-1
Cholesterol and Egg Activation
By Li Wang B.S. Biotechnology Peking University, China, 2012 Submitted to the Department of Biology in Partial Fulfillment of the Requirement for the
Degree of Doctor of Philosophy at the
Massachusetts Institute of Technology June 2019
0 2019 Massachusetts Institute of Technology All rights reserved
Signature redacted
Signature of A uthor:... Department Li Wang of Biology C ertified by : ... A ccepted by: ... MASSACHUSES WNTIUE OF TEC~j~jGAPR Z9- 2019
Signature redacted
\\ onty Krieger Whitehead Professor of Molecular Genetics Thesis SupervisorSignature redacted...
Ic
Amy E. K'atingProfessor of Biology Co-Director, Biology Graduate Committee
Cholesterol and Egg Activation
By Li Wang
Submitted to the Department of Biology in Partial Fulfillment of the Requirement for the Degree of Doctor of Philosophy
Abstract
The high-density lipoprotein (HDL) receptor SR-BI controls the structure and fate of plasma HDL. The SR-BI knockout (KO) females are infertile, apparently due to their abnormal,
cholesterol-enriched HDL particles. In this thesis, my colleagues and I examined the growth and meiotic progression of SR-BI KO oocytes and found that they underwent normal germinal vesicle breakdown; however, SR-BI KO eggs, which had accumulated excess cholesterol in vivo, spontaneously activated; they escaped metaphase II (MII) arrest and progressed to pronuclear, metaphase III and anaphase/telophase III stages. Eggs from fertile, wild-type mice were activated when loaded in vitro with excess cholesterol using a cholesterol/methyl--cyclodextrin complex, phenocopying SR-BI KO oocytes. In vitro cholesterol loading of eggs induced elevation of intracellular calcium (the [Ca2]i spike), reduction in MPF and MAPK activities, extrusion of a second polar body and progression to meiotic stages beyond MI. These results suggest the infertility of SR-BI KO females is due, at least in part, to excess cholesterol in eggs inducing premature activation, and that cholesterol can activate wild-type mouse eggs to escape from MII arrest.
In the Chapter 3, I studied the detailed mechanism of egg activation induced by excess
cholesterol. I showed that the [Ca2+]i spike induced by excess cholesterol was necessary for egg
activation and also sufficient for further development of the egg to the blastocysts stage. Excess cholesterol, in calcium free medium, did not induce changes in [Ca2+]i, indicating that
extracellular calcium was required for the [Ca2+]i spike and also suggesting the entry of extracellular calcium via plasma membrane channel(s). After screening of calcium channel inhibitors, single cell mRNA-sequencing and activation experiments using eggs from mutant females, I was able to show that co-inhibition of both the L-type calcium channel Cavl.3 and the transient receptor potential channel TRPC5, but not inhibition of either one alone, blocked the excess-cholesterol induced [Ca2+]i spike and egg activation. This result suggests that excess cholesterol activates the MII eggs by opening of Cavl.3 or TPRC5. Our results raise the possibility that excess cholesterol might also activate the same channels in other systems, and thus contribute to pathophysiology.
Thesis supervisor: Monty Krieger
Acknowledgements
I will always feel grateful for being admitted to the Department of Biology at MIT. It is here at MIT that I encountered many wonderful people and developed life-long relationships with some of the world's best scientists, mentors and friends.
Being at MIT has been great, and one of the best aspects of my stay at MIT was choosing to study in the laboratory of Monty Krieger. Monty is a great mentor both in research and in life. He spent much time and effort to guide me through difficulties in my projects, not only helping me on the big picture, but also on many details during my everyday experiments. Through our discussions, I learned to admire his capacity for critical thinking. I also learn from him writing and communication skills that help me better express myself in my second language. Most
importantly, he is always passionate and energetic with science. Even at times that I struggled, he kept me optimistic and encouraged me to overcome challenges.
My academic committee members, Prof. David Page and Prof. Michael Yaffe, also helped me during my years in the biology program. I am deeply grateful to them for their support and advice. I would also like to thank Prof. Catherine Racowsky for generously serving as a member of my thesis defense committee.
I would also like to thank the people with whom I worked in the Krieger lab. My project is based on previous findings of Dr. Ayce Yesilaltay, who also helped me to initiate my project. I thank Marsha Penman and Junmei Yao for technical help. And I want to thank Dalia Walzer, an MIT undergraduate who spent three years working with me, and our administrative assistant Cindy Woolley and the department program officer Betsey Walsh for their help.
My project would not have succeeded without help from a tremendous group of collaborators. Dr. Eliza Vasile helped me to set up the calcium imaging experiments in MIT's Koch Institute Microscope Facility. I did my RNA sequencing experiment under the supervision and help of Christopher Rodman, Dr. Daruis Przybylski, and Dr. Oriz Rozenblatt-Rosen at the Klarman Cell Observatory of the Broad Institute; Dr. David Baez from Dr. Jen Pan lab helped me to work with electrophysiology experiments not reported in this thesis. I want to give my special thanks to Prof. Jorge Stiessnig and Prof. Anna Greka for generously providing their knockout animals. Last but not least, I want to thank my family. The time and distance of my study abroad resulted in physical separation but our hearts will never be apart. My parents and my younger sister have always been my strongest supporters. Especially, I would like to thank my dear husband Chi, who accompanied me throughout my years at MIT, and shares with me my sadness and happiness for the past, present and future.
Table of Contents
Abstract
...
3
Acknowledgm ents ... 5 Table of Contents ... 7 List of Figures ... 12 List of Tables ... 15C hapter 1. Introduction... 17
Section 1.1. The Physiology of the Ovum ... 18
1.1.1. The Generation of Eggs ... 20
1.1.1.1. The Origin of Oocytes... 21
1.1.1.2. Folliculogenesis ... 23
1.1.1.3. GV Arrest and Oocyte M aturation... 25
1.1.1.4. M eiosis II Arrest ... 28
1.1.2. Egg Activation... 30
1.1.2.1. Sperm induced Egg Activation...31
1.1.2.2. Calcium Channels in Egg Activation...36
1.1.2.3. Parthenogenetic Activation and Its Applications... 38
Section 1.2. Cholesterol Metabolism, Transport and Homeostasis...41
1.2.1. Cholesterol Synthesis... 42
1.2.2. Cholesterol Absorption from the Diet ... 44
1.2.3. Circulation of Cholesterol in the Blood ... 45
1.2.4. Excretion of Cholesterol to the GI Tract... 49
1.2.5. Regulation of Cholesterol M etabolism ... 50
Section 1.3. Cholesterol Regulation of Channels... 54
1.3.1. Changes in Physical Properties of the M embrane... 56
1.3.2. Direct Protein-Cholesterol Binding ... 60
1.3.3. Examples of Cholesterol Sensitive Channels... 63
1.3.3.1. Inwardly-Rectifying K' Channels... 63
1.3.3.2. Voltage Gated Calcium Channels...65
1.3.3.3. Transient Receptor Potential Channels ... 67
Chapter 2. Excess cholesterol induces mouse egg activation and may cause
fem ale infertility...
