HAL Id: hal-02413453
https://hal.archives-ouvertes.fr/hal-02413453
Submitted on 2 Dec 2020HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Design of Protein-Coated Carbon Nanotubes Loaded
with Hydrophobic Drugs through Sacrificial Templating
of Mesoporous Silica Shells
Vincent Fiegel, Sebastien Harlepp, Sylvie Begin-colin, Dominique Begin,
Damien Mertz
To cite this version:
Vincent Fiegel, Sebastien Harlepp, Sylvie Begin-colin, Dominique Begin, Damien Mertz. Design of Protein-Coated Carbon Nanotubes Loaded with Hydrophobic Drugs through Sacrificial Templating of Mesoporous Silica Shells. Chemistry - A European Journal, Wiley-VCH Verlag, 2018, 24 (18), pp.4662-4670. �10.1002/chem.201705845�. �hal-02413453�
Design of protein-coated carbon nanotubes loaded
with hydrophobic drugs through sacrificial templating
of mesoporous silica shells
Vincent Fiegel
1,2, Sébastien Harlepp
1,3≠, Sylvie Bégin-Colin
1, Dominique Bégin
2*, Damien
Mertz
1*
1Institut de Physique et Chimie des Matériaux de Strasbourg (IPCMS), UMR-7504 CNRS-Université de Strasbourg, 23 rue du Loess, BP 34 67034, Strasbourg Cedex 2, France, §
2Institut de Chimie et Procédés pour l'Energie, l'Environnement et la Santé (ICPEES), UMR-7515 CNRS-Université de Strasbourg, 25 rue Becquerel, 67087 Strasbourg, Cedex 2, France,
3 INSERM UMR_S1109, MN3T, 67200 Strasbourg, France, ≠current address E-mails :dominique.begin@unistra.fr, damien.mertz@ipcms.unistra.fr
Abstract.
One important challenge in the fields of nanomedecine and tissue engineering is the design of nanoplatforms able to combine therapeutic and detection properties to monitor their therapeutic effect by imaging. Among current developed nano-objects, carbon nanotubes (CNTs) were found suitable to combine imaging and photothermal therapy and they can also be loaded with hydrophobic drugs. However, the main problem remains their resulting low hydrophilicity. To face this problem, an innovative method is addressed here, which consists in loading the surface of carbon nanotubes (CNTs) with drugs followed by a protein coating around them. The originality of this method relies on covering first CNTs by a templating sacrificial mesoporous silica (MS) shell grafted with isobutyramide (IBAM) binders on which a protein nanofilm is non-covalently but strongly cross-linked. Then silica dissolution allows this templating method forming tight human serum albumin (HSA) coating films around the CNTs. This concept demonstrated first without drug, is further improved for the suitable loading of hydrophobic drugs: curcumin (CUR) and camptothecin (CPT) which are retained between the CNTs and HSA layer. Such novel nanocomposites are expected very promising as new theranostic systems combining drug delivery, imaging and photothermal properties.Introduction
The development of CNTs or more generally carbon-based materials as innovative theranostics carriers for biomedical applications which may be used as components of implantable materials has importantly emerged this last decennia.[1–5] Indeed, current challenges in scaffold engineering rely in the design and in the incorporation of multifunctional nano-objects in order to improve their mechanical stability, to favor cell behavior and growth
through local delivery of growth factor (or drugs), and to follow the scaffold evolution by imaging. For two decades, several works reported the use of CNTs as carriers for the delivery of various drugs.[6–10] Furthermore, the property of CNTs to absorb light in the near infrared (NIR) window (range 750-1400nm) where biological tissues exhibit relative transparency and to convert these photons into local heat, makes them highly attractive for phototherapy applications This latter property makes them excellent candidates for drug release triggered by laser-induced hyperthermia. CNTs were also reported to act as contrast agents in different imaging modalities, like NIR Fluorescence, photo-acoustic imaging and Raman imaging[3,11] which can be combined with MRI and magnetic handling[12] by in situ functionalization of magnetic NPs inside their cavity CNTs. They are already used for the mechanical reinforcement of polymer-based scaffolds for tissue engineering and regenerative medecine[13] and were shown to display interesting osteo-inductive and osteo-blasts differentiation capacities, helping in the formation and regeneration of bone tissue.[14] Additionally, they were shown also to degrade in macrophages.[15]
However, given the hydrophobic nature of naked CNTs, huge efforts are implemented in developing strategies for biocompatible/biofouling coatings limiting toxicity and immunogenicity issues and favoring interactions of the CNTs with biological fluids such as blood plasma. Such strategies are highly needed to envision future developments to stabilize CNTs in aqueous solutions, by using either non-covalent adsorption of amphiphilic polymers or by using chemical surface modification followed by covalent grafting of hydrophilic polymers. The non-covalent coating is performed in general by using amphiphilic copolymers such as phospholipid-polyethyleneglycol (PL-PEG)[6,7,16] or F127 Pluronics[9] and even nucleic acids.[17] The covalent approach requires modifying the surface chemistry of the CNTs which can be done according to various strategies : acidic treatment for generating COOH functions, or dipolar cycloadditions for generating NH2 functions.[3] Then various polymers, including biocompatible synthetic polymers such as dendrimers[18] or polyvinylalcohol.[19] can be linked through usual chemical strategies Among biopolymer coatings, the coating of CNTs with proteins is highly attractive and suitable to avoid toxicity, opsonization, immune or inflammatory responses and may provide biological functions.[20] There are rare methods used to immobilize proteins, especially enzymes at the surface of CNTs which are based on the use of pyrene as non-covalent anchoring group followed by covalent binding with the protein through carbodiimidation.[21,22] However as these approaches are based on grafting-to methods, they may suffer from a low surface grafting density. Regarding the drug loading in such polymer-CNTs nanoconstructs, some approaches show that CNTs are suitable nanoplatforms to load hydrophobic drugs in their cavity[23,24] while other approaches of polymer grafted CNTs-polymers allowed the drug
loading by pi-pi stacking[6,7,25]. However these two methods may inhibit their further functionalization with proteins to render them biocompatible and biofunctional.
