The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters.
Citation Neisius, Ulf et al. "Aortic regurgitation assessment by
cardiovascular magnetic resonance imaging and transthoracic echocardiography: intermodality disagreement impacting on prediction of post-surgical left ventricular remodeling."
International Journal of Cardiovascular Imaging 36, 1 (August 2019): 91–100. © 2019 Springer Nature B.V.
As Published https://doi.org/10.1007/s10554-019-01682-x
Publisher Springer Science and Business Media LLC
Version Author's final manuscript
Citable link https://hdl.handle.net/1721.1/128533
Terms of Use Article is made available in accordance with the publisher's
policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use.
Author accepted manuscript
tricular remodeling
Cite this article as: Ulf Neisius, Connie Tsao, Thomas H. Hauser, Apranta D. Patel, Patrick Pierce, Eyal Ben-Assa, Reza Nezafat and Warren J. Manning, Aortic regurgitation assess-ment by cardiovascular magnetic resonance imaging and transthoracic echocardiography: intermodality disagreement impacting on prediction of post-surgical left ventricular remod-eling, The International Journal of Cardiovascular Imaging https://doi.org/10.1007/s10554-019-01682-x
This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Terms of use and reuse: academic research for non-commercial purposes, see here for full terms.https://www.springer.com/aam-terms-v1
Author accepted manuscript
Aortic regurgitation assessment by cardiovascular magnetic resonance imaging and transthoracic echocardiography: intermodality disagreement impacting on
prediction of post-surgical left ventricular remodeling
Ulf NeisiusPhD1, Connie Tsao MD1, Thomas H. HauserMD1, Apranta D. Patel MD1, Patrick PierceRT(MR)1, Eyal Ben-Assa MD1,2,
Reza Nezafat PhD1, Warren J. Manning MD1,3
1
Departments of Medicine (Cardiovascular Division) and 3Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, USA
2
Institute of Medical Engineering and Science, Massachusetts Institute of Technology, 77 Massachusetts Ave, Cambridge, USA
Address for correspondence: Warren J Manning, MD
Department of Medicine (Cardiovascular Division) Beth Israel Deaconess Medical Center
330 Brookline Ave Boston, MA 02215 Tel.: +1 (617) 667-2192 Fax: +1 (617) 975-5480
Email: wmanning@bidmc.harvard.edu
Word Count (Manuscript): 2861/4000 Acknowledgments:
Author accepted manuscript
The authors thank Beth Goddu, RT(MR) and Sophie Berg, RN for their help with CMR scanning and patient management.
Author accepted manuscript
Abstract
Purpose Transthoracic echocardiography (TTE) is the primary clinical imaging modality
for the assessment of patients with isolated aortic regurgitation (AR) in whom TTE’s linear left ventricular (LV) dimension is used to assess disease severity to guide aortic valve replacement (AVR), yet TTE is relatively limited with regards to its integrated semi-quantitative/qualitative approach. We therefore compared TTE and cardiovascular magnetic resonance (CMR) assessment of isolated AR and investigated each modality’s ability to predict LV remodeling after AVR.
Methods AR severity grading by CMR and TTE were compared in 101 consecutive
patients referred for CMR assessment of chronic AR. LV end-diastolic diameter and end-systolic diameter measurements by both modalities were compared. Twenty-four patients subsequently had isolated AVR. The pre-AVR estimates of regurgitation severity by CMR and TTE were correlated with favorable post-AVR LV remodeling.
Results AR severity grade agreement between CMR and TTE was moderate (=0.317,
P=0.001). TTE underestimated CMR LV end-diastolic and LV end-systolic diameter by
6.6 mm (P<0.001, CI 5.8-7.7) and 5.9 mm (P<0.001, CI 4.1-7.6), respectively. The correlation of post-AVR LV remodeling with CMR AR grade (=0.578, P=0.004) and AR volumes (R=0.664, P<0.001) was stronger in comparison to TTE (=0.511, P=0.011; R=0.318, P=0.2).
Conclusion In chronic AR, CMR provides more prognostic relevant information than
TTE in assessing AR severity. CMR should be considered in the management of chronic AR patients being considered for AVR.
Author accepted manuscript
Word Count (Abstract): 228/250Keywords: aortic regurgitation, aortic valve surgery, observer variation, remodeling, magnetic resonance imaging, transthoracic echocardiography.
