HAL Id: hal-02703309
https://hal.inrae.fr/hal-02703309
Submitted on 1 Jun 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Surface energy of clay-synthetic humic acid complexes
from contact angle measurement
Claire Jouany
To cite this version:
Claire Jouany. Surface energy of clay-synthetic humic acid complexes from contact angle measurement.
Clays and Clay Minerals, Clay Minerals Society, 1991, 39 (1), pp.43-49. �hal-02703309�
SURFACE FREE ENERGY COMPONENTS OF CLAY-SYNTHETIC HUMIC
ACID COMPLEXES FROM CONTACT-ANGLE MEASUREMENTS
CLAIRE JOUANY ~
I.N.R.A., Science du Sot, 78026 Versailles, Cedex, France
Abstract--The surface free energy components of clay-organic complexes were determined to assess to what extent an organic adsorbate modified the surface properties of the mineral, insofar as the stability of soil aggregates is concerned. Adsorption isotherms for two synthetic, humic acid-like polymers were determined on a Ca-montmorillonite. From contact-angle measurements performed on dry surfaces, the surface free energy properties of the clay-organic complexes were determined using the two-liquid-phases method (water and hydrocarbons). This method allows both the dispersive and nondispersive components of the solid surface free energy, 3,s D and 7 v, to be determined. The results show that a very small amount of polymer (1% by weight) adsorbed on the external surfaces of the montmorillonite decreased markedly the surface free energy components of the clay: 3,s D decreased from 75 to 28 m J / m ~ for polycondensate catechol (PC) and from 75 to 30 m J / m 2 for polycondensate catechol triglycine (PCT), whereas 3,s v ranged from 35 to 16 rrd/m 2 (PC) and from 35 to 17 m J / m 2 (PCT). Although their chemical compositions were different, both polymers similarly modified ~s D and -is P. Increasing the amount of polymer adsorbed (from 1% to 3.5% by weight) affected mostly 3,s v, which became as low as 5 mJ/m:; meanwhile, 3'~ decreased from 30 to 23 m J / m 2. Possibly, the molecular orientation of the adsorbate changed in the process of dehydration. Following adsorption of synthetic humic acid-like polymers, dry Ca-montmorillonite com- plexes displayed 3's values < 50 m J / m 2, which were consistent with the solid-water contact angles measured in air.
Key Words--Adsorption, Aggregate stability, Humic acid, Montmoriltonite, Surface free energy, Wettabil- ity.
R6sam6--Les composantes de l'6nergie libre de surface sont mesur~es pour des complexes organo-mi- n6raux. Cette &ude est men6e dans le but de v6rifier de quelle mani~re un rev~tement organique modifie les propri~t6s de surface du min6ral et peut jouer sur la stabilit6 structurale. On a d6termin6 les isothermes d'adsorption sur une montmofillonite calcique pour deux polym~res synth6tiques modules d'acides hu- miques. L'6nergie libre de surface des complexes organo-min6raux est calcul~e ~ partir de la mesure des angles de contact obtenus sur des surfaces d6shydrat6es avec la m&hode ~ deux phases liquides (eau et hydrocarbures). Cette m&hode permet de d6terminer ~ la fois "r~ et ~,~, qui sont respectivement la composante polaire et la composante dispersive de l'6nergie de surface 3's. Les r6sultats montrent qu'une quantit6 tr~s faible de polym6re (1% en poids) adsorb6 sur les surfaces externes de la montmorillonite diminue de mani~re importante les composantes de l'6nergie libre de surface du min6ral: 3,s D diminue de 75 ~t 28 m J / m 2 pour le polycondensat cat6chol (PC) et de 75 h 30 m J / m 2 pour le polycondensat cat6chol triglycine (PCT), alors que 3,s P varie de 35 ~ 16 m J / m 2 (PC) et de 35 ~t 17 m J / m 2 (PCT). Bien que leurs compositions chimiques soient diff6rentes, les deux polym6res modifient pareillement ~s P e t "y~'. Une augmentation sensible des quantit6s adsorb6es (de 1% ~t 3,5% en poids) affecte principalement 3,s v qui diminue jusqu'~ une valeur de 5 m J / m 2, aiors que "y~ passe de 30 h 23 m J / m 2. Ces modifications importantes sont attribu6es ~t un changement d'orientation des polym~res adsorb6s, susceptible de s'~tre produit au cours de la d6shydratation. Apr6s adsorption de polym~res synth&iques mod61es d'acides humiques, la montmorillonite calcique ~ l'6tat see pr6sente des valeurs de "Vs inf6rieures ~ 50 m J / m 2 qui sont en accord avec les angles de contact solide-eau-vapeur qui sont mesur6s.
I N T R O D U C T I O N
Physical fertility o f c u l t i v a t e d soils r e q u i r e s t h e or- g a n i z a t i o n o f soil aggregates i n t o water-stable units t h a t p r o m o t e a g o o d transfer for gas a n d w a t e r a n d t h e p r o p e r c o n d i t i o n s for seedling e m e r g e n c e . By i m m e r s - ing the aggregate in water, t h e pressure e x e r t e d by en- t r a p p e d air a n d t h e swelling o f t h e clay d i s r u p t a n d b r e a k d o w n aggregate units CYoder, 1936; C o n c a r e t ,
Present address: I.N.R.A., Station d'Agronomie, Chemin de Borde-Rouge, Auzeville, B.P. 27, 31326 Castanet-Tolosan Cedex, France.