Aside these grafting methods at the CNTs surface, the coating of a mesoporous silica (MS) shell is particularly interesting as MS brings valuable advantages such as biocompatibility, easy and versatile surface modification and cost-effective process. Single or multi-walled CNTs, have been previously coated with MS shell which may be used as platforms for further functionalization.[26–28] The coating of MS induces an important rise of the surface area of the material from several tens of m².g-1 for the CNT alone up to 500-1000 m².g-1 with mesopores having an average diameter in the range of 2-3 nm which makes this material particularly interesting for drug loading applications. However, despite the huge use of free MS or magnetic MS shells for hydrophilic drug loading[29,30], MS coated CNTs or other carbon based materials were scarcely reported for the loading of hydrophilic drugs, especially DOX [31–33] while the formulation of potent hydrophobic drugs was not reported to date in CNT@MS. In fact, the delivery of hydrophobic drugs is limited as a free injection because of the non-solubility of such molecules in the blood. These free hydrophobic drugs formulations for cancer treatment often need a structural modification like making salts of the drug to increase its water solubility but this lowers its antitumor activity.[34] Thus developing nanoconstructs that can encapsulate hydrophobic drugs to ensure a better transport is highly needed. MS materials appeared as a very good way to deliver the drug without their alteration while solving the water solubility problems. For the few studies reporting hydrophobic drug loaded MS materials[34,35], the release of the drug is not spontaneous and usually occurs when the particles are in contact with the hydrophobic environments within the cells.
To tackle the issue for loading hydrophobic drug in an hydrophilic carrier while ensuring protein functionalization, the idea here is to coat CNTs with a MS shell, load MS with hydrophobic drugs and add a protein layer. Indeed MS may be coated with proteins and further used as a suitable sacrificial matrix template for the formation of thin self-supported protein films. Recently we pioneered a new strategy based on the use of isobutyramide (IBAM) grafts and silica templates to form innovative non-covalent however strongly binded macromolecular nanoassemblies.[36,37] IBAM groups and their derivatives (BrIBAM) grafted at silica surfaces were shown to behave as versatile non-covalent surface binders to form physically stable cross-linked nanofilms through a single step adsorption of macromolecules. It was assumed that the driving force of the IBAM assemblies was attributed to significantly strong H-bonds. This original method allowed forming self-supported microcapsules and nanoparticles purely composed of proteins[38] and other biomacromolecules[37,39] after spherical silica template dissolution and without the need of an additional cross-linking or other adjuvant.
Herein, with the aim to develop a new method to coat protein around CNTs, we have at first investigated the use of a MS shell deposited at the surface of CNTs as a sacrificial template to ensure a tight protein capping around the CNTs after silica removal. Then, we have explored the possibility to load hydrophobic drugs in the pores of the MS shell to afford drug stacking after silica removal (Scheme 1). The design and characterization of MS-coated CNTs by sol gel process is here first reported according to well-established procedures. Then the coating of HSA layers at CNT@MS surface is investigated by applying the original IBAM-HSA strategy and by performing either one or several (n=5) HSA coating. Then subsequent formation of CNTs@HSA nanocomposites after silica dissolution is investigated in details. After the establishment of this protocol, loadings with two hydrophobic anti-tumoral drugs: Curcumin (CUR) and Camptothecin (CPT) are tested following with the HSA gate keeping and silica dissolution with the aim to form new CNTs@drugs@HSA nanocomposites that could be for instance incorporated within bioactive scaffolds.
Scheme 1. Procedure to form protein-coated CNTs loaded with hydrophobic drugs.
Experimental Section
Materials.
All the CNTs used were multi-walled. CNTs with a mean diameter of 60 nm were purchased from Pyrograf (Cedarville, OH, USA). CNTs were pre-treated first with nitric acid then heated at 900°C for 2h under argon flow. Tetraethyl orthosilicate (TEOS) (Sigma-Aldrich), cetyltrimethylammonium bromide (CTAB) (ROTH, >98%), aminopropyltriethoxysilane (APTES) (Sigma Aldrich), isobutyramide chloride (IBC) (Sigma Aldrich), ammonium hydroxide (25% in water) (Fluka), triethylamine (Sigma Aldrich), sodium hydroxide (Carlo Elba), ammoniumnitrate (Sigma Aldrich, >99%), human serum albumin (HSA) (Sigma Aldrich, >97%), rhodamine B isothiocyanate (RITC) (Sigma Aldrich), curcumin (Sigma Aldrich), camptothecin (CPT) (Enzo Life Science), dimethylformamide (DMF) (Carlo Elba) were used as received, without further purification. The HSA functionalized with RITC fluorophore (HSARITC) has been prepared according to previous protocols from Mertz et al.[36,37]
Procedures.
Procedure for the MS coating around the CNTs by sol-gel reaction. This method was
adapted from Bian et al[28]: CTAB (124 mg, 0.24 mmol) was dissolved in a mixture of H2O (30 mL) and EtOH (20 mL), stirred and heated at 60 °C during 2h. Then CNTs (24 mg) were added in the CTAB solution and dispersed by ultrasonication (2 x 10 min, P = 750 W, Amplitude = 40 %, T = 30 °C, Runs : 50’’ ON, 50’’ OFF, Vibracell 75043 from Bioblock Scientific). Sol-gel reaction starts after addition of TEOS (240 µL) and NaOH 1M (60 µL) and the mixture was stirred at room temperature on a mechanical wheel. After 15h of reaction, the composite was washed and centrifuged with EtOH (3 x 20 mL) and dispersed again in EtOH. CTAB extraction from the silica pores was done by mixing the material with NH4NO3 (25 mL at 20 mg/mL in EtOH), stirred and heated at 60 °C during 1h. The surface charge of the silica was measured by Zeta potential to follow the CTAB removal. This extraction was repeated until the CTAB is completely extracted (about 5 extractions are needed). After CTAB extraction, the composite was denoted CNT@MS.
Procedure for the HSA coating around the CNT@MS composite. This method was adapted
from Mertz et al.[36,37] CNT@MS composites were dispersed in EtOH (27mL), NH4OH (1.2 mL, 25 % in water) and APTES (5.0 mL) were added, then the mixture was stirred on the wheel at room temperature. After 2h, the amino-modified composites were isolated by centrifugation and washed with EtOH (20 mL) and DMF (2 x 20 mL). The nanocomposites were dispersed in DMF (16 mL) and Et3N (1.6 mL) was added. A solution containing IBC (2.2 mL) in DMF (16 mL) was separately prepared, and then slowly added to the previous solution. The whole mixture was stirred at room temperature on the wheel for 2h. After the reaction time, a little volume of H2O (ca. 2 mL) was added until the triethylamine salt was dissolved and the CNT@MS-IBAM composites were washed with DMF (2 x 20 mL). Finally, they were dispersed in DMF (1 mL, 5 wt%) and a HSA-RITC solution (10 mL at 0.25 mg/mL of H2O) was added to keep a solvent ratio of 1 : 10 between DMF : H2O. After 1h of stirring at room temperature and light protected, the composites were washed with H2O (20 mL) and dispersed in H2O again. Or, to pursue the HSA layering, washed with DMF (2 x 20 mL) after the H2O washing.
