Publisher’s version / Version de l'éditeur:
Journal of Power Sources, 205, pp. 10-23, 2012-01-20
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/j.jpowsour.2012.01.059
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
A review on performance degradation of proton exchange membrane
fuel cells during startup and shutdown processes : causes,
consequences, and mitigation strategies
Yu, Yi; Li, Hui; Wang, Haijiang; Yuan, Xiao-Zi; Wang, Guangjin; Pan, Mu
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=9fa1f4d8-9104-47e3-9e85-eb2eb58d372b
https://publications-cnrc.canada.ca/fra/voir/objet/?id=9fa1f4d8-9104-47e3-9e85-eb2eb58d372b
JournalofPowerSources205 (2012) 10–23
ContentslistsavailableatSciVerseScienceDirect
Journal
of
Power
Sources
j o ur n a l ho m e p age :w w w . e l s e v i e r . c o m / l o c a t e / j p o w s o u r
Review
A
review
on
performance
degradation
of
proton
exchange
membrane
fuel
cells
during
startup
and
shutdown
processes:
Causes,
consequences,
and
mitigation
strategies
Yi
Yu
a,b,
Hui
Li
a,∗,
Haijiang
Wang
a,∗∗,
Xiao-Zi
Yuan
a,
Guangjin
Wang
b,
Mu
Pan
baInstituteforFuelCellInnovation,NationalResearchCouncilCanada,4250WesbrookMall,Vancouver,BC,CanadaV6T1W5
bStateKeyLaboratoryofAdvancedTechnologyforMaterialsSynthesisandProcessing,WuhanUniversityofTechnology,Wuhan430070,PRChina
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5November2011
Receivedinrevisedform4January2012 Accepted5January2012
Available online 20 January 2012
Keywords:
Protonexchangemembranefuelcell Startupandshutdownprocess Hydrogen/airinterface Reversecurrent Systemstrategies
a
b
s
t
r
a
c
t
Performancedegradationduringstartupandshutdownisconsideredanimportantissueaffectingthe durabilityandlifetimeofprotonexchangemembranefuelcells(PEMFCs).Duetothehighpotentials experiencedbythecathodeduringstartupandshutdown,theconventionalcarbonsupportforthe cath-odecatalystispronetooxidationbyreactingwithoxygenorwater.Thispaperpresentsanoverviewof thecausesandconsequencesofperformancedegradationafterfrequentstartup–shutdowncycles. Miti-gationstrategiesarealsosummarized,includingtheuseofnovelcatalystsupportsandtheapplicationof systemstrategiestopreventperformancedegradationinPEMFCs.Itisfoundfromtheliteraturereview thatimprovementsincatalystsupportstopreventoxidationcomeattheexpenseofhighcost,andthe novelsupportsdevelopedtodatearenotsufficienttocompletelypreventcarbonoxidationinfuelcell engines.Systemstrategies,includingpotentialcontrolandreactiongascontrol,havebeendeveloped andappliedinfuelcellenginestoalleviateorevenavoidperformancedecay.Thisreviewaimsto pro-videaclearunderstandingofthemechanismsrelatedtodegradationbehaviorsduringthestartupand shutdownprocesses,therebyhelpingfuelcellmaterialorsystemdevelopersintheireffortstoprevent performancedegradationandprolongthelifetimeofPEMFCs.
Crown Copyright © 2012 Published by Elsevier B.V. All rights reserved.
Contents
1. Introduction... 11
2. Startupandshutdownprocesses... 11
2.1. Startupprocess... 11
2.2. Shutdownprocess... 12
3. DegradationofPEMFCduringstartupandshutdown... 12
3.1. Acceleratedlifetimetestsunderstartup–shutdowncycles... 12
3.2. RootcauseofPEMFCdegradationduringstartupandshutdown... 14
3.2.1. Reversecurrent... 14
3.2.2. Fuelstarvation... 16
3.2.3. Carbonoxidation... 16
3.2.4. Agglomerationand/ordissolutionofPtparticles... 17
4. Mitigationstrategies... 18
4.1. Alternativecatalystsupports... 18
4.2. Systemstrategies... 19
4.2.1. Gaspurge... 19
4.2.2. Auxiliaryloadwithpotentialcontrol... 19
4.2.3. Othersystemstrategies... 20
4.2.4. Summaryofsystemstrategies... 21
∗Correspondingauthor.Tel.:+16042213000. ∗∗Correspondingauthor.Tel.:+16042213038.
E-mailaddresses:hui.li@nrc.gc.ca(H.Li),haijiang.wang@nrc.gc.ca(H.Wang).
0378-7753/$–seefrontmatter.Crown Copyright © 2012 Published by Elsevier B.V. All rights reserved. doi:10.1016/j.jpowsour.2012.01.059
5. Concludingremarks... 21 Acknowledgements... 22 References... 22
1. Introduction
Afuelcellisanelectrochemicaldevicethatcandirectly con-verthydrogenenergytoelectricity.Amongthevarioustypesoffuel cell,theprotonexchangemembranefuelcell(PEMFC)isconsidered oneofthemostpromisingcleanenergysourcesofthetwenty-first centuryfortransportationandstationaryapplications,duetoits highenergyconversionefficiencyandpowerdensity,faststartup, andlow/zeroemissionlevel[1,2].Considerableresearchoverthe pastfewdecadeshassignificantlyadvanced PEMFCtechnology. However,sometechnological“bottlenecks”havelimitedits fur-thercommercialization.Forexample,therelativelyshortlifetime ofPEMFCs,inducedbymaterialsdegradation,isstillunsatisfactory forstationaryandautomotiveapplications.TheU.S.Departmentof Energy(DOE)lifetimetargetsfor2015are5000hfor transporta-tionpowersystemsand40,000hforstationarypowersystems[3], butcurrent PEMFC technologyyieldsonly1700hand 10,000h, respectively[4].Insufficientfuelcellsystemdurabilityiscaused bydegradationofthefuelcellcomponents.Thedurabilityofeach componentinaPEMFCisaffectedbymanyinternalandexternal factors,includingmaterialproperties,fuelcelloperatingconditions (suchashumidification,temperature,cellvoltage,etc.),impurities orcontaminantsinthefeeds,environmentalconditions(e.g., sub-freezingorcoldstart),operationmodes(suchasstartup,shutdown, potentialcycling,etc.),andthedesignofthecomponentsandthe stack.
For automobile applications, PEMFCs must operate under variousconditions,suchasloadchangingcycles,highpower con-ditions,idlingconditions,andstartupandshutdowncycles.Among thesevariousdynamicconditions,startupandshutdownprocesses presentauniquechallengeforPEMFCsystems,astheycausethe cathodepotentialtobecomeabnormallyhigh,atwhichpointthe catalystsupportispronetobeoxidized,resultinginadverseeffects forfuelcelldurability.Peiet al.investigatedthedurabilityof a PEMFCandevaluateditslifetimeunderstartupandshutdown con-ditions[5].Theresultssuggestedthatperformancedecayunder frequentstartupandshutdowncyclesisveryserious,buttheeffect ofstartupandshutdowncyclesonfuelcelllifetimecanbeignored ifthestackvoltageispromptlydispelledafterthefuelcellstops operating.However,quicklyandcompletelydispellingthestack voltage is not easy, due to residual gas in the flow field after shutdown.
Therecentliteraturecontains severalreviewpapers onPEM fuel cell durability/reliability issues. Wu et al. [6] published a comprehensivereview onPEMFC degradation mechanisms and mitigationstrategies,inwhichdurabilitytestsundersteadystate
[7–17]and dynamic state [18–30]conditions were also briefly summarized.Inaddition,Wuet al.[6]alsodiscussedthemajor failuremodesand mitigationstrategies ofdifferentcomponents inPEMFCs.Borupetal.[2]publishedanimportantreviewpaper contributedby56researchersfromnationallaboratoriesand uni-versitiesintheUnitedStatesandJapanwhoparticipatedinaPEMFC DurabilityWorkshopfundedbytheUnitedStatesDepartmentof Energy(DOE).Thisreviewprovidedcomprehensivediscussionson fundamentaland scientificaspectsof PEMFCdurability,suchas operationaleffectsonfuelcelldurability,butitsmainfocuswas onthedegradationofcomponents,includingthemembrane, cata-lystlayer,andgasdiffusionlayer.Vahidietal.[31]intheirreview introducedthemain parametersinfluencing thelong-term per-formanceanddurabilityofPEMFCs.Zhangetal.[32]provideda
reviewofPt-basedcatalystlayerdegradationinPEMFCs, includ-ingaverydetaileddiscussionofthecarboncorrosionmechanisms under gross fuel starvation and the air/fuel boundary; mitiga-tionstrategieswerealsointroduced,amongthemcarbonsupport improvementandsystemstrategies.
Allthesereviewshave touchedonperformancedegradation and carbon oxidation during thestartup and shutdown cycles, butthereisnocomprehensivereviewofPEMFCdurabilitystudies andmitigationstrategiesunderstartupandshutdownconditions. Over thelast fewyears, in anefforttoenhance thelifetimeof PEMFCsystemsforautomobileapplicationsundergoingfrequent startup and shutdown, significant progress has been made in thedevelopmentofnovelcatalystsupports.Inaddition,various systemstrategiesfortacklingstartupandshutdownissueshave beendevelopedandreportedthroughaconsiderablenumberof papers and patents.There isthus a need for a detailed review ofdegradationmechanismsandallknownmitigationstrategies, includingmaterialsimprovementandsystemstrategiesforstartup andshutdownprocesses,tohelpfuelcellmaterialdevelopersor fuelcellsystemdevelopersintheireffortstopreventperformance degradationandprolongthelifetimeofPEMFCsforautomotive applications.
Thepurposeofthisreviewisthereforetosummarizethestudies conductedbyacademicandindustrialresearchersonthe durabil-ityofPEMFCsduringstartupandshutdown.First,adescriptionof startupandshutdownprocessesisprovidedforaclear understand-ingofwhathappenstofuelcellsduringtheseprocesses.Second, accelerated lifetimetests under startup–shutdown cycle condi-tions,conductedbybothacademicandindustrialresearchers,are summarizedanddiscussed.Inaddition,themajorfailuremodes androotcausesofdegradationduringstartupandshutdown pro-cessesarediscussedindetail.Thereviewconcludeswithadetailed introductionofrecentlydevelopedmitigationstrategies,including materialsimprovementandsystemstrategies,basedonan exhaus-tivesurveyofjournalpapersandpatents.
2. Startupandshutdownprocesses
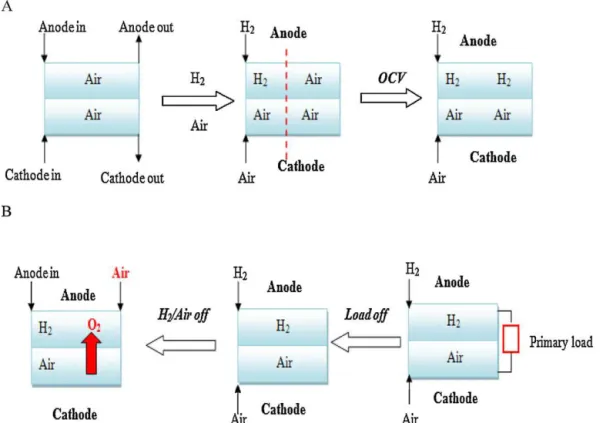
Bothstartupandshutdownaredynamicprocessesthatafuel cellinevitablymustconfrontinautomobileapplications.Compared tosteady-stateprocesses,startupandshutdownprocesses experi-encedifferentprofilesunderoperatingconditions.Forexample,the celltemperature,gashumidity,andlocalgasmixturearedifferent thanundersteady-stateconditions—forexample,increasing tem-peratureandhumidityduringstartup,anddecreasingtemperature andhumidityduringshutdown.However,themajorfeature dur-ingstartupandshutdown,afeaturethatisalsothemajorcause ofperformance degradationduringthose processes,isthelocal gasmixtureattheanode,whichiscommonlycalledthe hydro-gen/airinterfaceorthefuel/airinterface.Toclearlyunderstandthe acceleratedlifetimetestsanddegradationmechanismsassociated withstartupandshutdownprocesses,abriefintroductiontothese processesisgiven belowandaschematicgraphis presentedin
Fig.2.