95
2.1. Abstract...97
2.2. Introduction...98
2.3. Results...102
2.3.1. Oocyte follicular growth ... 103
2.3.2. M eiotic resumption and extrusion of the first polar body... 105
2.3.3. Spontaneous progression beyond M II arrest... 107
2.3.4. Abnorm ally high cholesterol deposition in SR-BI KO eggs... 112
2.3.5. Effects of excess cholesterol loading on wild-type eggs ... 114
2.4. Discussion...120
2.4.1. Cholesterol accumulation in SR-B 1 KO eggs contributes to their abnormal phenotypes ... 122
2.4.2. HDL is the likely source of excess cholesterol in SR-BI KO eggs ... 124
2.4.3. Excess cholesterol and egg activation ... 127
2.4.4. Implications for human infertility... 128
2.5. M aterials and M ethods...130
2.6. Acknowledgm ents...131
2.7. References...132
2.8. Supporting Inform ation ... 141
2.8.1. Supplem ental M ethods... 141
2.8.2. Supplem ental References ... 151
2.8.3. Supplem ental Figures... 153
Chapter 3. Excess-Cholesterol Activates MIT Arrested Eggs via Opening of
C av1.3 and TR PC 5...161
3.1. Abstract...162
3.2. Introduction...163
3.3. Results...167
3.3.1. Suppression of the [Ca2 +], Spike... 168
3.3.2. Embryonic Development after Parthenogenetic Activation ... 170
3.3.3. Egg Activation in Calcium Free M edia ... 172
3.3.4. Identification of Excess-Cholesterol Activated Calcium Channels ... 175
3.3.5. Channel Expression in M I1 Arrested Eggs... 181
3.3.6. Ca,1.3 L-type Channel Knockout Eggs... 183
3.3.7. Multi-channel Hypothesis for Excess-Cholesterol Induced Egg Activation ... 187
3.4. Discussion...190
3.4.1. Two-Channel M odel for Cholesterol-Induced Egg Activation... 191
3.4.2. Cholesterol and Ion Channels... 194
3.4.3. Calcium Channels Involved in SrCl2 Induced Egg Activation ... 195
3.4.4. Off-target Effects of Channel Blockers ... 196
3.5. Acknowledgem ents...197
3.6. M ethods...198
3.7. Supplem ental Figures and Tables...203
Chapter 4: Conclusions and Future Directions...223
4.1. Key Conclusions ... 223
4.2. Future Directions...224
4.2.1. Genetic Validation of the Two-Channel Model of Excess-Cholesterol-mediated Egg Activation...224
4.2.2. Cholesterol Induced Channel Activation ... 226
4.2.3. Hypercholesterolemia and Human Infertility...228
List of Figures
Fig. 1.1. M eiosis. ... 19
Fig. 1.2. Development from the Primordial Germ Cell to the Preovulatory Follicle...20
Fig. 1.3. M igration of PGCs to the Gonadal Ridge. ... 21
Fig. 1.4. The Formation and Breakdown of the Cyst... 22
Fig. 1.5. Folliculogenesis. ... 23
Fig. 1.6. The GV Arrest and M eiotic Resumption ... 27
Fig. 1.7. M II Arrest Held by Em i2 ... 29
Fig. 1.8. Egg Activation by Sperm ... 32
Fig. 1.9. [Cal'] Oscillations in M II Eggs during in vitro Fertilization... 33
Fig. 1.10. Cholesterol Synthesis ... 43
Fig. 1.11. Structure of a Lipoprotein Particle... 46
Fig. 1.12. Cholesterol Homeostasis ... 46
Fig. 1.13. Sizes and Lipid Compositions of Different Lipoproteins ... 48
Fig. 1.14. SCAP Regulation of SREBP ... 51
Fig. 1.15. Structure of Cholesterol... 57
Fig. 1.16. Cholesterol and Its Analogs... 57
Fig. 1.17. Change of Reaction Energy... 58
Fig. 1.18. Kir Subunit ... 64
Fig. 1.19. Topology of the Voltage Gated Calcium Channels ... 66
Fig. 1.20. xI Subunit of the Ca, Channel ... 66
Fig. 1.21. Topology of TRP Channels ... 68
Fig. 2.3. Meiotic progression of oocytes from control SR-BI+'- and SR-BI KO mice assessed by deconvolution m icroscopy. ... 110 Fig. 2.4. Cholesterol levels in SR-BI"'* and SR-BI KO eggs...113 Fig. 2.5. Effects of cholesterol loading on [Ca2+ ] in eggs from wild-type C57B1/6 females...115 Fig. 2.6. Additional effects of cholesterol loading and SrCl2 treatment on eggs from wild-type C 57B1 /6 fem ales. ... 118 Supplemental Fig. 2.Sl. Visualization of spontaneous pronucleus formation in SR-BI KO eggs.
... 1 5 3 Supplemental Fig. 2.S2. Visualization of spontaneously formed second polar bodies in SR-B! K O eg g s... 154 Supplemental Fig. 2.S3. MPF and MAPK kinase activities of ovulated eggs from control and S R -B I K O m ice. ... 155 Supplemental Fig. 2.S4. Relative levels of cholesterol in eggs determined by filipin fluorescence.
... 1 5 6 Supplemental Fig. 2.S5. SrCl2-induced oscillations in [Ca2+], in a WT C57B1/6 egg...157 Supplemental Fig. 2.S6. Effects of cholesterol loading for 25 minutes on the relative [Ca2+ of eggs from w ild-type C 57B1 /6 fem ales...158 Supplemental Fig. 2.S7. Effects of the cholesterol loading on viability and parthenogenetic activation of control SR -B I+/- eggs. ... 159 Fig. 3.1. Excess Cholesterol Activates MIL Eggs and Induces a [Ca2+ ] Spike... 166 Fig. 3.2. BAPTA-2AM Treatment Suppressed the Rise in [Ca]2+] (A) and Egg Activation (B). 169 Fig. 3.3. Blastocyst Development of Eggs activated by Excess Cholesterol (A,B) or SrCl2 (C,D)
... '7... 7
Fig. 3.4. Extracellular Calcium Influence on the Excess-cholesterol-induced [Ca2+], Spike .... 174 Fig. 3.5. SKF-96365 and Isradipine Prevented Cholesterol-Induced Egg Activation...179 Fig. 3.6. D ifferent Treatm ent w ith Isradipine ... 180
Fig. 3.7. Activation of Cav1.3 Knockout Eggs by Cholesterol or SrCl2... 185
Fig. 3.8. Isradipine Inhibited SrCl2-Induced Egg Activation and [Ca2+]i Oscillations... 186
Fig. 3.9. Double Inhibition by Isradipine and AC 1903. ... 189
Fig. 3.10. Four Different Models for the Formation of [Ca2 +], spike. ... 193
Supplemental Fig. 3.S1. Inhibitor Experiment in Different Culture Dishes...203
Supplemental Fig. 3.S2. The Oil-Dish and IVF-Dish Methods for Egg Culturing...204
Supplemental Fig. 3.S3. L-type Channel and Its Blockers ... 205
Supplemental Fig. 3.S4. Egg Activation Inhibited by Different L-type Channel Blockers...206
List of Tables
Table 2.1. Quantitative morphologic analysis of spontaneous meiotic progression beyond MII arre st... 11 1
Table 2.S 1. C om pletion of m eiosis I ... 160
Table 3.1. m RNA Expression in W T M I1 Eggs ... 182
Supplemental Table 3.S1. Inhibitor Screening for Cholesterol Induced Egg Activation...208
Supplemental Table 3.S2. Messenger RNA Expression of Calcium Channels ... 209
Table 4.1: Cholesterol binding motifs CARC on mouse Ca, 1.3 channel...229
Table 4.2: Cholesterol binding motifs CRAC on mouse Cav1.3 channel...231
Table 4.3: Cholesterol binding motifs CARC on mouse TPRC5...232
Section 1.1. The Physiology of the Ovum
It was in the year 1827 when Karl Ernst von Baer discovered the mammalian ovum under a microscope and accurately described it in his letter to the Imperial Academy of Sciences of St. Petersburg, 'On the Genesis of the Ovum of Mammals and of Man'. 1,2 This finding facilitated in vitro and in vivo studies of oogenesis and zygotic development in the next century. In 1944,
Rock and Menkin reported the first 'successful' in vitro fertilization (IVF) experiment in the human. They matured oocytes in vitro, inseminated them with sperm and successfully cultured the fertilized eggs to the two-cell stage.3 In 1978, 34 years after Rock and Menkin's pioneering work, Louise, the first IVF baby was born in England.1