Procedure for the silica dissolution with HF. This method was adapted from Mertz et al.[37]
Mesoporous silica coated materials dispersed in aqueous solution were mixed with HF / NH4F (2/ 7 M) in 1 : 1 proportion and let the dissolution occurs during 10 min. Then the material was washed by ultrafiltration with H2O (4 x 2 mL) and finally dispersed in H2O.
Drug loading. Typically, CNT@MS-IBAM composites (0.5 mL at 10 mg/mL in DMF) and drug
(1.5 mL, at the wished concentration in DMF), were stirred on the wheel at room temperature. After 16h, the mixture was centrifuged and the supernatant was removed. By using UV/vis spectroscopy, the absorption measurements of the original drug solution and the supernatant were compared to determine the amount of drug that was loaded inside the mesoporous silica of the composite. The CNT@MS-IBAM@drug material is then coated with HSA according to a similar procedure as described above and is then washed with H2O (3 x 2 mL) and dispersed in H2O (1 mL).
Characterization methods
TEM microscopy : Morphology of the different nanocomposites were characterized by
transmission electron microscopy (TEM) with a TOPCON 002B ultra high resolution microscope operating at 200 kV at IPCMS. TEM samples were prepared by depositing one drop of the nanocomposite in solution on a carbon-coated copper grid, then let the solvent evaporate a few hours before observation. The thickness of the silica or the protein layers were determined using ImageJ software on the TEM pictures and the results are indicated as mean layer thickness (nm) ± standard deviation (nm). When the composites were dispersed in aqueous solution, the carbon-coated copper grid is pre-coated with PEI (polyethylene imine) polymer a few tens of second then washed two times with distilled water before depositing the sample.
Zeta potential : zeta potential measurements of the nanocomposites at different stages of the
synthesis was measured by using a Zetasizer nano ZS by Malvern Instruments. The measurements were performed using DTS1070 folded capillary cells in which 10 µL of suspension was diluted in 1 mL of water. The pH of the measured solution was always set between pH 6 and 8, adjusted by using HCl or NaOH 100mM.
Nitrogen adsorption / BET analysis: Mesoporous silica characteristics were determined by
nitrogen adsorption-desorption analysis through Brunauer-Emmett-Teller (BET) method to determine surface area and Barrett-Joyner-Halenda (BJH) method to determine the pore size distribution. All the analyses were done on a Tristar Gas Adsorption Analyzer by Micromeritics Instruments. Before the tests, the samples were outgassed under vacuum at 150°C for about 4h.
TGA : Thermal gravimetric analysis (TGA) was carried out on a Q5000 Automatic Sample
under the hood, then 48h in a sterilizer at 80°C. For the runs, the samples were heated from 25 to 1000°C at a rate of 40°C / min.
UV/Vis spectroscopy : The UV-vis spectra, to determine the amount of drug loaded into the
composite through the supernatant titration, were recorded with a Lambda 950 UV/vis Spectrometer by Perkin Elmer at IPCMS. For all the measurements, the samples were diluted in a UV/vis quartz cell and into a final volume of 3 mL of solution. The solutions and the quartz cell were always protected from light using aluminum foil until the measurement was done.
Optical fluorescence microscopy. Fluorescent nanocomposites : CNT@MS@HSARITC and CNT@MS@drug@HSA, and after silica removal CNT@HSARITC and CNT@drug@HSA were observed by optic fluorescence microscopy on a Olympus IX73 microscope with a Olympus X100 oil NA 1.3 objective and a Hamamatsu Orca flash Cmos camera. For the fluorescence excitation, a Xcite 110 led lamp was used combined with filter sets from Omega optical (Dapi, DsRed and GFP fluorescence filter sets). Typically, 10 µL of the dispersed composite was dropped onto a microscope slide, then after waiting of the solvent evaporation, the material is excited at the suitable wavelength during a time between 10 and 500 ms and finally the fluorescence images are captured. All the images where analyzed and processed with ImageJ software.
Results and Discussion
1. Coating of carbon nanotubes (CNTs) with small pore mesoporous silica (MS)
MS coatings were performed around most open-ended CNTs having an average diameter of 60 nm and 100 – 1000 nm length (see sol-gel reaction in Scheme 1). These CNTs (TEM image in
Figure 1.A) were pre-treated with nitric acid (80°C under reflux) to remove iron catalyst traces
from the CNTs synthesis and then heated to 900°C under inert atmosphere to remove the oxygenated moieties resulting from the acid treatment and to favour their CTAB coating. Indeed following the procedure from Bian et al.[28] the CNTs were dispersed in a mixture of water/EtOH/CTAB. The CTAB is a surfactant molecule and plays also the role of structuring agent of silica mesopores. It forms “rod-like” micelles in solution and interacts with the hydrophobic CNT surface with its alkyl chains, allowing a better CNTs dispersion in aqueous/EtOH solution prior to the incoming sol-gel. The polymerization of the TEOS precursor around the CTAB micelles and CNTs is catalyzed by adding a small amount of NaOH. The layer thickness of silica around the CNTs depends on the amount of reagents (TEOS precursors and CTAB surfactants) and also on the reaction time. TEM images reveal that after 15h of sol-gel reaction, a very uniform porous silica layer with a shell thickness of 50 ± 9 nm was obtained around the CNTs (TEM image on Figure 1.B in a large zone). TEM observations showed opened
CNTs closed (Figure 1.B inset) by the silica coating and a zoomed TEM image (Figure1.C) evidences clearly the porous structure of the silica shell.