Author accepted manuscript
Abbreviations
AR aortic regurgitation
ASE American Society of Echocardiography AVR aortic valve replacement
CMR cardiovascular magnetic resonance
LVEDD change of LVEDD
LVEDV change of LVEDV
LVESV change of LVESV
ICC Intraclass correlation coefficient LV left ventricle/left ventricular LVEDD LV end-diastolic diameter LVEDV LV end-diastolic volume LVESD LV end-systolic diameter LVESV LV end-systolic volume
RV Right ventricle/right ventricular TTE transthoracic echocardiography
Author accepted manuscript
Introduction
Transthoracic echocardiography (TTE) is the clinical standard to assess aortic regurgitation (AR) severity [1]. Contemporary guidelines [1, 2] recommend cardiovascular magnetic resonance imaging (CMR) in patients with significant disease and suboptimal TTE images, which acknowledges CMR’s superior capacity to quantify AR volume and regurgitant fraction by direct measurement of aortic blood flow [3, 4] and to accurately compare right ventricular (RV) and left ventricular (LV) stroke volumes [3]. However the cited evidence is predominantly based on prospective recruitment of unselected patients assessed by TTE [5] and the investigation of a patient cohort where CMR assessment of AR severity was deemed clinically necessary could provide further evidence.
Accurate quantification of AR severity and LV dimensions is crucial in this patient cohort considering the recommendation for aortic valve replacement (AVR) in asymptomatic patients with severe AR with a reduced LV ejection fraction or increased LV dimensions [1, 6]. The improved reproducibility of AR volume and regurgitant fraction measurements by CMR [5] and CMR being the non-invasive gold-standard of ventricular volumes and systolic function assessment [7], indicate a potential routine diagnostic role of CMR for anatomical LV assessment in these patients. In the absence of CMR based evidence and taking into account that CMR and TTE LV dimension measurements can differ [8], both modalities’ relationship of LV dimensions is relevant for CMR interpretation.
Author accepted manuscript
identified a group of patients referred for CMR assessment of chronic AR to compare TTE and CMR AR grading as well as LV dimension measurements. We hypothesized that CMR grading would be a better predictor of favorable LV remodeling [9] after AVR in this cohort and as such would provide evidence for contemporary guideline recommendations [1, 2].
Material and Methods
One-hundred and one consecutive patients referred for CMR to assess isolated chronic AR severity and who had a contemporary (<6 months) TTE were identified from our electronic CMR reporting database. All CMR scans were conducted at Beth Israel Deaconess Medical Center, Boston, US between July 2001 and November 2017. The cohort contained no patient with more than mild aortic stenosis or primary cardiomyopathies. For post-surgical LV-remodeling assessment, the time interval was extended to 12 months. Cases with more than mild mitral regurgitation (n=16) were excluded. Surgery was indicated in the context of aortic root dilatation or isolated AR. The study was approved by the hospital’s Committee for Clinical Investigations/Institutional Review Board which waived informed consent.
TTE. TTE was conducted with conventional two-dimensional guided color flow and
pulse-wave Doppler imaging in accordance with American Society of Echocardiography guidelines [2]. AR severity was assessed by an integrated approach [1, 6] and interpreted by a level 2 or 3 trained reader who had passed the National Board of Echocardiography Special Competence in Echocardiography examination. AR was graded on TTE images according to American Heart Association/American College of
Author accepted manuscript
Cardiology guidelines [1]. TTE files were re-evaluated to allow interobserver agreement (C.T., 10 years of TTE experience), LV volume (Simpson’s biplane method) assessment, and AR volume assessment (U.N., 11 years of TTE experience). The latter was calculated based on comparison of mitral valve with LV outflow tract stroke volume by pulse wave Doppler or the proximal isovelocity surface area technique, as appropriate. Proximal isovelocity surface area was measured in the apical five-chamber or parasternal long axis view with lower Nyquist limit set at 40 cm/s [2]. Peak AR jet velocity and velocity time integral were determined using continuous-wave Doppler across the aortic valve.
CMR. CMR was performed using a commercial 1.5T scanner (Achieva, Philips
Healthcare, Best, The Netherlands) equipped with a 5- or 32-element cardiac coil. Breath-hold, retrospectively electrocardiogram-gated cine, balanced steady state free-precision images were acquired with in the LV 2- and 4-chamber long-axis views, and a short-axis stack covering the entire LV (8-mm slices with 2-mm gaps), as previously described [10]. A free breathing, electrocardiogram-triggered, phase-contrast velocity-encoded CMR sequence of the cross-sectional aorta was acquired in the axial plane at the level of the pulmonary artery bifurcation. Sequence parameters were repetition time 15 ms, echo time 6.5 ms, flip angle 30°, field-of-view 320 x 210 mm2, matrix 120x120. Respiratory motion compensation was accomplished with the use of multiple signal averages (NSA=4).