Copyright 9 1991, The Clay Minerals Society
1967). H i g h organic m a t t e r c o n t e n t s are f r e q u e n t l y re- p o r t e d to be r e s p o n s i b l e for t h e high stability o f soil aggregates ( H a m b l i n a n d G r e e n l a n d , 1977; C h a n e y a n d Swift, 1984). O r g a n i c m a t t e r generally increases aggre- gate stability on i m m e r s i o n by t w o m e c h a n i s m s : (1) l o w e r i n g the w e t t a b i l i t y a n d (2) increasing t h e c o h e s i o n o f aggregate units ( M o n n i e r , 1965; Chassin, 1979; G i o v a n n i n i et al., 1983).
I n a d d i t i o n to strongly h y d r o p h o b i c m o l e c u l e s (e.g., lipids, waxes, chitins) w h i c h m a y cause s e v e r e recla- m a t i o n p r o b l e m s , h u m i c substances s h o w little affinity for w a t e r a n d r e p r e s e n t the organic fraction c o m m o n l y associated w i t h a high aggregate stability ( C h a n e y a n d 43
44 Jouany Clays and Clay Minerals
Figure I. Equilibrium contact angle in a solid (S)-liquid 1 (W)-liquid 2 (H) system.
Swift, 1986; Tschapek et al., 1973; Chassin, 1979; Giovannini and Lucchesi, 1984; Le Bissonnais, 1989). Thus, in soils, this organic fraction is capable o f m o d - ifying the wetting properties o f the high surface free energy hydrophilic minerals (Theng, 1982). The hy- d r o p h o b i c action o f h u m i c substances was suggested by R o b i n s o n and Page (1950) on the basis o f contact- angle d e t e r m i n a t i o n s on clay fractions extracted from high organic-matter content soils.
Chassin et al. (1986) d e t e r m i n e d % for C a - m o n t - morillonite. I n the present study, changes in 3's were measured after a d s o r p t i o n o f a synthetic h u m i c acid on C a - m o n t m o r i l l o n i t e , and the extent by which the a m o u n t o f adsorbate influenced the measured values was noted. F o r that purpose, the surface free energy c o m p o n e n t s o f the clay a n d clay complexes were de- t e r m i n e d from contact-angle measurements using the two-liquid-phases method.
M E T H O D
Two-liquid-phases m e t h o d
M o n t m o r i l l o n i t e surfaces are extremely hygroscopic, and the adsorption o f liquid or v a p o r on t h e m severely decreases the surface free energy c o m p o n e n t s o f the solid (Chassin et al., 1986; Chibowski and Staszczuk, 1988). Furthermore, because o f the high surface free energy o f m o n t m o r i l l o n i t e , a finite contact angle for water on that mineral cannot be measured. To this author's knowledge, one o f the few tensiometric meth- ods that is suitable for surface characterization o f such high energy solids is the two-liquids method, inasmuch as the h y d r a t i o n status for the clay mineral surface is controlled in this m e t h o d ( T a m a i et aL, 1967; Schultz
et al., 1977). Thus, the bare surface can be p r o b e d a n d the m a n n e r by which the surface free energy c o m p o - nents v a r y for increasing a m o u n t s o f adsorbate on the mineral can be determined.
Generally, solid-liquid molecular interactions are thought to originate from London-dispersion forces and polar forces, which include dipole-dipole, dipole in- duced-dipole, hydrogen bond, and II bonding. As a
consequence, the solid surface free energy can be ex- pressed as the sum o f the dispersive 3`~' and the polar 3,s P c o m p o n e n t o f 3,s:
3,s = 3,s ~ + 7~. (1) I f a d r o p liquid is in equilibrium on the solid surface (S) in a two-liquid phases system (Figure 1) o f water (W) and h y d r o c a r b o n (H), i f the spreading pressure, 1-I~, on the solid surface is assumed to be equal to zero, Young's general equation becomes:
"rsH = 3`sw + 3`.w cos Osw/H, (2) where 3'sw is the solid-water interracial energy, Ysn is the solid-hydrocarbon interfacial energy, 3,,w is the interfacial tension between water and hydrocarbon, and 0sw/. is the solid-water-hydrocarbon contact angle. This equation is valid only i f water r e m o v e s the hydrocar- bon film at the solid surface. H a m i l t o n (1972) a n d Schultz et al. (1977) considered this assumption to be verified in similar experimental conditions. Shanahan
et aL (1982) confirmed the validity o f this hypothesis
from calculations o f the energy o f adhesion o f the liq- uids to the surfaces.
Fowkes (1964) gave an empirical expression for the interfacial free energy (%2) between liquid 1 a n d liquid 2; he assumed that only L o n d o n - d i s p e r s i o n interac- tions take place at the liquid-liquid interface, i f one liquid is non-polar:
3`~2 = 3,~ + 3`2 - 2(3`9 3`~)'/=, (3) where 3,1 and "/2 are the liquid surface tension, and 3'P and 3`p are the dispersive c o m p o n e n t s o f the liquid surface tension.