Next, the CTAB confined in the silica mesopores was extracted by washing with NH4NO3 (20mg.mL-1) in EtOH. The CTAB extraction was followed by Zeta potential (ZP) measurements for CNTs@MS particles in water at pH 7 (S1). The isoelectric point of silica being ca. 4, ZP values decreased progressively from positive (CTAB charge) to negative (silica charge) values reaching a plateau after five successive extractions with NH4NO3 leading to CTAB-free MS coated CNTs. After removal of the CTAB template, the mesoporous silica was characterized by nitrogen adsorption-desorption isotherms. Pristine CNTs exhibited a type II adsorption isotherm (S2) with a narrow hysteresis loop, a BET surface area of ca. 31 m2.g-1 and a mean pore size of ca. 2.5 and 60 nm corresponding respectively to CNTs interstices and the pore diameter of the CNTs.
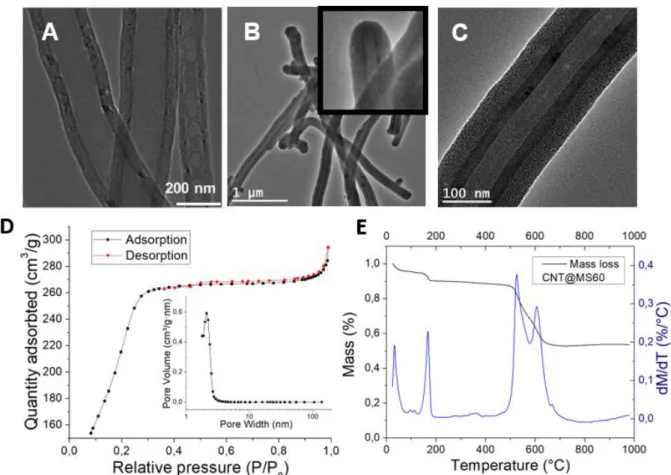
Figure 1. TEM images of (A) uncoated CNTs, (B) MS coating around CNTs (large zone) and
closed ends in inset and (C) a zoomed image showing the mesoporosity of the MS coating. (D) Nitrogen adsorption-desorption isotherms and pore size distribution of CNT@MS.(E) TGA analysis from CNT@MS.
For CNT@MS, its adsorption-desorption isotherm curve showed a type IV adsorption isotherm characteristic of mesoporous materials, with a BET surface area reaching 886 m2.g-1, a pore volume of 0.46 cm3.g-1 and an average pore size distribution of ca. 2.1 nm corresponding to the
silica mesopores (Figure 1.D). Let us note that the pore size contribution at 60 nm seen for CNT alone disappeared, confirming that the opened-CNTs ends were closed by the coated silica layer around the CNTs. TGA analysis was also performed under air with the CNT@MS composites (Figure 1.E). The TGA curve revealed three weight losses, the most significant weight losses were found at ca. 525 and 605°C (double peaks) and refers to the CNT burning . This enabled to deduct the proportion of silica coated around the CNTs, which was estimated at ca. 53 wt%. The two others initial weight losses at 32°C and 167°C refer to the physisorbed water and the chemisorbed water evaporation respectively.
HSA coating around the CNT@MS by IBAM
binders and silica removal
Then, we coated HSA on the surface of the CNT@MS by using the IBAM strategy. The construction steps of the CNT@MS-HSA are described in Scheme 2 where a CNT@MS composite is represented in a cross section view with the silanol moieties at its surface. A first step is the silanisation of the MS shell with APTS, in the presence of a small amount of NH4OH to catalyze the reaction of APTS condensation at the surface. The silica surface shows thus amine functions which can be further functionalized by isobutyrylchloride (IBC) leading to the formation of isobutyramide (IBAM) terminal functions alongside the CNT@MS composite. This IBAM moiety at the silica surface acts as a precursor layer to bind non-covalently HSA through strong assumed hydrogen bonds. This original coating method was shown to ensure a high degree of surface coverage (up to 300 ng.cm-2) and a non-covalent cross-linking of the protein film around the composite. If the IBAM functionalization and HSA coating steps are sequentially repeated, a thicker HSA film can be grown and deposited on the silica material as previously reported.[37]
Scheme 2. Strategy towards CNT@MS-HSA n=1 and n=5.
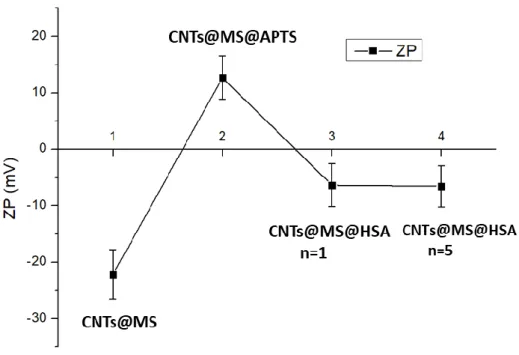
Thus, after each coating step of HSA, the amine residues (Lys, Arg) were converted into IBAM functions and again coated with a new layer of HSA, until that five HSA coating steps have been done (denoted n=5). To characterize the effective build-up of the IBAM-HSA film onto the surface of the CNTs@MS, Zeta potential (ZP) was measured in water at pH=7 after each step: bare, APTS, IBAM HSA n=1 and n=5 (Figure 2). The ZP being the electrical potential at the slipping plane of the double ionic layer of the coated surface, in a first approximation it gives an indication of the surface charge. ZP measurements showed thus a first charge reversal from the bare CNTs@MS at ca. -22 mV to the APTS-modified CNTs@MS modification at ca. +13 mV. Indeed, as expected, a bare silica surface is negatively charged in water given its IEP usually close to pH ca. 3, while APTS modified- CNTs@MS bearing amine functions are usually protonated below its pKa (ca. 8-9) which results in a positive value of ZP. A second charge reversal is also obtained when IBAM-HSA n=1 is deposited on APTS with a ZP measured at ca. – 6 mV. This results is in agreement with the IEP of HSA which is close to IEP=4.7 and then it explains the negative value of HSA coated on CNT@MS in water. Further, HSA coatings up to n=5 are shown not to influence the ZP values as indicated in the graph ZP measured at ca. – 7 mV.
Figure 2. Follow-up of the HSA coating building by ZP measurements in water at pH 7, of
CNTs@MS, CNTs@MS@APTS, CNTs@MS@IBAM HSA n=1 and n=5.