An ovum is defined as the haploid female gamete that can fuse with the male gamete to generate the zygote. A female gamete is also called an oocyte before maturation, or an egg when
it is mature and competent for fertilization.4 The ova that Karl Ernst von Baer found in the
fallopian tube were actually eggs and those in the ovary were oocytes. Different from the diploid somatic cells with two copies of the genome (2N) and two sets of chromatids (2C) each, the egg is haploid, with only one copy of the genome (IN). The mammalian egg originates from the primordial germ cell (2N2C) in the embryonic epiblast. The primordial germ cells proliferate via mitosis and remain diploid while migrating to the gonad, where they are stimulated to initiate meiosis (Fig 1.1) and become oogonia. The oogonium stops at the prophase I of meiosis (or GV arrest) after genome duplication, becoming an oocyte (2N4C). The oocyte finishes the first meiotic division during maturation in the follicle and stops again at metaphase II (M1I). The mature oocyte, or the egg, will be activated by sperm insemination to resumes meiosis, finish the second meiotic division and produce the female pronucleus(1N1C) to be fused with the male
pronucleus from the sperm (1NIC), generating a diploid (2N2C) zygote as the very first cell of a new life.4'5
The generation of the ovum, or oogenesis, is a tightly regulated process with multiple temporal controls. Both mitotic and meiotic processes are strictly controlled to guarantee the generation of healthy gametes and zygotes.4'6 In this part of chapter 1, I will briefly review the
process of oogenesis, from the embryonic development of the primordial germ cell to the
oogonium, then to the immature oocyte arrested at prophase I of miosis (or GV arrest), finally to the mature oocyte, or the egg, that is arrested at metaphase II of meiosis. (1.1) I will also discuss the meiotic resumption of the MII eggs at fertilization or under parthenogenesis (1.2).
Oogonium GV arrest Metaphase I Mil arrest Fertilized egg
Mitochondria PB1 PB2
2N2C 2N4C 2N4C 1N2C 1N1C*2
Fig. 1.1. Meiosis.
A sketch of meiotic division. The upper panel: the genomic DNA is half from maternal and half paternal. The mitochondrial DNA is maternal only. The lower panel: the changes in one pair of chromosomes in the oocyte during meiosis. The orange and blue indicating two different maternal chromosomes, the purple in the fertilized egg is a chromosome from the sperm. N: copy number of the genome, C: copy number of
1.1.1. The Generation of Eggs
The early development of eggs in humans is difficult to study because of the limited accessibility to experimental materials. Luckily, this process is evolutionally conserved in mammals and many histologic and genetic studies in dogs, rabbits and mice have mapped early oogenesis (Fig.
1.2).47 In this section, I will review the generation of pre-ovulatory eggs, with an emphasis on the regulation of multiple arrests and resumptions.
Mitosis PGCs Cyst Meiosis Pri F Follicle Oocyte Activation Maturation nordial Primary ollicle Follicle Preovulatory Follicle
Fig. 1.2. Development from the Primordial Germ Cell to the Preovulatory Follicle
A sketch of early oogenesis. The big, round, dark purple cell is the germ cell, the light purple cells are the
granulosa cells, and the blue, flat cells in the outer layer of the primary follicle and the preovulatory follicle are the theca cells. PGC: primordial germ cell. Redrawn from Ref. 4.4
1.1.1.1. The Origin of Oocytes
The specification of germline cells starts at embryonic day 6.25 (E6.25) in the mouse proximal epiblast8. About 40 cells are stimulated by the bone morphogenetic protein (BMP) signal9 to express the transcriptional regulator Blimp] (B-lymphocyte-induced maturation protein
I )/PrdmI (Pr domain containing protein 1)9 and Prdm 1411. These cells proliferate and become the primordial germ cells (PGC) specified by Stella expression.'2" Blimp], Prdm14 and
Stella-Primord positive PGC suppress the expression of somatic
Germ
Mouse mesodermal differentiation genes, restore
Embryo
pluripotency and start the PGC specific genome
HsHindgut
Hd wide epigenetic modifications.8',12",3 At E7.5, PGCs
Gut
\
G a Rkeep proliferating and start to migrate from the/-
posterior primitive streak to the endoderm. Then, they migrate via the hindgut and finally arrive and Fig. 1.3. Migration of PGCs to the colonize at the gonadal ridge at E10.5 (Fig. 1.3). Gonadal Ridge.Adapted from Ref. 14.'1 PGCs off the path will undergo apoptosis4,6,8"14. After arriving at the gonadal ridge at E 10.5, the PGCs keep proliferating and the progeny cells from one PGC form a germline cyst because of incomplete cytokinesis (Fig. 1.4). By E14.5, mitotic divisions of PGCs have ceased and they initiate meiosis. The cysts, before E14.5 and afterwards, partially fragment to smaller cysts. Some of the fragments directly form nests and others aggregate with cysts generated from other PGCs (Fig. 1.4).15 At the same time, the female gonad ridge is developing into the ovary and secreting retinoic acid, inducing the PGC-to-oogonium transition via two independent pathways, Stra8 and Rec8.16-18 The oogonia undergo homologous recombination and stop at the diplotene stage of prophase I of meiosis (MI) where
they enter into a prolonged resting phase and become the primary oocytes (Fig. 1.1, germinal vesicle, GV arrest). Oogonia derived from the same cyst at E 14.5 are strongly synchronized at their meiotic division even if they are relocated in different nests.'5
The final step of early ovarian development is the breakdown of the germ-cell nests associated with a decrease of estradiol after birth4,19,20. During the breakdown of nests, granulosa cells encircle each single oocyte, and eventually primordial follicles (PFs) are formed (Fig. 1.2). The activation of Notch signaling in granulosa cells adjacent to the nests is required for the nest breakdown and PF formation.9 20 In humans, the formation of PFs starts around 20 weeks of gestation.2' Each of the primordial follicles contains a single primary oocyte and a single layer of
flat granulosa cells on its surface (Fig. 1.2).
PGC Mitosis Granulosa CYST cell Fragment and Aggregate
Primordi al Breakdown NEST
Follicle nW.
S
-* Germ cell
from a different PGC
Fig. 1.4. The Formation and Breakdown of the Cyst.
One PGC, after mitosis, forms a cyst. The cyst from one PGC fragments and aggregates with other cyst fragments (green) to form a nest. The nest breaks down by fragmentation and apoptosis during development to become individual cells. Each of the surviving cells will recruit granulosa cells to form a primordial follicle. Redrawn from Ref. 15."
