1798 exp expS exp * Ocinebr ellus inornatus (Récluz, 1851) exp expN exp exp Pseudominolia nedyma (Melvill, 1897) exp expS est Pyrunculus fourierii (Audouin, 1826) cas expS est Rapana venosa (V alenciennes, 1846) unk inv cas est inv est est Rhinoclavis kochi (Philippi, 1848) cas expS est Sepioteuthis lessoniana
Férussac [in Lesson], 1831
est expS est Syrnola fasciata Jickeli, 1882 est expS est * Aquilonastra burtoni (Gray , 1840) exp exp Diadema setosum (Leske, 1778) est expS est Ophiactis savignyi (Müller & Troschel, 1842) cas expS est Ascidiella aspersa (Müller , 1776) exp exp est exp est exp exp est est Botrylloides giganteum (Pérès, 1949) exp unk exp * Botrylloides nigrum Herdman, 1886 exp exp expS expS exp Ciona r obusta Hoshino & Tokioka, 1967 exp exp exp exp est exp exp exp est Diplosoma listerianum (Milne Edwards, 1841) exp exp est exp est exp exp exp est * Ecteinascidia thurstoni Herdman, 1890 exp exp exp exp exp exp exp Her dmania momus (Savigny , 1816) est expS est Microcosmus exasperatus Heller , 1878 exp expS est Micr ocosmus squamiger Michaelsen, 1927 exp exp est exp exp Phallusia nigra Savigny , 1816 est expS est * Polyclinum constellatum Savigny , 1816 exp expS exp Species AL BG GR HR IT ME RO SL TR
* Rhodosoma tur cicum (Savigny , 1816) exp expS exp Styela clava Herdman, 1881 exp exp exp exp exp exp exp exp unk Styela plicata (Lesueur , 1823) exp exp exp est est exp exp est exp Symplegma brakenhielmi (Michaelsen, 1904) est expS est Apogonichthyoides pharaonis (Bellotti, 1874) exp est expS est Atherinomorus forskalii (Rüppell, 1838) est expS est Cheilodipterus novemstriatus (Rüppell, 1838) exp expS est Cynoglossus sinusarabici (Chabanaud, 1931) exp expS est Decapterus russelli (Rüppell, 1830) exp expS est Etrumeus golani DiBattista, Randal l & Bowen, 2012 est cas est Fistularia commersonii Rüppell, 1838 exp inv cas est cas exp inv Jaydia smithi Kotthaus, 1970 exp expS est Lagocephalus sceleratus (Gmelin, 1789) exp inv est est cas exp inv Lagocephalus suezensis
Clark & Gohar
, 1953 est expS est Liza carinata (V alenciennes, 1836) cas unk expS est Par exocoetus mento (V alenciennes, 1847) cas est expS est Pempheris r homboidea
Kossmann & Räuber
, 1877 est expS est Planiliza haematocheila (T emminck & Schlegel, 1845) est est expS inv est Plotosus lineatus (Thunber g, 1787) exp expS est Pteragogus trispilus Randall, 2013 est expS est Pterois miles (Bennett, 1828) exp est/inv cas exp est/inv Sar gocentr on rubrum (Forsskål, 1775) est expS est Saurida lessepsianus
Russell, Golani &
Tikochinski, 2015 cas est cas est Scomber omorus commerson (Lacepède, 1800) est expS est Siganus luridus (Rüppell, 1829) inv est est cas inv Siganus rivulatus
Forsskål & Niebuhr
, 1775 inv cas cas inv Sillago suezensis
Golani, Fricke &
Tikochinski, 2013 exp expS est Sphyraena chrysotaenia Klunzinger , 1884 exp est cas expS est Sphyraena flavicauda Rüppell, 1838 est expS est Stephanolepis diaspr os Fraser -Brunner , 1940 est exp est cas cas est Upeneus moluccensis (Bleeker , 1855) est cas est Upeneus pori Ben-T
uvia & Golani, 1989
est cas est Species AL BG GR HR IT ME RO SL TR
ing: 2), whereas P. miles was the only species that had solely been introduced through the Suez Canal (Table A, on-line supplement). There is an expected likelihood of all species to fur-ther expand their distribution in the ESENIAS countries, either unaided or through shipping, a possibility that in some cases is medium to high (Table 2). Out of the ten selected species, six have already impacts on the ESENIAS coun-tries, mainly on ecosystems/biodiversity and human health (Table 2), yet they are all expected to have new/additional impacts (Table 2), as in other invasive areas, that should be addressed/ mitigated to the best possible extent.
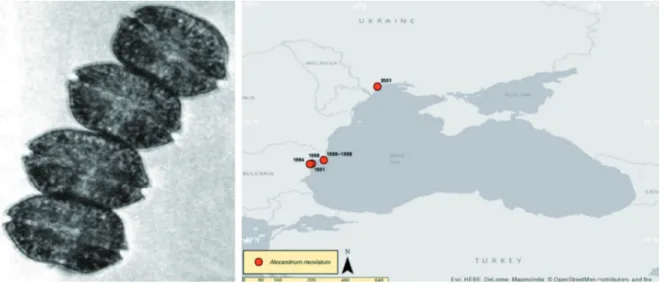
Alexandrium monilatum (J.F.Howell) Balech
(Fig. 2)
Alexandrium monilatum is a thecate,
chain-forming dinoflagellate. It is a coastal and estu-arine planktonic species of warm temperate and tropical environments (TAYLOR et al., 1995;
STEIDINGER & TANGEN, 1996). The species is
found in the Atlantic littoral of the USA as fol-lows: Gulf of Mexico (CONNELL & CROSS, 1950;
RAY & ALDRICH, 1967); Florida (HOWELL, 1953);
Chesapeake Bay (MAY et al., 2010). in the
Carib-bean Sea: Venezuela (HALIM, 1967), and in the
tropical Pacific Ocean off Ecuador (BALECH,
1995). It was reported for the first time at the
Bul-garian coast of the Black Sea in 1991
(MONCHE-VA et al., 1995), where it was also later observed
(MONCHEVA et al., 2001; NESTEROVA et al., 2008).
In 2001, the species has been detected also in Odessa port, Ukraine (ANONYMOUS, 2015).
Alexandrium monilatum has been associated
with bloom formation (HOWELL, 1953; PERRY et
al., 1979; HARDING et al., 2009) and fish kills due
to ichthyotoxins (GATES & WILSON, 1960; RAY &
ALDRICH, 1967). Whole cells and crude extracts
of A. monilatum have been shown experimen-tally to be lethal to mice, rats, fish, shellfish and cockroaches (GATES & WILSON, 1960; ALDRICH
et al., 1967; CLEMONS et al., 1980a, b; ERKER et al.,
1985; MAY et al., 2010). It was reported that A.
monilatum produce PSP toxins (HSIA et al., 2006),
yet, to date, the species has not been related to toxic events in the Black Sea. It has been reported in blooming concentration along the Bulgarian Black Sea coast (MONCHEVA et al., 1995, 2001). Blooming species cause water discoloration, especially in late summer, as well as community changes (MONCHEVA & KAMBUR-SKA, 2002; MONCHEVA et al., 1995, 2001; VELIKOVA
et al., 1999). Other socio-economic effects include beach water aesthetics with a negative impact on recreation (KATSANEVAKIS et al., 2014).