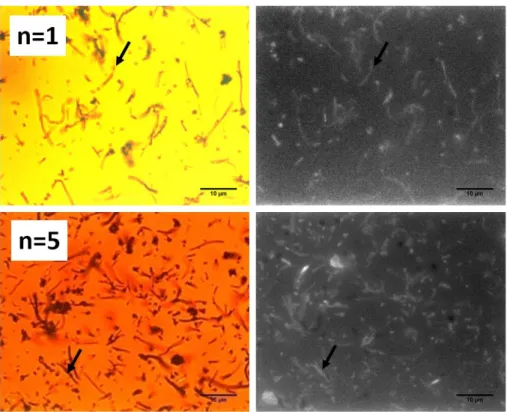
HSA was also labeled with a fluorophore, RITC (red fluorescence) which allowed confirming the presence of the protein coating around the CNT@MS composites by fluorescence microscopy. As seen in Figure 3, optical transmission and fluorescence images show the
HSA-RITC coatings on CNT@MS having n=1 and 5 HSA coating steps. These images revealed that in both cases (n=1 and n=5), the fluorescence is uniformly co-localized with the CNTs@MS, indicating a successful and homogeneous protein coating at CNT@MS surface with of course a more fluorescence intensity observed for n=5. These IBAM-HSA films at n=1 and n=5 HSA coating steps were then characterized by TGA to estimate the amount of HSA incorporated (S3). Comparing the mass loss in each curve (CNT@MS@HSA n=1 and n=5) with the TGA curve of CNT@MS used as a baseline, the mass of IBAM-HSA coated around the composite was estimated at ca. 60 and 100 µg HSA per mg of CNTs@MS respectively for CNT@MS@HSA n=1 and n=5.
Figure 3. Optical transmission (at the left) and fluorescence (at the right) images of
CNTs@MS-HSARITC with n=1 and n=5.
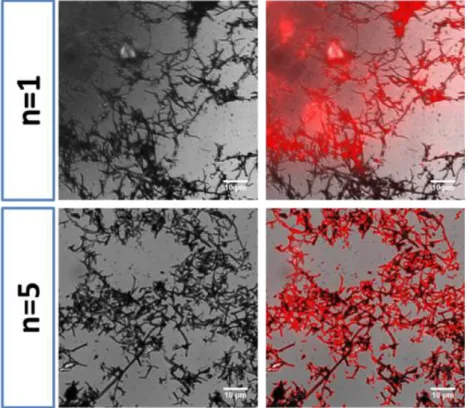
As discussed in the introduction section, MS shells can be used as sacrificial templates to form original protein nanoshells. Thus, the dissolution of the silica coating was done using HF buffered with NH4F (stock solution at 2/7 M) with a very short kinetic of dissolution (less than 10 min was needed for an entire silica dissolution). The suspension containing the resulting CNTs@HSA was then purified from the residual fluoride ions and silicates molecules by ultrafiltration. These CNTs@HSA nanocomposites after silica removal were then re-dispersed in water and were observed by optical transmission and fluorescence microscopies (top and bottom panels respectively for n=1 and n=5 in Figure 4). As it was the case for CNT@MS@HSA, the obtained images indicated an uniform co-localization between the CNTs structure and
HSA-RITC distribution (see fluorescence and transmission co-localized images). Furthermore, TEM analysis (Figure 5.A and B,) confirmed the total dissolution of the MS layer around the CNTs and the presence of an uniform elastic–like HSA coating at the CNT surface. Some neighboring CNTs were found to stick to each other’s on the grid during TEM observation (5.B). Moreover, the thickness of the HSA coating adhering on the CNT walls after n= 5 coating steps was measured with an average values of respectively 8 ± 2 nm. Thus, these data demonstrated the efficacy of the silica templating to form a protein film at the CNT surface.
Figure 4. (A) Top line : from the left to the right : optical transmission and superposed
transmission + fluorescence of CNT@HSA (n=1) dispersed in water after silica dissolution and showing the uniform HSA coating. (B) Bottom line : same imaging for n=5.
Figure 5. TEM images of CNT@HSA (n=5) dispersed in water after silica dissolution and
showing the uniform HSA coating around CNTs (A) and the gum-like elastic nature of the HSA coating between two neighboring CNTs (B).
2. Drug loading in CNT@MS@HSA coating and silica dissolution.
Then, we investigated the possibility to load hydrophobic anti-tumor drugs within the CNT@MS@HSA composites followed by the subsequent silica dissolution. We assessed the loading of two hydrophobic anti-tumor drugs: curcumin (CUR) and camptothecin (CPT) within CNTs@MS. These two drugs have absorption and fluorescence properties with wavelength absorption/emission (exc/em) intensities respectively at 420/550 nm for CUR and 370/434 nm for CPT ensuring suitable detection and visualization by spectroscopy and microscopy methods. Typically, the loading process starts by incubating CNT@MS-IBAM composites (ca. 5 mg) with a given amount of drugs (ca. 500 µg) dissolved in DMF. The drug loading within the CNTs@MS was performed overnight (ca. 16h) and the suspension was directly centrifugated to separate the drug in solution from the amount of drug loaded in the silica mesopores. Then, parameters of the drug loading within the MS were determined : the drug loading capacity (DLC) which is the amount of drug loaded versus the mass of composites and the drug loading efficacy (DLE), which corresponds to the amount of drug loaded versus the total mass of drug introduced in the reaction. For that, standard calibration curves representing maximum UV-visible absorbance vs drug concentrations were traced in DMF for both CUR and CPT. The curve profiles were found both perfectly linear (S4) and their equation model was used for the determination of the mass of drugs in the supernatant which after subtraction from the initial drug loaded mass allowed calculating the DLC. The DLC and DLE loading parameters were afterward calculated as defined above. For both CUR and, CPT, the DLC found were in the range of 0.4-0.7 %, and DLE in the range of 4-6.4 % (see experiment table assays in S5). Standard MSNs loaded with various hydrophobic anticancer drugs have been reported, including Camptothecin,[34,40–42] 5-Fluorouracile,[43] Paclitaxel,[41,44] Docetaxel,[45] Curcumin.[46] withdrug loading capacity of a few percent, mainly between 0.06 and 2.4%, which in fact are standard values for the loading of hydrophobic molecules in MS nanosystems.[34,41]
Furthermore, to afford a biocompatible HSA coating, the drug loaded CNT@MS-IBAM composites after removal of the drug supernatant, were brought in contact with a solution of HSA (at 0.25 mg/mL). In this case, the HSA used was not labelled with a fluorophore, in order to
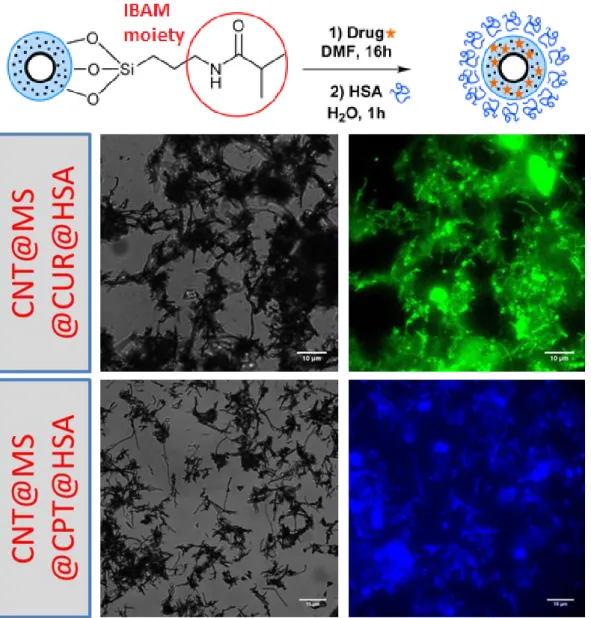
visualize only the drug fluorescence by microscopy. The fluorescence images showed thus emission of the corresponding drugs (green for CUR, blue for CPT, see Figure 6).