2.1. Startupprocess
Thestartupprocessforthefuelcellsreferredtointhispaper excludescold start belowfreezingtemperatures. In thenormal operationoffuelcellengines,airfillstheanodeflowfieldafter
extendedshutdown,duetopermeationbyatmosphericairfrom theanodeexhaustoracrossthemembrane.Thefirststepofthe startupprocessistointroduceairoroxygenintothecathodeand hydrogenintotheanode. Becauseof thepresenceof air inthe anode,thereisahydrogen/airinterfaceintheanodeflowfield dur-inghydrogenintroduction.Ashydrogenissuppliedcontinuously duringthestartupprocess,theairisdispelledfromtheanodeinlet totheanodeoutlet,resultinginafloatinghydrogen/airinterface. Thefasterthehydrogenissupplied,themorebrieflyisthis inter-facepresent.Eventually,alltheairintheanodeisdispelledbythe hydrogen,andtheair/hydrogeninterfacedisappears,leadingtothe stateoftheopencircuitvoltage(OCV)forfuelcells.Inalaboratory test,topreventfuelcellsfromformingthishydrogen/airinterface, theanodeandcathodeflowfieldsarepurgedwithnitrogento pro-tectthefuelcellsbeforethestartupprocess.However,inthereal operationoffuelcellengines,anitrogensupplyisnotpractical, resultinginanair/hydrogeninterfaceduringstartup.
Inaddition,ifthefuelcellexperiencesbadgasflow distribu-tionduringthestartupprocess(calledlocalfuelstarvation),oxygen couldcrossthemembranefromthecathodetotheanodeunder apressureorconcentrationgradient,resultinginahydrogen/air interface.
2.2. Shutdownprocess
Thesamesituationofahydrogen/airinterfacecanalsooccurin theshutdownprocess.Whentheprimaryloadisshutoff,thefuel cell’sshutdownprocessbegins.Afterthehydrogenand air sup-pliesareshutoff,residualgaswillremaininthegaschannelsat theanodeandcathode.Becauseofthegasconcentrationdifference betweentheanodeandcathode,theoxygenatthecathodecrosses themembranetotheanode,resultinginahydrogen/airinterface attheanode.Moreover,afterextendedshutdown,atmosphericair willpermeatethefuelcellfromtheanodeoutletduetoseal fail-ure.Theair/hydrogeninterfacewillremainforamuchlongertime thanwhenitisintroduceddirectlyviathehydrogensupplyinthe startupprocess,duetoslowairpermeationduringshutdown.
Toinvestigatetheeffectthatthehydrogen/airinterfacehason theperformanceofPEMfuelcellsduringthestartupandshutdown processes,manyresearchershavefocusedonacceleratedlifetime testsunderstartup–shutdowncycleconditionsinthelaboratory. 3. DegradationofPEMFCduringstartupandshutdown 3.1. Acceleratedlifetimetestsunderstartup–shutdowncycles
Inrecentyears,significantresearcheffortshavebeenfocused ondegradationbehaviorsunderstartupandshutdownconditions, asindicated by thenumber of publications shown in Fig.1. In thesepublications, mostofthedurability testswereconducted underacceleratedconditions,duetothecomplexityanddifficulty ofmimickingandcontrollingstartupandshutdownprocedures.To conductdurabilitytestsforstartupandshutdowncycles,the hydro-gen/airinterfacemustbeintroducedattheanode,whichdemands highstandardsoftestconditioncontrolsandsafetycontrolsinthe laboratory.Asaresult,durabilitytestsunderstartupandshutdown conditions arecostly,as therequisiteexperiments are lengthy. Acceleratedtestsarethereforecommonlyused.United Technolo-gies Corporation (UTC) and a group at the Fuel Cell Research Center,KoreaInstituteofScienceandTechnology,haveconducted a considerableamountworkonacceleratedteststoinvestigate degradationmechanismsanddevelopsystemstrategiesforstartup andshutdownprocesses.
AsshowninTable1,Choetal.fromtheFuelCellResearchCenter attheKoreaInstituteofScienceandTechnologyhaveinvestigated
Fig.1.Publicationhistoryofarticlesafter2004onthestartupandshutdown pro-cessesofPEMFCs.
several operating parameters [33–39] that can have an impact onthedegradationrateofPEMFCsunderstartupandshutdown cycles,includingcathodehumidity[37,38],celltemperature[33], applicationofa dummyload[35,36],andgassupplysequences
[34]. The hydrogen/air interface during the startup and shut-downprocesseswasmimickedbypurgingtheanodeandcathode channelswithair aftercontinuousair and hydrogenwere sup-plied.Duringthispurgingprocess,thetestconditions—including celltemperature,gashumidity,andgassupplysequences—were changed toinvestigate theresulting effects onthedegradation behaviors of fuel cells. The results indicated that performance degradationwasalleviatedatlowerhumidityandlowercell tem-perature with the dummy load during startup and shutdown. Similarly,Leeetal.[39]alsoinvestigatedtheeffectofthe hydro-gen/air interface onfuelcell performance degradation. In their study,thehydrogen/airinterfacewascreatedbyreplacing hydro-genwithairattheanode,toinvestigatecarboncorrosionatthe cathode.Itwasconcludedthattopreventthedegradationof PEM-FCscausedbyresidualgases,hydrogenshouldberemovedfrom theanodegaschannelbyairpurging,whichwasfoundtobevery effective.
Takagi et al. [40] investigated the effect that the shutoff sequence ofhydrogenandair hadonthefuelcellperformance degradation rate.Thestartup and shutdownprocess was oper-atedasfollows:(1)hydrogenandairweresuppliedtotheanode andcathode,respectively,andthecellwasoperatedfor1–1.5min underaconstantloadcurrent.(2)Theloadcurrentwasturnedoff tomaintaintheOCVstate,andsimultaneouslyeithertheairorthe hydrogensupplywasshutoffatthatpoint.(3)Whenthecellvoltage droppedto0.2V,theothergassupplywasshutoff.(4)Afterleaving thecellshutdownfor5minwhilemaintainingthecelltemperature atthesamelevelashadbeenusedduringoperation,both hydro-genandairweresuppliedsimultaneouslyandtheloadcurrentwas appliedagaintooperatethecell.Itwasconcludedthatinthe inter-estofsystemsafetyandfuelefficiency,itwouldbemorebeneficial toshutoffthehydrogensupplybeforetheairsupply.Nevertheless, topreventperformancedegradationduringtheshutoffprocess,the airsupplyshouldbeclosedpriortothehydrogensupplyto pre-ventairpermeationtotheanode,whichwouldotherwiseresultin ahydrogen/airinterfaceattheanodeduringtheshutdownprocess. Inukaietal.[41]simulatedstartup/shutdowncyclesbyexchanging hydrogenandairattheanode.Duringthisgasexchange,the dis-tributionofoxygenpartialpressuresattheanodewasvisualized
Fig.2.Descriptionofthestartup(A)andshutdown(B)processes. usingareal-time/spacevisualizationsystem,whichclearlyshowed
thelocationofH2-richandO2-richareasalongthegas-flowchannel
fromtheinlettotheoutlet.Theyobservedthatthegasexchange ratewasmuch slowerthanwhatwouldbepredictedfrom sim-plereplacement,andthatit correlatedwiththeprotontransfer derivedfromcarboncorrosionofthecathodecatalystlayer.From
thesevisualizationresultstheyfoundthattheshutdownprocess resultedinmoreseriouseffectsthanthestartupprocess.
Inadditiontothosestudyingthestartupandshutdown pro-cessesbyreplacinghydrogen/airorexchanginggasestointroduce thehydrogen/air interfaceat the anode,other research groups havestudiedtheprocesses usinga reformattedgassuppliedto Table1
SummaryofPEMFCdurabilitytestsunderstartupandshutdowncycles.
Authors Experimentalfactors Numberofcycles Degradationrate Reference Qietal. Air/fuelboundary 80 5mVdroppercycleat400mAcm−2 [42]
Darlingetal. Localizedfuelstarvation 100h Severedamagetothecatalystlayer [44] Takagietal. Shutoffsequenceofhydrogenandairwithout
adummyload
40 Outputpowerdeclinedby17%at1.0Acm−2 [40]
Peietal. Start-stopcyclingwithnitrogenpurge 80h Cellvoltagedecayedby0.00196%at100Apercycle with280cm2activeareaPEMFCstack [5]
Owejanetal. GraphitizedcarboninMPL 25h 63%lossincurrentdensityat0.6Vwithoutgraphitized carbon,25%improvementinvoltagedegradationat 1.2Acm−2
[30] Limetal. Shutdownprocess 200 0.31mVdroppercycleat80◦Cand400mAcm−2 [35]
Sakamotoetal. 50–90Vdroppercycle [22]
Choetal. Airpurgingeffect 50 4.2mVdroppercyclewithair/hydrogenpurge 2.0mVpercyclewithair/airpurge
[39] Applyingthedummyload 1200 Cellvoltagedecayedby0.030%withdummyloadbut
by0.068%withoutdummyload
[36] Cathodeinletrelativehumidity(RH) 1500 0.186mAcm−2droppercyclefor0%RH
0.240mAcm−2droppercyclefor50%RH
0.266mAcm−2droppercyclefor100%RH
[37,38] Supplysequenceofhydrogenandair 1200 0.68mAcm−2droppercyclewithconcurrentairand
hydrogensupply;0.47mAcm−2percyclewith
hydrogensuppliedpriortoair
[34] Fuelcelltemperature 1200 0.13mVdroppercycleat40◦C
0.24mVdroppercycleat65◦C
0.31mVdroppercycleat80◦Cat400mAcm−2
[33] Ettingshausenetal. Start/stopcycling 500 About0.66mVdroppercycleat1000mAcm−2 [45]
Yuetal. Cathodeexhaustcondition 1500 0.024mVdroppercycleforclosedcell;0.093mVdrop percycleforopen-endedcellat1000mAcm−2 [43]
Inukaietal. Gasexchangecycling 500 About0.9mVdroppercycleat200mAcm−2 [41]
Knightsetal. RelativehumidityonRudissolutionand
Fig.3.Potentialdistributionalonganodeflowpathduringreverse-currentconditions. ReprintedwithpermissionfromRef.[47].Copyright2005,TheElectrochemicalSociety.
theanode,orusinganopencathodeexhaustend.Insteadofpure hydrogenfortheanodegassupply,Qietal.[42]usedareformate gascomposedof10ppmCO,49%H2,and17%CO2,balancedby
N2.A2%airbleedwasusedattheanodesidetomimica
hydro-gen/airinterface.Theresultsindicatedadegradationrateofabout 5mVpercycleat400mAcm−2after80cycles.However,10ppm
COand17%CO2inthereformattedgasalsoaugmentedthePEMFC
degradationrate,whichshouldnotbeignored.Yietal.[43] stud-iedtheeffectofcathodeexhaustconditionsonthedegradation behaviorsofPEMFCsduringtheshutoffprocess.Thehydrogen/air interfacewassimulatedbyusingtheopenexhaustatthecathodeto leadtoatmosphericairpermeation.Theyconcludedthattheclosed cathodeexhaustwasbeneficialforPEMFCdurability.