Owens and W e n d t (1969) extended Fowkes expres- sion to solid-liquid systems, in which polar as well as dispersive interactions exist at the interface:
3,1z = 3,~ + 3`2 - 2(3,P 3,2D) '/2 -- I~2, (4) where I~'2 represents the polar interaction energy term between phase 1 and phase 2.
The expression for 3'sH, the interracial free energy between solid and hydrocarbons, results from Eq. (4): 3,sr~ = % + 3,n - 2(3's D 3,.) v2. (5) Because hydrocarbons are non-polar, no polar in- teraction exists between the solid a n d the liquid; hence IG = 0 and 3`~ = 3'i,.
F r o m Eq. (4), the solid-water interfacial free energy can be obtained as well:
3,sw = 3`s + 3,w - 2(ys D 3'g) '/' - I~w. (6) Replacing "Ys. and 3,sw by their expressions, respec- tively Eq. (3) and (4), in Eq. (2), the general equation for a two-liquid-phases system results:
3,w - 3"H + 3"nW COS Oswm = 2((YsO)'h((3"DW) '/= -- (3".)*)) + I~w, (7)
Table 1. Surface and interracial tensions for hydrocarbons and water (m j/m2). Liquid ~L ~ ~ ~Lw Hexane 18.4 18.4 -- 51.1 Heptane 20.3 20.3 -- 51 Octane 21.3 21.3 -- 51 Decane 23.4 23.4 -- 51 Dodecane 25.35 25.35 -- 51.15 Hexadecane 27.1 27.1 -- 51.3 Water 72.6 21.6 51.0 --
"rL = surface tension of the liquids. ~/L D = dispersive com- ponent of the surface tension of the liquids. 3,, P = polar com- ponent of the surface tension of the liquids. ~'LW = water- hydrocarbon interracial tensions.
i From Schultz et al. (1977).
o r :
Y = a X + b, (8) where "/H, "rHw, and 3'v~ are known and given in Table 1. The m e a s u r e m e n t o f the contact angle o f water under a series ofalkanes, 0SW/H, allows Eq. (7) to be calculated for each liquid and a straight line to be drawn whose slope equals 2 (3,~) '/2 and which has a y-intercept o f IPw (Figure 2).
W u (1973) suggested that the h a r m o n i c - m e a n equa- tion is m o r e c o n v e n i e n t than the geometric-mean equation to express IP2 for high surface energy solids, because in a solid-water system:
I~w = 4 ~/s~'yP/(~/~v + "ysP), (9)
o r :
P t, P
3's P = ~/wlsw/(43'w - IPw), (10) where 3'ge is known and given in Table 1.
This m e t h o d was used to determine the dispersive and polar c o m p o n e n t s o f the surface free energy o f solids equilibrated at 0% R H . F o r each solid, water contact angles were d e t e r m i n e d by using the same tech- nique described by Chassin et al. (1986). Contact angles were measured with a R a m 6 - H a r t telegoniometer equiped with a thermostatic chamber; samples were kept at 20~ A n average value o f 0 corresponds to a m i n i m u m o f 30 determinations. High-purity alkanes were used without further purifications.
M A T E R I A L S
Ca- montmorillonite
SWy- 1 Wyoming montmorillonite obtained from the Source Clays Repository o f The Clay Minerals Society was used in this work. The <2.0-~tm fraction o f this Crook County, Wyoming, m o n t m o r i l l o n i t e was puri- fied according to the m e t h o d given by Chassin et aL
(1986) and washed three times with a 1.0 M CaCI2 solution to transform the clay to a h o m o i o n i c form. The suspension was then extensively washed with deionized water on a tangential ultrafiltration device
Figure 2. Plots of Eq. (5) for Ca-montmorillonite and its complexes with polycondensate catechol (PC) and polycon- densate catechol-triglycine (PCT); x = (~/Dw)~ -- (~/nD) '2 and y = 3~w -- 2/H + "YW~ COS Osw/n.
until the solution was in equilibrium with a saline con- centration < 10 -4 M. The final suspension contained 25 g clay/liter.
Synthetic humic acid
T w o synthetic h u m i c acids were prepared according to the m e t h o d o f A n d r e u x et al. (1980) by oxidation o f catechol only, polycondensate catechol (PC), and o f catechol in the presence o f triglycine, polycondensate catechol-triglycine (PCT). These products differed mainly by their molecular weight and N content; their solubility in water was m u c h higher for P C T because o f incorporation o f polypeptidic chains into the poly- condensate; the pK~ o f the m a i n acidity was lower for PCT. These complexes have chemical compositions, functional groups, and molecular weights similar to those obtained for soil-extracted h u m i c acids (Table 2). The PC polymer was used as a m o d e l for highly d e c o m p o s e d and stable aromatic compounds, whereas the P C T represented less humified and m o r e aliphatic h u m i c substances; these differences are evidenced from infrared spectra (Andreux et al., 1980).
Clay-synthetic humic acid complexes
Adsorption isotherms were constructed at 20~ as follows: 125 mg o f C a - m o n t m o r i l l o n i t e was reacted for 16 hr with 25 ml o f organic solutions at concentrations ranging between 0 and 3.0 mg/ml. T h e organic solu- tions had a 0.05 p p m concentration in N a N 3 to prevent microorganisms growth; the N a N 3 did not appear to interfere with p o l y m e r adsorption. Adsorption took place in unbuffered media; the initial p H o f the reacting solution ranged between 2.8 and 3.0.