Figure 6. At the left, transmission and at the right, fluorescence images of CNT@MS-IBAM-HSA
composites loaded with (A) CUR (green fluorescence), and (B) CPT blue fluorescence.
In each case, the drug fluorescence was well co-localized with the CNTs, confirming the drug loading inside the composites. Furthermore, during the HSA coating, the UV/Vis absorbance of the HSA supernatant was measured and no significant signal at CUR or CPT maximum absorptions were measured indicating no significant drug leakage in the aqueous solution. Likewise, four weeks after the MS drug loading and HSA coating, the same conclusion could be made (i.e) the drugs did not leak from the composites. Then, to afford silica free CNTs@drugs@HSA composites, the silica dissolution with HF/NH4F buffer (pH =5) following the same procedure as seen above was performed. Our aim was to design a nanocomposite which
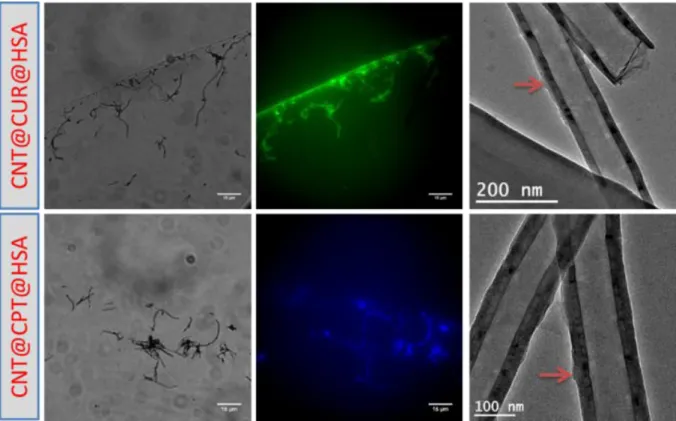
had a drug stacked between the CNT and the HSA layer. Fluorescence microscopy images indicate that after silica dissolution, the CNT@drug@HSA composites as well for CUR or CPT payloads remained highly fluorescent, confirming that the drug remained stacked between the CNT and the protein layers. Morever, the TEM images of the loaded composites displayed at the right to the microscopy images (Figure 7) confirmed the full dissolution of the silica coating. A small organic layer composed of HSA and the drug was clearly seen (red arrows) on these TEM images.
Figure 7. CNTs@drug@HSA loaded composites with CUR and CPT by optical transmission,
fluorescence microscopy and TEM.
Conclusion
In this work, we have developed an original method to form tight HSA coating films around CNTs based on the use of sacrificial templating mesoporous silica shells. First, CNT@MS nanocomposites were successfully synthesized, characterized and covered by an homogenous protein layer (HSA) to increase its biocompatibility through to the IBAM-mediated assembly. Then, the silica dissolution allowed to form original and homogenous protein-coated CNTs nanocomposites as showed by optical fluorescence and TEM microscopies. The porosity of the silica was shown beneficial for the loading of two hydrophobic antitumoral drugs: curcumin and camptothecin. At least, the silica dissolution did not affect the drug retention between the CNTs and the protein layer and allowed creating novel drug loaded biocompatible nanocomposites
made of CNTs, adsorbed stacked drugs and HSA layer denoted as CNT@drug@HSA nanocomposites. Hence, for future works, we anticipate such nanocomposites will be used for phototherapy and NIR-light activated drug release.
References
[1] C. Cha, S.R. Shin, N. Annabi, M.R. Dokmeci, A. Khademhosseini, Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering, ACS Nano, 7 (2013) 2891–2897.
[2] S. Augustine, J. Singh, M. Srivastava, M. Sharma, A. Das, B. D. Malhotra, Recent advances in carbon based nanosystems for cancer theranostics, Biomater. Sci., 5 (2017) 901–952. [3] K. Kostarelos, A. Bianco, M. Prato, Promises, facts and challenges for carbon nanotubes in
imaging and therapeutics, Nat. Nanotechnol., 4 (2009) 627–633.
[4] A. Bianco, K. Kostarelos, C.D. Partidos, M. Prato, Biomedical applications of functionalised carbon nanotubes, Chem. Commun., (2005) 571–577.
[5] L. Lacerda, A. Bianco, M. Prato, K. Kostarelos, Carbon nanotubes as nanomedicines: from toxicology to pharmacology, Adv. Drug Deliv. Rev., 58 (2006) 1460–1470.
[6] Z. Liu, X. Sun, N. Nakayama-Ratchford, H. Dai, Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery, ACS Nano, 1 (2007) 50–56.
[7] Z. Liu, A.C. Fan, K. Rakhra, S. Sherlock, A. Goodwin, X. Chen, Q. Yang, D.W. Felsher, H. Dai, Supramolecular Stacking of Doxorubicin on Carbon Nanotubes for In Vivo Cancer Therapy, Angew. Chem. Int. Ed., 48 (2009) 7668–7672.
[8] W. Wu, R. Li, X. Bian, Z. Zhu, D. Ding, X. Li, Z. Jia, X. Jiang, Y. Hu, Covalently Combining Carbon Nanotubes with Anticancer Agent: Preparation and Antitumor Activity, ACS Nano, 3 (2009) 2740–2750.