Fig. 2. Alexandrium monilatum (J.F.Howell) Balech (left; figure from WALKER & STEIDINGER (1979)) and its distribu-tion in the Black Sea (TRICHKOVA et al., 2017)
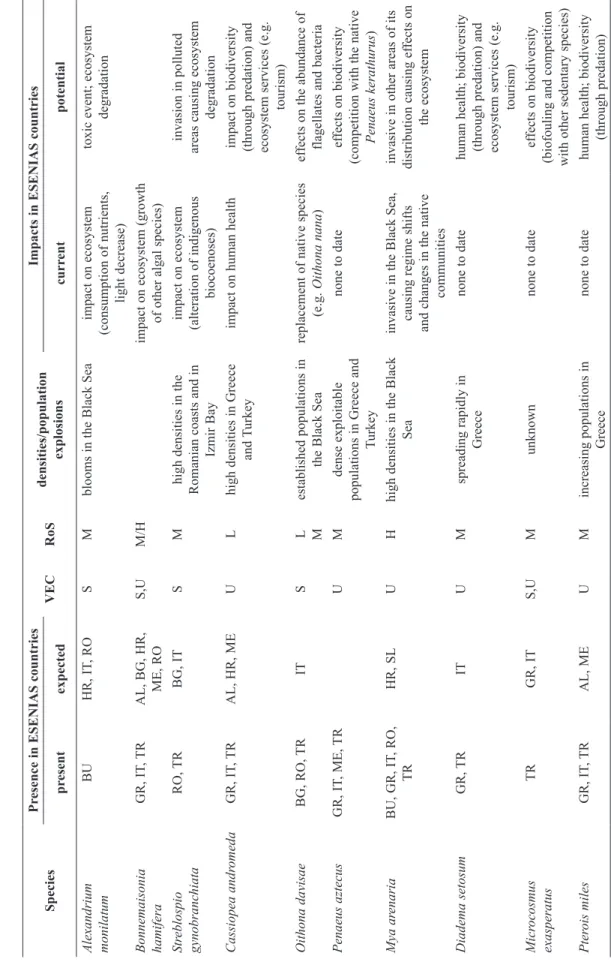
Table 2. Selected
featur
es used for horizon scanning and risk assessment, for the ten species that fact sheets wer
e compiled within the ESENIAS-T ools pr oject. AL=Albania, BG=Bulgaria, GR=Gr eece, HR=Cr oatia, IT=Italy , ME=Montenegr o, RO=Romania, SL=Slovenia, TR=T urkey . VEC= potential
vector of expansion, S=shipping, U=unaided,
RoS= risk of spr
ead, M=Medium, H=high, L=low
Species Pr esence in ESENIAS countries VEC RoS densities/population explosions Impacts in ESENIAS countries pr esent expected curr ent potential Alexandrium monilatum BU HR, IT , RO S M
blooms in the Black
Sea impact on ecosystem (consumption of nutrients, light decrease) toxic event; ecosystem degradation Bonnemaisonia hamifera GR, IT , TR AL, BG, HR, ME, RO S,U M/H impact on ecosystem (growth of other algal species) Str eblospio gynobranchiata RO, TR BG, IT S M
high densities in the
Romanian coasts and in Izmir Bay impact on ecosystem (alteration of indigenous biocoenoses) invasion in polluted
areas causing ecosystem
degradation Cassiopea andr omeda GR, IT , TR AL, HR, ME U L
high densities in Greece
and T urkey impact on human health impact on biodiversity (through predation) and ecosystem services (e.g. tourism) Oithona davisae BG, RO, TR IT S L M established populations in the Black Sea replacement of native species (e.g. Oithona nana ) ef
fects on the abundance
of flagellates and bacteria Penaeus aztecus GR, IT , ME, TR U M dense exploitable populations in Greece and Turkey none to date ef fects on biodiversity (competition
with the native
Penaeus kerathurus ) Mya ar enaria BU, GR, IT , RO, TR HR, SL U H
high densities in the Black
Sea
invasive
in the Black Sea,
causing regime
shifts
and changes in the native
communities
invasive
in other areas of its
distribution causing ef fects on the ecosystem Diadema setosum GR, TR IT U M spreading rapidly in Greece none to date human health; biodiversity (through predation) and ecosystem services (e.g. tourism) Micr ocosmus exasperatus TR GR, IT S,U M unknown none to date ef fects on biodiversity (biofouling and competition
with other sedentary species)
Pter ois miles GR, IT , TR AL, ME U M increasing populations in Greece none to date human health; biodiversity (through predation)
Bonnemaisonia hamifera Hariot (Fig. 3)
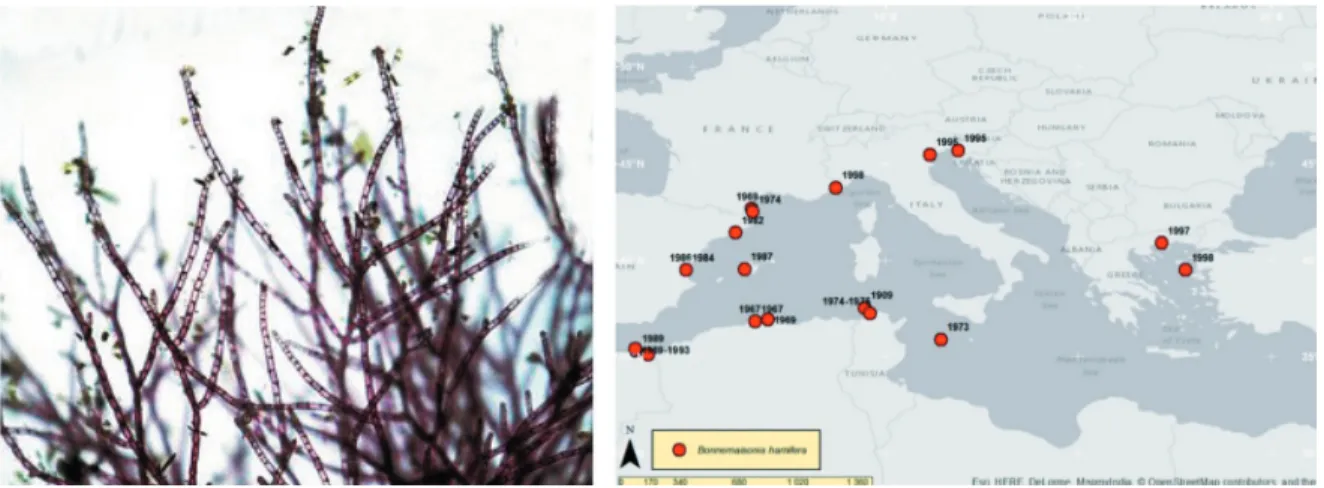
Bonnemaisonia hamifera is a red alga with
a heteromorphic cycle with alternation between a large, coarsely branched gametophyte and a delicate filamentous sporophyte, originally described as a separate species (Trailliella
intri-cata Batters). Native in the North West Pacific, B. hamifera was first recorded in Europe in the
British Isles (Dorset) in 1890. The species was probably introduced by shipping. Nowadays, it is widely distributed on southern and western coasts to Shetland Isles, and can be abundant in certain locations, notably where there are large, lagoon-like lower intertidal pools. In the Medi-terranean Sea, the first record was as “Trailliella
intricata” in 1909 from Tunisia. The
gameto-phytic phase seems to have been observed only in Spain, close to Gibraltar and in Catalonia, whereas the sporophytic phase has been record-ed all around the basin (VERLAQUE et al., 2015).