Image analysis. CMR images were analyzed using ViewForm software (Release 4,
Philips Healthcare). LV and RV volumes were evaluated by manual segmentation of the short-axis CMR images. LV dimensions were measured in the short-axis view at the
Author accepted manuscript
level of the chordae. LV volumes were measured by manual tracing the end-diastolic and end-systolic endocardial contours in each slice and applying a summation of discs method. The LV ejection fraction was calculated as: [LV end-diastolic volume (LVEDV) – LV end-systolic volume (LVESV)] / LVEDV. Aortic flow values were measured with the resident semi-automated algorithm. AR volume was directly calculated from the aortic flow curve by integrating the diastolic reverse flow. AR fraction was taken from the phase contrast data as the ratio of AR volume and LV stroke volume. In addition to CMR categories suggested by Gelfand et al. (mild, 15%; moderate, 16 to <26%; moderate-severe, 26 to 48%; severe, >48%) [10], AR fraction was categorized using TTE thresholds as recommended by the American Society of Echocardiography/Society for Cardiovascular Magnetic Resonance (ASE/SCMR) (mild, <30%; moderate, 30 to <50%; severe 50%) [2]. Threshold values best reflecting on TTE AR grades were used for subsequent analyses. Readers blinded to the patients’ clinical data and the initial study interpretation assessed the interobserver variability of AR severity in a subset of 60 (TTE) and 43 (CMR) patients.
Statistical Analysis. Data were analyzed using SPSS software (version 25.0,
International Business Machines Corp., Armonk, New York, USA). Normality of data distribution was determined using the Kolmogorov-Smirnov test and visual inspection of Q-Q plots. The two-sample Student’s t test or the Mann Whitney U-test were conducted as appropriate for comparison of continuous variables between groups. For comparison of categorical data, the Chi-squared test was employed. Linear regression analysis (Pearson correlation) was used to assess the association between LV dimensions measured by TTE or CMR followed by Bland-Altman plots comparison. Spearman’s
Author accepted manuscript
rank-order correlation was used to evaluate the relationship between AR categories and continuous variables. Correlation coefficients were compared using Fisher’s Z-test. The intraclass correlation coefficient (ICC) for a 2-way random-effects model with absolute agreement was calculated to assess the concordance between TTE and CMR for AR severity quantification, as well as the interobserver reproducibility. ICCs were categorized as excellent (ICC≥0.75), good (ICC 0.6-0.74), fair (ICC 0.4-0.59) or poor (ICC<0.40) [11, 12]. One-way analysis of variance or the Kruskal-Wallis test by ranks was used as appropriate to compare continuous variables among multiple groups. A 2-sided P<0.05 was considered statistically significant.
Results
Subject characteristics and CMR findings. The clinical characteristics and baseline
TTE/CMR parameters are summarized in Table 1 and Supplementary Table 1. Of the 101 chronic AR patients and the 24 patients proceeding to AVR, 84% and 100% had single-valve disease (Table 1), 40% and 46% had eccentric AR jets (Figure 1) and 37% and 42% had bicuspid aortic valves, respectively. The median time between TTE and CMR was 44 days (range 0 – 181) for AR and 37 days (range 0-210) for AVR patients.
