Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
ACI SP, N91, 2, pp. 1445-1462, 1986
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=bf239155-7666-4b34-9ccf-353f050e1a22
https://publications-cnrc.canada.ca/fra/voir/objet/?id=bf239155-7666-4b34-9ccf-353f050e1a22
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Carbonation of granulated blast furnace slag cement concrete during
twenty years of field exposure
C
CounsHWrUd.
*--Qrud.
1
N21d
no.
1398
Institute for
lnstitut de
,
c.
2
Research in
recherche en
B19G
Construction
construction
Carbonation of Granulated
Blast Furnace Slag Cement
Concrete during Twenty
Years
of Field Eicposum
I
by G.G. Litvan and A. Meyer
A N , ~ L \ [ Z E D
Reprinted from
"Fly Ash, Silica Fume, Slag, and Natural Pouolans
in Concrete"
Proceedings Second International Conference
Madrid, Spain, 1986
ACI, SP-91, Vol. 2, p. 1445-1462
(IRC Paper No. 1398)
Price $2.00
NRCC 26203
1
N!?C
-
CISTIg r a n u l e (CLG), o n t t c o n s t r u i t e s dans des c o n d i t i o n s soigneusement c o n t r a l 6 e s e t documentks. AprBs 20 ans d ' e x p o s i t i o n , d e s c a r o t t e s o n t 8 t B a n a l y s e e s e t une c a r b o n a t a t i o n importante s u r 40 ma de profondeur a 6 t 6 d6cel6e p a r a n a l y s e thermogravimgtrique e t par a n a l y s e chimique. F a i t p l u s important, on n ' a guBre trouvd de Ca(OH)* dans l e beton de CLG,
B
quelque niveau que c e f a t , de s o r t e que t o u t e armature d ' a c i e r d e v r a i t 8 t r e c o n s i d Q C conme a u j e t t eB
corrosion.D'aprBs l e s r d s u l t a t e obtenus grace au porosim8tre
B
mercure,l a p o r o s i t e du bdton de CPO a diminu6 a p r e s
l a
c a r b o n a t a t i o n mais c e l l e du beton de CLG n'a pas changd.De
p l u s , l e grossissement des pores a e n t r a f n 6 un accroissement de l a p e r k b i l i t g du beton de CLG, a p r s s c a r b o n a t a t i o n , e t l a r e s i s t a n c eB
l a t r a c t i o n de l a zone de s u r f a c e - ~ a diminusBlast Furnace Slag Cement Concrete
During Twenty Years of Field Exposure
by
G .
G .
Litvan and A. Meyer
S y n o p s i s : Two e x p e r i m e n t a l h o u s e s , one of o r d i n a r y p o r t l a n d cement (OPC) c o n c r e t e and t h e o t h e r o f g r a n u l a t e d b l a s t f u r n a c e s l a g cement (GBFSC) c o n c r e t e , w e r e b u i l t u n d e r c a r e f u l l y c o n t r o l l e d and documented c o n d i t i o n s . A f t e r 20 y e a r s of e x p o s u r e , c o r e s were a n a l y s e d and s i g n i f i c a n t c a r b o n a t i o n t o 40 mm i n d e p t h was d e t e c t e d by TGA and t h e w e t c h e m i c a l method. More s i g n i f i c a n t l y , l i t t l e Ca(OH)2 was f o u n d i n t h e GBFSC c o n c r e t e a t a l l l e v e l s , s o t h a t a n y r e i n f o r c i n g s t e e l would h a v e t o be c o n s i d e r e d s u s c e p t i b l e t o c o r r o s i o n . According t o Hg p o r o s i m e t r y r e s u l t s , t h e p o r o s i t y o f OPC c o n c r e t e d e c r e a s e d a f t e r c a r b o n a t i o n b u t t h a t of GBFSC remained unchanged. I n a d d i t i o n , i n c r e a s e d p e r m e a b i l i t y o f GBFSC c o n c r e t e w i t h c a r b o n a t i o n was i n d i c a t e d by c o a r s e n i n g of t h e p o r e s , and t h e t e n s i l e s t r e n g t h of t h e s u r f a c e r e g i o n s u f f e r e d a l a r g e d e c r e a s e . Keywords: b l a s t f u r n a c e s l a g ; c a r b o n a t i o n ; c h e m i c a l a n a l y s i s ; e x p o s u r e ; f i e l d t e s t s ; p o r t l a n d c e m e n t s ; p o r t l a n d s l a g cements; r e i n f o r c e d c o n c r e t e ; t e n s i l e s t r e n g t h .
G.G. L i t v a n i s a s e n i o r r e s e a r c h o f f i c e r of t h e D i v i s i o n of B u i l d i n g Research, N a t i o n a l Research Council of Canada. His r e s e a r c h d e a l s w i t h d u r a b i l i t y problems of b u i l d i n g m a t e r i a l s . Dr.-Ing. A. Meyer i s p r o f e s s o r , Dept. C i v i l E n g i n e e r i n g , Technische Hochschule Darmstadt, and D i r e c t o r of Research and Development, H e i d e l b e r g e r Zement AG, H e i d e l b e r g , West Germany. His r e s e a r c h i n t e r e s t i s i n t h e a r e a of b u i l d i n g m a t e r i a l s , c o n c r e t e technology, f i b r e c o n c r e t e , t i m b e r c o n s t r u c t i o n and b u i l d i n g p h y s i c s .