Bonnemaisonia hamifera can be found on rocks
and on various benthic organisms.
In the north-eastern Atlantic, B. hamifera can have a negative impact as an ecosystem engineer by forming dense epiphytic growth on host algae (JOHANSSON et al., 1998), thus reducing
light and nutrient availability for those algae, in addition to preventing competing algae to colo-nize (SVENSSON et al., 2013). By its high ratio of
surface to volume, it has a greater potential for rapid uptake of nutrients in comparison to their host algae (LITTLER & LITTLER, 1980).
Native herbivores strongly preferred native algae to B. hamifera. Bonnemaisonia hamifera produces chemical grazer deterrents, mainly the secondary metabolite 1,1,3,3-tetrabromo-2-heptanone not known from the native algae of the invaded area and the importance of the chemical defence was underlined by the feeding preference of herbivores for B. hamifera indi-viduals with an experimentally depleted content of 1,1,3,3-tetrabromo-2-heptanone (ENGE et al.,
2012). Herbivores used B. hamifera as a refuge
for fish predation. As a result, the presence of herbivores decreases the performance of neigh-bouring native algae and increases growth and relative abundance of B. hamifera (ENGE et al.,
2013). The 1,1,3,3-tetrabromo-2-heptanone also
works as an allelopathic compound that prevents settlement of epiphytic organisms and that can be transferred from B. hamifera to a native host algal species by direct contact, with an active and unaltered function, i.e. in inhibiting recruit-ment of native competitors (SVENSSON et al.,
2013). The secondary metabolite affects the
natu-ral fouling community by altering the composi-tion, and changed the diversity by increasing the evenness and decreasing the density, indicating a broad specificity of this metabolite against bacterial colonization (e.g. PERSSON et al., 2011).
Optimal experimental conditions for the biomass production of the sporophytic phase are represented by a combination of temperatures of 15-20°C, photon irradiances of 20-30 µmol photons m-2 s-1 and long daylengths (16:8 h L:D)
(NASH et al., 2005).
Fig. 3. Bonnemaisonia hamifera Hariot (left; Photo: Marjan Richter) and its distribution in the Mediterranean Sea (TRICHKOVA et al., 2017). Scale bar=1 cm
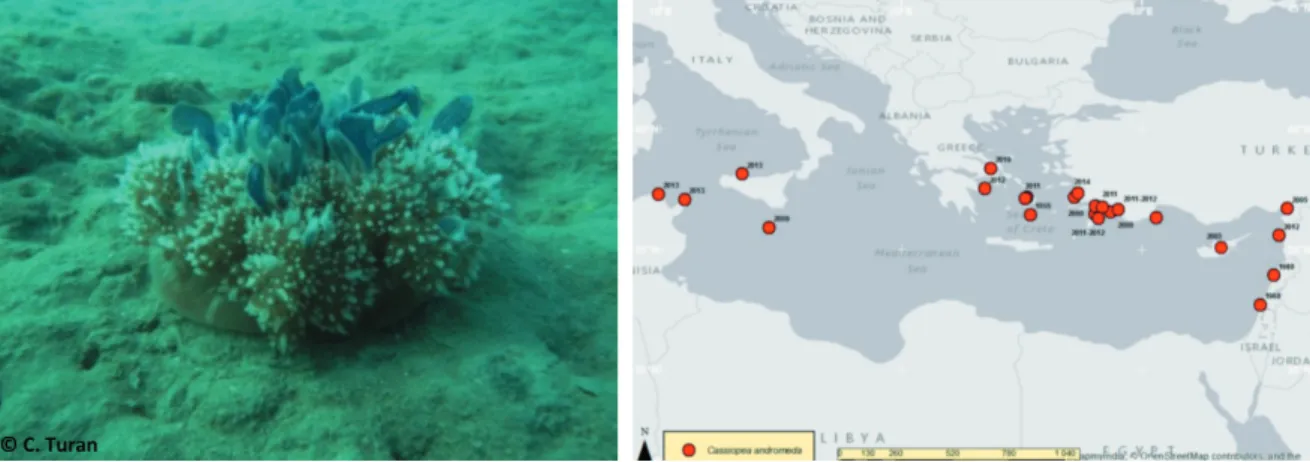
Cassiopea andromeda (Forsskål, 1775) (Fig. 4) Cassiopea andromeda is a globally
dis-tributed in warm coastal regions semi-sessile, planktonically dispersed scyphomedusa, typi-cally found in shallow lagoons, intertidal sand or mud flats, and around mangroves in Florida, and in the Caribbean. Its native distribution extends from the West Indian Ocean to West Pacific Ocean, and the tropical Atlantic (HOLLAND et al.,
2004). The presence of C. andromeda in the
Med-iterranean Sea dates prior to 1900 (MAAS, 1903).
Cassiopea andromeda is venomous
(PALO-MARES & PAULY, 2016) and can kill smaller fish
and other marine organisms with which they come into contact, and as such is a threat to the ecosystem functioning. The species might cause an imbalance in a closed area like a bay or lagoon, but its motility is very limited, and is unlikely to spread very much.
Cassiopea andromeda is not highly
danger-ous because its sting is very mild. Although there has not been any record on hospitalized events of C. andromeda stings, this jellyfish stinging cell can possibly cause discomfort on thin or sensitive skin, as well as the eyes and lips. Moreover, C. andromeda do not normally swim in the water but lie on the bottom with their umbrella facing and touching the sub-stratum and their arms, which are short and shrubby, pointing towards the surface. Swim-ming over the jellyfish (especially using swim fins) may cause the transparent, essentially invisible, sheets of the mucus to rise in the water
column. The stings, appearing in the form of a red rash-like skin irritation, are known for being extraordinarily itchy.
Due to its appearance and nature it may bring people snorkelling or diving into the areas in which the jelly lives, adding to the local economy.
Cassiopea andromeda feeds on copepods,
cladocerans, mollusc larvae and on pelagic fish eggs and larvae (PALOMARES & PAULY, 2016). The
predation upon fish larvae and eggs could poten-tially cause impacts on fisheries, especially in the case of consumption of commercial species, yet this is a hypothesis that needs to be further investigated.
No risk assessment analysis on biodiversity, ecosystem services or economy has been con-ducted. However, its presence in Turkish and Greek coastal areas that are favourite tourist destinations may lead to significant loss of eco-system services.
Mya arenaria Linnaeus, 1758 (Fig. 5)
Mya arenaria is a bivalve that lives
bur-rowed in muddy, sandy and gravelly sediments. It adapts well to a wide range of salinities and temperatures, and can survive both in pristine and disturbed/polluted habitats, including oxy-gen depleted environments, down to ≥200 m deep.