The adsorption process progressed with a p H in- crease (equilibrium p H between 3.0 and 4.0) and dis- solution o f Ca, resulting from cation exchange between the Ca ions o f the m o n t m o r i l l o n i t e and H from the polycondensate. Afterwards, the suspension was cen-
46 Jouany
Table 2. Characteristics of the polymers used.
Clays and Clay Minerals
C c o n t e n t O c o n t e n t H c o n t e n t N c o n t e n t T o t a l acidity (%) (%) (%) (%) M o l e c u l a r w e i g h t ~ ( m e q / g )
PC 51 35.3 2.9 -- 10,000 4.2
PCT 47.8 36 4 11 30,000 5
Humic acid Theng (1979) 55-60 33-36 -- 4 5000-100,000 6-10 PC = polycondensate catechol. PCT = polycondensate catechol-triglycine.
Relative molecular weight, as determined on a dextran gel column calibrated using globular proteins. trifuged at 20,000 r p m for 15 min; the resulting su-
pernatant was decanted and analyzed for C and Ca. The clay-polymer slurry was washed several times with deionized water until the supernatant was free o f polymer; the resulting materials were t e r m e d the b i n d - ing complexes (Andreux, 1981). The C contents o f the supernatant solutions and binding complexes were de- t e r m i n e d b y dry combustion, the evolved CO2 being measured by coulometry; the C content values were converted into p o l y m e r content b y using the p o l y m e r C content given in Table 2. Ca in solution was deter- m i n e d by atomic absorption.
The a m o u n t o f p o l y m e r a d s o r b e d on the clay was estimated b y difference between the initial and the equilibrium p o l y m e r concentration. Isotherms were constructed by plotting the a m o u n t o f p o l y m e r ad- sorbed and the a m o u n t o f p o l y m e r b o u n d (on the basis o f 100 g o f dry clay) against the equilibrium p o l y m e r concentration.
A 1% (by weight) clay-polycondensate complex was prepared for b o t h p o l y m e r s from the results obtained for binding isotherms (Figure 4), a n d a 3.5% complex was prepared for PCT only, because a m a x i m u m o f 1% o f the polycondensate PC could be fixed on the clay. T h e a m o u n t o f Ca desorbed in the process o f adsorption was 20% o f the exchangeable cation on the clay. Unfortunately, s m o o t h films from PC or P C T solutions were impossible to prepare: hence, the surface free energy c o m p o n e n t s o f the p o l y m e r itself could not be determined.
Oriented deposits on glass slides, prepared from a 25 g/liter suspension o f the given complex, were al-
20 0 Figure 3. % 9 l t13 rr~ 9 ~11 i ~ I O O I I 1 2 3 Equilibritlm concentration (mg/ml)
Adsorption isotherm for polycondensate catechol (PC) and polycondensate catechol-triglycine (PCT) on Ca- montmorillonite.
lowed to equilibrate for several weeks in desiccators at 0% R H (controlled with P205). X - r a y p o w d e r diffrac- tion (XRD) patterns obtained on oriented deposits equilibrated at 0% R H showed no p o l y m e r intercala- tion in the interlayer.
R E S U L T S
Adsorption isotherms
A d s o r p t i o n isotherms were shown in Figure 3. Ca- m o n t m o r i l l o n i t e a d s o r b e d twice as m u c h PCT as PC. These observations agree with the general b e h a v i o r o f p o l y m e r towards adsorption, the a m o u n t s a d s o r b e d increasing with the size and molecular weight o f the p o l y m e r (Theng, 1982). These results were similar to those reported by Evans and Russell (I 959), Levy and Francis (1976), and Theng and Scharpenseel (1976), who obtained linear isotherms for similar equilibrium concentration ranges, the a m o u n t s a d s o r b e d being o f the same order o f magnitude as those reported here for synthetic PC and PCT. Unlike Chassin
et al.
(1977), no adsorption plateau was noted. Previously, F. A n - dreux (CPB-CNRS, BP 5, 54501 Vandoeuvre-les-Nan- cy Cedex, France, personal c o m m u n i c a t i o n ) obtained linear isotherms for P C T a d s o r p t i o n on C a - m o n t m o - rillonite. Possibly, changes in the shape o f the isotherm for concentrations > 1.0 m g / m l originated from floc- culation processes o f the p o l y m e r on the mineral sur- face, taking place together with adsorption.Binding isotherms
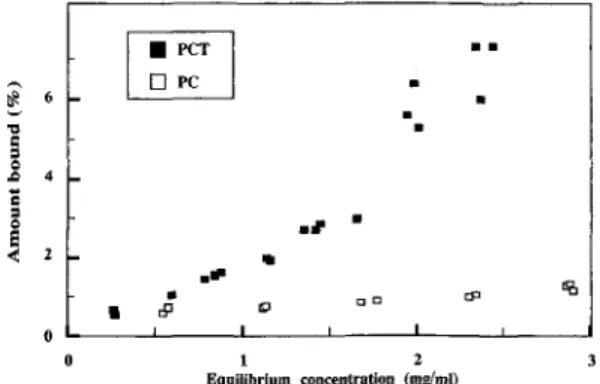
Binding isotherms are shown in Figure 4. F o r b o t h polycondensates, extensive washing led to r e s o r p t i o n o f substantial a m o u n t s o f polymer. Because no poly- m e r intercalation was detected with X R D , this high retention on the mineral was p r o b a b l y not due to trap- ping in the interlayer.
