Publisher’s version / Version de l'éditeur:
Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011),
2011-07
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Experimental study on the possible factors that affect the saturation of
gas hydrate in natural sediments
Ukita, Toshiyasu; Noguchi, Satoshi; Shimada, Tadaaki; Lu, Hailong;
Moudrakovski, Igor; Ripmeester, John; Ratcliffe, Chris
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=80f8deea-2476-48bc-9b94-813bdd09f703 https://publications-cnrc.canada.ca/fra/voir/objet/?id=80f8deea-2476-48bc-9b94-813bdd09f703
EXPERIMENTAL STUDY ON THE POSSIBLE FACTORS THAT AFFECT
THE SATURATION OF GAS HYDRATE IN NATURAL SEDIMENTS
Toshiyasu Ukita
, Satoshi Noguchi, Tadaaki Shimada
Technology and Research Center
Japan Oil, Gas, and Metals National Corporation
1-2-2 Hamada, Mihama-Ku, Chiba 261-0025
Japan
Hailong Lu, Igor Moudrakovski, John Ripmeester, Chris Ratcliffe
Steacie Institute for Molecular Sciences
National Research Council Canada
Ottawa, Ontario K1A 0R6
Canada
ABSTRACTNatural gas hydrate exists in sediment, a complex system with various constituents, and its saturation is subject to the influence of sediment. Through a series of experimental investigations, particle size is identified as the most important factor that controls the saturation of gas hydrate in sediment. Studies also revealed that sorting of sediment and mineral composition also play certain roles in affecting hydrate saturation in sediments. The investigation about the proton relaxations of water in sediment found that the sediment control on hydrate saturation is realized through its influence in water states in sediment, some water in sediments not available for hydrate formation.
Keywords: gas hydrate, saturation, sediments, particle size, clay, water state
Corresponding author: Phone: +81 43 276 9289 Fax +81 43 276 4062 E-mail: ukita-toshiyasu@jogmec.go.jp
INTRODUCTION
Except for those occurring at seafloor and in fracture zones, natural gas hydrates exist in sediments. The geophysical and geochemical studies have found that the saturations of gas hydrate in sediments are related to their types: seemly enriched in coarse sediments, such as sand, but less saturated in fine sediments, such as silt and clay. However as limited by the investigation method and the special property of gas hydrate, being stable only at relatively high pressure and low temperature, how the sediment controls hydrate saturation has not been well understood. Natural sediment is a complex system with various constituents (minerals, organic debris, fossil
remains, pore water, etc), so it is difficult to specify the role of each factor on hydrate saturation with bulk natural sample. As a result, artificial sediments, which were prepared with various minerals and the particles with various sizes, have been employed to investigate the various factors that exert their influence on hydrate saturation in natural sediments. Here we report the results obtained.
METHOD
Among the parameters that are used to describe a sediment, particle size distribution is the most important, because natural sediments are categorized to various types based on particle size
Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, Scotland, United Kingdom, July 17-21, 2011.
distribution. To investigate the particle size effect, a series of artificial sediments and a set of natural sediments, which are with various particle size distributions, were employed. The saturations of methane hydrate in both artificial and natural sediments were investigated and the relationship between hydrate saturation and particle size distribution in sediment was explored.
To investigate the mineral effect on gas hydrate formation, artificial sediments with various mineral combinations were prepared and hydrate saturation in these sediments were determined. To study the mechanism of how sediments control hydrate saturation, the proton relaxations of water in both artificial and natural sediments were measured with an AVANCE 200 MHz Bruker NMR spectrometer. The measurements were carried out at ambient conditions.
The sediments were saturated with 3.5% NaCl before test. The synthesis of methane hydrate in sediments was implemented at the temperature of 3 °C, and the pressure was about 120 bar in the beginning and about 100 bar at the end of experiments.
The hydrate saturation is calculated with the equation V(hydrate)/V (pore space). V(hydrate) is obtained from the mass amount of hydrate M(hydrate) and the density of hydrate (generally taken as 0.92 g/cm3), while the amount of hydrate
M(hydrate) is calculated from the amount of gas in hydrate with a hydration number of 6.
The water conversion stands for the amount of water that was enclathrated into hydrate, which is computed from the gas amount released from hydrate with a hydration number of 6 (CH4 6H2O).
RESULTS AND DISCUSSION Particle-size effect
Previous studies with silica sands have found that the saturation of methane hydrate has close relation with particle size (Lu et al., 2011).
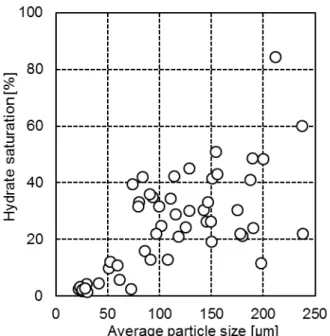
Figure 1 shows the results obtained from our studies with the sediments from Nankai Trough. Similar to the results obtained with silica sands, certain relationship between hydrate saturation and the average particle size can be recognized. Compared with the results obtained with silica sands, the results obtained with natural sediments seem more dispersed. This is mainly because the particle size of natural sediments is generally in a larger range than that of artificial silica sand.
