S1
Supporting Information
Poly-Carboxylated Dextran as a Multivalent Crosslinker:
Synthesis and Target Recognition of the Antibody-Nanoparticle
Bioconjugates in PBS and Serum.
Filip Kunc
a, Colin Moore
b, Rachel E. Sully
c, Andrew J. Hall
cand Vladimir
Gubala
c*a) National Research Council Canada, 100 Sussex Drive, Ottawa, Ontario,
K1N 0R6, Canada
b) Italian Institute of Technology, 30 Via Morego, 16163 Genoa, Italy
c) Medway School of Pharmacy – Universities of Greenwich and Kent, Anson
Building, Central Avenue, Chatham ME4 4TB, UK
*Corresponding author. E-mail:
V.Gubala@kent.ac.uk
; Tel: +44-1634-202952
Pages: 4
Figures: 3
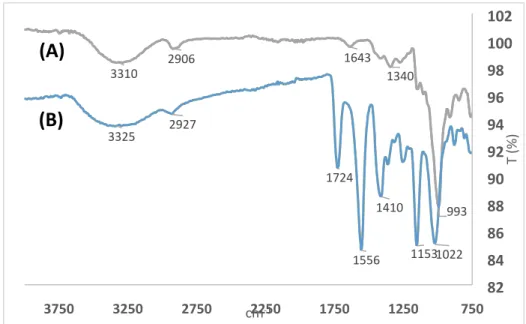
S2 Characterization of Dextran 40 kDa modification with succinic anhydride
Figure S1: 13C NMR spectra and a proposed reaction scheme of 3-bromopropionic side reaction
observed after the attempt to use it for 40 kDa dextran carboxylation.
3310 2906 1643 1340 3325 2927 1724 1556 1410 11531022 993 750 1250 1750 2250 2750 3250 3750 82 84 86 88 90 92 94 96 98 100 102 cm-1 T ( % )
(A)
(B)
Figure S2: FTIR spectra of plain 40 kDa dextran before the chemical modification (a), and ‘40Dex-2×’ derivative modified with 2 × molar excess of succinic anhydride to hydroxyl groups.
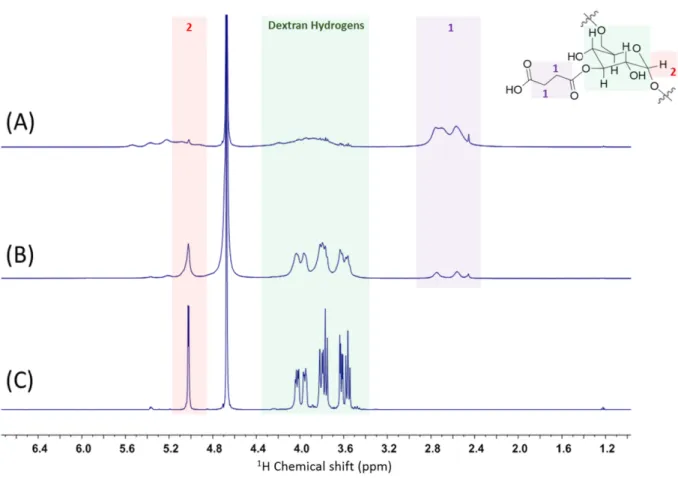
S3 Figure S3: 1H NMR spectra of 40 kDa Dextran (a) 40Dex-0.1× (b), and 40Dex-2× (c). All measurements
were conducted at concentration 30 mg/mL in D2O at 40 ˚C (100 scans, 5 s relaxation delay). (ADD
STRUCTURES)
Table S1: The study of various succinic anhydride excesses in reaction with 40 kDa plain dextran. The estimates from 1H NMR spectra are based on the comparison of the integrals corresponding to the
anomeric hydrogen at 5.02 ppm and new resonances at 2.56 and 2.75 ppm corresponding to the succinate moiety. Product Name No of hydroxyl groups per dextran No of SA molecules per dextran in reaction No of attached SA molecules per dextran (gravimetric estimate) No of attached SA molecules per dextran (Acid-Base Titration) 40Dex-0.1× 667 67 28 19 40Dex-2× 667 1330 345 129 40Dex-5× 667 3340 416 51
S4 Table S2: S/N value represents the signal-to-noise ratio. It is measured as a ratio between signal
intensities representing specific binding ‘signal’ and signals representing non-specific, background ‘noise’. S/N PBS Whole serum Adsorption 9.5 11 PAMAM 32 52 6Dex 81 22 40Dex 54 12 70Dex 59 1.1