A m o n g several m e c h a n i s m s reported by Theng (1979), water-bridging (outer-sphere complexes) inter- actions between the anionic or neutral functional groups o f the p o l y m e r and the exchangeable cations on the clay through a water molecule seems to be the leading m e c h a n i s m for adsorption o f h u m i c acid on divalent m o n t m o r i l l o n i t e . Other b o n d i n g mechanisms, how- ever; should still be considered, inasmuch as adsorp- tion was conducted in unbuffered m e d i a (equilibrium p H ranged from 3.0 to 4.0).
6
i
.~ 4 < 2 Figure 4. m m s t 9 ml# 9 c~ ~ t~ta t~a I I ~ | I 1 2 E q u i l i b r i u m concentration (mg/ml)Binding isotherm for polycondensate catechol (PC) and polycondensate catechol-triglycine (PCT) on Ca-mont- morillonite.
edges, a n d A1 a n d M g can be released by h y d r o l y s i s o f t h e o c t a h e d r a l layer (readily e x c h a n g e a b l e w i t h t h e C a ions o n t h e clay). M i n e r a l h y d r o l y s i s was c o n f i r m e d by n o t i n g the d a y ' s c a t i o n e x c h a n g e c a p a c i t y (CEC) a n d t h e e x c h a n g e a b l e cations after a d s o r p t i o n o f 1% P C T . (1) T h e d a y ' s C E C c h a n g e d f r o m 92 to 84 m e q / 1 0 0 g; n o n - e x c h a n g e a b l e A1 p o s s i b l y r e p l a c e d initial Ca o n t h e clay. (2) T h e resulting c o m p l e x c o n t a i n e d 6 m e q / 100 g o f e x c h a n g e a b l e Mg. H e r e , c a t i o n - b r i d g i n g (in- n e r - s p h e r e c o m p l e x ) a n d a n i o n - e x c h a n g e i n t e r a c t i o n s were likely as well (Sposito, 1984). T h e n g a n d Scharp- enseel (1976) d e s o r b e d as m u c h as 85% o f t h e h u m i c a c i d a d s o r b e d o n d i v a l e n t m o n t m o r i l l o n i t e ; w h e r e a s o n l y 70% was r e m o v e d by a d s o r p t i o n o n a t r i v a l e n t clay (A1 o r Fe), i f i n n e r - s p h e r e c o m p l e x e s w e r e likely to be f o r m e d . P o l y m e r s e g m e n t s a d s o r b e d t h r o u g h in- ner-sphere c o m p l e x e s are less readily r e m o v e d by water t h a n those i n t e r a c t i n g t h r o u g h w e a k w a t e r - b r i d g i n g c o m p l e x e s . T h i s p h e n o m e n o n m u s t be c o n s i d e r e d in n o t i n g t h a t a n a v e r a g e o f 40% o f p o l y m e r a d s o r b e d r e m a i n s fixed o n t h e m o n t m o r i l l o n i t e after e x t e n s i v e I 0 0 ,o 40 2o C a . m o n t m o . C a - m o n t m o . C a . m o n t m o . Ca-rnontmo. A l - m o n t m o . + I%PC + 1%PCT + 3 . 5 % P C T
Figure 5. Polar 7s P and dispersive 3,s D components of the sur- face free energy 7s (Ts = "Y~ + 7~) for Ca-montmorillonite and its complexes with polycondensate catechol (PC) and poly- condensate catechol-triglycine (PCT); vertical bar on the col- umn refers to standard error.
washings. T h i s ratio t e n d e d to decrease, h o w e v e r , w i t h the e q u i l i b r i u m c o n c e n t r a t i o n , suggesting an e v o l u t i o n o f t h e i n t e r a c t i o n m e c h a n i s m s b e t w e e n t h e p o l y m e r a n d the surface.
Surface free energy components of dry complexes
T h e surface free energy c o m p o n e n t s for C a - m o n t - m o r i l l o n i t e a n d the c o m p l e x e s w e r e c o m p u t e d o n t h e basis o f t h e e x p e r i m e n t a l d a t a g i v e n in T a b l e 3. T h e results r e p o r t e d in F i g u r e 5 s h o w t h a t 1% (by weight) o f the p o l y m e r b o u n d to t h e m i n e r a l surface d e c r e a s e d 3's significantly; b o t h 3'~ a n d -ys e were affected. 7~ de- creased f r o m 75 to 28 m J / m 2 (PC) a n d f r o m 75 to 30 m J / m 2 (PCT), w h e r e a s 7s P c h a n g e d f r o m 31 to 16
m J~
m 2 (PC) a n d f r o m 31 to 17 m J / m 2 (PCT).
I n c r e a s i n g t h e a m o u n t o f p o l y m e r a d s o r b e d affected m o s t l y the n o n d i s p e r s i v e t e r m of'ys, w h i c h was as l o w as 5.0 m J / m 2, w h e r e a s 7~ was e q u a l to 23 m J / m 2. As a c o n s e q u e n c e , t h e surfaces o f clay organic c o m p l e x e s h a d 3's v a l u e s < 5 0 m J / m 2.