Theacceleratedtestingresultsdiscussedabovenotonly demon-stratetheadverseeffectsthatstartupandshutdownprocessescan haveonthedurabilityofaPEMFC,butalsoprovideinformationon howtoalleviatetheseadverseeffectsbychangingoperating condi-tions,suchashumidity,temperature,etc.However,thecondition changesmentionedinthosepapersarenotpracticalforrealfuelcell operation.Forexample,theperformancedegradationcausedby startupandshutdowncouldbemitigatedwithlowertemperatures andlowergashumidityduringthestartupandshutdowncycles. However,underrealconditionsitwouldbedifficulttocontrolthe temperatureandhumiditybeforestartinguporshuttingdownthe system.Furthermore,itwouldtakealongtimetolowerthe tem-peratureandhumidityfromtheirworkingpointstotheirshutdown points.Anotherimpracticaltacticwouldbeshuttingofftheair sup-plypriortothehydrogensupply;althoughthis wouldmitigate theadverseeffectsofthestartupandshutdownprocess,itisnot
advisableforeithersystemsafetyorfuelefficiency.Inaddition,as showninTable1,althoughmosttestsconductedunderoptimized operatingconditionsandfrequentcyclesdemonstratedsomelevel ofimprovementindegradationrates,theseimprovementswere stillnotenoughtomeettheDOElifetimetargets.
Hence,morepracticalandeffectiveproceduresshouldbe devel-opedtomitigatetheperformancedegradationcausedbystartup andshutdowncycles.InSection4.2,weexploreindepthafew sys-temstrategiesputforwardbyUTCthatcouldbepracticallyapplied inrealfuelcellsystemsandresultinmuchbetterdurability. 3.2. RootcauseofPEMFCdegradationduringstartupand shutdown
Asdiscussedearlier,thehydrogen/airinterfaceformedduring thestartupandshutdownprocessesistherootcauseofthenegative effectsthattheseprocesseshaveonPEMFCdurability.Inthispart wewilldiscusstheunderlyingdegradationmechanismscausedby thehydrogen/airinterfaceinaPEMFC.
3.2.1. Reversecurrent
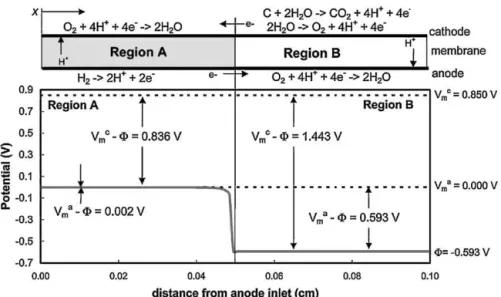
ThereversecurrentmechanismwasfirstproposedbyUTCin 2005[47].AsdiscussedinSection2,theypointedoutthata hydro-gen/airinterfaceintheanodewascreatedduringboththestartup andshutdownprocesses.Whenthishappens,asshowninFig.3, partoftheanodeisfilledwithhydrogenandtherestisfilledwith air,leading toa regionwhereoxygenreductionoccursonboth thecathodeandanodesides,resultinginahighpotential differ-ence(about 1.44V) atthe cathode.This highcathodepotential
Fig.4.Schematicofadualcellconfigurationusedtosimulatethereverse-currentcondition. ReprintedwithpermissionfromRef.[47].Copyright2005,TheElectrochemicalSociety.
Fig.5.Intensitypotentialcurvesfortwoelectrodesofanelectrochemicalcell. ReprintedfromRef.[48]withpermission.
temporarilyreversesthecurrentwhereairispresentintheanode, causingseverecarbonoxidation(alsoshowninFig.3).They pro-posedusinghighcathodepotentialand“reversedcurrent”,based onthesignificantdecreaseinthecatalystlayerthattheyobserved inasingle-cellexperiment.However,theydidnotdirectly mea-surethevalueofthehighcathodepotential;instead,theyuseda dualcellconfigurationtoestimatethecathodevoltagetobearound 1.44V.AsshowninFig.4,intheirdualcellconfiguration,thetwo singlecellswereconnectedbyconductivewires,whichwould cer-tainlyleadtosomevoltagelossattheinterfaceofthetwosingle cells.However,undertherealconditionofasinglecell,Cell1and Cell2inFig.4wouldbeconnectedbyanelectrolyteandan elec-trode,sotheresistancebetweenthetwocellsshouldbenegligible. Therefore,theaccuracyoftheirestimated1.44Vcathodepotential isdebatable.
Basedonthesamepremiseof“reversedcurrent”andhigh cath-odepotential,othergroupsalsoconductedsimilaranalysesand tests.Yousfi-Steiner[48]explained theobserveddegradationat thecathodebyheterogeneitiesincurrentdistribution,withlocal currentclosetozerointheareafedwithnitrogenordiluted oxy-gen.AccordingtoYousfi-Steiner’sassumption,sincethetwocellsin thisconfigurationareconnectedtoneitheranelectronicloadnor apotentiostat,andsincetheyareonlyexternallyconnected,the setuppresentedinFig.4mostlikelyrepresentsagenerator(Cell1) connectedinseriestoanelectrolysiscell(Cell2),ratherthantwo singlefuelcellsconnectedinparallel[49].Analyzingthecurrent flowandcalculatingthevoltageinthetwodevicesyieldsthe con-clusionthatthepositiveelectrodeofCell2canreachhighenough potentialsforfastcarbonandPtcorrosiontooccur,asshowninthe givenformulasandFig.5.
Similarly,Owenjanetal.[30]haveconsideredthePEMFCunder thestartupand shutdowncyclesastwo shortedcells. The sec-tionofthecellwhereairwaspresentinboththeanodeandthe cathodewasconsideredanair/aircell,andthesectionwherethe cathodewasfilledwithairandtheanodewasfilledwithhydrogen wasconsideredanormalPEMFC.Theirassumptionwassimilarto Yousfi-Steiner’s,buttheydidnotpredictormeasurethevalueof thecathodicpotentialduringthestartupandshutdownprocesses. Reversecurrenthasbecomeacommonlyacceptedmechanism toexplainthedegradationinducedbythestartupandshutdown processes.Asaresult,manyresearcheffortshavebeendirected tomeasuringorpredictingthehighpotentialatthecathode.The followingparagraphssummarizetheexperimentalmeasurements andmodelpredictionsofthecathodepotentialduringstartupand shutdown.Duetotheinstantaneousnatureofthehydrogen/air interfaceat the anode,the occurrence of high potentialat the cathodeisalsoinstantaneous,whichpresentsgreatchallengesfor
correctlymeasuringthispotential.Inrecentpapers,several meth-odshavebeenreportedtotestthecathodepotential:
•Dualcellconfiguration:Twosinglecellswereconnected exter-nallybyelectricwires.Theanodeandthecathodeofthefirst singlecellweresuppliedwithhydrogenandair,respectively,as inanormalPEMFC.Intheothercell,airfilledtheanodeand cath-odetosimulateanelectrolysiscelloradrivencell[42,47,48].The cathodepotentialofthesecondcellwasthemaximumcathode potentialofaPEMFCexposedtoreversecurrent.Asdiscussed above,intherealconditionofasinglecell,Cells1and2wouldbe connectedbyanelectrolyteandanelectrode,notexternalwires, whichproducedavoltagelossattheinterfaceofthetwosingle cells.Amuchsmallervoltagelosswouldoccurinarealsituation. Consequently,someerrorsprobablyresultedfromtheexternal connection.
•Referenceelectrodemethod:Sinceitisnotpossibleto distin-guishbetweentheanodeandcathodehalf-cellreactionswith asegmentedfuelcellassembly,thereferenceelectrodemethod hasbeendevelopedandemployedbyseveralresearchgroups
[50–55].Measurementofthecathodepotentialwasachievedby introducingaconstantpotentialintothetestcell,utilizinga refer-enceelectrode,andthentheanodeandcathodepotentialscould bedeterminedrelativetothisconstantpotential.Inthismethod, asmallstripofmembraneintheMEAwassoakedinsulfuricacid withamercurysulfatereferenceelectrode[50]orahydrogen ref-erenceelectrode[56].Boththeanodesideandthecathodeside oftheMEAwereconsideredtheworkingelectrode.Thecathode potentialcouldbemeasuredasthepotentialdifferencebetween theworkingelectrodeandthereferenceelectrode.However,it shouldbepointedoutthatthereferenceelectrodemethodalso hasitsdrawbacks.Becausethereferenceelectrodeisplacedata specialpointonthemembrane,thepotentialmeasuredatthis particularpointmaynotcorrectlyreflect therealpotentialof thewholemembrane.Therefore,itwouldbemoreaccurateto developatestdevicethatcanevaluatethepotentialofthewhole membrane,ratherthanthepotentialofa specialpointonthe membrane.
•Modelprediction:Toovercomethedisadvantagesinthe experi-mentalmeasurementsmentionedabove,someresearchershave developedmathematicalmodelstoobtaintheelectrolyte poten-tialprofileforthisphenomenon[47,57,58].Theresultscanassist researchersinclearlyunderstandingdegradationmechanisms.
Table 2 summarizes the methods used to measure cathode potentialduringstartupandshutdown,asreportedinrecent pub-lications. Theresearchers atPlug Powermeasuredthe valueof thecathodicpotentialwithreformatehydrogenasthefuel[42]. Byusingadualcellconfiguration,acathodepotentialashighas 1.75Vwasmeasured,whichwasdifferentfromthecalculatedvalue reportedbyReiseretal.[47].Baumgartneretal.[50]obtaineda cathodeelectrodepotentialofupto1.5Vwithfourreference elec-trodesunderhydrogenstarvationconditions,whichalsoresultedin aH2/airboundaryintheanode.Shenetal.[56]measuredthe
cath-ode,anode,andmembranepotentialsversusahydrogenreference electrodeusingaspecialelectrodedesign,asshowninFig.6;the resultsindicatedthattheinterfacialpotentialdifferencebetween thecathodeandthemembranewasashighasabout1.6V.Allthese experimentsprovethathighpotentialdoesexistatthecathodeof aPEMFCduringthestartupandshutdownprocesses.
However,Sidik etal. [57] proposeda different viewonthe maximumpotentialaPEMFCcathodecouldexperienceduetothe formation of a hydrogen/air interface atthe anode. They clari-fiedthatthemaximumpotentialwasabouttwicethepotential observedwhenoneconnectedadrivingfuelcelltoadrivencell, basedontheButler–Volmerequationandtheexperimentaldata
Table2
Summaryofthecathodepotentialmeasurementsorpredictionsforthestartupandshutdownprocesses,asreportedinrecentpublications.
Authors Testmethods Cathodepotentialvalue Reference
Qietal. Dualcellconfiguration 1.75V [42]
Reiseretal. Modelprediction 1.44V [47]
Baumgartneretal. Fourreferenceelectrodes 1.5V [50]
Shenetal. Hydrogenreferenceelectrode 1.6V [56]
Taketal. Dynamichydrogenelectrode(DHE) 1.4V [59]
Sidiketal. Theoreticanalysis TwicetheOCVofthedrivingcell [57]
Ohsetal. Modelingstudy >1.2V [58]
fromPEMFCs(InFig.3,thedrivingcellisCell1andthedrivencell isCell 2.).Becauseofsomesimplificationsemployedin Reiser’s model, the predicted cathode potentialwas 1.44V, which was lowerthantwicetheOCV.