Archaeological evidence seems to suggest that Mya arenaria originated in the Pacific Ocean during the Miocene, and extended its range in the early Pliocene to the Atlantic.
Fig. 4. Cassiopea andromeda (Forsskål, 1775) (left; Photo: Cemal Turan) and its distribution in the Mediterranean Sea (TRICHKOVA et al., 2017; modified)
However, the Pacific and European popula-tions became extinct during the Pleistocene glaciations, leaving only the Northwest Atlantic population alive. Mya arenaria only recolonized both areas in historical times, and now occurs in a wide North East Atlantic area from the Bar-ents Sea to Portugal, including the Baltic Sea
(STRASSER, 1999; CROSS et al., 2016; LASOTA et al.,
2016). It was also recently sighted in the
Medi-terranean and Black Sea, including the Turkish Straits (Bosphorus and Marmara Sea), where its presence is confirmed in five ESENIAS coun-tries (Fig. 5). There are no convincing records from the Dardanelles and the Turkish Black Sea so far, presumably due to lack of field studies.
Mya arenaria local abundances and
estab-lishment success vary within the different ESE-NIAS countries. It is established in the Mediter-ranean Sea and the Turkish Straits, but with very low abundances and/or restricted ranges except France, where the species spread and become abundant in the Gulf of Lion (ZIBROWIUS, 2002;
CROCETTA & TUROLLA, 2011). In the Black Sea,
soon after its first record from Ukraine (1966)
(BESHEVLY & KALYAGIN, 1967), the species
estab-lished and became dominant in the north west-ern and westwest-ern parts of the Black Sea, and in the Sea of Azov (ZOLOTAREV, 1996). On the Bulgarian shelf, M. arenaria is widely distribut-ed on sandy sdistribut-ediments in low salinity waters and at some sites it reaches densities of 4860 indi-viduals m-2(MARINOV, 1990). In the north western part of the Black Sea it is especially numerous in the coastal zones on muddy sediments, where its biomass exceeds 1 kg m-2 (ZOLOTAREV, 1996).
Mya arenaria impacts vary within the
differ-ent ESENIAS countries. No impacts have been reported for the Mediterranean and the Turkish Straits, but the taxon shows invasive properties in the Black Sea dominating the soft substratum communities, causing regime shifts and structur-al changes in native communities/invaded habi-tats, and affecting sediment and water-column characteristics (KATSANEVAKIS et al., 2014).
In its native area, M. arenaria is considered a delicacy, and is harvested by commercial fish-ery for cookfish-ery purposes. On the contrary, to date, the species is not commercially exploited in Europe. It is also a food source for migrating shorebirds and for a wide number of sea inhabit-ants (fish, sandworms and crabs, among others)
(COHEN, 2005). As a suspension feeder, it plays
a crucial role in filtering and cleaning water sources, and can be used as a tool in regulating and enforcing pollution standards in water qual-ity control. It sequesters carbon in the form of calcium carbonate used for shell creation (KAT-SANEVAKIS et al., 2014).
Streblospio gynobranchiata Rice & Levin, 1998
(Fig. 6)
Streblospio gynobranchiata is a
shallow-water tube-dwelling polychaete species, found in muddy sediments of estuaries, coastal areas and harbours, at depths ranging from 0.5 to 35.6 m, where it forms dense aggregations. CINAR et
al. (2005b) reported densities of up to 34,740 individuals m-2. It prefers the upper layer of the muddy sediments with high levels of organ-ic enrorgan-ichment, rorgan-ich in hydrogen sulphide and
Fig. 5. Mya arenaria Linnaeus, 1758 (left; Photo: Edoardo Turolla) and its distribution in the Mediterranean and Black Seas (TRICHKOVA et al., 2017; modified)
organic nitrogen (RADASHEVSKY & SELIFONOVA,
2013; BOLTACHOVA et al., 2015). The tolerance
of S. gynobranchiata to low concentrations of dissolved oxygen contributes to the colo-nization of substrates in severely polluted and physically degraded environments. Streblospio
gynobranchiata is considered as one of the most
successful pioneer and opportunistic species. The native distribution area of this species is the western Atlantic (RICE & LEVIN, 1998;
RADASHEVSKY & SELIFONOVA, 2013). It is one of
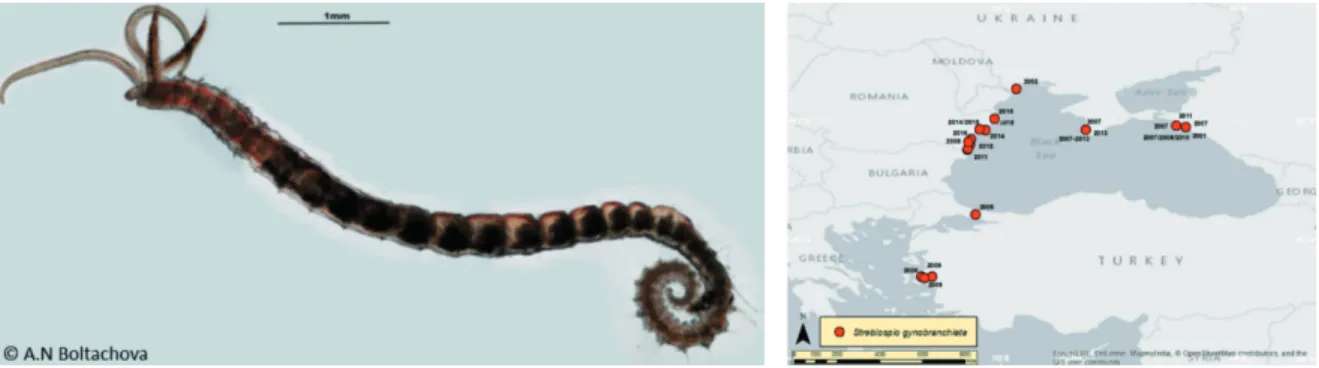
the worst invasive species in the Mediterranean basin, where it was probably introduced via ships ballast waters (ÇINAR et al., 2005a, b). It was
first mentioned in the ESENIAS geographic area from the Aegean Turkish littoral (Izmir Bay), where it has been established since 2000 (ÇINAR
et al., 2005a, b; 2006; DAĞLI et al., 2011). This species
established large and dense populations in the Sea of Marmara (Istanbul area, Golden Horn Estuary, Bosporus Strait; ÇINAR et al., 2009). It
was also reported in the Black Sea (MURINA et al., 2008; BOLTACHOVA, 2008; RADASHEVSKY & SELI-FONOVA, 2013; BOLTACHOVA et al., 2015; TEACĂ et
al., 2015) and the Caspian Sea (TAHERI et al., 2008).
Recently, in 2015, this species was mentioned from the north-eastern part of the Azov Sea, from Taganrog Bay and from the Don River Delta (SYOMIN et al., 2017).
To date, the impact in the ESENIAS area is a local one, with populations establishing especially in harbour areas and polluted or degraded habitats. The dense populations of the polychaete change the indigenous biocoenoses, especially in polluted soft-bottom benthic habi-tats (ÇINAR et al., 2005b, 2011).
