High-Cell-Density Phorbol Ester and Retinoic
Acid Upregulate Involucrin and Downregulate
Suprabasal Keratin 10 in Autocrine Cultures
of Human Epidermal Keratinocytes
Yves Poumay,* Franc¸oise Herphelin,* Patrick Smits,† Isabelle Y. De Potter,* and Mark R. Pittelkow‡
*De´partement Histologie-Embryologie, Faculte´s Universitaires Notre-Dame de la Paix, B-5000 Namur, Belgium; †Laboratory of Molecular Biotechnology, Department of Biochemistry, UIA, B-2610 Wilrijk, Belgium; and ‡Department of Dermatology, Mayo Clinic, Rochester, Minnesota 55905
Received October 5, 1999
Autocrine growth of human epidermal keratino-cytes is initiated in subconfluent cell cultures in the absence of exogenous growth factors, at low calcium concentration of the medium and with sufficient cell density. Culture confluence inhibits keratinocyte pro-liferation and upregulates expression of early, keratin 10 (K10), and late, involucrin, markers of differen-tiation. In this report, the phenotype of autocrine keratinocytes was studied at high cell density (post-confluence), specifically after treatment with 12-O-tetradecanoylphorbol 13-acetate (TPA), or all-trans retinoic acid (RA). At postconfluence, K10 is decreased but not involucrin. TPA upregulates involucrin ex-pression, but not K10 in subconfluent keratinocytes. Treatment of confluent keratinocytes with RA down-regulates K10, but updown-regulates involucrin. This in
vitro culture model, unlike others, simulates for the
first time the in vivo effects of RA, a member of the retinoid family which potently modulates keratino-cyte differentiation and the expression of selected gene products. It thus can be developed to further examine epidermal differentiation. © 1999 Academic Press
Stratification and differentiation of keratinocytes in the human epidermis is a process that generates and maintains the stratum corneum, an impermeable bar-rier made up of keratinized anucleate cells and isolates the organism from its environment, including the abil-ity to protect against chemicals and pathogens that come into contact with the skin surface (1). The con-tinuous renewal and progressive maturation of kera-tinocytes during their upward migration through the epidermis creates the distinct basal, spinous, granular and cornified layers constitutive of this tissue, and
abnormalities in epidermal differentiation can directly be linked to dermatological diseases (1, 2). The avail-ability of in vitro models of human and rodent keratin-ocyte culture during the last two decades has allowed investigation of the cellular and molecular mecha-nisms regulating differentiation (1– 4). However, each model has been recognized as having specific advan-tages as well as disadvanadvan-tages such as the presence of serum, feeder layers or comparison of human and mouse keratinocytes (2, 4). Consequently, when initi-ating in vitro investigations of the epidermal differen-tiation process in keratinocyte, one model must be se-lected, ideally taking into account the particular process or environmental conditions the study will be dealing with.
Autocrine cultures of human epidermal keratino-cytes were recently described, and can be established in a defined culture medium at low calcium concentra-tion, when cell density is sufficient to provide factors that sustain cell proliferation (5, 6). Autonomously growing autocrine keratinocyte cultures reach conflu-ence where upon terminal differentiation is induced within the cell population. We have previously shown that growth-arrest induced by confluence of the culture is associated with strong induction of the early differ-entiation markers, suprabasal keratins 1 (K1) and 10 (K10), and occurs independently of the medium cal-cium concentration (7). Thus, these specific autocrine conditions are particularly suited for studies of the effects of individual growth factors on the initiation of epidermal differentiation (7, 8).
Among the genes expressed by keratinocytes during the epidermal differentiation program in vivo are those expressed early in the process, such as K1 and K10, and others that are expressed later (1, 3). A represen-tative later marker is involucrin, a component of the
Article ID mcbr.1999.0165, available online at http://www.idealibrary.com on
138 1522-4724/99 $30.00
cornified envelope, that characterizes keratinized cells of the stratum corneum and is expressed late in differ-entiation program in vivo, in the upper spinous and granular epidermal layers (1, 3, 4). Interestingly, whereas the early markers K1 and K10 are induced at confluence of autocrine keratinocyte cultures (7), re-cent studies have further shown that postconfluent cultures (i.e., cultures maintained under autocrine growth conditions for several days after confluence) continue to follow the program of epidermal differenti-ation and trigger expression of later markers (9) sug-gesting that not only the onset of epidermal differenti-ation, but also the regulation of later phenomena, can be studied in this model.