1.1.1.2. Folliculogenesis
The follicle is an importance structure that supports and nurtures the development of
oocytes4
,6,14
. In mammals, primordial follicles represents the entire pool of primary oocytes for
potential egg production over the entire reproductive life.21'22 While some recent studies claimed
the existence of ovarian stem cells (OSCs) in postnatal mice and reproductive-age women23,2 4, the physiological significance of the OSCs in replenishing the germ cell pool in vivo remains controversial2 ,26.
The primordial follicles are continuously recruited for folliculogenesis (Fig. 1.5) right after their formation and during the whole reproductive life4,27,28. During the initial recruitment, a
cohort of PFs start to grow while others are synergistically held at their primordial stage by the
PTEN/FOXO3 and TSC/mTORCI pathways. 29-3
1 Some have suggested that the order of PFs to
be initiated is based on the order of their formation but a detailed mechanism is still not fully understood.3 2
Follicle FSH,
O
Activation LHPrimordial Primary Secondary Follicle Follicle Follicle
Antral Preovulatory
Follicle Follicle
Fig. 1.5. Foiliculogenesis.
In the preovulatory follicle, the granulosa cells next to the oocyte are called cumulus cells and those next to the theca cells, surrounding the antrum are called mural cells. FSH: follicle stimulating hormone; LH: luteinizing hormone. See Fig. 1.2 for more information. Redrawn from Ref. 4.4
Once entering the growing pool, the PF grows first to the primary follicle that has cubical granulosa cells and a single layer of theca cells on the surface, then to the secondary follicle where granulosa cells proliferate to a multilayer complex and the oocyte is encapsulated by a layer of glycoprotein polymer called zona pellucida, and finally to the antral follicle (AF) with a fluid-filled cavity (Fig. 1.5).4,6 The AF granulosa cells differentiate to two different group of cells, cumulus cells connected with the oocyte and mural cells connected with the theca cells. While pre-antral follicles do not respond to gonadotropins (e.g. FSH and LH), AFs do require FSH and LH for further development. Most of the AFs without an FSH surge will undergo atresia, a granulosa cell regulated apoptotic process. After puberty, a cohort of AFs can be rescued by a transient elevation of FSH. FSH induces the expression of the luteinizing hormone (LH) receptor (LHR) in mural cells in preparation for the LH surge that is responsible for maturation and ovulation of the oocyte.3 3-5
The oocyte, though it remains arrested at the prophase I of meiosis (MI or GV arrest), increases in size and volume during folliculogenesis.,6,3 2 The oocytes and the granulosa cells
interact via paracrine signals and the more direct gap junctional communications (GJCs), forming an electrophysiological syncytium.36 With the help of granulosa cells, the oocyte
accumulates an astonishing amount of proteins and RNAs in preparation for maturation,
ovulation and fertilization. After re-entering into meiosis, the oocyte stops transcription because of the inaccessibility of the condensed DNA, and the regulation of cell function will depend mostly on post-transcriptional regulation such as protein phosphorylation and relocation, mRNA
1.1.1.3. GV Arrest and Oocyte Maturation
The oocyte in the antral follicle is competent for meiotic resumption but it is held at the germinal vesicle (GV) stage by the granulosa cells until stimulation by a high dose of luteinizing hormone (LH).3 8-43 When removed from the follicle (and association with the granulosa cells), the oocyte undergoes spontaneous maturation.40'41 Cyclic-nucleotides (e.g., cAMP and cGMP) play a
crucial role in the maintenance of GV arrest; incubation with cAMP inhibits the maturation of isolated oocytes.4 2 A high concentration of cAMP activates the cAMP dependent protein kinase
A (PKA, not shown in the figure) which suppresses the maturation-promoting factor (MPF) required for egg maturation (Fig 1.6, top).434 4
As shown in Figure 1.6, the GV arrested oocyte synthesizes cAMP from ATP by the adenyl cyclase (not shown in the figure) stimulated by a constitutively active G-protein receptor, GPR3 in mice.45'46 A sufficient amount of cAMP is produced in the oocyte to prevent
maturation.47 A high level of cAMP in the GV oocyte is also maintained by the inhibition of the
cyclic nucleotide phosphodiesterase 3A (PAD3E), which hydrolyzes cAMP48. How is PAD3E
inhibited? It appears that during follicular development, the FSH surge (not shown in the figure) to the antral follicle induces the expression of a mural cell specific C-type natriuretic peptide (CNP).49 Both granulosa cells, cumulus and mural, express the CNP receptor/guanylyl cyclase NPR2 that converts GTP to cGMP.50-2 The high concentration of cGMP synthesized in
granulosa cells, enters the oocyte through the GJCs and competitively inhibits the activity of PAD3E. 5 Together, the constitutive activation of GPR3 and the inhibition of PAD3E by high cGMP, are able to maintain a high cAMP concentration in GV oocyte and prevent its
A surge of luteinizing hormone (LH) to the follicle reduces the cAMP level in the oocyte and activates the MPF (Fig. 1.6, lower).4'38 3 9 How does LH lower the cAMP level in the oocyte? Surprisingly, LH binding to the LHR (a GPCR) activates the adenylyl cyclase and induces a cAMP spike in mural cells rather than a reduction.16
,7 As a result of the mural cell cAMP spike, the NPR2 is inactivated by de-phosphorylation and the cGMP phosphodiesterase PDE5 (not shown in the figure) is activated by phosphorylation.2' 3 With the decreased production by
NPR2 and increased degradation by PDE5, the cGMP level in granulosa cells drops
dramatically, so does translocation of cGMP to the oocyte. Also, the closure of GJCs followed by the cAMP spike further lowers the diffusion of cGMP.58 Thus, the oocyte cGMP level drops
below the threshold for an effective inhibition to the cAMP phosphodiesterase PDE3A.54
,58 The
cAMP level in oocyte decreases, PKA is inhibited, MPF activated, and the oocyte will resume meiosis, finish the first cell division, extrude a first polar body, and arrest again at the metaphase II of meiosis. The mature egg will be ovulated for fertilization. (Figure 1.1, GV arrest to MII arrest)
CNP -~ - -~~~ GTP NN CNP CNP ATP cGMP GV arrest JRP8gmo high cAMP GTP cGMP
high cGMP high -- PAD3E
>
I
MPF inactivecGMP cGMP MPF active
Maturation low X cGMP low
cAMP -- I CNP PAD3E--
cAMP
LHR
LH LRATP
LH
AT
- - PR2
Mural Cells Cumulus Cells Oocyte
Fig. 1.6. The GV Arrest and Meiotic Resumption
Upper: high cGMP in mural cells and cumulus cells enters the oocyte via gap junctions to inhibit PAD3E, so cAMP in the oocyte is high and can inhibit the MPF. Lower: High cAMP induced by LH and its receptor in mural cells reduces CNP and the level of cGMP in mural and cumulus cells, and closes the gap junctions, leading to low cGMP in the oocyte. Reduced cGMP cannot inhibit PAD3E, so active PAD3E reduces cAMP and activates MPF. The light blue boxes in mural and cumulus surface are CNP receptors, NPR2s; the orange and blue cylinders represent the GPCRs GPR3 and LHR and the attached circles represent their cognate G-proteins ("G"); the black crosses in the bottom panel between mural-cumulus and cumulus-oocyte indicate the closed gap junctions; the red crosses indicate blocked activation or inhibition. MPF: maturation-promoting factor; LH: luteinizing hormone; LHR: LH receptor; CNP: C-type natriuretic peptide; PAD3D: cAMP phosphodiesterase. Redrawn from ref. 4.4
1.1.1.4. Meiosis II Arrest
The mature oocyte, or egg, which is fully competent for fertilization, however, it is arrested at the metaphase 1I of meiosis (MII) by a cytostatic factor (CSF)9-62. Keith T. Jones and his group
established that the endogenous meiotic inhibitor 2 (Emi2) was the CSF in mice (Fig. 1.7).63
Emi2 establishes and maintains MII arrest by inhibiting the activity of the anaphase promoting
complex/cyclosome64
1,6 (APC/C), an E3 ubiquitin ligase required for degradation of cyclin B6 6
(part of MPF) and exit from metaphase to anaphase67. Biochemical and structural analyses have shown that Emi2 bound to the APC activator protein Cdc20 and dissociated it from the APC/C complex68 70. It is also found in Xenopus that Emi2 could competitively inhibit the E2
ubiquitin-conjugating enzyme Ube2S from binding to APC/C, further inhibiting the APC/C activity.7' The release of an egg from M1I arrest, a process called egg activation, occurs during fertilization. I will discuss the details of egg activation in the next subsection (1.2).