Dense populations and high biomass of S.
gynobranchiata and the position it holds in the
food-web as a deposit feeder in areas highly polluted with organic matter have an obvious ecological impact. In such habitats, S.
gyno-branchiata prove to be a dominant species, as
well as a pollution indicator. As a result of the species biology and the large ecological toler-ance of S. gynobranchiata, harbour areas with huge traffic and narrow gulfs with muddy bot-toms are the most sensitive areas where this spionid polychaete could establish and develop large and dense populations replacing native species. The only measures to avoid the estab-lishment of this species could be maintaining low pollution and eutrophication levels and avoiding discharge of ballast waters in the coastal areas.
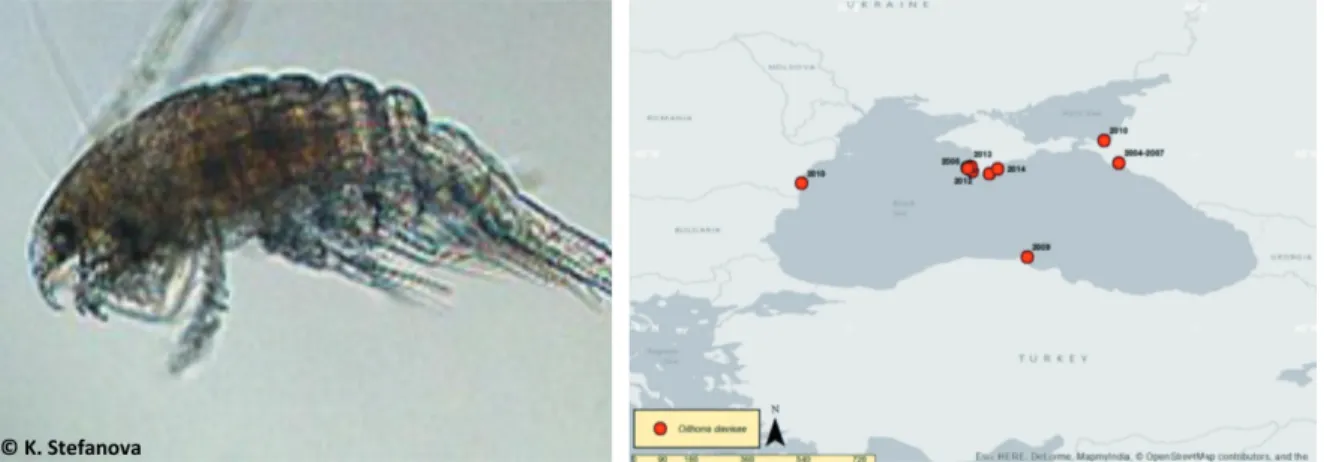
Oithona davisae Ferrari & Orsi, 1984 (Fig. 7) Oithona davisae is a pelagic cyclopoid
cope-pod which could be the most abundant meso-zooplankton species in its native habitat. It is widely spread around the Black Sea: during the first years the species developed mainly in the coastal waters but now could reach also deep waters above the depth 1000 m. In the coastal and open Black Sea this new Oithona taxon occupied the niche of the disappeared Oithona
nana Giesbrecht, 1893 (SHIGANOVA et al., 2012).
It is widely accepted that O. davisae is origi-nally endemic to the temperate coastal waters of East Asia and its occurrence in other remote regions is due to anthropogenic introduction, mainly through ballast waters (FERRARI & ORSI,
1984; NISHIDA, 1985; HIRAKAWA, 1988). Oithona
Fig. 6. Streblospio gynobranchiata Rice & Levin, 1998 (left; Photo: Natalya A. Boltachova) and its distribution in the Mediterranean and Black Seas (TRICHKOVA et al., 2017)
davisae usually inhabits eutrophic embayments
(UYE & SANO, 1995; ALMEDA et al., 2011) and is
indigenous to Japan and the China Seas, and many coastal areas (RAZOULS et al., 2012), but it
is invasive along the USA west coast (FERRARI &
ORSI, 1984) and the Spanish Mediterranean (SAIZ
et al., 2003). The invasive successful establishment
and expansion of the thermophilic copepod O.
davisae in the cold Black Sea is evidence of the
extremely high adaptive plasticity of this spe-cies. The successful establishment of O. davisae to the Black Sea seems related to phytoplankton structure changes including a prevalence of small flagellates due to climate-driven effects
(NESTEROVA et al., 2008; MAVRODIEVA, 2012). It
appears the species has expanded its distribution in the Azov Sea, as it: it has been reported in the Temruk Bay in 2010 (SVISTUNOVA, 2013).
At present, O. davisae successfully com-petes with the larger copepods Acartia tonsa Dana, 1849 and Acartia clausi Giesbrecht, 1889 in the Black Sea. It is still not clear why O.
davisae successfully occupied the ecological
niche of the disappeared O. nana, while the lat-ter is not successful to return. Because of the specific features of O. davisae, e.g. feeding on flagellates and not on diatoms and detritus, their mass development could affect the abundance of flagellates and bacteria (ROFF et al., 1995).
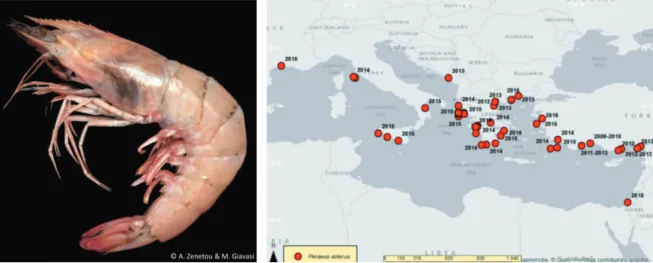
Penaeus aztecus Ives, 1891 (Fig. 8)
Penaeus aztecus is a shrimp species, an
active swimmer and burrower (SMITHSONIAN
MARINE STATION AT FORT PIERCE, 2016), with
the juveniles living also in estuarine habitats. It is found from the coastline up to 160 m deep, on muddy or sandy-muddy bottoms, sometimes mixed with sand, clay or broken shells. The adults are mostly active at night, burying in the substrate at daytime (HOLTHUIS, 1980; TAVARES, 2002).
The native distribution of the species is in the western Atlantic (PALOMARES & PAULY,
2016). Penaeus aztecus has also been introduced
to New Caledonia and French Polynesia in the 1970s for aquaculture purposes (PALOMARES &
PAULY, 2016). The first record of the species in
the Mediterranean Sea was in 2009 in Antalya
Bay (DEVAL et al., 2010), and by 2013 the
spe-cies had a well-established population in the area (GÖKOĞLU & ÖZVAROL, 2013; ÖZVAROL
& GÖKOĞLU, 2014). Nowadays, P. aztecus has
been reported from various areas throughout the Mediterranean, revealing the fast spreading of the species in the basin.
The mode of introduction of P. aztecus in the Mediterranean is still not clear. Some authors consider that it was most likely transferred from the USA through ballast waters (DEVAL et al.,
2010; KEVREKIDIS, 2014), whilst more recent
dis-cussions (CRUSCANTI et al., 2015; GALIL et al., 2016)
suggest that it may be a result of illegal introduc-tions (an aquaculture release/escapee). Yet, both hypotheses need further investigation.