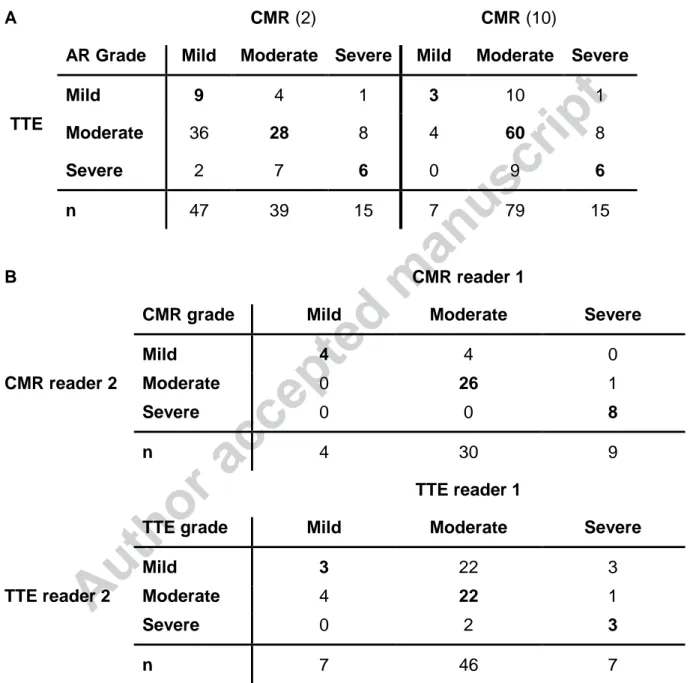
Intermodality and -observer variability. The comparison of CMR (ASE/SCMR or
Gelfand thresholds) and TTE AR grading is shown in Table 2A. For ASE/SCMR criteria, there was agreement with TTE in 43%, whilst Gelfand criteria had an agreement with TTE in 69% of patients (P<0.001). Subsequent analyses were therefore performed with the latter. There was a modest but significant correlation between TTE and CMR grades (=0.314, P=0.001; ICC=0.478, 95% CI 0.227-0.647, P=0.001). The interobserver
Author accepted manuscript
variabilities of AR grades by TTE and CMR are shown in Table 2B. CMR’s reproducibility was excellent (ICC 0.908, 95% CI 0.821-0.951, P<0.001), whilst TTE reproducibility was poor (ICC 0.229, 95% CI -0.181-0.513, P=0.113).
AR grading association with LV dilatation. LV volume indexes by CMR were
correlated with AR volume (LVEDV/Body Surface Area, R=0.699; LVESV/body surface Area, R=0.492, P<0.001 for both) and AR fraction (LVEDV/body surface area, R=0.599; LVESV/body surface area, R=0.462, P<0.001 for both) (Suppl. Figure 1). CMR AR severity better reflected CMR baseline LV dilatation (LVEDV, 179 ml [166;201], 238 ml [202;282], 342 ml [270;367], P<0.001; LVESV, 70 ml [49;79], 100 ml [83; 131], 153 ml [125;178], P<0.001) in comparison to TTE AR severity (LVEDV, 208 ml [173;244], 236 ml [201;286], 315 ml [253;367], P<0.001; LVESV, 82 ml [64;115], 101 ml [82;137], 153 ml [113;181], P=0.004) (Supplementary Table 1). The correlation between CMR (vs TTE) AR grade and these markers was stronger (LVEDV, =0.468 vs. =0.389, both
P<0.001; LVESV, =0.438 vs. =0.327, both P<0.001).
Comparison of CMR and TTE. LV dimensions and AR volumes. TTE and CMR
measures of LV end-diastolic dimension (LVEDD) and LV end-systolic dimension (LVESD) correlated moderately, R=0.795 and R=0.613 (P<0.001 for both), respectively. Both CMR LVEDD and LVESD were greater than TTE measurements (LVEDD 658 mm vs. 597 mm, P<0.001; LVESD 448 mm vs. 387 mm, P<0.001). LVEDD and LVESD values were larger by CMR in 94% (94 of 99) and 85% (47 of 56) of cases, respectively (Figure 2).
Author accepted manuscript
LV remodeling after AVR. Twenty-four patients underwent TTE follow-up after single
valve correction (surgical AVR, n=21; transcutaneous AVR, n=1; aortic valve resuspension after aortic root repair, n=2). Nine (38%) of these patients had pre-operative severe AR by CMR and 5 (21%) had severe AR by TTE. The median time interval between presurgical TTE or CMR and AV surgery was 126 days (range 21 -320) and 72 days (range 16 – 304), respectively. The median time interval between AVR and post-surgical TTE was 116 days (range 34 – 554). The post-surgical decrease in TTE (biplane Simpson or Teichholtz formula) LVEDV (∆LVEDV), LVESV (∆LVESV) and LVEDD (LVEDD) was 6145 ml, 2927 ml and 98 mm, respectively. Post-AVR LV changes correlated with CMR AR volume (∆LVEDV, R=0.667, P<0.001; ∆LVESV, R=0.474, P=0.022; ∆LVEDD, R=0.463, P=0.023) and AR fraction (∆LVEDV, R=0.626,
P=0.001; ∆LVESV, R=0.477, P=0.021; ∆LVEDD, R=0.593, P=0.002) (Figure 3). The
∆LVEDV was different between patients with moderate and severe AR by CMR (6) (P=0.004) but not for TTE (P=0.057). The correlation of post-surgical LV changes with CMR grades (∆LVEDV, =0.578, P=0.003; ∆LVESV, =0.620, P=0.002; LVEDD,
=0.529, P=0.008) was stronger in comparison to TTE (∆LVEDV, =0.511, P=0.011; ∆LVESV, =0.484, P=0.019; LVEDD, =0.349, P=0.095). Figure 3 also shows no correlation between AR volume by TTE and LVEDV (R=0.318, P=0.2) or LVESV (R=0.224, P=0.442).