Table 3. Measured contact angles (degrees).
L i q u i d C a - m o n t m o r i l l o n i t e 1.0% P C C a - m o n t m o r i l l o n i t e 1.0% P C T 3.5% P C T 0sw/n Hexane 54.7 _+ 1.6 ~ -- 422 Heptane -- 90.6 _+ 53 Octane 61.5 _+ 1.7 92.4 _+ 38 48 Decane 61.3 + 1.6 91.8 + 47 43 Dodecane -- 91.7 + 38 Hexadecane 64.8 + 1.4 -- 53 Osw~v -- 0 59.7 _+ 32 90.0 _+ 1.3 128.7 _ 2.2 84 27 1.5 90.2 _+ 1.5 -- 33 1.8 89.7 _-. 1.5 129.2 + 2.7 61 30 1.5 91.9 _+ 1.5 130.9 + 2.4 66 27 1.6 -- -- 91.1 _+ 1.4 128.3 + 2.1 77 26 2.7 51.5 _+ 3.5 70.9 ___ 2.3 34 47
0swm = solid-water-hydrocarbon contact angle; 0sway = solid-water-air contact angle; PC = polycondensate catechol. PCT = polycondensate catechol-triglycine.
' -+ Average standard deviation.
48 Jouany Clays and Clay Minerals
D I S C U S S I O N
In this study, C a - m o n t m o r i l l o n i t e showed large val- ues for "rs, which are characteristic for high surface- free-energy solids; these results are similar to those reported for similar surfaces by Giese et al. (1989) and by Chibowski and Staszczuk (1988) for smectites and kaolinites, respectively.
After a severe drying (0% RH), b o t h organo-mineral complexes exhibited similar surface properties (Figure 5). The adsorption o f synthetic h u m i c acid on Ca- m o n t m o r i l l o n i t e modified m a i n l y the dispersive c o m - ponent o f 3's, which decreased by 60% and was as low as 30 mJ/m2; such low values have been frequently obtained, for example, for synthetic organic polymers: N y l o n 6.6 or p o l y m e t h y l metacrylate (Gerson, 1981). The low values obtained for 3's are consistent with the chemical nature o f the p o l y m e r s PC and PCT, which contain m a i n l y C and O. A b s o l o m and N e u m a n n (1988), reported similar "rs values for dried protein layers, h u m a n serum a l b u m i n (HSA), a n d b o v i n e se- r u m a l b u m i n (BSA), all o f which gave "rs values be- tween 28 and 52 m J / m 2. Lee and Luner (1972) o b ' rained ~s values between 46 and 56 m J / m 2 for lignins, the principal precursors o f humus.
Because the molecular structure o f PC and PCT a n d their h y d r o d y n a m i c radii are not precisely known, the exact extent to which the mineral surfaces are covered is difficult to ascertain. Assuming a spherical structure for the polycondensates (Gosh a n d Schnitzer, 1980) a n d a h y d r o d y n a m i c r a d i u s o f a few tens o f Angstroms (as reported by Cornell et aL, 1986) a n d a BET specific surface o f 30-40 m2/g for C a - m o n t m o r i l l o n i t e , the 1% organo-mineral complexes prepared for b o t h p o l y m e r s should not have presented surfaces fully covered by an adsorbate monolayer; at best, 50% o f the surface should have been covered, whereas 100% o f the surface should have been covered for the 3.5% complex, assuming no shrinkage o f the adsorbate in the drying process.
Dispersive component o f q's
According to Young (1958) and as was reported by Chassin et aL (1986), dispersive forces on C a - m o n t - morillonite originate from the silicate tetrahedra. In- creasing the a m o u n t o f adsorbate on the m o n t m o r i l - lonite decreased the 3'SO c o m p o n e n t o f ~s. C o m p a r e d with results reported for a l k y l a m m o n i u m - m o n t m o r i l - lonite complexes by J o u a n y a n d Chassin (1987), the "/s ~ value obtained for the 1% complexes corresponds to that measured for an h e x y l a m m o n i u m - m o n t m o r i l - lonite, which showed a 68% coverage o f the surface. I f the a m o u n t o f PCT was increased from 1% to 3.5%, 3,s D decreased from 30 to 23 m J / m 2. This difference is similar to that reported b y J o u a n y and Chassin (I 987), i f the percentage o f the mineral surface covered with a l k y l a m m o n i u m chains increases from 68% to 100%. The changes in 2~s observed i f the a m o u n t o f adsorbate
increased correlate well with the calculated percentage o f the surface covered b y a l k y l a m m o n i u m ions.
Polar component o f "ys
F o r C a - m o n t m o r i l l o n i t e equilibrated at 0% R H , 2r P originated m a i n l y from the partially h y d r a t e d cations (Chassin et aL, 1986; Jaficzuck a n d Biakopiotrowicz, 1988); each organo-mineral surface displayed smaller values of3,s v c o m p a r e d with the untreated clay, because the initial Ca ions were c o m p l e x e d by the p o l y m e r or, in the process o f adsorption, they were exchanged b y A1, which presented h y d r a t i o n energies lower t h a n Ca. Both situations are likely.