[9] C. Samorì, H. Ali-Boucetta, R. Sainz, C. Guo, F. Maria Toma, C. Fabbro, T. da Ros, M. Prato, K. Kostarelos, A. Bianco, Enhanced anticancer activity of multi-walled carbon nanotube – methotrexate conjugates using cleavable linkers, Chem. Commun., 46 (2010) 1494–1496. [10] S.Y. Madani, N. Naderi, O. Dissanayake, A. Tan, A.M. Seifalian, A new era of cancer treatment:
carbon nanotubes as drug delivery tools, Int. J. Nanomedicine, 6 (2011) 2963.
[11] H. Gong, R. Peng, Z. Liu, Carbon nanotubes for biomedical imaging: the recent advances, Adv. Drug Deliv. Rev., 65 (2013) 1951–1963.
[12] X. Liu, I. Marangon, G. Melinte, C. Wilhelm, C. Ménard-Moyon, B.P. Pichon, O. Ersen, K. Aubertin, W. Baaziz, C. Pham-Huu, Design of covalently functionalized carbon nanotubes filled with metal oxide nanoparticles for imaging, therapy, and magnetic manipulation, ACS Nano, 8 (2014) 11290–11304.
[13] J. Ayutsede, M. Gandhi, S. Sukigara, H. Ye, C. Hsu, Y. Gogotsi, F. Ko, Carbon nanotube reinforced Bombyx mori silk nanofibers by the electrospinning process, Biomacromolecules, 7 (2006) 208– 214.
[14] G. Cancian, G. Tozzi, A.A.B. Hussain, A. De Mori, M. Roldo, Carbon nanotubes play an important role in the spatial arrangement of calcium deposits in hydrogels for bone regeneration, J. Mater. Sci. Mater. Med., 27 (2016) 1–10.
[15] D. Elgrabli, W. Dachraoui, C. Ménard-Moyon, X.J. Liu, D. Bégin, S. Bégin-Colin, A. Bianco, F. Gazeau, D. Alloyeau, Carbon Nanotube Degradation in Macrophages: Live Nanoscale Monitoring and Understanding of Biological Pathway, ACS Nano, 9 (2015) 10113–10124. [16] Z. Liu, K. Chen, C. Davis, S. Sherlock, Q. Cao, X. Chen, H. Dai, Drug Delivery with Carbon
Nanotubes for In vivo Cancer Treatment, Cancer Res., 68 (2008) 6652–6660.
[17] M. Zheng, A. Jagota, E.D. Semke, B.A. Diner, R.S. Mclean, S.R. Lustig, R.E. Richardson, N.G. Tassi, DNA-assisted dispersion and separation of carbon nanotubes, Nat. Mater., 2 (2003) 338–342.
[18] N. Zhu, H. Gao, Q. Xu, Y. Lin, L. Su, L. Mao, Sensitive impedimetric DNA biosensor with poly (amidoamine) dendrimer covalently attached onto carbon nanotube electronic transducers as the tether for surface confinement of probe DNA, Biosens. Bioelectron., 25 (2010) 1498–1503. [19] N.G. Sahoo, H. Bao, Y. Pan, M. Pal, M. Kakran, H.K.F. Cheng, L. Li, L.P. Tan, Functionalized
carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: a comparative study, Chem. Commun., 47 (2011) 5235–5237.
[20] C. Ge, J. Du, L. Zhao, L. Wang, Y. Liu, D. Li, Y. Yang, R. Zhou, Y. Zhao, Z. Chai, others, Binding of blood proteins to carbon nanotubes reduces cytotoxicity, Proc. Natl. Acad. Sci., 108 (2011) 16968–16973.
[21] K. Besteman, J.-O. Lee, F.G. Wiertz, H.A. Heering, C. Dekker, Enzyme-coated carbon nanotubes as single-molecule biosensors, Nano Lett., 3 (2003) 727–730.
[22] R.J. Chen, Y. Zhang, D. Wang, H. Dai, Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization, J. Am. Chem. Soc., 123 (2001) 3838–3839. [23] S. Hampel, D. Kunze, D. Haase, K. Krämer, M. Rauschenbach, M. Ritschel, A. Leonhardt, J.
Thomas, S. Oswald, V. Hoffmann, others, Carbon nanotubes filled with a chemotherapeutic agent: a nanocarrier mediates inhibition of tumor cell growth, Nanomed., 3 (2008) 175–182. [24] U. Arsawang, O. Saengsawang, T. Rungrotmongkol, P. Sornmee, K. Wittayanarakul, T.
Remsungnen, S. Hannongbua, How do carbon nanotubes serve as carriers for gemcitabine transport in a drug delivery system?, J. Mol. Graph. Model., 29 (2011) 591–596.
[25] H. Ali-Boucetta, K.T. Al-Jamal, D. McCarthy, M. Prato, A. Bianco, K. Kostarelos, Multiwalled carbon nanotube–doxorubicin supramolecular complexes for cancer therapeutics, Chem. Commun., (2008) 459–461.
[26] M. Zhang, X. Zhang, X. He, L. Chen, Y. Zhang, A facile method to coat mesoporous silica layer on carbon nanotubes by anionic surfactant, Mater. Lett., 64 (2010) 1383–1386.
[27] K. Ding, B. Hu, Y. Xie, G. An, R. Tao, H. Zhang, Z. Liu, A simple route to coat mesoporous SiO 2 layer on carbon nanotubes, J. Mater. Chem., 19 (2009) 3725–3731.
[28] S.-W. Bian, Z. Ma, L.-S. Zhang, F. Niu, W.-G. Song, Silica nanotubes with mesoporous walls and various internal morphologies using hard/soft dual templates, Chem. Commun., (2009) 1261– 1263.
[29] D. Mertz, O. Sandre, S. Begin-Colin, Drug releasing nanoplatforms activated by alternating magnetic fields, Biochim. Biophys. Acta BBA-Gen. Subj., (2017).
[30] N.Ž. Knežević, J.-O. Durand, Large pore mesoporous silica nanomaterials for application in delivery of biomolecules, Nanoscale, 7 (2015) 2199–2209.
[31] Y. Wang, K. Wang, J. Zhao, X. Liu, J. Bu, X. Yan, R. Huang, Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma, J. Am. Chem. Soc., 135 (2013) 4799–4804.