Discussion
The current retrospective study aimed to compare TTE and CMR assessment of chronic AR, hypothesizing that CMR has numerous advantages. The results provide several
Author accepted manuscript
important insights into multimodality AR assessment: (1) there is frequent disagreement between TTE and CMR grading; (2) the threshold values of AR volume and AR fraction differ between TTE and CMR; (3) LV dimensions by TTE underestimate CMR measurements; and (4) CMR AR grades are numerically stronger associated with post-surgical LV remodelling, which supports a role for multimodality imaging in pre-AVR assessment.
Disagreement between TTE and CMR AR garding. We found only a modest
agreement between TTE and CMR AR grading. Of the 15 patients with severe AR by TTE, only 6 (40%) had severe AR by CMR, independent of the two CMR AR grading system used. There are numerous explanations for this finding. Popovic et al. summarized the caveats of TTE, such as jet width underestimation, measurement error accumulation when calculating the regurgitation volume based on LV outflow tract and mitral valve inflow volumes, and difficulties to assess the proximal regurgitant isovelocity surface area, just to name a few [13]. These may explainthe considerable interobserver variability for TTE AR grading observed in our study. Related, the interobserver variability of AR relevant parameters [5, 14], and a referral bias towards inconclusive TTE cases possibly affected our results. Considering the excellent reproducibility of CMR AR grading in our cohort and other retrospective [15] and prospectively recruited patients [5, 16], our findings provide further support for the contemporary recommendation to proceed with CMR in cases of suboptimal TTE assessment [1, 2].
CMR specific cut-off values for AR grading. In the absence of sufficient CMR
outcome data on AR patients, the ASE/SCMR recommends thresholds for CMR AR grading by AR volume or AR fraction equivalent to TTE cut-off values [2]. As we have
Author accepted manuscript
shown, this leads to frequent mismatch between moderate AR by TTE and mild AR by CMR (n=36, 50% of all moderate AR by TTE). Considering the clinical importance of this differentiation, CMR specific cut-off values appear better suited to provide reasonable overlap with TTE grading [10, 17, 18]. Accordingly, AR volume or AR fractionvalues allowing optimal prediction of AVR in small cohorts of asymptomatic AR patients were found 20 ml or 17% lower than the TTE threshold for severe AR [19, 20]. Similarly, Polte et al. [21] found CMR AR volume >40 ml and AR fraction >30% to be the best discriminator between patients with severe AR qualifying for guideline recommended surgery [6] and patients with moderate AR, both assessed by TTE.
Differences in LV dimension by TTE and CMR. Noteworthy for the clinical application
of CMR is also the systematic relative underestimation of LVEDD and LVESD by TTE in our AR cohort. As supported by clinical recommendation [22, 23], TTE and CMR assessment of LVEDD and LVESD differ amongst others in location (tip of the mitral valve leaflets vs. basal to the tips of papillary muscles), timing (LVEDD: maximal diameter on CMR vs. onset of the R-wave on TTE; LVESD: minimal diameter vs. minimal cross-sectional area), and image axis (parasternal short axis vs. short axis). Regarding the latter, Puntmann et al. observed a similar relationship between CMR and TTE parameters where LV dimensions on parasternal long axis CMR images were smaller and more conform with TTE than those on short axis images [8]. Contemporary guidelines recommend (Class IIa-b; Level B-C) AVR in asymptomatic patients with LVESD >5.0 cm [1, 6] or LVEDD >6.5 cm [1]/ >7.0 cm [6] based on TTE data. Our findings suggest that CMR and TTE measurements in agreement with the contemporary clinical standard are not directly interchangeable in the treatment decision process.