INTRODUCTION
I n a s u r v e y of t h e long-term behaviour of c o n c r e t e made from g r a n u l a t e d b l a s t f u r n a c e s l a g cement (GBFSC), Schr6der and Smolczyk (1) r e p o r t e d c o n f l i c t i n g c o n c l u s i o n s i n t h e p e r t i n e n t l i t e r a t u r e . On t h e one hand, no s i g n i f i c a n t d i f f e r e n c e s were observed between r a t e and e x t e n t of c a r b o n a t i o n of o r d i n a r y p o r t l a n d cement (OPC) and GBFSC. On t h e o t h e r , a tendency was observed f o r GBFSC t o c a r b o n a t e a t a h i g h e r r a t e t h a n OPC, p a r t i c u l a r l y a t f i r s t . They e x p l a i n e d t h e disagreement a s f o l l o w s :
"a. So f a r we have t o o few long-time c a r b o n a t i o n s t u d i e s w i t h c o n c r e t e a v a i l a b l e , s o t h a t we t o o o f t e n e x t r a p o l a t e t h e r e s u l t s of few y e a r s on t o much l o n g e r t i m e s p a n s . A l l we know about c a r b o n a t i o n f u n c t i o n s s o f a r can only be regarded a s rough approximations and s o q u i t e i n a d e q u a t e f o r e x t r a p o l a t e d c o n c l u s i o n s and t h e e x a c t d e f i n i t i o n of d i f f e r e n t behaviour.
"b. The e x p e r i m e n t a l c o n d i t i o n s of many t e s t s a r e not s t r i c t l y comparable. Since c o n c r e t e q u a l i t y , e.g., i t s d e n s i t y , i s a d e c i s i v e f a c t o r , i t must be k e p t uniform i f we want t o measure t h e i n f l u e n c e of chemical cement composition."
I n t h e l a s t decade i n t e r e s t i n t h e t o p i c h a s i n c r e a s e d
c o n s i d e r a b l y a s use of a d d i t i o n a l cementing m a t e r i a l s such a s GBFSC a s a replacement f o r OPC h a s been made even more a t t r a c t i v e by t h e e s c a l a t i n g c o s t of energy. But i n s p i t e of i n t e n s e a c t i v i t y i n t h e f i e l d , few long-term e x p e r i m e n t s d e s i g n e d t o c l a r i f y u n d e r s t a n d i n g of t h e d u r a b i l i t y of GBFSC c o n c r e t e have been performed.
A t t h e time of t h e SchrGder and Smolczyk s t u d y (1) a f i e l d exposure program had been i n p r o g r e s s f o r some s i x y e a r s i n Germany. Those e x p e r i m e n t a l h o u s e s , b u i l t under c o n t r o l l e d c o n d i t i o n s , have now been monitored f o r 20 y e a r s . An a n a l y s i s of t h e accumulated r e s u l t s i s p r e s e n t e d h e r e .
EXPERIMENTAL
The r e i n f o r c e d c o n c r e t e house w i t h OPC was b u i l t 2 3 September 1963 and t h a t w i t h GBFSC on 17 September 1964, b o t h i n Beckum, W e s t p h a l i a , F.R.G. No i n d u s t r y o t h e r t h a n a cement p l a n t c o u l d have a f f e c t e d t h e s u r r o u n d i n g atmosphere. These s m a l l houses had n o windows and were n e i t h e r h e a t e d n o r i n h a b i t e d . The p r o p e r t i e s of t h e cements and c o n c r e t e s a r e l i s t e d i n T a b l e s 1 and 2. TWO c o r e s of 15-cm d i a m e t e r were d r i l l e d from t h e n o r t h w a l l of e a c h e x p e r i m e n t a l house, l e n g t h s of 15 cm (GBFSC) and 17 cm (OPC) r e p r e s e n t i n g t h e e n t i r e t h i c k n e s s of t h e w a l l s . The c o r e s were h a l v e d l e n g t h w i s e i n t h e l a b o r a t o r y by means of a water-cooled diamond saw and b o t h e n d s of t h e s e m i - c i r c u l a r p i e c e s were c u t i n t o t e n 4-m t h i c k s l i c e s . The s e c t i o n s were t h e n d r i e d i n vacuo a t room t e m p e r a t u r e and s t o r e d i n a d e s i c c a t o r . Before t e s t i n g , t h e c o n c r e t e was c r u s h e d w i t h a p e s t l e i n a n a g a t e m o r t a r and any c o a r s e and i d e n t i f i a b l e f i n e a g g r e g a t e s were removed.