Itcanbeconcludedfromthemeasuredorpredictedcathode potentialsdiscussedabovethat,inspiteofthedifferentmethods, themaximumpotentialvalueatthecathodeismuchhigherthan theOCV.Atsuchahighpotential,thecarbonsupportofaPt-based catalyst is proneto oxidation, resulting in PEMFC performance degradation.
Inadditiontomeasuringormathematicallypredictingthehigh cathodepotentialexperiencedduringthestartupandshutdown processes,researchershavealsoconductedotherrelatedtests,such asmeasuringthecurrentdistributioninsegmentedcells[60–62], monitoringthe concentrationof CO or CO2 during startup and
shutdown[59,63],evaluatingtheeffectofcelldesign(flowfield structure andGDLthickness)onstart–stopphenomena [64,65], andconducting modelingstudies[58,66–73]onwater manage-mentandcarbon-corrosionduringstartupandshutdown.Allthe testresultshaveconfirmedperformancedegradationduringthe startupandshutdownprocessesofPEMFCs.
3.2.2. Fuelstarvation
Localizedfuelstarvationcanhappenduringnormaloperation duetopoordistributionofreactantsortowaterflooding.However, duringunprotectedfuelcellstartupandshutdown,ahydrogen/air boundaryforms,whichinsomecasesleadstoasituationsimilarto fuelstarvation.Thissectiondiscussesinageneralwaythe conse-quencesthatfuelstarvationcanhaveonfuelcellperformanceand durability.Butithastobepointedoutthatfuelstarvationisnot limitedtostartupandshutdown.
InthenormaloperationofPEMFCs,allthereactantsare suffi-cientlysuppliedtotheanodeandthecathode,resultingineven distributionattheelectrode surface.However,ifheterogeneous
Fig.6. SchematicsoftestMEAwithreferencehydrogenelectrodeandthincopper wiressandwichedbetweentwomembrane.
ReprintedfromRef.[56]withpermission.
fueldistributionoccurs,airoroxygenatthecathodewillcrossthe membranetotheanode,duetothepressuredifferencebetween theanodeandcathode. In2008,Ofstad[74]reportedcreatinga transienthydrogen/air interfacein theanodelayer.InJingwei’s modeling study[73],the resultsindicated thatthe rateof car-boncorrosionatthecathodesideofthefuelstarvationregionwas stronglydependentonoxygendiffusionthroughthemembrane. Theconcentrationgradient acrossthemembrane isthedriving forceforoxygentopermeatefromthecathodetotheanode, accord-ingtoFick’sfirstlaw.Ifwesupposeafuelcellsysteminasteady state,theconcentrationofoxygenremainsconstantatallsurfaces ofthemembrane[75].Underthisassumption,theone-dimensional diffusionequationfromFick’sfirstlawisexpressedas[76]: JO2 =−DO2 dCO2 dz =DO2 CcathodeO 2 −C anode O2 z (1)
whereJO2istheoxygenpermeationrate,DO2istheoxygendiffusion
coefficient,COcathode
2 andC
anode
O2 aretherespectiveoxygen
concen-trationsonthesurfaceofthemembraneatthecathodeandanode, andzisthemembranethickness.Asaresult,ahydrogen/air inter-faceisproducedattheanode.Accordingtothe“reverse-current mechanism”[47],thisinterfaceisnotbeneficialtothedurabilityof thecarbonsupportinPEMFCs.
Someresearchershaveinvestigatedthecurrentdistributionof PEMFCsduringlocalfuelstarvation[50,77–83],andYousfi-Steiner et al.[48] providedan excellentreview ofthecausesand con-sequences of starvation issues. So in this review, we will turn fromperformancedegradationduetolocalfuelstarvationarising fromahydrogen/airinterface,andfocusinSection4on materi-alsimprovementandsystemstrategiestomitigatecatalystdecay duringthestartupandshutdownprocesses.
3.2.3. Carbonoxidation
Catalystdegradationatthecathodeisconsideredamajorfailure mode for PEMFCs when the catalysts are exposed to reverse-currentconditionsduringstartupandshutdown.
In thecatalyst layersof PEMFCs,platinum nanoparticlesare always dispersed on supports to increase the active area and therebylowermaterialcosts[84–86].AsconcludedinRef.[87], theidealcatalystsupportshouldhavethefollowingproperties: •Reasonablyhighelectricalandthermalconductivity.
•Goodcorrosionresistanceintheelectrolyte.
•Dimensionalandmechanicalstabilitywithreasonablestrength. •Highsurfacearea.
•Availabilityatlowcostandwithreasonablyhighpurity. •Easydispersionofsmallcatalystparticles.
•Easyfabricationintoelectrodes. •Absenceofadversecorrosionproducts.
Becauseofitsgoodelectronicconductivityandlowcost, car-boniswidelyusedasafuelcellcatalystsupport.Althoughcarbon hasmostoftheaboveproperties,itissusceptibletooxidationor
Fig.7.Quantitativeanalysisofcathodeoutletgaseswithchangeincathode poten-tial.
ReprintedfromRef.[59]withpermission.
corrosion at high temperature and high oxygenconcentration, yieldingCO2orCO,asinthefollowingequations[42,63]:
C+2H2O →CO2+4H++4e− (2)
C+H2O→ CO+2H++2e− (3)
Shaoetal. [88]investigatedthethermodynamicpotentialof carbonoxidationintoCO2.Theirresultsindicatedthatoncethe
potentialwashigherthan+0.207Vvs.RHE,thecarbonwouldbe oxidizedtoCO2,accordingtoreaction(2).Carboncanalsobe
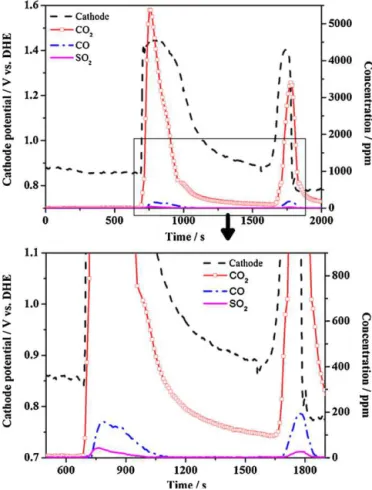
oxi-dizedtoCOat0.518Vunderstandardconditions[63,89,90]. Innormalfuelcelloperation,thecarbonoxidationrateisslow duetotheslowkineticsofreactions(2)and(3)atPEMFC operat-ingtemperatures.However,carbonoxidationoccursmuchfaster underdynamicprocessessuchasstartupandshutdown,because thecathodepotential is much higher than thethermodynamic potentialofcarbonoxidation.Ithasbeenreportedthatalthoughthe kineticsofthecarbonoxidationreactionissluggishatlow poten-tialandlowtemperature,thepresenceofPtparticleswillaccelerate carbonoxidation[90].Kimetal.[59]directlymeasuredand ana-lyzedexhaustgasusingFT-IRduringstart/stopoperation.Asshown inFig.7,theamountofCO2evolvedwasproportionaltothecathode
potentialatvaluesabove1.0V.Inaddition,atpotentialshigherthan 1.2V,COandSO2weregenerated,havingdetrimentaleffectson
fuelcellperformance.Similarly,S.MassmeasuredtheCO2andCO
concentrationsincathodeexhaustusingnon-dispersiveinfrared spectroscopy(NDIR)[63].Moredirectly,Inukaietal.usedscanning transmissionelectron microscopy (STEM) imagestoinvestigate catalyst-layerdegradation at thecathode during500 start/stop cycles.Theyobservedseveralholesatthecatalystlayer,withno
Fig.8.STEMimagesneartheinlet,center,andoutletofanMEAatthecathodeafter degradation.
ReprintedfromRef.[41]withpermission.
carbonsupport,indicatingadrasticcorrosionofthecarbonsupport
[41](Fig.8).
Nano-particlessuchasPtparticlesinthecatalystlayerhavethe inherenttendencytoagglomerateintobiggerparticlestoreduce theirhighsurfaceenergy[91,92].Carbonoxidationorcorrosion weakenstheattachmentofPtparticlestothecarbonsurfaceand eventuallyleadstostructuralcollapseanddetachmentfromthe carbonsupport,resultingintheagglomerationand/ordissolution ofPtparticlesintotheelectrolytewithoutredeposition.Moreover, Ptmaybetransportedthroughtheelectrolyteand/orthroughthe ionomerafterbeingdetached,causingareductioninelectrolyte conductivity[93].Therefore,carbonoxidationpromotedbyhigh cathodepotentialduringthestartupandshutdownprocessesis themaincauseofcatalystdegradationinthecathodeofthefuel cell[43].
3.2.4. Agglomerationand/ordissolutionofPtparticles
ThedegradationmechanismsforPtcatalystsincludethe follow-ing[32,48]:
•Ptparticleagglomerationandparticlegrowth. •Ptlossandredistribution.
•Poisonouseffectsarousedbycontaminants.
Once oxidation of the catalyst support happens during the startupandshutdownprocesses,thedegradationof Ptparticles followsthefirsttwomechanisms.However,thereisno consen-susonthedominanceofthesetwomechanisms.Someresearchers haveinvestigatedthedegradationprocessofaPtcatalystduring extendedoperationunderOCV[92,93].Theresultsindicatedthat Ptparticlesdissolvedintheionomerandthengrewtolarger par-ticles.OtherresearcherssuggestedthatPtparticleswoulddetach fromtheoxidizedcarbonsupportanddissolveintheelectrolyte
[94,95].
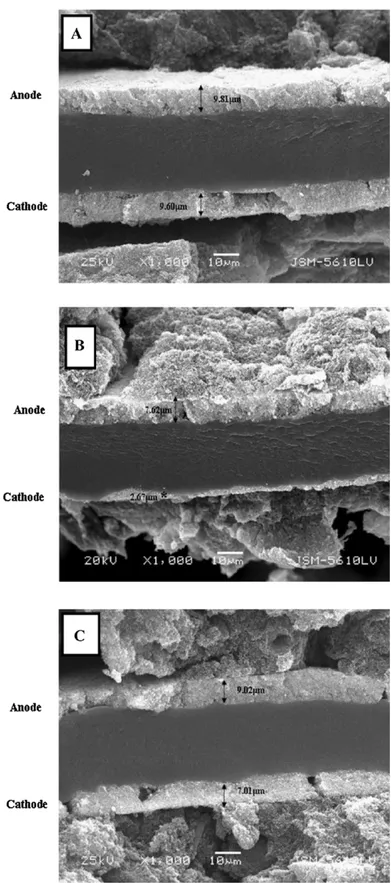
Cross-sectionalscanningelectronmicroscopy(SEM)and trans-mission electron microscopy (TEM) have often been used to characterizePt/Ccatalystssubjectedtofrequentstartupand shut-downcycles.Yireported[43]thatthethicknessesofthecatalyst layers in theanode and cathode of fresh MEAs were9.81 and 9.60m,whichdecreasedto7.02and2.47m,respectively,after 1500frequentstartupandshutdowncyclesforanopen-endedfuel cell(seeFig.9).Ettingshausenetal.[45]observedthegrowthofPt particlesbyusingTEMandhighresolutionTEMtoanalyzeaPt/C catalystthathadexperiencedstart/stopcyclesandhighcathode potential.Afterthestart/stopcycles,therelativenumberof parti-cleswithadiameter≤2.5nmdeclinedfrom57.20%inafreshMEA to1.92%.Choetal.combinedtheresultsofcross-sectionalSEMand TEMtoevaluatePt/Cdegradation[33,34,36,38,39,45,59].