Multiple meiotic arrests and resumptions occur during oogenesis temporally control pregnancy and embryonic development in mammals. As the pool of all potential eggs, the primordial follicles need to be strictly controlled to ensure that most eggs with the best quality are activated during the period of reproductive life. PFs that matured too early are discarded because the female is not sexually mature, and those too late also cause problems because the aged female cannot carry or deliver babies. The GV arrest is the regulation for each menstrual cycle, e. g., the uterus needs to be prepared for implantation so only a narrow time window is available for ovulation. And last, but not least, the MII arrest controls the timing for meiosis-to-mitosis transition so the embryonic development can start simultaneously with sperm-egg fusion. Eggs that are spontaneously activated before ovulation (without sperm insemination) can resume meiosis and initiate mitosis before ovulation, causing ovarian teratomas.72-74
Cdc20 4b Emi2
--APC/C - MPF -I
M11 Arrest
Activated
Fig. 1.7. MI1 Arrest Held by Emi2
Emi2 and MPF hold MII arrest and Cdc20, APC/C activate eggs. Emi2: endogenous meiotic inhibitor 2;
APC/C: anaphase-promoting complex/cyclosome; MPF: metaphase-promoting complex (CDKI/Cyclin B). In
the egg icons, the green lines represent microtubes, blue represents DNA, the blue oval represents the male pronucleus and red dots represent centromeres.
1.1.2. Egg Activation
In productive fertilization, ovulated, MII arrested eggs are activated by sperm for further
development.75 76 The egg activation process includes three major events in mice and humans: 1)
exocytosis of cortical granules to prevent polyspermy77, 2) completion of meiosis to extrude a
second polar body (2PB) and produce the haploid female pronucleus41, and 3) an increase of metabolism and protein synthesis for the egg-to-embryo transition.7 78 All of these can be
induced by an increase of intracellular, cytosolic calcium concentration ([Ca2
+],) in the egg79-84. How sperm induces the increase of [Ca2
+] and all of the activation events will be reviewed
below. I will also discuss different methods for the parthenogenetic (without sperm) activation and their applications in in vitro fertilization (IVF).
1.1.2.1.
Sperm induced Egg Activation
In 1981, Cuthbertson et. al., observed for the first time oscillations in [Ca2
1], in sperm
inseminated mouse eggs using a calcium sensitive photoprotein aequorin.85 Actually, the sperm induced [Ca2+]i rise had been hypothesized for decades to be responsible for egg activation in
many different species86 87, and Barbara P. Fulton and D. G. Whittingham in 1978 had reported that intracellular injection of calcium could activate the MII arrested eggs in mice.88 Using the calcium chelator BAPTA, Douglas and Joanne Kline were able to show that blocking changes in [Ca2+], inhibited either sperm or parthenogenetic chemicals-induced egg activation.89 So, the [Ca2
+]i rise is not only sufficient but also necessary for egg activation.
After years of efforts, researchers have identified a sperm-specific phospholipase C (PLC() as the sperm factor that stimulates the [Ca2+]i rise in mouse eggs (Fig. 1 .8).90-92 During fertilization, PLCC enters the egg and hydrolyzes phosphatidylinositol- 4, 5- bisphosphate (PIP2) to inositol- 1,4,5- triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3R) on the endoplasmic reticulum (ER) membrane and releases ER calcium, increasing the
intracellular, cytosolic calcium concentration ([Ca2+ ).75
,76,90,93
-96 In response to the increased
cytosolic calcium, calcium ATPases pump excess cytosolic calcium back into the ER or into the extracellular space and sodium/calcium exchangers can move the calcium into mitochondria or the extracellular space. These responses drive the [Ca2+], to the normal baseline, and the process repeats itself. As a result, the [Ca2+]i oscillates with the presence of constant IP3.93 In fact, the
sperm stimulated change of [Ca2+], yields oscillations for as long as hours until the formation of
MII-arrested eggs stimulates [Ca2+]i oscillations, while a mutant version of cDNA that encode
non-catalytical PLCC does not induce changes in [Ca2+].90 Also, immunodepletion of PLC or antibody inhibition of IP3R prevents the [Ca2
+], change and its subsequent egg activation after
fertilization by sperm.90'97
Sperm
PLCC PIP2 1P3Ca
2.
.*
/
g2
0R
Granular +- PKC CamKll active
exocytosisAPC
Emi2 Egg
APCC active
-
MAPK-+ Mitotictransition
Metaphase Anaphase
Fig. 1.8. Egg Activation by Sperm
Pathway of sperm induced egg activation. PLC activates PKC for granular exocytosis, activates APC/C for meiosis resumption and inhibits MAPK for mitotic transition. The blue cylinder is the IP3 receptor on the ER membrane, the green dots indicate calcium ions. See 1.2.1 for more abbreviates. See Fig. 1.7 for more about the metaphase and anaphase chromosomes.
-I sperm
1.0
-0.8
-C) Co CO) C)
'0.6-0 CD C JQ (U U- L,
0.2-10 min
0.0-Fig. 1.9. ICa2+], Oscillations in M11 Eggs during in vitro Fertilization.
Fura-2 ratio indicates cytosolic calcium concentration (arbitrary unit). Adapted from Ref. 96.96
As an important second messenger, calcium can regulate many cellular processes via calcium dependent kinases. For eggs, all three activation events can be triggered by the calcium signal stimulated by sperm PLCC.8 9 The sperm stimulated [Ca2 ]i oscillation has a frequency of
10-20 rises per hour of about 1 min duration for each rise, and a much longer (-5 min) first rise as shown in Fig. 1.9.98 Only the first [Ca2]i rise is required to trigger the granular exocytosis and prevent polyspermy while more oscillations (-60-90 min) are required for resumption of meiosis and extrusion of the second polar body. Prolonged oscillations (>180 min) are critical for later activation events, including pronucleus (PN) formation, new protein synthesis and chromosome
modifications.8 1,929 9 ER calcium is the initial calcium source for [Ca2+ ] rises but longer
oscillations require the entry of extracellular calcium.'00
Two hypotheses for the mechanism of the long-lasting oscillations during egg activation in the mouse have been proposed. The first is based on the well-established knowledge that the [Ca2+], oscillations are strictly tuned in many different cells to fulfill signaling functions.'0 1 In
this hypothesis oscillation is the required pattern (with unique frequency and amplitude) of [Ca2+], for egg activation, and prolonged oscillations are critical for late activation events such as
chromosome remodeling. The other hypothesis is that it is not the pattern and duration of [Ca2], oscillations but the overall magnitude of the [Ca2+]i rise - integrated increase in [Ca2+]i, that is necessary for the events of egg activation to occur.'00 And hypothesis one is one specific case of the second hypothesis that accumulates calcium increase with prolonged oscillations. The fact that embryos generated from eggs activated with reduced oscillations have deficiency in further development agrees with both hypotheses.0 2 I will discuss how both hypotheses have been examined using parthenogenetic egg activators later in this section (1.2.4).