P h e n o l p h t h a l e i n T e s t
2% p h e n o l p h t h a l e i n i n e t h y l a l c o h o l was u s e d t o d e t e r m i n e t h e d e p t h of c a r b o n a t i o n ( 2 ) on t h e c o r e s immediately a f t e r t h e y were sawn l e n g t h w i s e ( s p l i t ) .
Thermogravimetry
lOO-mg specimens were h e a t e d i n a Dupont Model 1090 i n s t r u m e n t a t 20°C/min i n a s t r e a m of n i t r o g e n gas.
Chemical A n a l y s i s of C a r b o n a t e s
Approximately 0.5 g was d i g e s t e d i n 3 M H2S0,+ and t h e
l i b e r a t e d C02 gas f l u s h e d by N2 s t r e a m was absorbed i n 0.1 N Ba(OH)2. The amount of n e u t r a l i z e d Ba(OH)2 was d e t e r m i n e d by t i t r a t i o n w i t h 0.1 N HC1.
Pore S i z e A n a l y s i s
P o r o s i t y and p o r e s i z e d i s t r i b u t i o n were d e t e r m i n e d by mercury i n t r u s i o n p o r o s i m e t r y . The maximum p r e s s u r e a p p l i e d i n t h e AMINCO i n s t r u m e n t was 410 MPa ( 6 0 000 p s i ) .
Compressive S t r e n g t h
P r e p a r a t i o n and t e s t i n g of t h e specimens were c a r r i e d o u t i n c o n f o r m i t y w i t h German S t a n d a r d D I N 1048.
T e n s i l e S t r e n g t h
A SATTEC M a t e r i e l i n s t r u m e n t was u s e d t o measure t h e f o r c e r e q u i r e d t o b r e a k , i n t e n s i o n , t h e s u r f a c e r e g i o n of t h e c o n c r e t e below a s t e e l p l a t e (35-m diam) a f f i x e d t o t h e specimen w i t h epoxy r e s i n . T e n s i l e s t r e n g t h of t h e specimens was determined i n August 1982, i . e . , a t 19 and 18 y e a r s , r e s p e c t i v e l y .
Freezing and Thawing
Specimens 10 mm wide and 25 mm l o n g were c u t from t h e 4-mm t h i c k s e c t i o n s . Length changes were determined d u r i n g t e m p e r a t u r e c y c l e s (+8 and -lg°C) i n a homemade e x t e n s o m e t e r ( 3 ) p l a c e d i n a microcomputer-governed t e m p e r a t u r e l h u m i d i t y t e s t chamber
(Thermotron Model SM.8C).
RESULTS
The p h e n o l p h t h a l e i n - t r e a t e d c o n c r e t e s u r f a c e s were t r a c e d from c o l o u r photographs f o r e a s e of r e p r o d u c t i o n and a r e shown i n Fig. 1. Thicknesses of t h e c a r b o n a t e d l a y e r s a t t h e two e n d s of t h e specimens a r e not t h e same, presumably owing t o d i f f e r e n c e s i n humidity and Cop c o n c e n t r a t i o n of t h e e x t e r i o r and i n t e r i o r
atmospheres t o which t h e house was exposed. The w i d t h of t h e c o l o u r l e s s l a y e r s i s 6 mm and 3 mm, r e s p e c t i v e l y , f o r OPC c o n c r e t e , and 22 mm and 13 mm, r e s p e c t i v e l y , f o r GBFSC c o n c r e t e .
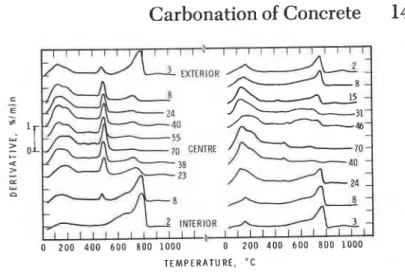
The r e s u l t s of t h e r m o g r a v i m e t r i c a n a l y s i s a r e g i v e n i n Fig. 2. The c o n c e n t r a t i o n of CaCOJ and l i m e was determined by i n t e g r a t i o n of t h e a r e a s below t h e peaks (Fig. 3). The, peak of t h e d e r i v a t i v e o c c u r r i n g between 100 and 200°C i s caused by l o s s of w a t e r from t h e c a l c i u m s i l i c a t e h y d r a t e s . That between 450 and 550°C i s due t o decomposition of Ca(OH)2 i n h y d r a t e d C S ( 4 ) . That a t
3
approximately 780°C s i g n i f i e s decomposition of CaC03 ( 5 ) . The f o l l o w i n g o b s e r v a t i o n s were made:
- a t a l l d e p t h s t h e l i m e c o n t e n t of t h e OPC c o n c r e t e was f a r
g r e a t e r t h a n t h a t of t h e GBFSC c o n c r e t e ;-