To date, no impacts have been reported for the Mediterranean, but as the species shares the same niche and has similar biological traits with
Fig. 7. Oithona davisae Ferrari & Orsi, 1984 (left; Photo: Kremena Stefanova) and its distribution in the Black Sea (TRICHKOVA et al., 2017)
the indigenous commercial Penaeus kerathurus (Forskål, 1775) (KEVREKIDIS, 2014, 2015) the
interaction amongst the two species should be monitored and assessed. In the Mediterranean,
P. aztecus is caught with both bottom trawlers
and trammel nets, along with P. kerathurus. Due to the low catches, it is not yet marketed nor reported separately. Nevertheless, the larger size it can attain compared to that of P. kerathurus, and given the fact that its presence in the catches is constantly increasing (GÖKOĞLU & ÖZVAROL,
2013), may result in its commercial exploitation
in the Mediterranean Sea. Yet, the fact that P.
aztecus is infested by the parasitic isopod Epipe-naeon ingens (Nobili, 1906), combined with the
fact that parasitic bopyrid isopods as E. ingens have been found to affect its growth and repro-duction (KORUN et al., 2013 and references therein),
might lead to a natural control of P. aztecus populations. However, this hypothesis needs to be monitored and further investigated.
Diadema setosum (Leske, 1778) (Fig. 9)
Diadema setosum is a venomous sea urchin
(needlespined urchin, long-spined sea urchin) with distinctively delicate hollow spines that inhabits hard substrata, rocks covered with veg-etation, and coral reefs. It is widely distributed in the West Indo-Pacific, from the Red Sea, Arabian Gulf, East coast of Africa, to India, Australia and Japan at depths ranging from 0 to 70 m. In the Red Sea, Gulf of Thailand and other native regions, it is the most common sea urchin and one of the most abundant benthic invertebrates.
Fig. 8. Penaeus aztecus Ives, 1891 (left; Photo: Argyro Zenetos & Maria Giavasi) and its distribution in the Mediterranean Sea (TRICHKOVA et al., 2017)
Fig. 9. Diadema setosum (Leske, 1778) (left; Photo: A. Liami) and its distribution in the Mediterranean Sea (TRICHKOVA et al., 2017; modified)
The tropical sea urchin fauna, which sur-vived the Messinian crisis, but the sudden cool-ing that started with the Arctic glaciation 2.58 million years ago, led to the end of the tropical fauna of the Mediterranean during the third Pliocene phase (POR, 2009). Yet, Diadema,
re-col-onized the Mediterranean after an interruption of more than two million years (YOKES & GALIL,
2006). Nowadays, the species is established in the
eastern Mediterranean Sea (Fig. 9).
No impact on biodiversity has been reported for the Mediterranean. It is a food source for many reef fishes. The most notable predators of adult sea urchins in the tropical Indo-Pacific are certain large reef fishes, particularly the larger wrasses, triggerfishes, puffers and por-cupine fishes. In coral reef aquaria, it offers useful microhabitats to many organisms and it is considered an excellent algae controller. The risk of possible overgrazing phenomena in the ESENIAS countries deserves further study.
Diadema setosum is of medical importance.
The species requires particular care when han-dling or working around it. Its long, slender spines may inflict painful injuries on the unwary swimmers, divers and fishermen. The spines are brittle and hollow, with barbed tips that penetrate the skin and remain imbedded in the flesh, releasing venom from their tissue and lumen. The venom may cause redness, swell-ing, and acute pain, which subsides after a few hours; however, spine fragments are difficult to
remove, and healing may take several weeks
(YOKES & GALIL, 2006). The venom of D.
seto-sumis not at all fatal tohumans.
Edible and eaten in some native areas, but it is not very palatable. Diadema setosum is also involved in the marine aquarium trade.
Microcosmus exasperatus Heller, 1878 (Fig. 10) Microcosmus exasperatus is a solitary
ascid-ian with long siphons and leathery bright orange tunic with a few encrustations around the base. It typically attaches to natural and mostly arti-ficial marine hard substrates, forming dense aggregations, which may be heavily fouled by a numerous epibionts.
Microcosmus exasperatus shows a
circum-tropical distribution, being widely recorded from both the Atlantic and the Indo-Pacific regions, including the Red Sea. According to
RAMOS-ESPLA et al. (2013) M. exasperatus was first
recorded from Djerba Island (south of Tunisia) in 1998 and then it was observed on the coasts of Lebanon and Israel. It is commonly considered as being a Lessepsian immigrant due to multiple records from its easternmost shores. However,
Microcosmus taxa are difficult to be identified,
and therefore M. exasperatus worldwide iden-tifications and possible spread patterns may be re-assessed by molecular taxonomy and phylo-geography (genbank number: KT387604). Its alien Mediterranean distribution only includes
Fig. 10. Microcosmus exasperatus Heller, 1878 (left; Photo: Noa Shenkar) and its distribution in the Mediterranean Sea (TRICHKOVA et al., 2017)
one ESENIAS country and one main sea (Tur-key, Mediterranean Sea), where the taxon is only known on the basis of a single specimen
(RAMOS-ESPLÁ et al., 2013). Studies that are more
recent have revealed that it is established in the region (ME Cinar, unpubl. Info.). However, M.
exasperatus was recently recorded from Cyprus
(GEWING et al., 2016), and its known distribution
may be easily concealed by taxonomic impedi-ments and low ascidian research effort.
Native Microcosmus taxa are commercially exploited in the Mediterranean and consumed in France, Italy and Greece due to their strong iodine taste. The high nutritional values and the most likely similar taste of the alien taxon would suggest a possible use for culinary purposes. The typical dense populations and heavy epibi-ont coverage of M. exasperatus may cepibi-ontribute to its establishment as an ecosystem engineer species, as already reported for other worldwide tunicates, and may cause problems when associ-ated with other fouling species.
Pterois miles (Bennett, 1828) (Fig. 11)
A tropical marine fish species, Pterois miles is a reef associated species, living depth range 25-85 m, in a variety of habitats, including natural hard bottom, artificial structures, wrecks, bridge pilings, and seagrass (FROESE & PAULY, 2016).
Outside its native range, the species is reported along the southeastern United States coast from Florida to North Carolina, from Ber-muda, Bahamas, and is becoming established in
the Gulf of Mexico and the Caribbean, including northern South America, and Brazil (SCHOFIELD, 2010; FERREIRA et al., 2015).
In the Mediterranean, a single record from Israel in 1991 (GOLANI & SONIN, 1992), had no
follow up for about 20 years. However, a wave of P. miles invasion struck the eastern Medi-terranean in the 2010s and is continuing at an increasing rate. In 2012 it was reported from Lebanon (BARICHE et al., 2013), and a year later
from Cyprus (EVRIPIDOU, 2013) where, in just
one year, it had colonized nearly all of Cyprus’ southeastern coast (JIMENEZ et al., 2016). In the
following years, the species was sighted in sev-eral Mediterranean locations (Fig. 11).