In Figure 5, u n p u b l i s h e d results obtained on AI- treated C a - m o n t m o r i l l o n i t e , for which all o f the Ca had been exchanged by AI, show a 3'~ value (4.5 m J / m 2) very similar to that obtained for the 3.5% P C T complex (5.1 mJ/m2). The 1% complexes displayed an i n t e r m e d i a t e value (16 and 17 mJ/m2), meaning that Ca r e m a i n e d at the solid-air interface available for po- lar interactions with water.
Significant differences in 3,s v were expected, however, between the different adsorbates, inasmuch as the PCT complexes contained hydrophilic nitrogeneous func- tional groups, quite able to interact strongly with water. This p o l y m e r is also m u c h m o r e soluble in water than the catechol polycondensate. Apparently, either the m e t h o d used to probe the surface was not precise enough to detect the differences in the chemical c o m p o s i t i o n o f the adsorbates or, m o r e likely, in the process o f d e h y d r a t i o n , the c o n f o r m a t i o n o f t h e m o l e c u l e s changed. This last scenario has already been p r o p o s e d by several authors to explain the changes in wetting properties o f soil organic m a t t e r after a severe drying (e.g., F a r m e r , 1978). Because o f the flexibility o f the organic molecules, hydrogen b o n d s occur between po- lar functional groups that are r e m o v e d from the solid- air interface, the organo-mineral surface becoming hard to rewet.
Changes in the molecular orientation o f the adsor- bates are confirmed by the ~/~ values for the two PCT complexes. Regardless o f the a m o u n t o f functional groups present on the surface and Drone to interact strongly with water, drying r e m o v e d t h e m from the solid-air interface, and the surface displayed properties o f a h y d r o p h o b i c low surface free energy solid. Ac- tually, solid-water-air (Osw/v) contact angles were m e a - sured on oriented deposits o f the three complexes, pre- viously equilibrated at 0% R H for several weeks. These measurements (Table 3) confirm that after drying the organo-mineral surfaces displayed a distinctly hydro- phobic character; the largest angle was measured for the greatest a m o u n t o f adsorbate.
In conclusion, the very small a m o u n t o f synthetic h u m i c acid a d s o r b e d on C a - W y o m i n g m o n t m o r i l l o n - ire decreased its surface free energy, "rs, and trans- f o r m e d the hydrophilic mineral into a h y d r o p h o b i c
m i n e r a l . F u r t h e r m o r e , the c h e m i c a l c o m p o s i t i o n o f the a d s o r b a t e h a d v e r y little influence o n the surface p r o p - erties o f o r g a n o - m i n e r a l c o m p l e x e s p r e p a r e d in this i n v e s t i g a t i o n . T h e m i n i m u m a m o u n t o f p o l y m e r n e c - essary to t r a n s f o r m the C a - m o n t m o r i l l o n i t e i n t o a l o w energy a n d h y d r o p h o b i c surface still r e m a i n s to be es- t i m a t e d . A f t e r drying, no differences w e r e a p p a r e n t b e - t w e e n P C a n d P C T , b u t w h e t h e r the c o m p l e x e s w o u l d s h o w the s a m e t r e n d s w h e n h y d r a t e d is n o t k n o w n .
A C K N O W L E D G M E N T S
T h e a u t h o r expresses h e r gratitude to F. A n d r e u x for his h e l p in t h e p r e p a r a t i o n o f t h e p o l y m e r s a n d his v a l u a b l e c o m m e n t s o n the m a n u s c r i p t a n d to D. P o n e s for his c o l l a b o r a t i o n o n t h e graphics.
R E F E R E N C E S
Absolom, D. R. and Neumann, A. W. (1988) Modification of substrate properties through protein adsorption: Colloids
and Surfaces 30, 25-45.
Andreux, F. (1981) Utilisation de molrcules modules de synth~se dans l'&ude des processus d'insolubilisation et de biodrgradation des polycondensats humiques: Bull. Ass. Fr.
Etude Sol. Sci. Sot 11, 271-291.
Andreux, F., Golebiowska, D., and Metche, M. (1980) Po- lymrrisation oxydative des O-diphrnols en prrsence ou non d'amino-acides. Cas des systrmes (catrchol-glycocolle) et (catrchol-diglycylglycine): C.R. Ass. G~n. Groupe Polyphe-
nols. Logrono. 9, 178-188.
Chaney, K. and Swift, R. S. (1984) The influence of organic matter on aggregate stability in some British soils: J. Soil
Sci. 35, 223-230.
Chaney, K. and Swift, R. S. (1986) Studies on aggregate stability: J. Soil Sci. 37, 337-343.
Chassin, P. (1979) D&ermination de l'angle de contact aci- des humiques-solutions aqueuses de diols. Consrquences sur rimportance relative des mrcanismes de destruction des agrrgats: Ann. Agron. 30, 481-491.
Chassin, P., Le Berre, B., and Nakaya, N. (1977) Influence des substances humiques sur les propri&rs des argiles: Clay
Miner. 12, 261-271.
Chassin, P., Jouany, C., and Quiquampoix, H. (1986) Mea- surement of the surface free energy of Ca-montmorillonite:
Clay Miner. 21, 899-907.
Chibowski, E. and Staszezuk, P. (1988) Determination of surface free energy of kaolinite: Clays & Clay Minerals 36, 455-461.