t h e r e was no lime i n an a p p r o x i m a t e l y 20-mm t h i c k l a y e r below t h e i n t e r i o r and none i n a 10- t h i c k l a y e r below t h e e x t e r i o r s u r f a c e of t h e GBFSC c o n c r e t e ;-
even a t t h e c e n t r e of t h e GBFSC c o n c r e t e o n l y a v e r y s m a l l amount of l i m e was p r e s e n t ;-
t h e calcium c a r b o n a t e c o n t e n t of t h e OPC c o n c r e t e was s i g n i f i c a n t i n t h e v i c i n i t y of t h e exposed s u r f a c e ; t h e c e n t r e , 38 mm below t h e s u r f a c e , was e s s e n t i a l l y c a r b o n a t e f r e e ;-
CaC03 was d e t e c t e d i n a l l s l i c e s of GBFSC c o n c r e t e e x c e p t two, which o r i g i n a t e d 40 mm and 70 mm (marked c e n t r e on Fig. 2) below t h e i n t e r i o r s u r f a c e ;-
i n both c o n c r e t e s c a r b o n a t i o n p r o g r e s s e d from t h e e x t e r i o r s u r f a c e a t a s l o w e r r a t e t h a n from t h e i n t e r i o r ., Chemical A n a l y s i s
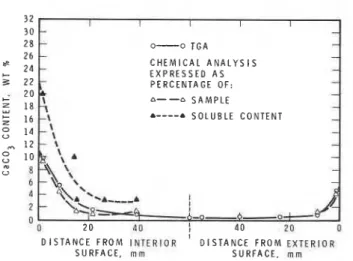
CaC03 c o n c e n t r a t i o n s a t v a r i o u s d e p t h s from t h e exposed s u r f a c e s a r e shown i n Fig. 4 f o r GBFSC c o n c r e t e and i n Fig. 5 f o r t h e r e f e r e n c e OPC c o n c r e t e . Agreement between chemical a n a l y s i s and TGA r e s u l t s can be c o n s i d e r e d good i n view of t h e inhomogeneity of t h e samples, caused by t h e f i n e a g g r e g a t e t h e y c o n t a i n e d . The weight of t h e cement i n t h e g r o s s sample was not w e l l d e f i n e d and i s t h e r e f o r e a major s o u r c e of d i s c r e p a n c y . When, i n s e v e r a l
i n s t a n c e s , t h e same method of a n a l y s i s was r e p e a t e d , d e v i a t i p n s of 10 t o 15% were found ( s e e , f o r example, Fig. 4 . ) . I n a n a t t e m p t t o overcome such u n c e r t a i n t y , specimens were d i s s o l v e d i n a c i d and t h e c o n c e n t r a t i o n s e x p r e s s e d a s p e r c e n t a g e s of t h e a c i d s o l u b l e f r a c - t i o n of t h e specimen. These v a l u e s a r e shown i n F i g s . 4 and 5.
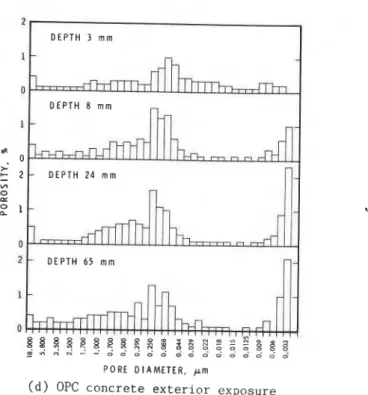
Pore S i z e D i s t r i b u t i o n
R e p r e s e n t a t i v e r e s u l t s f o r t h e two t y p e s of p a s t e ( a t v a r i o u s d e p t h s from t h e exposed s u r f a c e s ) a r e p r e s e n t e d a s h i s t o g r a m s (Fig. 6a-d). T h e i r r e p r o d u c i b i l i t y i s q u i t e good, a s may be s e e n from t h e v a l u e s i n Table 3, which l i s t s t h e f r a c t i o n of p o r o s i t y i n s e l e c t e d r a n g e s of p o r e s i z e ; i n s e v e r a l i n s t a n c e s two o r more specimens from t h e same l e v e l were analysed.
Because t h e p o r o s i t y v a l u e s a r e e x p r e s s e d a s p e r c e n t a g e s o f sample volume, which a l s o c o n t a i n s t h e non-porous sand a g g r e g a t e , t h e t o t a l p o r o s i t y v a l u e s l i s t e d i n Table 3 mst be c o n s i d e r e d nominal and a r e g i v e n o n l y f o r comparison. With i n c r e a s i n g
c a r b o n a t i o n t h e t o t a l p o r o s i t y of GBFSC c o n c r e t e does n o t a p p e a r t o be a f f e c t e d , b u t t h a t of OPC c o n c r e t e a p p e a r s t o d e c r e a s e
(Table 3).
The p o r e s i z e d i s t r i b u t i o n of GBFSC c o n c r e t e undergoes changes w i t h i n c r e a s i n g c a r b o n a t i o n , mainly i n t h r e e p o r e s i z e ranges: p o r e d i a m e t e r 3 s m a l l e r t h a n 0.009 pm; t h o s e between 0.019 and 0.35 pm; and t h o s e g r e a t e r t h a n 18
um.