Figure 1 The saturation of methane hydrate and the average particle size in the sediments from Nankai Trough
Sorting effect
Upon studying the sediments from Nankai Trough, Uchida and Tsuji (2004) found that the sediments with good sorting were generally with high hydrate saturation. For a better understanding of this issue, studies with artificial sediments, which were with various sorting values and prepared with the combinations of silica sands with various particle sizes and kaolinite.
Figure 2 The plot of sorting value vs the degree of water conversion in sediments (centered around 75-90 µm)
Figure 2 shows one of the results obtained. Clearly sorting is one factor affecting water conversion into hydrate in sediments.
Similar results have also been obtained in other test specimens, which were centered around different particle sizes. However, when we plot the water conversion versus average particle sizes (Figure 3), stronger relationship between water conversion into hydrate and average particle size can be found. As a result, although sorting is one factor affecting gas hydrate formation in natural sediments, particle size is still the main factor controlling the saturation of gas hydrate in sediments.
Figure 3 The relationship between the degree of water conversion and the average particle size (phi) in sediments
Clay effect
In addition to the small particle size (generally < 2 µm), having charge on the surface is another important characteristics of clay mineral. Previous studies indicated that clay was an important factor affecting the saturation of gas hydrate in sediments (Kawasaki et al., 2009). However, it is reasonable to wonder that the clay-effect is solely from the small particle size or also with the contribution of other characteristics of clay minerals.
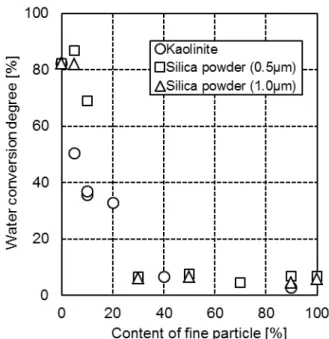
Figure 4 Degree of water conversion into hydrate in the mixtures of 250-500 µm silica sand and clay size silica sand
To clarify this question, investigations were carried out with clay-size silica sands (~1µm). The results obtained are depicted in Figure 4. It can be seen that indeed certain difference can be observed, clay mineral playing a more significant role than clay-size silica in affecting water conversion into hydrate, especially when the fraction of fine particle component is low. However, this difference becomes smaller with the increase in fine particle component. The results imply that clay-effect is not only due to its small particle size but also with the contribution of other characteristics of clay, although particle size plays the major role.
Proton relaxations of water vs water conversion into hydrate in sediments
Our previous studies with silica sands have found that some water in sediments cannot be converted into hydrate, and it was named as unclathratable water (Lu et al., 2011). Since then further investigations have been carried out with both artificial and natural sediments.
Figure 5 The Relationship between water conversion and proton relaxations in artificial sediments
Figure 5 shows the relationship between water conversion into hydrate and proton relaxations, longitudinal (T1) and transverse (T2), in artificial sediments that were prepared by mixing the silica sands with various sizes and kaolinite in various ratios. Similar to the results obtained with silica sands, the relationship between water conversion
into hydrate and proton relaxations of water in sediments can be clearly recognized.
Figure 6 The relationship between water conversion and the proton relaxations of water in the sediments from Nankai Trough
Figure 6 shows the relationship between water conversion into hydrate and the proton relaxations of water in the sediments from Nankai Trough. Similar to the results obtained on artificial sediments, certain relationship can be identified easily, although the data points are more dispersed
as compared with the results obtained on artificial sediments. This is mainly due to the complex composition, especially iron containing components, and the complicated particle size distribution in natural sediments.
It can be seen that the degree of water conversion into hydrate ranges from several percent to about 80%. Obviously some water is not converted into hydrate, confirming the results obtained on silica sands: some water unclathratable for hydrate formation.
CONCLUSION
Based on the results obtained, the following conclusions can be drawn.
1) Particle size is the main factor that controls the saturation of gas hydrate in sediments,
2) Although sorting can play a certain role in affecting hydrate saturation in sediments, particle size is still the main factor,
3) Clay effect is mainly from its small particle size, but the characteristics of clay does contribute into its influence on hydrate saturation in sediments,
4) Some water in sediments is not available for hydrate formation, and the saturation of hydrate in sediments is related to the amount of this unclathratable water.
ACKKNOWLEDGEMENT
This research was carried out as a part of the research organized by The Research Consortium for Methane Hydrate Resources in Japan (Mh21), consisting JOGMEC and AIST (National Institute of Advanced Industrial Science and Technology) with financial support from METI (Ministry of Economy, Trade and Industry).
REFERENCES
[1]
Lu, H., Kawasaki, T., Ukita, T.,
Moudrakovski, I., Fujii, T., Noguchi, S.,
Shimada, T., Nakamizu, M., Ripmeester, J.,
Ratcliffe, C. Particle-size effect on the
saturation of methane hydrate in sediments –
Constrained from experimental results.
Marine and Petroleum Geology, 2011; DOI:
10.1016/j.marpetgeo.2010.11.007.
[2] Uchida, T., and Tsuji, T. Petrophysical
properties of natural gas hydrates-bearing sands and their sedimentology in Nankai Trough.
Resources Geology 2004; 54: 79-88.
[3]