Little or no transcription is associated with egg activation, but a big shift of the proteome is required for the egg to embryo transition.78 Thus, the calcium induced changes in protein
composition during egg activation are mostly regulated post transcriptionally. Maternal mRNA adenylation and degradation, protein phosphorylation and degradation, together with new-protein translation from maternal mRNA, mediate the egg to embryo transition.75", 03104
The [Ca2+] oscillations stimulated by sperm during egg activation are initially transduced into downstream phosphorylation signals via the calmodulin-dependent protein kinase Ily
(CaMKIIy). (Fig. 1.8)105 CaMKIIy is a serine/threonine protein kinase that can be activated by calcium and the calcium-sensing, regulatory molecule calmodulin. During fertilization, the
activity of CaMKI1y fluctuates with the [Ca2+]i oscillations.' 6,07 The activation of CaMKIIy by the increase of [Ca2+] leads to the phosphorylation and degradation of Emi2, releasing its
inhibition of APC/C.82,05 Once the E3 ubiquitin ligase APC/C is activated, it can ubiquitinate many proteins that are involved in the cell cycle arrest, e.g., cyclin B of the MPF.82 (Fig. 1.8) Another important protein to be degraded is the securin that holds the metaphase sister chromatids together.105 The activation of CaMKIIy also regulates the activation of PKC for granular exocytosis and the deactivation of the MAP kinase for proteome reprogramming. [Ca2
+],
oscillations failed to activate eggs from CaMKIIy knock out mice, and egg activation could be successfully rescued by injecting a constitutively active form of CaMKIIy, indicating that CaMKIIy is not only required but also sufficient for egg activation.' 051 08 Calcineurin is another
calcium/calmodulin-dependent protein kinase that plays a critical role in egg activation for the degradation of cyclin B in Xenopus but no evidence has shown that it also functions in
1.1.2.2. Calcium Channels in Egg Activation
As mentioned earlier, the calcium of the initial sperm-induced oscillations is provided by the ER via IP3 receptors (IP3Rs). The IP3Rs are expressed early in GV oocytes but they are insensitive to IP3 stimulation, only in post maturation eggs can the receptors be activated by the IP3 induced by sperm.II0 IP3R inhibitors can inhibit the sperm induced [Ca2+], oscillations and egg activation, and IP3 alone can activate eggs, indicating that the activation of IP3R is critical to induce egg activation.97 However, the fact that PLC knock out (KO) males are not sterile raises the
possibility that, when the normal PLC -dependent transduction pathway is not available, there is an alternative pathway for egg activation under physiological conditions.I As Hachem et al. reported, egg activation and zygote development induced by the PLC KO sperm were delayed for hours, and the changes in [Ca2+], , if any, for such a long term have not been measured. I Further experiments are required to specify whether the alternative activation pathway requires a [Ca2
+] raise and whether IP3R is involved.
Because extracellular calcium is also required for later [Ca2+ ] oscillations in sperm induced egg activation, the mechanism(s) by which extracellular calcium enters the egg has also been extensively studied." 2 Three of the potential pathways are, 1) via the store-operated
calcium entry (SOCE) to replenish the ER calcium, 2) via the voltage-gated calcium channels (VGCs) or 3) the transient potential channels (TRPs) to directly increase [Ca2
+],. Store-operated
calcium entry (SOCE) has been reported to be involved in egg activation in pigsI
L3,
but knocking out of neither STIM, the ER membrane-resident calcium sensor of SOCE, nor ORAL, the channel protein of SOCE that interacts with STIM, changes the fertilization [Ca2+ ] oscillations in mouse eggs compared with those of the wildtype (WT). And both of the single KO mice are fertile." 4activation but the T-type channel KO females are fertile with only a very minor decrease in the litter size."5 The TRPV3 and TRPM7 (See 1.3.3 for more channel details) channels are also expressed in the MII eggs but both KOs are fertile.96"'6 It was recently reported that the double
KO of T-Type channel Cav3.2 and the TRPM7 resulted in female subfertility in terms of litter size, and their eggs could not respond to extracellular calcium or magnesium. But the litter size change for the double KO females was very small and the suppression of [Ca 2 ]i oscillation was
partial, suggesting there were other mechanisms responsible for the calcium entry during fertilization.' 16 I will address the role of other calcium channels in excess cholesterol-mediated
1.1.2.3.
Parthenogenetic Activation and Its Applications
Parthenogenetic egg activation refers to egg activation without sperm. As we have reviewed earlier, the [Ca2+] oscillations stimulated by the sperm PLC is the key for egg activation; activation of CaMKIIy generates the phosphorylation signals in response to the [Ca2+]i changes; and the final meiosis resumption and proteome remodeling depend on the activation of APC/C and the reduction of cyclin B. So, parthenogenetic activation is usually assessed by 1) increase of the [Ca2+1i, 2) activation of the CamKIIy and 3) decrease of cyclin B.
The most commonly used parthenogenetic activation methods stimulate rise(s) in [Cai2+ SrCl2 is a widely used chemical activator that can induce [Ca2+ ] oscillations in eggs in various species, including the mouse, rat, pig and bovine etc., but not human.17-122 Another way to induce repetitive [Ca2+], spikes is using electrical pulses and this method can simulate any pattern of [Ca2
+] by controlling the number of pulses, extracellular calcium concentration or the current
for each pulse.8 Many other stimulators do not induce [Ca2+], oscillations but rather just a single,
usually large, [Ca2]i spike. Ethanol 2 3, ionomycin'10,124, the ion calcium 125 and the calcium
ionophore A2318789,126 all fall in this category.
The two different kinds of parthenogenetic activators have been used to examine whether the oscillatory pattern or the integrated, net accumulation of calcium is the key for egg
activation. In mouse eggs, the single large [Ca2+] rise (single 'spike') induced by ethanol, can activate eggs as efficiently as the [Ca2
+], oscillations induced by SrCl2. Eggs activated by either
activation method followed by injection of 'inactivated' sperm heads (activation incompetent) can generate an equal number of embryos and produce an equivalent percentage of off springs from those embryos.123,12 7 So, additional evidence is required to determine if the oscillatory pattern is required for physiologically relevant, viable embryo-producing, egg activation.