I n t h e c e n t r e s e c t i o n , where t h e p a s t e i s c a r b o n a t e d l e a s t , 6.5% of t h e volume, o r 55% of t h e t o t a l p o r o s i t y , i s i n p o r e s of d i a m e t e r (0.009; i n t h e s u r f a c e l a y e r 0.6% of t h e volume, o r 3% of p o r o s i t y , i s i n p o r e s of d i a m e t e r (0.009 Dm (Table 3). A s t h e volume f r a c t i o n of s m a l l p o r e s d e c r e a s e s w i t h i n c r e a s i n g p r o x i m i t y t o t h e exposed s u r f a c e , t h e volume f r a c t i o n of medium s i z e pores i n t h e 0.019-0.35 pm r a n g e and of t h o s e w i t h d i a m e t e r s >18 pmi n c r e a s e s . T h i s i s t r u e f o r b o t h e x t e r i o r and i n t e r i o r exposed s u r f a c e s .
The v a l u e s f o r p o r e s i z e d i s t r i b u t i o n i n OPC c o n c r e t e , a s l i s t e d i n Table 3 and p l o t t e d i n Fig. 6, r e v e a l s i m i l a r t r e n d s , b u t o n l y t o t h e e x t e n t t h a t t h e s m a l l p o r e f r a c t i o n ((0.009 pm)
d e c r e a s e s w i t h i n c r e a s i n g c a r b o n a t i o n . Behaviour of OPC c o n c r e t e d i f f e r s from t h a t of GBFSC c o n c r e t e i n two i m p o r t a n t r e s p e c t s : 1 ) t h e change i n p o r e s i z e d i s t r i b u t i o n i s much less d r a m a t i c , and 2) t h e t o t a l p o r o s i t y d e c r e a s e s w i t h i n c r e a s i n g c a r b o n a t i o n . Thus, t h e e f f e c t s of c o a r s e n i n g of t h e pore s t r u c t u r e i n r e l a t i v e terms a r e m i t i g a t e d by t h e s m a l l e r o v e r a l l p o r e volume.
Compressive S t r e n g t h
The OPC and GBFSC c o n c r e t e c o r e s o b t a i n e d i n t h e c o u r s e of m o n i t o r i n g a t 12 and 11 y e a r s of age, r e s p e c t i v e l y , were t e s t e d and found t o be 40.4 and 52.8 MPa.
T e n s i l e S t r e n g t h
Table 4 l i s t s t h e v a l u e s f o r t h e s u r f a c e r e g i o n of t h e two t y p e s of c o n c r e t e core.
F r e e z i n g and Thawing
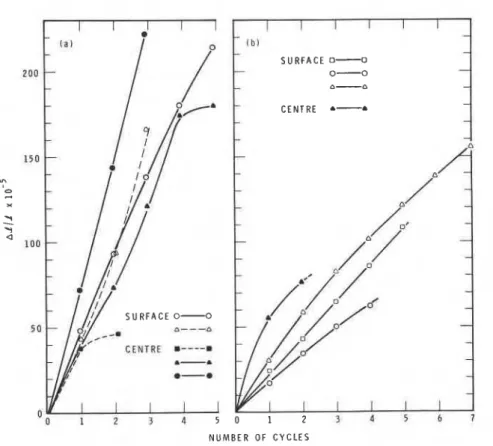
R e s i d u a l l e n g t h changes of t h e exposed s u r f a c e and c e n t r e s e c t i o n s of t h e GBFSC and OPC c o n c r e t e specimens a r e shown i n Fig. 7 a s a f u n c t i o n of t h e number of f r e e z e - t h a w c y c l e s . These r e s u l t s must be c o n s i d e r e d a s merely a n i n d i c a t i o n of a t r e n d owing t o u n c e r t a i n t i e s i n t r o d u c e d by t h e v a r y i n g amounts of c o a r s e
a g g r e g a t e i n t h e specimens. Aggregates do n o t normally c o n t r i b u t e t o e x p a n s i o n and t h e r e f o r e have a d i l u t i n g e f f e c t . N e v e r t h e l e s s , i t seems t o be c l e a r t h a t t h e s l o p e s of r e s i d u a l e x p a n s i o n v e r s u s number of freeze-thaw c y c l e r e l a t i o n s a r e s i g n i f i c a n t l y g r e a t e r f o r GBFSC samples (20015 = 42) t h a n f o r OPC samples (12015 = 24). T h i s behaviour i n d i c a t e s v e r y much g r e a t e r freeze-thaw s u s c e p t i b i l i t y f o r GBFSC p a s t e s t h a n f o r OPC p a s t e s .