Pterois miles is native to the Indo-Pacific
realm (FROESE & PAULY, 2016), and it is thought to
have been introduced to the western Atlantic in the mid 1990’s by aquarists (HARE & WHITFIELD,
2003). In the Mediterranean, the most likely
introduction vector is considered to be the Suez Canal either through ballast water released from vessels crossing the canal (even though glob-ally successful introductions of Scorpaenidae through ballast water have not been reported;
see HARE & WHITFIELD, 2003) or by adult
migra-tion. However, secondary introductions through additional aquarium release events cannot be ruled out, and genetic connectivity studies are required to trace the origin of the Mediterranean populations. A recent genetic study presents evidence, albeit from a limited sample size, that supports the immigration of a few individuals from the Red Sea via the Suez Canal as the most
Fig. 11. Pterois miles (Bennett, 1828) (left; Photo: Gerasimos Kondylatos) and its distribution in the Mediterranean Sea (TRICHKOVA et al., 2017; modified)
likely introduction mechanism (BARICHE et al,. 2017).
In the west Atlantic invaded range, lionfish have reached densities that are far higher than those reported from their native range
(KUL-BICKI et al,. 2012), exhibit extraordinary predation
rates and are having dramatic impacts on the Caribbean ecosystem by displacing native spe-cies and disrupting food webs (LESSER & SLAT-ERY 2011; ALBINS 2013).
In the Mediterranean, owing to the young age of the invasion, strong impacts on ecosys-tems have not been reported yet, as is the case in the invaded Atlantic distribution (for details see ALBINS & HIXON, 2008; LESSER & SLATERY, 2011; GREEN et al., 2012, 2014; ALBINS, 2013; FALETTI
et al., 2013; BENKWITT, 2015; BALLEW et al., 2016;
PALMER et al., 2016) but are the subject of ongoing
research. In addition, the full extent of the socio-economic damage potentially caused by lionfish is yet to be realised, since the full spectrum and intensity of its ecological impacts and interac-tions with native species in not fully known.
Pterois miles has a direct impact on human
health. The sting from its venomous spines can cause irritation, inflammation, pain and even serious complications in the case of an allergic reaction, putting fishermen, divers and other potential stakeholders at risk. On the other hand, it appears to be very attractive to divers, many of who specifically request dives in areas where the lionfish is known to be present (JIMENEZ
et al., 2017), at least in the Eastern
Mediterra-nean, where the species is still a novel sighting, similar to what was observed in the Atlantic in the early stages of the invasion. Regardless of its venomous spines, the species is a popular aquarium fish and may create an extra source of income for fishermen who capture and supply the aquarium trade. Moreover, it is consumed in subsistence fisheries in its native area and can provide an alternative fish stock and food source
in the invaded range. Its consumption is already promoted in some countries as a means of intro-ducing control measures (NUÑEZ et al., 2012).
CONCLUSIONS
This is the first effort to compile a list of marine invasive/potential invasive species in the ESENIAS countries. Such lists are an important initial step for the development of an early warn-ing system, through a horizon scannwarn-ing process
(ROY et al., 2015). Hence, based on the
informa-tion presented here identificainforma-tion of future inva-sions in the ESENIAS countries is possible. It is essential, as a way forward for the countries referred herewith in, to further plan/enforce effective early warning and rapid response mechanisms, as well as take management meas-ures for species that are already widely spread, in compliance with the EU Regulations (EU,
2014). Yet, such a list should be regularly revised
and updated so that the management measures enforced would be effective.
ACKNOWLEDGMENTS
This study was conducted in the frame of the project entitled “East and South European Net-work for Invasive Alien Species - a tool to sup-port the management of alien species in Bulgaria (ESENIAS-TOOLS)”, an EEA funded project (Contract No. Д-33-51/30.06.2015). The authors would also like to thank the following col-leagues for providing photos: M. RICHTER (photo
of Bonnemaisonia hamifera), C. TURAN (photo
of Cassiopea andromeda), E. TUROLLA (photo
of Mya arenaria), N.A. BOLTACHOVA (photo of
Streblospio gynobranchiata), A. LIAMI (photo of
Diadema setosum) and G. KONDYLATOS (photo
of Pterois miles). Finally, special thanks to L.
FILIPOV for creating the ESENIAS maps, and
REFERENCES
ALBINS, M.A. 2013. Effects of invasive Pacific
red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish com-munities. Biol. Invasions, 15: 29-43.
ALBINS, M.A. & M.A. HIXON. 2008. Invasive
Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reeffishes.Mar. Ecol. Prog. Ser.,367: 233-238.
ALDRICH, D.V., S.M. RAY & W.B. WILSON. 1967.
Gonyaulax monilata: population growth and
development of toxicity in cultures. J. Proto-zool., 14: 636-639.
ALMEDA, R., M. ALCARAZ, A. CALBET & E. SAIZ.
2011. Metabolic rates and carbon budget of
early developmental stages of the marine cyclopoid copepod Oithona davisae. Lim-nol. Oceanogr., 56(1): 403-414.
ANONYMOUS. 2015. Alexandrium monilatum.
In: AquaNIS. Editorial Board, 2015. Infor-mation system on Aquatic Non-Indigenous and Cryptogenic Species. World Wide Web electronic publication. http://www.corpi.ku.lt/ databases/index.php/aquanis/introductions/view/ id/4053. Accessed on 2017-06-26
BALECH, E. 1995. The genus Alexandrium Halim
(Dinoflagellata). Publ. Sherkin Island marine station Sherkin Island Co. Cork Ireland, 151 pp.
BALLEW, N.G., N.M. BACHELER, G.T. KELLISON
& A.M. SCHUELLER. 2016. Invasive lionfish
reduce native fish abundance on a regional scale. Sci. Rep., 6: 32169.
BARICHE, M., M. TORRES & E. AZZURRO. 2013. The
presence of the invasive Lionfish Pterois
miles in the Mediterranean Sea. Medit. Mar.
Sci., 14(2): 292-294.
BARICHE, M., P. KLEITOU, S. KALOGIROU & G.
BERNARDI. 2017. Genetics reveal the
iden-tity and origin of the lionfish invasion in the Mediterranean Sea. Sci. Rep. 7: 6782. doi:10.1038/s41598-017-07326-1.
BENKWITT, C.E. 2015. Non-linear effects of
inva-sive lionfish density on native coral-reef fish communities. Biol. Invasions, 17: 1383– 1395.
BESHEVLY, L.E. & V.A. KALYAGIN. 1967. The
find-ing of the mollusc Mya arenaria L.
(Bival-via) in the northwestern part of the Black Sea. Vestnik Zool., 3: 82–84 (in Russian).
BOLTACHOVA, N.A. 2008. Finding of new alien
species Streblospio gynobranhiata Rice et Levin, 1998 (Polychaeta: Spionidae) in the Black Sea. Mar. Ecol. J., 7(4): p. 12 (in Rus-sian).
BOLTACHOVA, N.A., E.V. LISITSKAYA & D.V.
POD-ZOROVA. 2015. The population dynamics and
reproduction of Streblospio gynobranchiata (Annelida, Spionidae), an alien polychaete worm, in the Sevastopol Bay (the Black Sea). Ecol. Mont., 4: 22-28.