Apparently,asupportmaterialthatismorestablethanthe cur-rentcarbonsupportforPEMFCcatalystsisdesirabletoalleviatethe degradationcausedbythestartupandshutdownprocesses.Butif thecurrentcatalyst-supporttechnologyhastobeemployed,there isanurgentneedtoapplyeffectivesystemstrategiesforstartup andshutdowntomitigateoxidationofthecarbonsupportunder highcathodepotential.
4. Mitigationstrategies
Many journal publications and patents have focused onthe development of strategies to mitigate the performance decay causedbystartupandshutdown.Thestrategiescanbeclassified intotwomajorcategories:
•Materialimprovementformorestablecatalystsupports. •Systemmitigationstrategiesforconventionalcarbon-black
sup-ports.
4.1. Alternativecatalystsupports
Replacing conventional carbon supports with corrosion-resistantmaterialshasbeenone important mitigationstrategy. Yuetal.[66]reportedthatusinggraphitizedcarbonasasupport yieldeda5timeslowerdegradationratethanaconventionalcarbon supportafter1000startup/shutdowncycles.Owenjanetal.[30]
alsoreporteda25%reductioninthestartup/shutdowndegradation rateat1.2Acm−2withtheimplementationofagraphitizedcarbon
inthemicroporouslayers(MPL).Theyexplainedthattheuseof graphitizedcarbonresultedinareducedmasstransportlimitation of the gasdiffusion layer,in comparison withthe mass trans-portlimitationenhancedbyconventionalcarbonoxidationatthe MPL/electrodeinterface.Inadditiontographitizedcarbon,other carbonmaterials,suchascarbonnanotubesorcarbonnanofibers
[96–99],andcarbonaerogelandxerogel[100–102],havealsobeen consideredascatalystsupportsbecauseoftheirmorestable elec-trochemicalbehaviors[32].However,thehighcostofsynthesizing
Fig.9. Cross-sectionalSEM images:(A)freshMEA withoutstartup–shutdown cycles;(B)MEAinopen-endedcellafter1500cycles;(C)MEAinclosedcellafter 1500cycles.
ReprintedfromRef.[43]withpermission.
thesecarbonmaterialsincreasedtheburdenonthecommercial developmentofPEMFCs.Inadditiontocarbon-typesupports,many non-carbonsupports[63,103–108],suchassubstoichiometric tita-niumoxide[107],tungstencarbide [104],andindium tinoxide
[105], have been investigated toreplace the conventional car-bonsupportsandachievegreaterstabilityduringlong-termtests.
Ioroietal.[107]testedasinglecellusinga Pt/Ti4O7 cathodeat
300mAcm−2 for more than350hwithfully humidifiedH 2/O2.
Stablevoltagewasobservedthroughoutoperation,demonstrating thatPt/Ti4O7wasapossibleoxidation-resistantcatalystmaterial
foraPEMFCcathode.However,noreportshavebeenpublishedon durabilitytestsofPEMFCsunderstartupandshutdowncyclesusing non-carbonsupports.
Asaresult,systemstrategiesseemmorepracticalatthepresent time,whenconventionalcarbonisbeingusedasacatalystsupport. 4.2. Systemstrategies
Asdiscussedinprevioussections, thehydrogen/airinterface causingreversed currentand highcathodepotentialistheroot causeforPEMFCdegradationduringstartupandshutdown.Allthe reportedorpatentedsystemstrategiesweredevelopedtoprevent ahydrogen/airinterfaceattheanodeandeliminatehighpotential atthecathodeduringstartupandshutdown.
UTC,GeneralMotorsCorporation(GM),andotherautomotive companiessuchasFord,Toyota,Nissan,andDaimlerChryslerhave beenresearchingsystemstrategiesforthestartupandshutdown processes.Table 3lists importantpatents onsystemstrategies, including:
•Gaspurgetoanodebeforestartupandaftershutdown.
•Auxiliaryloadappliedtoconsumeresidualoxygenatthecathode withpotentialcontrol.
•Exhaustgasrecycleaspurginggasorreactiongas. •Electronicshorttoeliminatehighpotentialatthecathode.
4.2.1. Gaspurge
Gaspurgingisaneffectivewaytopreventahydrogen/air inter-faceattheanode.Itcanalsominimizethetimethataninterface exists.
Fortheshutdownprocessoffuelcells,Margiott[115]andReiser
[116]atUTChaveproposedthefollowingprocedurewithair purg-ing:supplyingtheairthroughtheblowerintotheanodetodispel theresidualhydrogenattheanode,afterdisconnectingtheprimary loadandstoppingthehydrogensupply.Reiser’slong-term durabil-itytest[120]revealedanaveragevoltagelossof0.2Vwithoutair purgingafterabout250startupandshutdowncycles;however, theperformancedegradationwasnotsosevere,withonlyabout 0.055Vvoltagelossafternearly500cycleswithairpurging(see
Table4).YuandWagneratGeneralMotorsCompany[128–130]
reportedthat air purgingduringthe shutdownprocess wasan effectivemethodtopreventperformancedegradationinfuelcells. However,theyproposedthatitwasmoreeffectivetoemploythe airpurgeuntilthetemperatureofthestackwasreducedbelowa predeterminedtemperature.
Withregardtothestartupprocess,Balliet[119]andReiser[120]
inventedasafetystrategythatconsistedofastartupsystemand methodforafuelcellpowerplantusingapurgingofthecathode flowfieldwithahydrogen-rich,reducingfluidfueltominimize corrosionofthecathodeelectrode.Theirstrategyincludedthe fol-lowingsteps:(1)purgingthecathodeflowfieldwiththereducing fluidfuel;(2)directingthereducingfluidfueltoflowthroughan anodeflowfield;(3)terminatingtheflowofthefluidfuelthrough thecathodeflowfieldanddirectinganoxygen-containingoxidant toflowthroughthecathodeflowfield;and(4)connectingaprimary loadtothefuelcell.Inaddition,GeneralMotorsalsopatentedtheir inventionofastartupprocesswithgaspurging,whichincluded introducinghydrogengasintotheanodeandthecathodeto con-sume/purgeoxygeninboth[127].
Itshouldbenotedthatinimplementingtheabovementioned systemstrategies,theblowersandotherdevicesusedtomovethe gasesthroughthesystemshouldbeproperlyselectedtoachieve
thedesiredspeedforgasdisplacementtooccurinlessthanabout 1.0s,andpreferablylessthan0.2s,tominimizethetimethatthe hydrogen/airinterfaceexists.
Nitrogenisasafeandinertgasthathasbeenwidelyusedasa purgativeforfuelcells,butitisnoteasilymadeavailableinreal fuelcelloperations.Tohavenitrogenasapurginggasforstartup andshutdown,FordMotorCompanyhasinventedapurgingsystem withaseparatorthatremovesoxygenfromtheexhaustgasatthe cathode.Afterseveralcyclesofcathodeexhaustgas,areformate gaswithahighnitrogenconcentrationandlowoxygen concen-tration canbe usedas thepurge gas [139]. Thampan invented astrategy thatusedamembrane-basedhumidifiertoprovidea N2-rich exhaustasthepurginggas[151].Themembrane-based
humidifierwasusedtotransfermoisturefromamoisture-laden exhauststreamtothedryairfeed,andwasalsousedinashutdown modeinwhichthemembrane-basedhumidifierwasimplemented topermeatemoistureandoxygenfromthemoisture-ladenexhaust stream,therebyprovidingnitrogen-richgas.Thesamemethodwas alsopatentedbyScotto[154,155].
4.2.2. Auxiliaryloadwithpotentialcontrol
Gaspurgingcanpreventa hydrogen/air interfaceduringthe startupandshutdownprocesses.However,itcannotcompletely dispelresidualgaspresentintheflowfieldoffuelcells,suchasin thegasdiffusionlayerorthecatalystlayer.Anothereffectiveway todispelresidualgasistointroduceanauxiliaryload(alsocalled adummyload).Kimetal.[36]haveinvestigatedhowapplyinga dummyloadaffectsfuelcelldegradation.Theirresultsindicated thatapplicationofadummyloadduringthestartupprocedure sig-nificantlyreducedperformancedecayandlossofelectrochemically activesurfacearea,andincreasedcharge-transferresistance,which resultedinadramaticimprovementindurability.InCondit’s[112]
andBalliet’s[113]invention,adummyloadwasappliedto pre-ventcellreversalbyconsumingtheairatthecathodeduringthe shutdownprocess.Yu[129]appliedanauxiliaryloadtoconsume theoxygenatthecathode,leavingnitrogenandhydrogeninthe cathodeandanode,respectively,duringtheshutdownprocess.
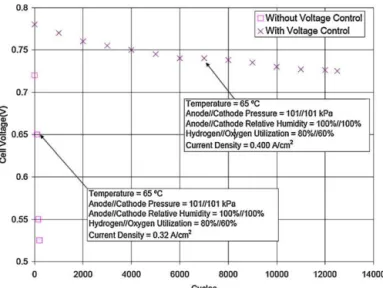
Anauxiliaryloadhasalwaysbeenusedinthecompanyofan anodeexhaustrecycleloop.Yangproposedastartupprocedurefor afuelcellsystemhavingananodeexhaustrecycleloop[121].First, theauxiliaryloadwasconnectedacrossthecelltoreducethecell voltage.Thenthelimitedhydrogenfuelfromtheanodeexhaust, througharecycleloop,wasprovidedtotheanode.Theaddedfuel reactedwiththeoxygenthatremainedintherecirculationgases untilvirtuallynooxygenremainedwithintherecycleloop.Finally, fuelandaircouldbesuppliedatthenormaloperatingflowrates intotheanodeandcathode,respectively,aftertheauxiliaryload wasshutoff.Fig.10showstheapplicationofadummyloadduring fuelintroductionatstartup[87].Thelosseswithoutadummyload weresevere,andtheperformancedecreasedby∼100Vcycle−1. Withadummyloadtocontrolthecellvoltage,performancedecay wasreducedtoapproximately4Vcycle−1.Yangetal.patented asimilarstrategythat appliedanauxiliaryloadwiththeanode recycleloop,andcouldbeusedintheshutdownprocesstoreduce thecellpotential[110,121].
Chanetal.atHyundaiMotor[149]havedesignedanapparatus foreffectivelypreventingcarboncorrosionfromoccurringatthe cathodeofafuelcell.Inthisstrategy,tocompletelyexhaustthe residualoxygenatthecathodeafterthefuelcellwasshutdown,a pressuresensorandanairdischargesolenoidvalvewereinstalled intheairdischargepipetodetecttheairpressureinthefuelcell andcontroltheairsupply.Underthefunctionofthepressure sen-sorandthesolenoidvalve,astheresidualoxygenatthecathode wascompletelyconsumed,thesolenoidvalvereceivedtheclosing signalandthedummyloadwasdisconnectedtoavoidanegative voltageinthefuelcell.
Table3
Summaryofpatentsonsystemstrategiesforstartupandshutdownprocesses.