Author accepted manuscript
Association between Post-surgical LV Remodelling and AR grading. The
integrative TTE and quantitative CMR approach are both recommended for assessment of AR severity [1, 2]. It remains uncertain which modality is more accurate. In our cohort proceeding with AVR, we observed a stronger correlation of LV remodeling with AR severity and AR volume measured by CMR. Acknowledging multiple influences on LV remodeling [24, 25], the linear relationship between LV volume and AR volume or AR fraction [15] and LVEDD, LVESD and LV ejection fraction parameters being in the normal range in the majority of our patients indicates a significant contribution of AR removal. The superior remodeling prediction of CMR AR grading and AR volume is also consistent with findings in patients with isolated mitral regurgitation proceeding to mitral valve surgery published by Uretsky et al. [26]. Furthermore, CMR assessment of asymptomatic patients with organic mitral regurgitation can better predict adverse outcome than TTE derived parameters [27]. Quantitative CMR mitral regurgitation grading is based on the subtraction of aortic forward flow volume from LV volumetric stroke volume, while CMR AR grading is based on a single measurement. In theory, CMR AR grading is therefore more accurate.
A potential overestimation of AR severity by TTE has important clinical implications. Current American College of Cardiology/American Heart Association [1] as well as European Heart Association guidelines [6] recommend surgery for asymptomatic patients with severe chronic AR in the presence of LV systolic dysfunction and/or increased LV dimensions. Therefore, patients with non-severe AR disease can potentially proceed to AVR if wrongly categorized as severe AR. Accuracy improvement
Author accepted manuscript
of AR grading in pre-surgical assessment will therefore influence timing and indication of surgeries significantly.
Limitations
The major limitation of our study is the retrospective approach, a non-simultaneous TTE and CMR acquisition, and the small study cohort. For instance , the latter contributed to the non-significant difference (P>0.05) between Spearman’s ρ coefficients when comparing association between TTE or CMR AR grades and post-surgical LV remodeling (i.e. ∆LVEDV). A large prospective multicenter trial is needed to establish if our results can be generalized. Based on current clinical practice, only post-surgical TTE were available. Also, in patients with aortic root dilatation surgery was not indicated for AR alone. Furthermore, our study is too small to incorporate outcome analysis.
Conclusion
Among chronic AR patients undergoing TTE and CMR, TTE and CMR grading is only modestly interchangeable and TTE LV dimensions underestimate corresponding CMR LV measurements. CMR AR grading (vs TTE) is better associated with LV dilatation. The stronger correlation between CMR AR severity (vs TTE) and post-surgical remodeling suggests that CMR improves its prediction. Our data suggest a benefit of a multimodality approach, especially for the pre-surgical assessment of chronic AR patients.
Author accepted manuscript
References1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary :A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 129:2440–2492
2. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ (2017) Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 30:303–371 . doi: 10.1016/j.echo.2017.01.007
3. Didier D, Ratib O, Lerch R, Friedli B (2000) Detection and quantification of valvular heart disease with dynamic cardiac MR imaging. Radiographics 20:1279-99;. doi: 10.1148/radiographics.20.5.g00jl111279
4. Dall’Armellina E, Hamilton C, Hundley G (2007) Assessment of blood flow and valvular heart disease using phase-contrast cardiovascular magnetic resonance. Echocardiography 24:207–216 . doi: 10.1111/j.1540-8175.2007.00377.x
5. Cawley PJ, Hamilton-Craig C, Owens DS, Krieger E V., Strugnell WE, Mitsumori L, D’Jang CL, Schwaegler RG, Nguyen KQ, Nguyen B, Maki JH, Otto CM (2013) Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 6:48–57 . doi: 10.1161/CIRCIMAGING.112.975623
6. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M, Bax JJ, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Von Segesser L, Badano LP, Bunc M, Claeys MJ, Drinkovic N, Filippatos G, Habib G, Pieter Kappetein A, Kassab R, Lip GYH, Moat N, Nickenig G, Otto CM, Pepper J, Piazza N, Pieper
Author accepted manuscript
PG, Rosenhek R, Shuka N, Schwammenthal E, Schwitter J, Mas PT, Trindade PT, Walther T (2012) Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 33:2451–2496 . doi: 10.1093/eurheartj/ehs109
7. Bellenger NG, Burgess MI (2000) Ray SG et al (2000) Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Hear J 21:1387–1396
8. Puntmann VO, Gebker R, Duckett S, Mirelis J, Schnackenburg B, Graefe M, Razavi R, Fleck E, Nagel E (2013) Left ventricular chamber dimensions and wall thickness by cardiovascular magnetic resonance: Comparison with transthoracic echocardiography. Eur Heart J Cardiovasc Imaging 14:240–246 . doi: 10.1093/ehjci/jes145
9. Ong G, Redfors B, Crowley A, Abdel-Qadir H, Harrington A, Liu Y, Lafrenière-Roula M, Leong-Poi H, Peterson MD, Connelly KA (2018) Evaluation of left ventricular reverse remodeling in patients with severe aortic regurgitation undergoing aortic valve replacement: Comparison between diameters and volumes. Echocardiography 35:142–147. doi: 10.1111/echo.13750
10. Gelfand E V., Hughes S, Hauser TH, Yeon SB, Goepfert L, Kissinger K V., Rofsky NM, Manning WJ (2006) Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson 8:503–507 . doi: 10.1080/10976640600604856
11. Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP (2015) Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol 84:840–848 . doi: 10.1016/j.ejrad.2015.02.002
12. Castillo E, Osman N, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke D, Lima J (2005) Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson 7:783–91. doi: 10.1080/10976640500295417
13. Popović ZB, Desai MY, Griffin BP (2018) Decision making with imaging in asymptomatic aortic regurgitation. JACC Cardiovasc Imaging 11:1499–1513. doi: 10.1016/j.jcmg.2018.05.027
Author accepted manuscript
Evaluation of the integrative algorithm for grading chronic aortic and mitral regurgitation severity using the current American Society of Echocardiography Recommendations: to discriminate severe from moderate regurgitation. J Am Soc Echocardiogr 31:1002–1012.e2. doi: 10.1016/j.echo.2018.04.002
15. Uretsky S, Supariwala A, Nidadovolu P, Khokhar SS, Comeau C, Shubayev O, Campanile F, Wolff SD (2010) Quantification of left ventricular remodeling in response to isolated aortic or mitral regurgitation. J Cardiovasc Magn Reson 12:32. doi: 10.1186/1532-429X-12-32
16. Dulce M, Mostbeck G, O’Sullivan M, Cheitlin M, Caputo G, Higgins C (1992) Severity of aortic regurgitation: interstudy reproducibility of measurements with velocity- encoded cine MRI imaging. Radiology 185:235–40
17. Ley S, Eichhorn J, Ley-Zaporozhan J, Ulmer H, Schenk JP, Kauczor HU, Arnold R (2007) Evaluation of aortic regurgitation in congenital heart disease: Value of MR imaging in comparison to echocardiography. Pediatr Radiol 37:426–436. doi: 10.1007/s00247-007-0414-4
18. Kutty S, Whitehead KK, Natarajan S, Harris MA, Wernovsky G, Fogel MA (2009) Qualitative echocardiographic assessment of aortic valve regurgitation with quantitative cardiac magnetic resonance: A comparative study. Pediatr Cardiol 30:971–977. doi: 10.1007/s00246-009-9490-6
19. Harris AW, Krieger E V., Kim M, Cawley PJ, Owens DS, Hamilton-Craig C, Maki J, Otto CM (2017) Cardiac magnetic resonance imaging versus transthoracic echocardiography for prediction of outcomes in chronic aortic or mitral regurgitation. Am J Cardiol 119:1074–1081. doi: 10.1016/j.amjcard.2016.12.017 20. Myerson SG, D’arcy J, Mohiaddin R, Greenwood JP, Karamitsos TD, Francis JM,
Banning AP, Christiansen JP, Neubauer S (2012) Aortic regurgitation quantification using cardiovascular magnetic resonance: Association with clinical outcome. Circulation 126:1452–1460. doi: 10.1161/CIRCULATIONAHA.111.083600
21. Polte CL, Gao SA, Johnsson ÅA, Lagerstrand KM, Bech-Hanssen O (2017) Characterization of Chronic Aortic and Mitral Regurgitation Undergoing Valve Surgery Using Cardiovascular Magnetic Resonance. Am J Cardiol 119:2061– 2068. doi: 10.1016/j.amjcard.2017.03.041
22. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St. John Sutton M, Stewart W (2006) Recommendations for chamber quantification. Eur J
Author accepted manuscript
Echocardiogr 7:79–108. doi: 10.1016/j.euje.2005.12.01423. Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA (2015) Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 17:29. doi: 10.1186/s12968-015-0111-7
24. Stone PH, Clark RD, Goldschlager N, Selzer A, Cohn K (1984) Determinants of prognosis of patients with aortic regurgitation who undergo aortic valve replacement. J Am Coll Cardiol 3:1118–1126. doi: 10.1016/S0735-1097(84)80168-0
25. Bonow RO, Rosing DR, McIntosh CL, Jones M, Maron BJ, Lan KK, Lakatos E, Bacharach SL, Green M V, Epstein SE (1983) The natural history of asymptomatic patients with aortic regurgitation and normal left ventricular function. Circulation 68:509–17 . doi: 10.1161/01.CIR.68.3.509
26. Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, Gurram S, Jain K, Subero M, Jang JJ, Cohen R, Wolff SD (2015) Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: A prospective multicenter trial. J Am Coll Cardiol 65:1078–1088. doi: 10.1016/j.jacc.2014.12.047
27. Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp G (2017) Prognostic implications of magnetic resonance - derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with Doppler echocardiography-derived integrative approach. Circulation 137:1349–1360. doi: 10.1161/CIRCULATIONAHA.117.029332
Author accepted manuscript
Table 1 Patient Clinical and Demographic Data including Baseline for Surgical Group
AR cohort Aortic valve
replacement cohort
n=101 n=24
Age, years 54.814.7 44.0 [39.3; 62.8]
Gender, m (%) 75 (74.3) 18 (75.0)
Caucasian, n (%) 82 (81.2) 16 (66.7)
Systolic Blood Pressure, mmHg 13117 13419 Diastolic Blood Pressure, mmHg 6512 6316
Heart Rate, bpm 6813 7213
Body Mass Index, kg/m2 27.46.2 29.55.4 Hypertension, n (%) 53 (52.5) 11 (45.8) Diabetes Mellitus, n (%) 9 (8.9) 5 (20.8) Dyslipidemia, n (%) 39 (38.6) 9 (37.5) Coronary Artery Disease, n (%) 15 (14.9) 3 (12.5)
Stroke, n (%) 0 (0) 0 (0)
Dyspnea, n (%) 21 (20.8) 12 (50.0)
NYHA Heart Failure classification - Stage II, n (%) - Stage III, n (%) - Stage IV, n (%) 15 (14.9) 4 (4.0) 2 (2.0) 9 (37.5) 2 (8.3) 1 (4.2) Transthoracic Echocardiography (TTE)
Aortic Regurgitation, n (%) - Mild - Moderate - Severe 14 (13.9) 72 (71.3) 15 (14.8) 2 (8.3) 17 (70.8) 5 (20.8) Eccentric Regurgitant Jet, n (%) 40 (39.6) 11 (45.8) Mild Aortic Stenosis, n (%) 19 (18.8) 4 (16.7) Mitral Regurgitation, n (%) - None/Trivial - Mild 44 (43.7) 38 (37.6) 8 (33.3) 16 (66.7)
Author accepted manuscript
- Moderate 16 (15.8) 0 (0)
Cardiovascular Magnetic Resoncane (CMR)
Bicuspid Aortic Valve, n (%) 37 (36.6) 10 (41.7) Aortic Regurgitation, n (%) (10) - Mild - Moderate - Severe 7 (6.8) 79 (75.7) 15 (13.6) 0 (0) 15 (62.5) 9 (37.5) Regurgitant Volume, ml 4027 5228 Regurgitant Fraction, % 3215 4013 Mitral Regurgitation, n (%) (24) - None - Mild - Moderate 8 (7.9) 82 (81.1) 11 (10.9) 0 (0) 21 (87.5) 3 (12.5) AR, aortic regurgitation; NYHA, New York Heart Association.
Author accepted manuscript
Table 2 Intermodality comparison for AR grading agreement (A) (ASE/SCMR (2) &
Gelfand criteria (10)) and interobserver variability (B).
A CMR (2) CMR (10)
AR Grade Mild Moderate Severe Mild Moderate Severe
Mild 9 4 1 3 10 1
TTE Moderate 36 28 8 4 60 8
Severe 2 7 6 0 9 6
n 47 39 15 7 79 15
B CMR reader 1
CMR grade Mild Moderate Severe
Mild 4 4 0
CMR reader 2 Moderate 0 26 1
Severe 0 0 8
n 4 30 9
TTE reader 1
TTE grade Mild Moderate Severe
Mild 3 22 3
TTE reader 2 Moderate 4 22 1
Severe 0 2 3
n 7 46 7
Intermodality agreement (A) for TTE with CMR grades by ASE/SCMR (2) (ICC 0.426, 95% CI 0.148-0.613, P<0.001) and by Gelfand (10) (ICC 0.478, 95% CI 0.227-0.647, P<0.001) was fair. ASE/SCMR, American Society of Echocardiography/Society for Cardiovascular Magnetic Resonance; CI, confidence interval; CMR, cardiovascular
Author accepted manuscript
magnetic resonance; ICC, intraclass correlation coefficient; TTE, transthoracic echocardiography.