Concaret, J. (1967) Etude des mrcanismes de la destruction des agrrgats de terre au contact de solutions aqueuses: Ann.
Agron. 18, 65-144.
Cornell, P. K., Summers, R. S., and Robert, P. V. (1986) Diffusion of humic acid in dilute aqueous suspension: J.
Coll. Inter. Sci. 114, 149-164.
Evans, L. T. and Russell, E. W. (1959) The adsorption of humie and fulvic acids by clays: J. SoilSci. 10, 119-132. Farmer, V. C. (1978) Water on particle surfaces: in The
Chemistry of Soil Constituents, D. J. Greenland and M. H.
B. Hayes, eds., Wiley, New York, 405 pp.
Fowkes, F.M. (1964) Dispersion force contributions to sur- face and interracial tensions, contact angles, and heats of immersion: Adv. Chem. Ser. 43, 99-111.
Gerson, D. F. (1981) Methods in surface physics for im-
munology: in Immunological Methods 2, Academic Press,
New York, 105-138.
Giese, R. S., van Oss, C., Norris~ J., and Constanzo, P. M.
(1989) Surface energies of smectite clay minerals: in Pro-
gram Abstracts, Int. Clay Conf., Strasbourg, 1989, p. 158.
Giovannini, G. andLucchesi, S. (1984) Differentialthermal analysis and infrared investigations on soil hydrophobic substances: Soil Sci. 137, 457--463.
Giovannini, G., Lucchesi, S., and Cervelli, S. (1983) Water repellent substances and aggregate stability in hydrophobic soils: Soil Sci. 135, 110-113.
Gosh, K. and Schnitzer, M. (1980) Macromolecular struc- tures of humic substances: Soil Sci. 129, 266-276. Hamblin, A. P. and Greenland, D.J. (1977) Effect of organic
constituents and complexed ions on aggregate stability of some East Anglian silt soils: J. Soil Sci. 28, 410-416. Hamilton, W.C. (1972) A technique for the characterization
of hydrophilic solid surfaces: J. ColloM lnter. Sci. 40, 219- 222.
Jaficzuck, E. and Bialopiotrowicz, T. (1988) Components of surface free energy of some clay minerals: Clays & Clay
Minerals 36, 243-248.
Jouany, C. and Chassin, P. (1987) Determination of the surface energy of clay-organic complexes by contact-angle measurements: Colloids and Surfaces 27, 289-303. Le Bissonnais, Y. (1989) Analyse des processus de microfis-
suration des agr~gats ~ l'humectation: Bull. Ass. Fr. Etude
SoL Sci. Sol 27, 187-199.
Lee, S. B. and Luner, P. (1972) The wetting and interfaeial properties of lignins: Tappi 55, 116-121.
Levy, R. and Francis, C. W. (1976) Adsorption and de- sorption of cadmium by synthetic and natural organo-clay complexes: Geoderma 15, 361-370.
Monnier, G. (1965) Action de la mati~re organique sur la stabilit~ structurale des sols: Th~se de Docteur Ing~nieur, Universit6 de Paris, Paris, 140 pp.
Owens, D. K. and Wendt, R. C. (1969) Estimation of the surface free energy of polymers: J. AppL Polymer Sci. 13, 1741-1747.
Robinson, D. O. and Page J. B. (1950) Soil aggregate sta- bility: Soil Sci. Soc. Amer. Proc. 15, 25-29.
Schultz, J., Tsutsumi, K., and Donner, J. B. (1977) Surface properties of high-energy solids: J. Coll. Inter. Sci. 59, 272- 277.
Shanahan, M. E. R., Cazeneuve, C., Carre, A., and Schultz, J. (1982) Wetting criteria in three phases solid-liquid- liquid systems: J. Chim. Phys. 79, 241-245.
Sposito, G. (1984) The Surface Chemistry of Soils: Oxford University Press, Oxford, 234 pp.
Tamai, Y., Makuuchi, K., and Suzuki, M. (1967) Experi- mental analysis of interracial forces at the plane surface of solids: J. Phys. Chem. 71, 4176-4179.
Theng, B. K. G. (1979) Formation and Properties of Clay-
Polymer Complexes: Elsevier, Amsterdam, 262 pp.
Theng, B. K. G. (1982) Clay-polymer interactions summary and perspectives: Clays & Clay Minerals 30, 1-10. Theng, B. K. G. and Scharpenseel, H. W. (1976) The ad-
sorption of ~4C-labelled humic acid by montmorillonite: in
Proc. Int. Clay Conf., Mexico City, 1975, S. W. Bailey, ed.,
Applied Publishing, Wilmette, Illinois, 643-653. Tschapek, M., Pozzo Ardizzi, G., and De Busseti, S.G. (1973)
Wettability ofhumic acid and its salts: Z. Pflanzen. Bodenk. 1, 16-31.
Wu, S. (1973) Polar and nonpolar interactions in adhesion:
J. Adhes. 5, 39-55.
Yoder, R. E. (1936) A direct method for aggregate analysis of soils and a study of the physical nature of erosion losses:
J. Amer. Soc. Agron. 28, 337-351.
Young, C. J. (1958) Interaction of water vapor with silica surfaces: J. Coll. Sci. 13, 67-85.
(Received 1 March 1990; accepted 31 August 1990; Ms. 1987)