Inventors Year Systemstrategy Reference
UTC 2000 Makingthefuelcellsysteminertbycoolantfloodingduringshutdown [109] 2002 Shuttingdownthefuelcellsystemusingananodeexhaustrecycleloop [110]
2003 [111]
2003 Shuttingdownfuelcellswithanauxiliaryloadtoconsumetheoxygen [112] 2004 Shuttingdownfuelcellswithanauxiliaryloadtoconsumetheoxygen [113]
2004 Fuelpurgeofcascadedfuelcellstacks [114]
2004 Shuttingdownfuelcellswithanairpurgeintotheanode [115]
2005 [116]
2005 Cascadedanodeinletmanifolddesign [117]
2005 ReducingcathodepotentialfortheMEAwithanelectronicshort [118] 2005 Startingupfuelcellswithfuelpurgeintothecathode [119,120] 2006 Startingupafuelcellsystemusingananodeexhaustrecycleloop [121] 2006 Usingahydrogenreservoirtoreceiveandstorehydrogenduringfuelcelloperation,andto
releasehydrogenwheneverthefuelcellisshutdown
[122]
2011 Preventingairintrusionintohydrogenduringshutdown [123]
GMMotorsCorporation 2005 Shuttingdownandstartingupwithastoichiometricstagedcombustor [124]
2005 Shutdownandstartupwithacathoderecycleloop [125,126]
2006 Shutdownandstartupwithahydrogenpurge [127]
2006 Shuttingdownthefuelcellsystemusinganairpurgeinlowtemperature [128] 2008 Shuttingdownwithanauxiliaryloadtomakenitrogenandhydrogeninthecathodeandanode [129]
2008 Shuttingdownthefuelcellsystemusinganairpurge [130]
2008 Hydrogenpurgeintothecathodeforoperatingafuelcellstack [131] 2008 Specialelectrodedesignforreducingelectrodedegradation [132] 2009 Specialelectrodedesigncontainingoxygenevolutionreactioncatalysts [133] 2011 Fuelcelloperatingmethodsforoxygendepletionatshutdown [134]
PlugPower 2007 Gaspurgeforstartupandshutdown [135]
BallardPowerSystems 2005 Newcatalystdesigntoimprovevoltagereversaltolerance [136,137] 2006
2007 Recirculatingtheoxidantwithananodepurgepath [138]
FordMotorCompany 2009 Purgingsystemwithaseparatorthatremovesoxygenfromtheexhaustgasatthecathode [139] NissanMotor 2009 Fuelcellsystemwithvoltagesensorandaccurategas-supplycontrol [140–142]
2006 2005
DaimlerChrysler 2011 Aselectivelyconductingcomponentisincorporatedinelectricalserieswiththeanode componentsinthefuelcell
[143,144] 2009
HondaMotor 2010 Afuelcellsystemthatincludesanoxidantgassupplyapparatusandafuelgassupplier [145] ToyotaMotor 2009 Therestrictionontheoutputofthefuelcellislifted,andtheoutputofthefuelcellis
controlledaccordingtotherequestedoutput
[146–148] 2008
HyundaiMotor 2008 Apparatusforpreventingcarboncorrosionatthecathodeinthefuelcell [149] Anodesidehydrogen/oxygeninterfaceformationinhibitionstructure [150] Others 2010 Membrane-basedhumidifiertoprovideaN2-richexhaustforpurginggas [151]
2011 Shuttingdownwithcathodegasrecycle [152]
2010 Detectingtheanodegasamount [153]
2011 Generatingagasthatmaybeusedforfuelcellstartupandshutdown [154,155] 2008 Startupandshutdownwithanelectricalshortingdeviceforindividualcellshorting [156] 2006 Electricallyconnectingoutputterminalscoupledtothefuelcellwithabatteryaftershutting
downtoconsumetheoxygenandfuelgas [157]
2005 Startingupwithafuelpurge [158]
2010 Usingchemicalshortingduringstartupandshutdown [159]
2008 Detectingairpressuretopreventahydrogen/airinterface [160]
2002 Safetyprocessanddevicewiththeanodegascycle [161]
2007 Shuttingdownwithstoringfuelintheanodeandcathodechambers [162]
2009 Systemandmethodforstartingupandshuttingdown [163]
Anotherwayofapplyinganauxiliaryloadistouseanelectronic shortofthefuelcell.Bekkedahl[118]inventedaspeciallydesigned fuelcellstackwithapermanentshunt,adiode,andastripof con-ductivecarbonclothorblack.Theremovableshuntcouldberotated intoandoutofcontactwiththefuelcellanodesandcathodesto make aninternal auxiliaryload.Ramani [159]and Miller [156]
alsoappliedchemicalshortingduringthestartupandshutdown processes.
4.2.3. Othersystemstrategies
Inadditiontogaspurgingandauxiliaryloadstrategies,other systemstrategieshavebeendevelopedtoenhancethedurability
ofPEMFCsduringstartupandshutdown.Theyincludenovel cata-lystdesigntoimprovethecatalyst’stolerancetovoltagereversal
[136],unique electrode design to reduceelectrode degradation under startup and shutdown [132], use of an electrode that contains oxygenevolution reactioncatalyststopreventcurrent reversal[133], and applicationof a cascadefuel inlet manifold
[114,117] to achieve better hydrogen distribution in the flow filed.
SomeJapanese companies,suchasToyotaMotor [146–148], Seiko Instruments [164], Nissan Motor [140–142], Fuji Electric
[165],andDaihatsuMotor[166],haveproposedtheirownPEMFC systems with voltage sensors and accurate gas-supply control
Table4
Summaryofsystemstrategies,withdurabilitytestresultsforstartupandshutdownprocesses.
Inventors Systemstrategies Durabilitytestresult Reference Reiseretal. Uncontrolledstartupandshutdown Averagevoltageloss:0.195Vafter250cycles [116,120]
StartupandshutdownwithH2purgeintothe
cathode,withanauxiliaryload
Averagevoltageloss:0.055Vafter300cycles N2purgeduringthestartupandshutdown
cycles
Averagevoltageloss:0.04Vafter1550cycles Conditetal. ShutdownwithauxiliaryloadandH2recycle
loop;startupwithauxiliaryloadandN2purge
Averagevoltagedecreasedfrom0.760Vto0.695Vin576cyclesat
400mAcm−2 [112]
ShutdownwithauxiliaryloadandH2recycle
loop;startupwithH2andN2storedatthe
anodeandcathode
Averagevoltagerecoveredfrom0.695Vto0.755Vafter2315cyclesat 400mAcm−2
Bekkedahletal. Reducingfuelcellcathodepotentialwith electronicshort
Cellvoltagedecayedmuchlessafter230–256startupandshutdown
cyclesat108mAcm−2and325mAcm−2 [118]
Yuetal. Shuttingdownfuelcellsystembyusingair purgeatlowcelltemperature(after40cycles)
30◦C Voltageloss:0.0142Vat200mAcm−2 [128,130] Voltageloss:0.0322Vat800mAcm−2 50◦C Voltageloss:0.0327Vat200mAcm−2 Voltageloss:0.1486Vat800mAcm−2 80◦C Voltageloss:0.1937Vat200mAcm−2 Voltageloss:0.4092Vat800mAcm−2
Yuetal. ShutdownandstartupwithH2purge Cellvoltagedecreasedfrom0.79Vto0.78Vafter200cycles [127]
Tangetal. H2purgeforstartupandshutdown PerformancewithH2-purgeprotectionisbetterthanwithN2-purge
protectionafterstartupandshutdowncycles
[135] Thampanetal. Membrane-basedhumidifiertoprovidea
N2-richexhaustforpurginggas
NoN2purge Cellvoltagedecreasedfrom0.65Vto
0.47Vat600mAcm−2 [151]
WithN2purge Cellvoltagedecreasedfrom0.65Vto
0.55Vat600mAcm−2
Ramanietal. ChemicallyshortedMEAwithH2andairbleed
purge
Averagevoltagedecreasedfrom0.69Vto0.50Vat600mAcm−2after
350startupandshutdowncycles
[159] Paiketal. Purgesystemwithaseparatortoremove
oxygenfromtheexhaustgasatthecathode
Air/H2cyclingatanode Voltagedecreasedfrom0.78Vto
0.25Vat600mAcm−2after500cycles [139]
N2purgeatanode Voltagedecreasedfrom0.39Vto
0.25Vat600mAcm−2after639cycles
5%O2innitrogenpurgeatanode Voltagedecreasedfrom0.6Vto0.44V
at1000mAcm−2after500cycles
10%O2innitrogenpurgeatanode Voltagedecreasedfrom0.7Vto0.4Vat
500mAcm−2after40cycles
systemstoachieveadesirabledurabilityduringthestartupand shutdownprocesses.
4.2.4. Summaryofsystemstrategies
As shown in Table 4, recent patents have reported several durabilitytestsconductedwithdifferentsystemstrategiesunder startupandshutdowncycles.Theperformancedecaywasgreatly mitigated by various strategies, among which the most effec-tivewasthecombinationofhydrogenpurging,applicationofan
Fig.10.Effectofvoltagecontrolduringfuelintroductiononperformanceloss. ReprintedwithpermissionfromRef.[87].Copyright2006,TheElectrochemical Society).
auxiliaryload,anduseofavoltagecontroldevicetoprevent volt-agereversal.AllthesystemstrategieslistedinTable4arebasedon thefollowingtwoprinciples:
•Minimizingthetimethatthehydrogen/airinterfaceexists,such asbyusingfuelpurgingduringthestartupandshutdown pro-cesses,andproducingN2-richgaswithaspecialsystemdesign.
•Reducingthepotentialsduringthestartupandshutdown pro-cesses,suchasbyapplying anexternal auxiliaryloadand by creatinganinternalshortinthefuelcells.
Incomparison withmaterialsimprovementbyusing graphi-tized carbon or non-carbon supports, system strategies are relativelysimpleandcheaptoimplementinrealfuelcellengines. Itisurgentlynecessarytosetuprelevantproceduresanddevices tomeetthedurabilityrequirementsofPEMFCs.
5. Concludingremarks
Performancedegradationduringstartupand shutdownisan importantissuethataffectsthedurabilityandlifetimeofPEMFCs. Thisreviewpaperhassurveyedandanalyzedthedurabilitytests, degradationmechanisms,andsystemstrategiesforthestartupand shutdownprocesses.
Thehighpotentialat thecathode, introducedbythe hydro-gen/airinterfaceattheanode,isthemajorcauseofperformance degradationof PEMFCs.A greatdeal ofwork hasbeen doneto developalternativenovelcatalystsupportsandsystemstrategies tomitigatecatalystdegradation.Currently,severalstrategieshave greatlyenhancedthedurabilityofPEMFCsduringthestartupand
shutdowncycles,suchasapplyingahydrogenpurgeandan auxil-iaryload.
Furtherworkis neededtoapplytheseeffectivestrategies in morefuelcellsystems.Bycombiningsystemstrategieswithnovel catalystsupportsthathavebettercorrosionresistance,the perfor-mancedegradationcausedbythestartupandshutdownprocesses canbeavoidedtoachievelonglifetimesthatcanmeetthedurability requirementsforPEMFCs.
Acknowledgements
YiYuthankstheChinaScholarshipCounciland theNational ResearchCouncilCanada(NRC)forfinancialsupportthroughthe NRC-MOEResearchandPost-doctoralFellowshipProgram. References
[1]W.Vielstich,H.Yokokawa,H.A.Gasteiger,HandbookofFuelCells: Fundamen-tals,Technology,andApplications.AdvancesinElectrocatalysis,Materials, DiagnosticsandDurability,Part1,Wiley,2009.
[2] R.Borup,J.Meyers,B.Pivovar,Y.S.Kim,R.Mukundan,N.Garland,D.Myers, M.Wilson,F.Garzon,D.Wood,ChemicalReviews107(2007)3904–3951. [3]Hydrogen, Fuel Cells & Infrastructure Technologies Program
Multi-Year Research, Development and Demonstration Plan, August 2006, http://www1.eere.energy.gov/hydrogenandfuelcells/mypp.