Another parthenogenetic activator is the constitutively active form of CamKIIy that, when injected into eggs, can activate the eggs independently of calcium signals.105 Inhibitors of protein synthesis, e.g., cycloheximide, can also activate eggs downstream of calcium regulation. Cycloheximide activates eggs via inhibiting the synthesis of Emi2, cdc20, cyclin B and MAPK and reducing their activities.1 28 But the disadvantage of the protein synthesis inhibitors is that
they do not specifically inhibit the proteins that control cell cycle so they could cause deleterious effects on the later development of the embryos. To maximize the efficacy of egg activation, two or more activators (for example, SrCl2 together with ionophore, or ionophore together with cycloheximide) are usually combined to activate the egg in IVF labs.129
Since the birth of the first baby generated by in vitro fertilization 40 years ago, assisted reproductive technology (ART) has helped many infertile couples to have their own babies. Among all of the ART methods, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are the most two common methods employed.I30 Despite the success of ART, it has limitations -there is a failure rate of 1-3% percent of all cycles in ART even when there are a sufficient number of eggs (or mature oocytes) and motile sperm.'31 One major reason for the failure in both IVF and ICSI is defective egg activation. Assisted oocyte activation (AOA) was proposed to use chemical activators to overcome the failure in activation. The first birth after AOA was reported in 1997, in which case the sperm from the patient were not able to activate the mouse eggs so the human mature oocytes were injected with CaCl2 and spermatozoon during ICSI and treated twice with a calcium ionophore afterwards.' 25 Follow-up experiments have
scored the development of the AOA babies and the preliminary data currently available suggest that those children are in good mental and physical conditions.'31" 32 But these data were
collected by the hospitals with the potential to make profit from the clinical application of AOA. Prior to its general clinical application, additional work is required to assess the safety of AOA129
In this thesis, I will describe in Chapter 2 how my colleagues and I discovered that excess cholesterol was a parthenogenetic activator of mouse eggs that induces a single [Ca2+]i spike.133
Below in the next two sections of this chapter, I will review cholesterol metabolism and homeostasis and how cholesterol can affect the activity of ion channels.
A
Section 1.2. Cholesterol Metabolism, Transport and Homeostasis
Our interest in egg activation and female fertility developed as a consequence of our analysis of the high-density lipoprotein (HDL) receptor, scavenger receptor class B type 1 (SR-BI), which controls the structure and fate of plasma HDL.I34 As is described in greater detail in Chapter 2,
we discovered that excess-cholesterol can activate eggs (Chapter 2) as a consequence of previous
studies of homozygous null SR-BI knockout mice, whose females are infertile.'33,135-137 These
SR-BI knockout (KO) animals suffer dyslipidemia (hypercholesterolemia) because of a failure of cells to take up cholesterol (as cholesteryl esters) from circulating HDLs. As a consequence, the HDL particles are abnormally large and have an abnormally high unesterified cholesterol content in their polar shell (for structure of lipoprotein, see Fig. 1.12). The eggs in the female SR-BI KO mice accumulate with excess cholesterol that activates the eggs prematurely before fertilization leading to female infertility
Sterols, such as cholesterol in higher organisms, is a component of the plasma membrane, and of vital importance to all eukaryotes. Cholesterol is also the precursor to many important biomolecules such as bile acids, steroid hormones, vitamin D and oxysterols.' 38-140 It is required to maintain the membrane structure of every cell. Our body obtains cholesterol from the diet or endogenous synthesis, and the major routes of excretion of cholesterol and its metabolites are the bile or plasma secretion to the gastrointestinal (GI) track. Deficiency or surplus of obtainment or excresion will disrupt the cholesterol homeostasis. The liver is the most important organ in cholesterol synthesis and regulation; however, the brain, intestine and other peripheral tissues also make substantial contributions to whole body cholesterol metabolism. In this section, I will briefly review the synthesis, absorption, circulation and excretion of cholesterol, and then discuss the regulation of these processes that maintain the cholesterol homeostasis.
1.2.1. Cholesterol Synthesis
One major source of body cholesterol is endogenous synthesis, which occurs in virtually every nucleated cell.38- 41 In de novo synthesis, generation of each cholesterol molecule (27 carbons,
C27) consumes 18 acetyl-CoA (C2) molecules (Figure 1.10).141 Firstly, three acetyl-CoA
molecules are converted to one molecule of mevalonate (C6). One of the rate limiting steps, also a common target for cholesterol lowering drugs (e.g., statins), is the reduction of 3-HMG-CoA to mevalonate by HMG-CoA reductase (HMGCR).13 8 Then, one molecule of 30-carbon squalene is generated from six mevalonate molecules and cyclized to lanosterol (C30). After processed by 9 enzymes in 19 steps, lanosterol is converted to cholesterol in the endoplasmic reticulum (ER). The newly synthesized cholesterol will be translocated to other intracellular organelles via a non-vesicular mechanism, or to the plasma membrane to be deposited to its carriers in the blood stream, where it will be transported to peripheral organs and tissues to fulfill its function.'40 Excess cholesterol in the cell will be esterified by the enzyme, acyl-CoA acyltransferase (ACTA) to cholesterol esters, a more insoluble form of cholesterol that can be stored as lipid droplets.' 40
Many of the intermediates of cholesterol synthesis are biomolecules with important cellular functions including their roles as precursors for other metabolites. Blockage of
cholesterol synthesis might cause sever developmental problems caused by the accumulation of a particular intermediate or the lack of another.'42
',CoA Acetyl-CoA (C2) HOyyS'COA 3-HMG-CoA (C6) O HO 0 MUGCOA mfductaae Mevalonate (C6) 0 HO Squalene (C30) HO Lanosterol (C30) HO g H \H H ___ cholesterol (C27)
Fig. 1.10. Cholesterol Synthesis.
1.2.2. Cholesterol Absorption from the Diet
The other source of body cholesterol is intestinal absorption of cholesterol derived from the diet, bile, intestinal secretion, and dead epithelial cells. 143 A typical western diet provides 300-5 00 mg cholesterol per day and the intestine absorbs approximately 800-1200mg biliary cholesterol daily.14 4
-146 Because of their minimal solubility in aqueous environment, absorption of sterols in the GI track is facilitated by bile acids. Cholesterol and phytosterols (plant sterol) in food are first incorporated into micelles formed by bile salts. Together with the biliary cholesterol, dietary cholesterol as well as the plant sterols in the micelles enter the enterocytes through the brush border membranes (BBM). The entry of cholesterol was considered passive diffusion for decades but the Niemann-Pick C 1 Like 1 (NPC 1 L 1) transporter has been shown to transport cholesterol across the BBM .147150 (Fig. 1.11) Part of the sterols absorbed from the intestinal lumen will efflux back to the intestine by the ATP-binding cassette (ABC) transporter G5/G8
heterodimers.'51-15 3 Absorbed cholesterol can be esterified by the ACTA2 in the ER of enterocytes and incorporated into chylomicrons (CMs) for secretion into the lymph. The ABC transporter Al (ABCA1) can also mediate cholesterol movement from enterocytes high density lipoprotein (HDL) particles for transport through the blood. 143,144
1.2.3. Circulation of Cholesterol in the Blood
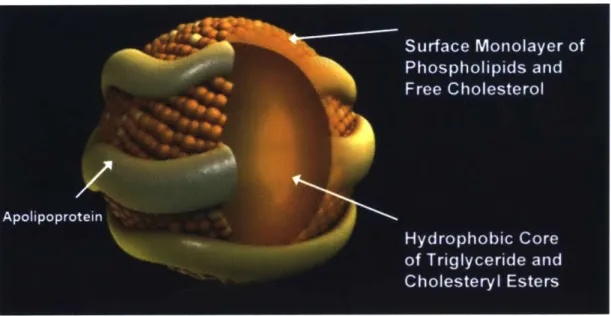
Cholesterol and its ester are highly hydrophobic so they are packed in lipoproteins for transportation in the blood stream (Fig. 1.12).111 Lipoproteins are complex particles with a
central core containing cholesterol esters and triglycerides surrounded by free cholesterol, phospholipids, and apolipoproteins, which facilitate lipoprotein formation and function (Fig.