DISCUSSION
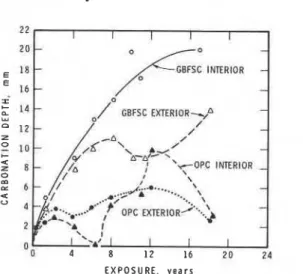
The p h e n o l p h t h a l e i n test i n d i c a t e d g r e a t e r c a r b o n a t i o n i n GBFSC t h a n i n OPC c o n c r e t e s , i n agreement w i t h some e a r l i e r r e p o r t s
( 2 , 6 ) b u t a t v a r i a n c e w i t h a t l e a s t one s t u d y ( 7 ) . T h i s method h a s l i m i t e d p r e c i s i o n , a s m a n i f e s t e d by t h e wide s c a t t e r of r e s u l t s i n t h e t e s t s e r i e s on t h e two e x p e r i m e n t a l h o u s e s o v e r a n 1 8 y e a r p e r i o d (Fig. 8 ) . It a l s o s u f f e r s from i n s e n s i v i t y , a s demonstrated by t h e TGA r e s u l t s ( F i g . 2) which i n d i c a t e s i g n i f i c a n t c a r b o n a t i o n of b o t h t y p e s of c o n c r e t e w e l l beyond t h e d e p t h d e t e c t e d by t h e i n d i c a t o r .
It h a s t o be remembered t h a t p h e n o l p h t h a l e i n i s a n a c i d - b a s e i n d i c a t o r , and t h a t c o l o u r change s i g n i f i e s n o t s o much t h e p r e s e n c e of c a r b o n a t e a s t h e a b s e n c e of l i m e . The r e d c o l o u r o f t h e p a i n t e d a r e a must n o t be i n t e r p r e t e d a s e v i d e n c e of no c a r b o n a t i o n .
The d e p t h of c a r b o n a t i o n of t h e GBFSC c o n c r e t e does n o t d i f f e r t o o much from t h a t of OPC c o n c r e t e a c c o r d i n g t o t h e r e s u l t s of TGA and c h e m i c a l a n a l y s i s ( F i g . 3). Any d e v i a t i o n below t h e e x t e r i o r s u r f a c e i s p r o b a b l y more a p p a r e n t t h a n r e a l , presumably caused by s c a t t e r of t h e r e s u l t s . An i m p o r t a n t f e a t u r e of t h e OPC c o n c r e t e c a r b o n a t i o n p r o c e s s i s t h a t d e p t h , X, i n c r e a s e s w i t h t h e s q u a r e r o o t of t i m e , t. Thus AX, t h e i n c r e m e n t a l c a r b o n a t i o n p e r u n i t t i m e , d e c r e a s e s w i t h a g e ( 8 , 9 ) because a l o g a r i t h m i c r e l a t i o n e x i s t s between r a t e and p a t h l e n g t h i n d i f f u s i o n - c o n t r o l l e d p r o c e s s e s . The v a r i o u s s e c t i o n s of t h e c o r e s a r e a s o u r c e of i n f o r m a t i o n , n o t o n l y a b o u t s p a t i a l v a r i a t i o n of t h e p r o p e r t i e s a t t h e time of t e s t i n g b u t a l s o a b o u t time-dependent changes (e.g., t h o s e due t o d i f f u s i o n of CO ) t h a t have o c c u r r e d . The c e n t r e s e c t i o n h a s p r o p e r t i e s s i m i f a r t o o r a p p r o a c h i n g t h o s e of t h e o r i g i n a l
non-carbonated concrete, while the surface sections are in the
final stage of the carbonation process.
The spatial distribution of the carbonate content found in OPC
concrete is consistent with the predictions of the logarithmic
relation; the relatively high degree of carbonation at the surface
decreases rapidly in the region below. In contrast, in GBFSC
concrete the carbonate content decreases gradually from the surface
towards the centre, indicating a relation between
X
and
tthat is
almost linear in the first 20 years of exposure. It is thus quite
possible that significant carbonation of GBFSC concrete will occur,
even in the central region, well within the expected service life
of the concrete. Data shown in Fig. 8 also point to this.
The reason for the difference in rates of carbonation of the
two types of concrete is suggested by the pore size distribution
analysis. The values in Table 3 indicate that carbonation of OPC
concrete caused a decrease in total porosity, from a nominal 15% to
11.55%, and a sharp reduction in pores with diameter less than
0.009
urn.At the same time the volume of large
( > l apm) and
mid-size pores remained relatively constant. Carbonation rate
therefore decreased because of increased resistance to diffusion
and longer path length.
Onthe other hand, the total porosity of
GBFSC did not decrease and the volume fraction of the large
(>18 pm) and mid-size (0.35-0.019 pm) pores increased in both
relative and absolute terms. This change more than compensates for
the longer path length.
The cumulative pore size distribution of the surface and
centre sections of the two types of concrete is shown in Fig. 9.
Although it does not have much greater total porosity than the
GBFSC centre section, the OPC centre section is quite permeable
owing to its large pores. After carbonation of its surface
section, the OPC concrete becomes more impermeable, although not to
the same extent as pristine GBFSC; the carbonated GBFSC has more
pores than the pristine OPC in almost all pore diameter regions.
Thus, the advantage of low permeability of GBFSC concrete is lost
with carbonation.
Carbonation of concrete is, in general, undesirable because
this reaction decreases alkalinity and the high pH that provides
good protection against corrosion of reinforcing steel is lost
(9-10).