[4]T.Payne,FuelCellsDurability&Performances,TheKnowledgePressInc.,US Brookline,2009.
[5]P.Pei,Q.Chang,T.Tang,InternationalJournalofHydrogenEnergy33(2008) 3829–3836.
[6]J.Wu,X.Z.Yuan,J.J.Martin,H.Wang,J.Zhang,J.Shen,S.Wu,W.Merida, JournalofPowerSources184(2008)104–119.
[7]T.R.Ralph,PlatinumMetalsReview41(1997)102–113.
[8] J.St-Pierre,D.P.Wilkinson,S.Knights,M.L.Bos,JournalofNewMaterialsfor ElectrochemicalSystems3(2000)99–106.
[9]K.Washington,ProceedingsofFuelCellSeminar2000,2000,pp.468–472. [10]S.Y.Ahn,S.J.Shin,H.Y.Ha,S.A.Hong,Y.C.Lee,T.W.Lim,I.H.Oh,Journalof
PowerSources106(2002)295–303.
[11] M.W.Fowler,R.F.Mann,J.C.Amphlett,B.A.Peppley,P.R.Roberge,Journalof PowerSources106(2002)274–283.
[12]J.St-Pierre,N.Jia,JournalofNewMaterialsforElectrochemicalSystems5 (2002)263–271.
[13] O.Yamazaki,M.Echigo,T.Tabata,ProceedingsofFuelCellSeminar2002, 2002,pp.105–108.
[14]X.Cheng,L.Chen,C.Peng,Z.Chen,Y.Zhang,Q.Fan,Journalofthe Electro-chemicalSociety151(2004)A48–A52.
[15] J.Scholta,N.Berg,P.Wilde,L.Jörissen,J.Garche,JournalofPowerSources127 (2004)206–212.
[16]S.J.C.Cleghorn,D.K.Mayfield,D.A.Moore,J.C.Moore,G.Rusch,T.W.Sherman, N.T.Sisofo,U.Beuscher,JournalofPowerSources158(2006)446–454. [17]E.Endoh,H.Kawazoe,S.S.Honmura,ProceedingsofFuelCellSeminar2006,
2006,pp.284–287.
[18]C.Sishtla,G.Koncar,R.Platon,S.Gamburzev,A.J.Appleby,O.A.Velev,Journal ofPowerSources71(1998)249–255.
[19]T.Isono,S.Suzuki,M.Kaneko,Y.Akiyama,Y.Miyake,I.Yonezu,Journalof PowerSources86(2000)269–273.
[20]H.Meada,A.Yoshimura,H.Fukumoto,Proceedingsofthe2000FuelCell Seminar,2000,pp.379–400.
[21]T.Nakayama,Proceedingsofthe2000FuelCellSeminar,2000,pp.391–394. [22]S.Sakamoto,A.Fuju,K.Shindo,S.Yoshida,K.Nakato,N.Nishizawa,
Proceed-ingsofFuelCellSeminar2000,2000,pp.85–94.
[23]M.Fowler,J.C.Amphlett,R.F.Mann,B.A.Peppley,P.R.Roberge,JournalofNew MaterialsforElectrochemicalSystems5(2002)255–262.
[24]E.Cho,J.J.Ko,H.Y.Ha,S.A.Hong,K.Y.Lee,T.W.Lim,I.H.Oh,Journalofthe ElectrochemicalSociety151(2004)A661–A665.
[25]M.Oszcipok,D.Riemann,U.Kronenwett,M.Kreideweis,M.Zedda,Journalof PowerSources145(2005)407–415.
[26]J.Xie,D.L.WoodIii,D.M.Wayne,T.A.Zawodzinski,P.Atanassov,R.L.Borup, JournaloftheElectrochemicalSociety152(2005)A104–A113.
[27]J.Yu,T.Matsuura,Y.Yoshikawa,M.N.Islam,M.Hori,Electrochemicaland Solid-StateLetters8(2005)A156–A158.
[28]B.Du,R.Pollard,J.Elter,ProceedingsofFuelCellSeminar2006,2006,pp. 61–64.
[29]H.Xu,M.Wu,Y.Liu,V.Mittal,R.Vieth,H.R.Kunz,L.J.Bonville,J.M.Fenton, ECSTransactions3(2006)561–568.
[30]J.E.Owejan,P.T.Yu,R.Makharia,ECSTransactions11(2007)1049–1057. [31]W.Schmittinger,A.Vahidi,JournalofPowerSources180(2008)1–14. [32] S.Zhang,X.Z.Yuan,J.N.C.Hin,H.Wang,K.A.Friedrich,M.Schulze,Journalof
PowerSources194(2009)588–600.
[33]Y.Y.Jo,E.Cho,J.H.Kim,T.H.Lim,I.H.Oh,S.K.Kim,H.J.Kim,J.H.Jang,Journal ofPowerSources196(2011)9906–9915.
[34]Y.Y.Jo,E.A.Cho,J.H.Kim,T.H.Lim,I.H.Oh,J.H.Jang,H.J.Kim,International JournalofHydrogenEnergy35(2010)13118–13124.
[35]H.J.Kim,S.J.Lim,J.W.Lee,I.G.Min,S.Y.Lee,E.A.Cho,I.H.Oh,J.H.Lee,S.C.Oh, T.W.Lim,JournalofPowerSources180(2008)814–820.
[36]J.H.Kim,E.A.Cho,J.H.Jang,H.J.Kim,T.H.Lim,I.H.Oh,J.J.Ko,S.C.Oh,Journal oftheElectrochemicalSociety156(2009)B955–B961.
[37]J.H.Kim,E.A.Cho,J.H.Jang,H.J.Kim,T.H.Lim,I.H.Oh,J.J.Ko,S.C.Oh,Journal oftheElectrochemicalSociety157(2010)B104–B112.
[38]J.H.Kim,Y.YeonJo,E.A.Cho,J.H.Jang,H.J.Kim,T.H.Lim,I.H.Oh,J.J.Ko,I.J. Son,JournaloftheElectrochemicalSociety157(2010)B633–B642. [39]S.Y.Lee,E.Cho,J.H.Lee,H.J.Kim,T.H.Lim,I.H.Oh,J.Won,Journalofthe
ElectrochemicalSociety154(2007)B194–B200.
[40]Y.Takagi,Y.Takakuwa,ECSTransactions3(2006)855–860.
[41] Y.Ishigami,K.Takada,H.Yano,J.Inukai,M.Uchida,Y.Nagumo,T.Hyakutake, H.Nishide,M.Watanabe,JournalofPowerSources196(2011)3003–3008. [42] H.Tang,Z.Qi,M.Ramani,J.F.Elter,JournalofPowerSources158(2006)
1306–1312.
[43] Y.Yu,Z.Tu,H.Zhang,Z.Zhan,M.Pan,JournalofPowerSources196(2011) 5077–5083.
[44] T.W.Patterson,R.M.Darling,ElectrochemicalandSolid-StateLetters9(2006) A183–A185.
[45]F.Ettingshausen,J.Kleemann,A.Marcu,G.Toth,H.Fuess,C.Roth,FuelCells 11(2011)238–245.
[46]P.He,T.T.H.Cheng,R.Bashyam,A.P.Young,S.Knights,ECSTransactions33 (2010)1273–1279.
[47]C.A.Reiser,L.Bregoli,T.W.Patterson,J.S.Yi,J.D.Yang,M.L.Perry,T.D.Jarvi, ElectrochemicalandSolid-StateLetters8(2005)A273–A276.
[48]N.Yousfi-Steiner,P.Moc¸otéguy,D.Candusso,D.Hissel,JournalofPower Sources194(2009)130–145.
[49]D.Devilliers,É.Mahé,Cellulesélectrochimiques:Aspectsthermodynamiques etcinétiques:Applicationsauxgénérateursetauxélectrolyseursindustriels 13(2003)31–40.
[50]W.R.Baumgartner,P.Parz,S.D.Fraser,E.Wallnöfer,V.Hacker,Journalof PowerSources182(2008)413–421.
[51]J.Ihonen,F.Jaouen,G.Lindbergh,A.Lundblad,G.Sundholm,Journalofthe ElectrochemicalSociety149(2002)A448–A454.
[52]M.Kunimatsu,H.Qiao,T.Okada,JournaloftheElectrochemicalSociety152 (2005)E161–E166.
[53]G.Li,P.G.Pickup,ElectrochimicaActa49(2004)4119–4126.
[54] G. Li, P.G. Pickup, Electrochemical and Solid-State Letters 9 (2006) A249–A251.
[55]Z.Siroma,R.Kakitsubo,N.Fujiwara,T.Ioroi,S.I.Yamazaki,K.Yasuda,Journal ofPowerSources156(2006)284–287.
[56] Q.Shen,M.Hou,D.Liang,Z.Zhou,X.Li,Z.Shao,B.Yi,JournalofPowerSources 189(2009)1114–1119.
[57]R.A.Sidik,JournalofSolidStateElectrochemistry13(2009)1123–1126. [58]J.H.Ohs,U.Sauter,S.Maass,D.Stolten,JournalofPowerSources196(2011)
255–263.
[59] J.Kim,J.Lee,Y.Tak,JournalofPowerSources192(2009)674–678. [60]D.Liang,Q.Shen,M.Hou,Z.Shao,B.Yi,JournalofPowerSources194(2009)
847–853.
[61] M.Dou,M.Hou,D.Liang,Q.Shen,H.Zhang,W.Lu,Z.Shao,B.Yi,Journalof PowerSources196(2011)2759–2762.
[62]D.Liang,M.Dou,M.Hou,Q.Shen,Z.Shao,B.Yi,JournalofPowerSources196 (2011)5595–5598.
[63]S.Maass,F.Finsterwalder,G.Frank,R.Hartmann,C.Merten,JournalofPower Sources176(2008)444–451.
[64]S.VonDahlen,G.G.Scherer,A.Wokaun,I.A.Schneider,ECSTransactions33 (2010)1365–1374.
[65]I.A.Schneider,S.VonDahlen,ElectrochemicalandSolid-StateLetters14 (2011)B30–B33.
[66]P.T.Yu,W.Gu,R.Makharia,F.T.Wagner,H.A.Gasteiger,ECSTransactions3 (2006)797–809.
[67] J.P.Meyers,R.M.Darling,JournaloftheElectrochemicalSociety153(2006) A1432–A1442.
[68]N.Takeuchi,T.F.Fuller,JournaloftheElectrochemicalSociety157(2010) B135–B140.
[69]J.Hu,P.C.Sui,S.Kumar,N.Djilali,ECSTransactions11(2007)1031–1039. [70]Z. Qi, H. Tang, Q. Guo, B. Du, Journal of Power Sources 161 (2006)
864–871.
[71]W.Gu, R.N.Carter,P.T. Yu,H.A. Gasteiger,ECS Transactions11(2007) 963–973.
[72]X.Wang,K.Tajiri,R.K.Ahluwalia,JournalofPower Sources195(2010) 6680–6687.
[73]J.Hu,P.C.Sui,S.Kumar,N.Djilali,ElectrochimicaActa54(2009)5583–5592. [74]A.B.Ofstad,J.R.Davey,S.Sunde,R.L.Borup,ECSTransactions16(2008)
1301–1311.
[75]K.D.Baik,M.S.Kim,InternationalJournalofHydrogenEnergy36(2011) 732–739.