1.11). 15455 There are 7-classes of lipoproteins in the plasma with different sizes, lipid
compositions and lipoproteins: Chylomicrons (CM), chylomicron remnants (not shown in the figure), very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL; VLDL remnants), low density lipoproteins (LDL), high density lipoproteins (HDL) and Lipoprotein (a) (Lp(a), not shown in the figure).
The cholesterol absorbed by enterocytes is deposited to HDL particles for direct
circulation in the blood, or esterified and packed into the CMs for lymph circulation and finally be taken up by the hepatocytes. 143 Hepatocytes secrete VLDL particles that are processed in the
circulation into LDL particles. Cholesterol from peripheral tissues will be secreted into HDL particles via ABCA 1. Circulating VLDL, LDL particles can exchange their triacylglycerol for cholesterol esters on HDL particles, an exchange mediated by the cholesterol ester transport protein (CETP).156-158 VLDL and LDL can be endocytosed into cells by LDL receptors, mainly in the liver but also into almost all other cells, including those in muscle, heart and adipose tissues.138 159 Lipid in the HDL can be utilized by selective uptake through the HDL receptor SR-BI, which is most highly expressed in hepatocytes and steroidogenic cells in the adrenal gland,
Fig. 1.11. Structure of a Lipoprotein Particle. Adapted from Ref. 154.154
Blood CETP SR-BI HDL
0
--- 4---* Cholesterol and e Triacylglycerol Intestine Cholesterol ABCAI absorption HDLO 4-f -- +-NPC1L1 CM ABCG -- Lymph ,Cholesterol excretion ster0
FecalFig. 1.12. Cholesterol Homeostasis
Cholesterol absorption in the intestine (bottom right, blue), processing in the liver (bottom left, yellow) and circulation in the blood stream(top, red). HDL: high-density lipoprotein; CM: chylomicron; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein. See Section 2.2, 2.3, and 2.4 for more abbreviates. Redrawn from Ref. 139139 and Ref. 160.160
VLDL LDL - Y LDLR Liver ABCG5/8 Bile Fecal
The lipoproteins move between plasma and interstitial fluid by passive ultrafiltration and transcytosis,160-162
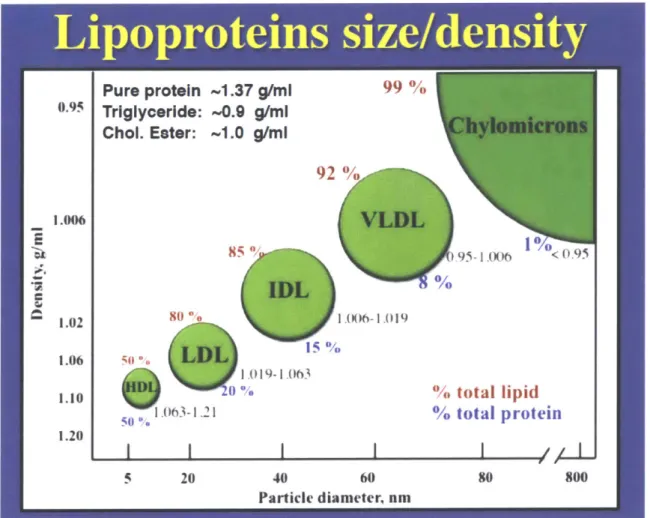
so the size of the circulating lipoproteins affect their ability to access the interstitial space. The largest particles, CMs and VLDLs, are limited to the blood or the lymph while the smaller ones, LDLs and HDLs can move cross the endothelial cells into the interstitial fluid (Fig 1. 13).161,161 The space between epithelial cells also controls the entrance of
lipoproteins into fluid compartments, for example, ovarian follicular fluid contains
predominantly HDLs with only traces of others because of the filtration of thecal blood.16-165 Another extreme example is the brain where the blood endothelial cells form tight junctions (the blood brain barrier, BBB) not even allowing the passage of HDLs, so the cholesterol metabolism of the brain is relatively isolated from that in other parts of the body.166-168 Degraded products of
cholesterol in the brain (oxysterols) are released into the circulation to remove excess cholesterol from the brain.' 67
Fig. 1.13. Sizes and Lipid Compositions of Different Lipoproteins
The figure shows major types of lipoproteins with different diameters and densities. Chylomicron (CM) has the largest size and lowest density. The diameters of CM, CM remnants (no shown), very-low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), low density lipoprotein (LDL) and high-density lipoprotein (HDL) decrease while the densities increase, indicating a declining of lipid composition (red). Adapted from Ref. 155.55
1.2.4. Excretion of Cholesterol to the GI Tract
Excess cholesterol and its metabolites are excreted out of the body. The excess cholesterol in peripheral tissues comes from excess synthesis and uptake, clearance of cholesterol metabolites and dead cells. The elimination of cholesterol has two major pathways, classical reverse
cholesterol transport (RCT)69 and transintestinal cholesterol excretion (TICE)170. RCT recycles cholesterol and its metabolites firstly to the liver where some of the cholesterol is converted into bile salts. Together, cholesterol and the bile salts are secreted by the hepatocytes into the bile and
later to the GI track (Fig. 1. 12). The TICE bypasses the liver; it can directly move plasma cholesterol in lipoproteins to the lumen of the GI tract via enterocytes. In both pathways, cholesterol would eventually be eliminated with fecal excretion. Another important source of cholesterol in the feces is the dead epithelial cells of the gastrointestinal tract. Patients after surgery or massive hemorrhage also loose a substantial amount of cholesterol in blood cells and platelets. 171
1.2.5. Regulation of Cholesterol Metabolism
Cholesterol homeostasis is maintained by the balance of its synthesis, absorption and excretion. There are two well-established regulators of cholesterol homeostasis, the sterol regulatory element-binding proteins (SREBPs) and liver X receptors (LXRs), both of which are transcription factors. 138-140,172
The SREBP on the ER membrane is in a complex with the SREBP cleavage-activating protein (SCAP), which can sense the ER cholesterol concentration.138 When the content of cholesterol falls below 5 mol% of the total ER membrane lipids (Fig. 1.14A), SCAP binds to the coat protein II (CopII).173"174 CopIL will escort the SCAP-SREBP complex to the Golgi where SREBP will be cleaved by the site-I and site-2 proteases. The cleavage of SREBP allows the release of its cytosolic portion (the basic helix-loop-helix, bHLH). The now free bHLH is translocated to the nucleus where it activates the genes that can help to increase the cellular concentration of cholesterol, including cholesterol synthesis (e.g., HMGCoAR) and uptake (e.g., LDL receptors).138 When the ER cholesterol concentration is high (Fig. 1.14B), cholesterol binds
to SCAP and changes the conformation of SCAP, which is further locked by the insulin-induced genes (INSIG) so that CopIl cannot bind to SCAP anymore. Thus, the SCAP-SREBP complex is anchored at the ER membrane by INSIG, retaining SREBP in their inactive form.
While SREBPs regulated genes are activated under a low cellular cholesterol level, LXRs are responsible for cholesterol removal under high cholesterol levels.17 2 The LXRs are activated
by cholesterol derivative oxysterols, as well as precursors in the cholesterol biosynthesis pathway, such as desmosterol but not cholesterol. When activated, LXRs can increase cholesterol efflux and reduce cholesterol uptake. In the enterocytes, the expression of




![Fig. 1.9. ICa 2 +], Oscillations in M11 Eggs during in vitro Fertilization.](https://thumb-eu.123doks.com/thumbv2/123doknet/14298882.493710/33.917.177.731.111.585/fig-ica-oscillations-m-eggs-vitro-fertilization.webp)