The pH of concrete is 13.5, and in this medium corrosion
rate is negligible. Strictly speaking, concern is not so much for
the quantity of CaC03 formed but for the quantity of Ca(OH)2
remaining after carbonation. Obviously, regardless of degree of
carbonation, a substantial lime reserve is what is needed. In this
respect the results shown in Fig. 3 are of concern. While OPC
concrete contains significant amounts of Ca(OH)2
even in carbonated
areas, the GBFSC is practically devoid of it. Clearly, no
protection against corrosion exists in a 23-30-mm thick layer,
depending on type of environment (interior or exterior), and the
small amount of lime still present in the central region may well
be converted before very long.
It has been known, of course, that slag containing cements
suffers from this potential problem owing to the small initial
concentration of Ca(OH)2.
Lime is produced during hydration of
portland cement and its concentration in blended cements, in which
a large fraction of cement is replaced by slag, is greatly reduced.
Furthermore, slag on curing does not produce lime; in fact, it
reacts with lime to result in a very small lime reserve. This, on
carbonation, can easily be exhausted.
The low permeability of pristine GBFSC concrete might be
viewed as a factor compensating for the potentially bad effects of
low lime content. The fact that carbonation of this type of
concrete not only reduces lime concentration but also increases
permeability defeats this argument.
The development of pores with diameter in the
0.35-2pm range
in the process of carbonation may imply that the freeze-thaw
resistance of GBFSC increases with age, as pores of this size.
were
shown (11) to protect the paste from damage.
The tensile strength results (Table 4) show a very dramatic
decrease due to carbonation of GBFSC. Further studies have to be
carried out to confirm the results.
Resistance to freezing and thawing could not be evaluated with
sufficient precision to assess the effect of carbonation.
CONCLUSIONS
Examination of GBFSC and OPC concrete from experimental houses
exposed in the field for
20and
21years, respectively, leads to
the following conclusions:
1.
The rate of carbonation in GBFSC significantly exceeds that in
OPC concrete.
2.
Carbonation increases the permeability of GBFSC because the
small pores originally present become larger.
3. Carbonation seriously decreases the tensile strength of GBFSC
concrete.
4. After
20years of exposure very little lime is left in GBFSC
concrete.
5.
Of the methods for determining extent of carbonation, TGA and
chemical analysis are far superior to the phenolphthalein
test.
ACKNOWLEDGEMENT
The chemical analysis of the cement, long-term monitoring of
experimental houses, and tensile strength determinations were
carried out by Zemlabor, Dr. Werner Loch. The authors gratefully
acknowledge this effort. They are also indebted to Messrs.
H. Schultz and G. Polomark for carrying out the measurements so
competently. This paper is a contribution from the Division of
Building Research, National Research Council of Canada.
REFERENCES
1.
Schrader, F. and Smolczyk,
H.G."Carbonation and protection
against steel corrosion." Principal Paper: Blast Furnace
Slags and Slag Cements. Proc., Fifth International Symposium
on the Chemistry of Cement, Tokyo, Vol. 5, 1968, p. 188.
2. Meyer,
A.
"Investigations on the carbonation of concrete,"
Proc. Fifth International Symposium on the Chemistry of
Cement, Tokyo, Supplementary Paper 111-52, 1968, p. 394.
3. Litvan,
G."Phase transitions of absorbates. 111. Heat
effects and dimensional changes in nonequilibrium temperature
cycles." J. Coll. Interface Sci., Vol. 38, No. 1, 1972,
pp. 75.
4. Ramachandran, V.S.
"Differential thermal method of estimating
calcium hydroxide in calcium silicate and cement pastes."
Cem. Concr. Res., Vol. 9, No.
6, 1979, pp. 677.
5.
Duval, C.
"Inorganic thermogravimetric analysis," Elsevier,
Amsterdam, 1963, p. 278.
6. Hamada,
M.
"Neutralization (carbonation) of concrete and
corrosion of reinforcing steel." Principal paper, Vol. 111,
Proc., Fifth International Symposium on the Chemistry of
Cement, Tokyo, 1968, p. 343.
. .7. Kleinschmidt,
H.-J."Investigation of the progress of
carbonation in concrete structures." Deutscher Ausschuss fiir
Stahlbeton, Vol. 170, 1965, p. 25.
8.
Smolczyk,
H.G.Discussion of "Carbonation of Concrete."
Proc., Fifth International Symposium on the Chemistry of
Cement, Vol. 111, Tokyo, 1968, p. 369.
9. Uhlig,
H.H.and Revie, R.W.
"Corrosion and corrosion
control."
(Third Ed.) Wiley, 1985, p. 96.
10. Tuutti, K.
"Corrosion of steel in concrete." Swedish Cem&nt
and Concrete Research Institute, Stockholm, Sweden, Research
Report Fo 4.82, 1982.
11. Litvan,
G.G."Air enterainment in the presence of
superplastizers." American Concrete Institute
J., No. 7-8,
1983, p. 326.