[76]J.Crank,TheMathematicsofDiffusion,ClarendonPress,Oxford,1970. [77]H.Sun,G.Zhang,L.J.Guo,H.Liu,JournalofPowerSources158(2006)326–332. [78]H.Sun,G.Zhang,L.J.Guo,S.Dehua,H.Liu,JournalofPowerSources168(2007)
400–407.
[79] T.Hottinen,M.Noponen,T.Mennola,O.Himanen,M.Mikkola,P.Lund,Journal ofAppliedElectrochemistry33(2003)265–271.
[81]Z.Liu,Z.Mao,B.Wu,L.Wang,V.M.Schmidt,JournalofPowerSources141 (2005)205–210.
[82]Z.Liu,L.Yang,Z.Mao,W.Zhuge,Y.Zhang,L.Wang,JournalofPowerSources 157(2006)166–176.
[83]F.Cocuret,IngénieriedesProcédesElectrochimiqucs(2003).
[84]M.Carmo,V.A.Paganin,J.M.Rosolen,E.R.Gonzalez,JournalofPowerSources 142(2005)169–176.
[85] E.P.Ambrosio,C.Francia,C.Gerbaldi,N.Penazzi,P.Spinelli,M.Manzoli,G. Ghiotti,JournalofAppliedElectrochemistry38(2008)1019–1027. [86] E.Antolini,AppliedCatalysisB:Environmental88(2009)1–24. [87]M.L.Perry,T.W.Patterson,C.Reiser,ECSTransactions3(2006)783–795. [88] Y.Shao,G.Yin,Y.Gao,JournalofPowerSources171(2007)558–566. [89]L.M.Roen,C.H.Paik,T.D.Jarvi,ElectrochemicalandSolid-StateLetters7
(2004)A19–A22.
[90] J.Willsau,J.Heitbaum,JournalofElectroanalyticalChemistry161(1984) 93–101.
[91]T.R.Ralph,S.Hudson,D.P.Wilkinson,ECSTransactions1(2005)67–84. [92]P.J.Ferreira,G.J.LaO,Y.Shao-Horn,D.Morgan,R.Makharia,S.Kocha,H.A.
Gasteiger,JournaloftheElectrochemicalSociety152(2005)A2256–A2271. [93] A.V. Virkar,Y. Zhou,Journalof theElectrochemicalSociety 154 (2007)
B540–B547.
[94] K.L.More,R.Borup,K.S.Reeves,ECSTransactions3(2006)717–733. [95]K.J.J.Mayrhofer,J.C.Meier,S.J.Ashton,G.K.H.Wiberg,F.Kraus,M.Hanzlik,M.
Arenz,ElectrochemistryCommunications10(2008)1144–1147.
[96]X.Wang,W.Li,Z.Chen,M.Waje,Y.Yan,JournalofPowerSources158(2006) 154–159.
[97]M.M.Waje,W.Li,Z.Chen,Y.Yan,ECSTransactions3(2006)677–683. [98] Y.Shao,G.Yin,Y.Gao,P.Shi,JournaloftheElectrochemicalSociety153(2006)
A1093–A1097.
[99]K.Lee,J.Zhang,H.Wang,D.P.Wilkinson,JournalofAppliedElectrochemistry 36(2006)507–522.
[100] A.Smirnova,X.Dong,H.Hara,N.Sammes,JournalofFuelCellScienceand Technology3(2006)477–481.
[101]N.Job,J.Marie,S.Lambert,S.Berthon-Fabry,P.Achard,EnergyConversion andManagement49(2008)2461–2470.
[102] C.D.Saquing,D.Kang,M.Aindow,C.Erkey,MicroporousandMesoporous Materials80(2005)11–23.
[103]H.Chhina,S.Campbell,O.Kesler,JournaloftheElectrochemicalSociety154 (2007)B533–B539.
[104] H.Chhina,S.Campbell,O.Kesler,JournalofPowerSources179(2008)50–59. [105] H.Chhina,S.Campbell,O. Kesler,JournalofPowerSources161 (2006)
893–900.
[106]N.Rajalakshmi,H.Ryu,M.M.Shaijumon,S.Ramaprabhu,JournalofPower Sources140(2005)250–257.
[107] T.Ioroi,H.Senoh,S.I.Yamazaki,Z.Siroma,N.Fujiwara,K.Yasuda,Journalof theElectrochemicalSociety155(2008)B321–B326.
[108]Y.Suzuki,A.Ishihara,S.Mitsushima,N.Kamiya,K.I.Ota,Electrochemicaland Solid-StateLetters10(2007)B105–B107.
[109] M.E.Gorman,U.S.Patent6,127,057(2000).
[110]D.Yang,M.M.Steinbugler,R.D.Sawyer,L.L.V.Dine,C.A.Reiser,U.S.Patent Appl.0102443A1(2002).
[111] L.L.V.Dine,M.M.Steinbugler,C.A.Reiser,G.W.Scheffler,U.S.Patent6,514,635 B2(2003).
[112]D.A.Condit,R.D.Breault,U.S.Patent6,635,370(2003). [113]R.J.Balliet,C.A.Reiser,U.S.Patent6,835,479(2004). [114]M.L.Perry,P.R.Margiott,U.S.Patent6,821,668(2004).
[115]P.R.Margiott,C.W.Callahan,M.L.Perry,G.W.Scheffler,U.S.Patent6,828,048 B2(2004).
[116]C.A.Reiser,D.Yang,R.D.Sawyer,U.S.Patent6,858,336B2(2005). [117]J.H.Whiton,T.J.Anderson,R.J.Guthrie,U.S.Patent6,924,056B2(2005). [118]T.A.Bekkedahl,L.J.Bregoli,R.D.Breault,E.A.Dykeman,J.P.Meyers,T.W.
Pat-terson,T.Skiba,C.Vargas,D.Yang,J.S.Yi,U.S.Patent6,913,845B2(2005). [119]R.J.Balliet,C.A.Reiser,T.W.Patterson,M.L.Perry,U.S.Patent6,838,199B2
(2005).
[120]C.A.Reiser,D.Yang,R.D.Sawyer,U.S.Patent6,887,599B2(2005).
[121] D.Yang,M.M.Steinbugler,R.D.Sawyer,L.L.V.Dine,C.A.Reiser,U.S.Patent Appl.0093879A1(2006).
[122] P.R.Margiott,F.R.Preli,G.W.Kulp,M.L.Perry,C.A.Resier,R.J.Balliet,U.S.Patent 6,984,464B2(2006).
[123] M.L.Perry,U.S.PatentAppl.0223495A1(2011).
[124] W.H.Pettit,S.G.Goebel,U.S.PatentAppl.0136304A1(2005). [125] S.G.Goebel,U.S.PatentAppl.0221148A1(2005).
[126] S.G.Goebel,U.S.PatentAppl.0058859A1(2005). [127] P.T.Yu,F.T.Wagner,U.S.PatentAppl.0046106A1(2006). [128] P.T.Yu,F.T.Wagner,U.S.PatentAppl.0040150A1(2006).
[129] P.T.Yu,F.T.Wagner,G.W.Skala,B.Lakshmanan,J.P.Salvador,U.S.PatentAppl. 0145716A1(2008).
[130] P.T.Yu,F.T.Wagner,U.S.PatentAppl.0003465A1(2008). [131] P.T.Yu,U.S.PatentAppl.0145717A1(2008).
[132] S.Swathirajan,B.Merzougui,P.T.Yu,U.S.PatentAppl.0166599A1(2008). [133] S.G.Yan,H.A.Gasteiger,P.T.Yu,W.Gu,J.Zhang,U.S.PatentAppl.0068541A1
(2009).
[134] T.W.Tighe,S.G.Goebel,G.M.Robb,A.B.Alp,B.Lakshmanan,J.N.Lovria,U.S. PatentAppl.0143241A1(2011).
[135] H.Tang,Z.Qi,M.Ramani,K.M.Rush,L.F.Wong,N.Miklas,U.S.PatentAppl. 0154742A1(2007).
[136] S.D.Knights,D.P.Wilkinson,S.A.Campbell,J.L.Taylor,J.M.Gascoyne,T.R. Ralph,U.S.Patent6,936,370(2005).
[137] U.M.Limbeck,R.Holger,PatentCA2517627(2006).
[138] B.Janusz,S.David,C.Anthony,H.Andrew,F.Richard,H.Steven,D.Michael, PatentWO2007044971A1(2007).
[139] C.Paik,J.A.Adams,G.S.Saloka,M.S.Sulek,U.S.PatentAppl.0023040A1 (2009).
[140] K.Mitsuhiro,S.Kazuo,M.Akira,S.Ryoichi,F.Takahiro,Y.Kenji,I.Takashi,T. Yasuhiro,K.Mitsunori,PatentJP2009181793(2009).
[141] Y.Masanari,PatentJP2006155917(2006).
[142] T.Yasuhiro,M.Shinichi,S.Hiromasa,PatentJP2005158557(2005). [143] B.Nicolae,B.Francine,F.Richard,H.Herwig,H.Yvonne,L.Andrew,P.Guy,R.
Joy,Y.A.Shunwen,PatentWO2011076396(2011). [144] S.Joerg,W.T.Markus,PatentDE102007048317(2009). [145] S.Seiji,M.Hiroshi,U.S.Patent2010081016A1(2010). [146] X.Gang,PatentWO2009031444(2009).
[147] S.Tsutomu,PatentWO2009010857(2009). [148] K.Hideaki,PatentWO2008146122(2008).
[149] O.S.Chan,L.J.Hyun,Y.J.Jin,K.Jun,K.Y.Min,S.I.Jae,PatentDE102007057488 (2008).
[150] O.S.Chan,PatentKR100821771(2008).
[151] T.M.K.Thampan,R.Benesch,U.S.PatentAppl.0248044A1(2010). [152] A.Knoop,U.S.PatentAppl.0045368A1(2011).
[153] T.Kamiyama,N.Nakajima,T.Yamashiro,K.Miyata,U.S.PatentAppl.0209793 A1(2010).
[154] M.V.Scotto,D.P.Birmingham,C.L.DeBellis,M.A.Perna,G.C.Rush,U.S.Patent Appl.0059376A1(2011).
[155] M.V.Scotto,D.P.Birmingham,C.L.DeBellis,M.A.Perna,G.C.Rush,U.S.Patent Appl.0059377A1(2011).
[156] D.P.Miller,J.A.Rock,C.M.Marsiglio,U.S.PatentAppl.0220294A1(2008). [157] D.M.Suh,U.S.PatentAppl.0228594A1(2006).
[158] G.W.Kulp,R.D.Breault,U.S.PatentAppl.0142399A1(2005).
[159] M. Ramani, R.A. Dross, L. Mao, B. Du, U.S. Patent Appl. 0035098 A1 (2010).
[160] S.C.Oh,J.H.Lee,J.J.Yoon,J.J.Ko,Y.M.Kim,I.J.Son,U.S.PatentAppl.0318099 A1(2008).
[161] P.Charlat,U.S.PatentAppl.0058167A1(2002).
[162] J.F.McElroy,D.T.Davis,M.D.Gasda,U.S.PatentAppl.0154752A1(2007). [163] U.M.Limbeck,M.Aberle,C.R.Louie,A.E.Nelson,U.S.PatentAppl.0258256A1
(2009).
[164] I.Noboru,I.Fumiharu,Y.Kazutaka,S.Takashi,O.Toru,Y.Takamasa,Patent JP2010055884(2010).
[165] K.Shigemi,W.Takanori,PatentJP2009117238(2009). [166] Y.Tomonori,Y.Naoya,PatentJP2008243500(2008).