CBD. 2014. Pathways of Introduction of Invasive
Species, their Prioritization and Manage-ment. UNEP/CBD/SBSTTA/18/9/Add.1. Secretariat of the Convention on Biological Diversity, Montréal. Pages??
ÇINAR, M.E., H. BALKIS, S. ALBAYRAK, E. DAĞLI
& S.A. KARHAN. 2009. Distribution of
poly-chaete species (Annelida: Polychaeta) on the polluted soft substrate of the Golden Horn Estuary (Sea of Marmara), with special emphasis on alien species. Cah. Biol. Mar., 50: 11-17.
ÇINAR, M.E., M. BILECENOĞLU, B. ÖZTÜRK, T.
KATAGAN, & V. AYSEL. 2005a. Alien species on
the coast of Turkey. Medit. Mar. Sci., 6(2): 119-146.
ÇINAR, M.E., M. BILECENOĞLU, B. ÖZTÜRK, T. KATAGAN, Μ.Β. YOKES, V. AYSEL, E. DAĞLI,
S. ACIK, T. ÖZCAN & H. ERDOGAN. 2011. An
updated review of alien species on the coasts of Turkey. Medit. Mar. Sci., 12(2): 257-315.
ÇINAR, M.E., Z. ERGEN, E. DAGLI & M.E.
PETERS-EN. 2005b. Alien species of spionid
poly-chaetes (Streblospio gynobranchiata and
Polydora cornuta) in Izmir Bay, eastern
Mediterranean. J. Mar. Biol. Assoc. U.K., 85(4): 821-827.
ÇINAR, M.E., T. KATAGAN, B. ÖZTÜRK, Ö. EGEMEN, Z. ERGEN, A. KOCATAS, M. ÖNEN, F. KIRKIM, K. BAKIR, G.KURT, E. DAĞLI, A. KAYMAKÇI, S.
AÇIK, A. DOGAN & T. ÖZCAN. 2006. Temporal
changes of soft-bottom zoobenthic commu-nities in and around Alsancak Harbor (Izmir
Bay, Aegean Sea), with special attention to the autecology of exotic species, Mar. Ecol., 27: 229-246.
CLEMONS, G.P., D.V. PHAM & J.P. PINION. 1980a.
Insecticidal acitivity of Gonyaulax (Dino-phyceae) cell powders and saxitoxin to the German cockroach. J. Phycol., 16: 305–307
CLEMONS, G.P., J.P. PINION, E. BASS, D.V. PHAM,
M. SHARIF & J.G. WUTOH. 1980b. A hemolytic
principle associated with the red tide dino-flagellate Gonyaulax monilata. Toxicon, 18: 323-326.
COHEN, A.N. 2005. Guide to the exotic species of San Francisco Bay. San Francisco Estu-ary Institute, Oakland, California. Available at: www.exoticsguide.org LAST ACCESSED - DATE
CONNELL, C.H. & J.B. CROSS. 1950. Mass
mortal-ity of fish associated with the protozoan
Gonyaulax in the Gulf of Mexico. Science,
112: 359.
CROCETTA. F. & E. TUROLLA. 2011. Mya arenaria
Linné, 1758 (Mollusca: Bivalvia) in the Mediterranean: its distribution revisited. J. Biol. Res.-Thessaloniki, 16: 188-193
CROSS, M.E., C.R. BRADLEY, T.F. CROSS, S. CULLO-TY, S. LYNCH, P. MCGINNICULLO-TY, R.M. O’RIORDAN,
S. VARTIA & P.A. PRODÖHL. 2016. Genetic
evidence supports recolonisation by Mya
arenaria (L.) of Western Europe from North
America. Mar. Ecol. Prog. Ser., 549: 99-112.
CRUSCANTI, M., G. INNOCENTI, J.R. ALVARADO
BREMER & B.S. GALIL. 2015. First report of
the brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Decapoda, Penaeidae) in the Tyrrhenian Sea. Mar. Biodiv. Records, 8: 1-4.
DAĞLI, E., M.E. ÇINAR & Z. ERGEN. 2011.
Spioni-dae (Annelida: Polychaeta) from the Aegean Sea (eastern Mediterranean). Ital. J. Zool., 78(S1): 49-64.
DEVAL, M.C., Y. KAYA, O. GÜVEN, M. GÖKOĞLU &
C. FROGLIA. 2010. An unexpected find of the
western Atlantic shrimp, Farfantepenaeus
aztecus (Ives, 1891) (Decapoda, Penaeidae)
in Antalya bay, Eastern Mediterranean Sea. Crustaceana, 83(12): 1531-1537.
ENGE, S., G.M. NYLUND, T. HARDER & H. PAVIA.
2012. An exotic chemical weapon explains
low herbivore damage in an invasive alga. Ecology,93(12): 2736-2745.
ENGE, S., G.M. NYLUND & H. PAVIA. 2013. Native
generalist herbivores promote invasion of a chemically defended seaweed via refuge mediated apparent competition.Ecol. Lett., 16(4): 487-492.
ERKER, E.F., L.J. SLAUGHTER, E.L. BASS, J. PINION
& J. WUTOH. 1985. Acute toxic effects in mice
of an extract from the marine algae
Gon-yaulax monilata. Toxicon, 23: 761-767.
ESSL, F., S. BACHER, T.M. BLACKBURN, O. BOOY, G. BRUNDU, S. BRUNEL, A.-C. CARDOSO, R. ESCHEN, B. GALLARDO, B. GALIL, E. GARCÍA-BERTHOU, P. GENOVESI, Q. GROOM, C. HAR-ROWER, P.E. HULME, S. KATSANEVAKIS, M. KENIS, I. KÜHN, S. KUMSCHICK, A.F. MARTI-NOU, W. NENTWIG, C. O’FLYNN, S. PAGAD, J. PERGL, P. PYŠEK, W. RABITSCH, D.M. RICH-ARDSON, A. ROQUES, H.E. ROY, R. SCALERA, S. SCHINDLER, H. SEEBENS, S. VANDERHOEVEN, M. VILÀ, JOHN R. U. WILSON, A. ZENETOS
& J.M. JESCHKE. 2015. Crossing frontiers in
tackling pathways of biological invasions. BioScience, 65 (8): 769-782. DOI 10.1093/ biosci/biv082
EU. 2008. Directive of the European
Parlia-ment and the Council Establishing a Frame-work for Community Action in the Field of Marine Environmental Policy (Marine Strat-egy Framework Directive). European Com-mission. Directive 2008/56/EC, OJ L 164
EU. 2011. Our life insurance, our natural
capi-tal: an EU biodiversity strategy to 2020. COM/2011/244, European Commission, Brussels, 16 pp.
EU. 2014. Regulation (EU) No 1143/2014 of the
European Parliament and of the 464 Council of 22 October 2014 on the prevention and management of the introduction and 465 spread of invasive alien species. Official Journal of the European Union, 57, 35.
EVRIPIDOU, S. 2013. Toxic lionfish makes its way
to Cyprus waters. http://www.cyprus-mail. com/cyprus/toxic-Lionfish-makes-its-way-cyprus waters/20130220.
FALETTI, M.E., R.D. ELLIS & F. BAY. 2013. Novel