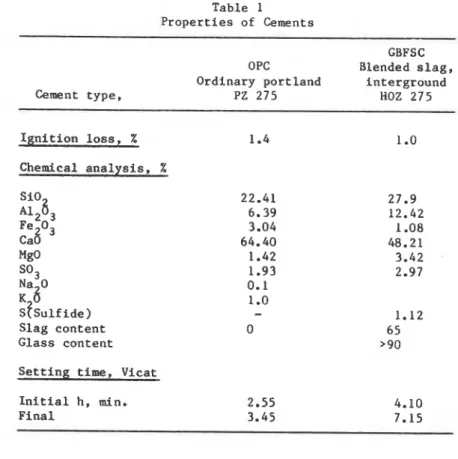
Table 1
Properties of Cements
-
GBFSC
OPC
Blended slag,
Ordinary portland
interground
Cement type,
PZ 275
HOZ 275
Ignition loss,
%Chemical analysis, %
:S3
Fe O3
ca8
MgO
S03F 6 O
~ P ~ u l f
ide)
Slag content
Glass content
Setting time, Vicat
Initial
h,
min.
Final
Table 2
Mix Design and Properties of Concrete
OPC
GBFSC
Cementitious material content, kg/m3
lb/cu yd
Sand content, kg/m3
lb/cu yd
Cement
:water:sand
Weight proportion
Compressive strength at 28 days
20
x20
x20- cubes
MPa
(psi)
Compressive strength in Sept. 1975
(at 12 and 11 yr, respectively)
MPa
(psi)
Table 3
Total P o r o s i t y and Fraction o f Pore Volume i n S e l e c t e d S i z e s of GBFSC and OPC Concrete a t Various Depths
from Exposed Surf ace
-
-Fraction o f volume i n p o r e s , diarn Depth Total
mm p o r o s i t y > 1 8 pm 0.35-0.019 pm t0.009 0.35-2 pm
GBFSC
Table 4
T e n s i l e Strength of t h e Surface Region of GBFSC and OPC Concrete a t 19 and 18 y e a r s , r e s p e c t i v e l y , MPa
Exposure
OPC GBFSC
Ext Int Ext Int
Avg 4.96 6.6 0.86 2.7
Fig. 1--Trace of photographs depicting ~henol~hthalein-treated
concrete surfaces. Hatched area represents purple coloured
T E M P E R A T U R E , " C
Fig. 2--Derivatives of thermogravimetric analysis curves of
sections of OPC (left) and GBFSC (right) concrete core samples.
Numbers indicate distance in mm between specimen and nearest
exposed surface of the wall
LllIN OPC 0-0
GBFSC .----a
GBFSC
DISTANCE FROM INTERIOR DISTANCE FROM EXTERIOR
SURFACE, mm SURFACE. rnm
Fig. 3--Concentration of CaC03 and Ca(OH)2, determined by TGA, of
OPC and GBFSC concrete specimens at various depths from exposed
surf
ace
9
-
I I I I I I I I I 8 - 0-0 TGA - 0 C H E M I C A L A N A L Y S I S 7-
R E R U N eP- I .----a E X P R E S S E D A S P E R C E N T A G EI
O F S O L U B L E C O N T E N T I-
I 1 I-
L I I-
I I 4 0/"-
; I , ,, ,
0 2 0 4 0 20 0 D I S T A N C E F R O M I N T E R I O R'
D I S T A N C E F R O M E X T E R I O R S U R F A C E , m m S U R F A C E , mmFig 4--CaCO3 concentration of GBFSC concrete specimen, determined
by chemical analysis, as a function of distance from exposed
surface. Results obtained by TGA shown for reference purposes
0-0 T G A C H E M I C A L A N A L Y S I S E X P R E S S E D A S P E R C E N T A G E O F : &--a S A M P L E &----a S O L U B L E C O N T E N T D I S T A N C E F R O M I N T E R I O R D I S T A N C E F R O M E X T E R I O R S U R F A C E , m m S U R F A C E , m m
Fig. 5--CaC03 concentration of OPC concrete specimen, determined by
chemical analysis, as a function of distance from exposed surface.
Results obtained by TGA shown for reference purposes
LA, z
(c) OPC concrete interior exposure
I
I I I I I-
Ibl - S U R F A C E 0-0 0-0-
a-a - - C E N T R E a-. L-
N U M B E R O F C Y C L E SFig. 7--Residual expansion of specimens a s a function of number of freeze-thaw cycles, (a)
GBFSC
(b)OPC
concrete8 1 2 16 20 24 E X P O S U R E , y e a r s
Fig. 8--Depth of carbonation determined b y phenolphthalein test as a function of years of exposure
I I I I t I r I l OPC CENTRE 60 mm GBFSC SURFACE 3 mm
----
GBFSC CENTRE 65 mm*
...
OPC SURFACE 3 mm....
...
...
...
....
...
348.8 2.9 1.3 0.58 a35 a055 0.m 0.016 0.012 0 . m P O R E D I A M E T E R , pmFig. 9--Cumulative pore size distribution of