[32] Y. Wang, K. Wang, R. Zhang, X. Liu, X. Yan, J. Wang, E. Wagner, R. Huang, Synthesis of core–shell graphitic carbon@ silica nanospheres with dual-ordered mesopores for cancer-targeted
photothermochemotherapy, ACS Nano, 8 (2014) 7870–7879.
[33] J. Liu, C. Wang, X. Wang, X. Wang, L. Cheng, Y. Li, Z. Liu, Mesoporous Silica Coated Single-Walled Carbon Nanotubes as a Multifunctional Light-Responsive Platform for Cancer Combination Therapy, Adv. Funct. Mater., 25 (2015) 384–392.
[34] J. Lu, M. Liong, J.I. Zink, F. Tamanoi, Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs, Small, 3 (2007) 1341–1346.
[35] D.P. Ferris, J. Lu, C. Gothard, R. Yanes, C.R. Thomas, J.-C. Olsen, J.F. Stoddart, F. Tamanoi, J.I. Zink, Synthesis of Biomolecule-Modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells, Small, 7 (2011) 1816–1826.
[36] D. Mertz, P. Tan, Y. Wang, T.K. Goh, A. Blencowe, F. Caruso, Bromoisobutyramide as an Intermolecular Surface Binder for the Preparation of Free-standing Biopolymer Assemblies, Adv. Mater., 23 (2011) 5668–5673.
[37] D. Mertz, J. Cui, Y. Yan, G. Devlin, C. Chaubaroux, A. Dochter, R. Alles, P. Lavalle, J.C. Voegel, A. Blencowe, others, Protein capsules assembled via isobutyramide grafts: sequential growth, biofunctionalization, and cellular uptake, ACS Nano, 6 (2012) 7584–7594.
[38] D. Mertz, H. Wu, J.S. Wong, J. Cui, P. Tan, R. Alles, F. Caruso, Ultrathin, bioresponsive and drug-functionalized protein capsules, J. Mater. Chem., 22 (2012) 21434–21442.
[39] D. Mertz, C. Affolter-Zbaraszczuk, J. Barthès, J. Cui, F. Caruso, T.F. Baumert, J.-C. Voegel, J. Ogier, F. Meyer, Templated assembly of albumin-based nanoparticles for simultaneous gene silencing and magnetic resonance imaging, Nanoscale, 6 (2014) 11676–11680.
[40] J. Lu, M. Liong, Z. Li, J.I. Zink, F. Tamanoi, Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals, Small, 6 (2010) 1794–1805.
[41] M. Liong, J. Lu, M. Kovochich, T. Xia, S.G. Ruehm, A.E. Nel, F. Tamanoi, J.I. Zink, Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery, ACS Nano, 2 (2008) 889–896. [42] D.P. Ferris, J. Lu, C. Gothard, R. Yanes, C.R. Thomas, J.-C. Olsen, J.F. Stoddart, F. Tamanoi, J.I.
Zink, Synthesis of Biomolecule-Modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells, Small, 7 (2011) 1816–1826.
[43] M.M. Ayad, N.A. Salahuddin, A.A. El-Nasr, N.L. Torad, Amine-functionalized mesoporous silica KIT-6 as a controlled release drug delivery carrier, Microporous Mesoporous Mater., (2016). [44] B. Chang, J. Guo, C. Liu, J. Qian, W. Yang, Surface functionalization of magnetic mesoporous
silica nanoparticles for controlled drug release, J. Mater. Chem., 20 (2010) 9941.
[45] H. Wu, G. Liu, S. Zhang, J. Shi, L. Zhang, Y. Chen, F. Chen, H. Chen, Biocompatibility, MR imaging and targeted drug delivery of a rattle-type magnetic mesoporous silica nanosphere system conjugated with PEG and cancer-cell-specific ligands, J. Mater. Chem., 21 (2011) 3037.
[46] S. Kumar, Balasubramanian, Ravindran Girija, Y. Nagaoka, Iwai, Suzuki, Kizhikkilot, Yasuhiko, T. Maekawa, Curcumin and 5-Fluorouracil-loaded, folate- and transferrin-decorated polymeric magnetic nanoformulation: a synergistic cancer therapeutic approach, accelerated by magnetic hyperthermia, Int. J. Nanomedicine, (2014) 437.
SUPPORTING INFORMATION
Design of protein-coated carbon nanotubes loaded with
hydrophobic drugs through sacrificial templating of
mesoporous silica shells
Vincent Fiegel
1,2, Sebastien Harlepp
1, Sylvie Bégin-Colin
1, Dominique Bégin
2*, Damien
Mertz
1*
1Institut de Physique et Chimie des Matériaux de Strasbourg (IPCMS), UMR-7504 CNRS-Université de Strasbourg, 23 rue du Loess, BP 34 67034
Strasbourg Cedex 2, France, §
2Institut de Chimie et Procédés pour l'Energie, l'Environnement et la Santé (ICPEES), UMR-7515 CNRS-Université de Strasbourg, 25 rue Becquerel, 67087 Strasbourg
Cedex 2, France,
S3 .Thermogravimetric analysis for HSA, CNTs, CNT60@MS, CNT60@MS-HSA n=1 (left), and
CNT60@MS-HSA n=5 (right) (bottom).
To estimate precisely the reference mass of CNTs in CNT@MS and the resulting masses of IBAM-HSA in CNTs@MS@IBAM-HSA (n=1 and 5), the weight loss masses contributions corresponding to the solvent evaporation (T≤200°C) changes were substracted from the CNTs and IBAM/HSA resulting masses losses. Hence, for that, the weight losses of the CNT burning in CNTs @MS and of CNTs HSA n=1 and n=5, CNT@MS@HSA (n=1 and n=5) were thus taken in the range (250- 800°C), the weight loss peak of HSA calcination in TGA being around 380°C.
S4 : Standard curves by UV/vis of the three different drugs in DMF : Curcumin (A), Camptothecin
(B)
A
S5. Experimental table assays summarizing the results of the CUR and CPT loading within
CNTs@MS@IBAM in DMF
Drug m(composite) m(incubated drug) m(drug
supernatant) m(loaded drug) DLC DLE CUR assay 1 5 mg 498 µg 466 µg 32 µg 0,64 % 6,4 % CUR assay 2 5mg 498 µg 473,5 µg 24,5 µg 0,50 % 5,0 % CPT assay 1 5 mg 470 µg 448 µg 22 µg 0,44 % 4,7 % CPT assay 2 5mg 470 µg 451 µg 19 µg 0,38 % 4,0 %