In the present work, we verify that the expression of early and late differentiation markers are differen-tially regulated by cell density in autocrine keratino-cyte cultures (9), and we demonstrate that autocrine cultures are sensitive to compounds known as modu-lators of epidermal differentiation. Most interesting, we find that all-trans retinoic acid (RA) upregulates involucrin in normal keratinocytes, providing in vitro data that parallel for the first time in vivo effects of RA (10).
MATERIALS AND METHODS
Cell Culture
Human adult normal skin samples were obtained at plastic surgery (Dr. B. Bienfait, Clinique St. Luc, Namur-Bouge). Keratinocytes were isolated by the trypsin float technique as described (11), and primary cultures were initiated in complete, 0.15 mM (low) calcium growth medium (KGM-2, BioWhittaker) con-taining growth factors and hormone supplements. The low calcium concentration was maintained throughout all the experiments reported here. Culture medium was renewed every two days. Keratinocytes harvested by trypsinization of primary proliferating subconfluent cultures were cryopreserved. After thawing, cells were
plated into secondary cultures at 53 103
cells/cm2 in complete medium, then, once approximately 40% of the culture substratum was covered by keratinocytes, cul-tures were washed repeatedly with KBM-2 medium prepared by excluding bovine pituitary extract, insu-lin, transferrin, epinephrine and EGF from complete culture medium. The KBM-2 medium is designated hereafter as basal medium. Washed keratinocytes were then incubated in the basal medium to initiate their autocrine growth at subconfluence (5) and termi-nal differentiation at confluence (7). Secondary auto-crine cultures were also refed every other day with
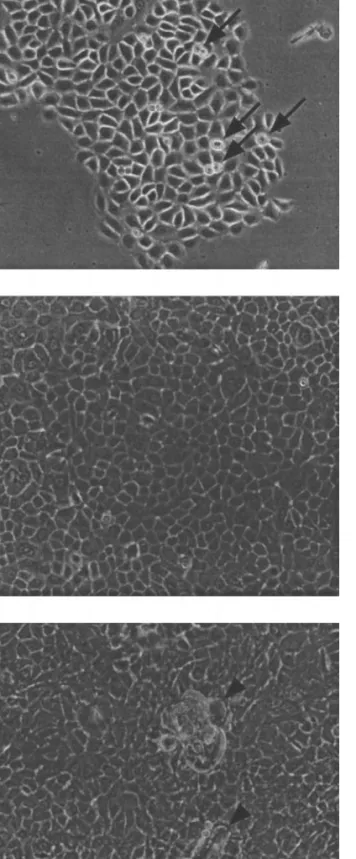
FIG. 1. Morphology of autocrine cultures of human epidermal keratinocytes at various cell densities. Keratinocytes were plated in secondary cultures in KGM-2 medium, then switched to basal me-dium when cells were covering approximately 40% of the substra-tum. Autocrine keratinocytes were photographed using inverted phase contrast microcopy at subconfluence after one day of autocrine growth (A), on the first day of complete confluence of the culture (B), and four days after confluence at postconfluence (C). Arrows indicate
cells undergoing mitosis and arrowheads indicate cellular stratifica-tion. Bar: 250mm.
basal medium. Autocrine subconfluent keratinocytes grown in basal medium were treated with 1, 10, or 100 ng/ml of 12-O-tetradecanoylphorbol 13-acetate (TPA, Sigma) for 18 h. Treatment of autocrine keratinocytes
cultured in basal medium with 1028-1026 M all-trans
retinoic acid (RA, Sigma) was performed for 18 h at subconfluence or confluence. Stock solutions of TPA and RA were prepared in dimethyl sulfoxide (DMSO). DMSO 0.01% was also included in control cultures. Representative experiments are shown on the figures, each experiment being reproduced at least three times with similar results.
Poly(A)RNA Isolation and Northern Blot Analysis
Poly(A)RNA from autocrine cultures was isolated and analyzed by Northern blotting as described previ-ously (7). The cDNAs specific for the human basal
keratin 14 (K14), suprabasal keratin 10 (K10) (12), or cDNA specific for human involucrin (13) were used to detect RNA expression of epidermal differentiation markers. The membrane was also hybridized with the housekeeping gene cyclophilin/1B15 cDNA to verify equivalent loading and transfer of RNA (14). The rel-ative gene expression was analyzed using densitomet-ric measurements performed by the NIH Image Anal-ysis software package based on comparison of each gene product with expression of cyclophilin/1B15.
Western Blot Analysis
Cell lysates were analyzed by Western blotting as described (7). Involucrin protein was detected by incu-bation for 2 h with 1:250 dilution of a rabbit polyclonal antibody (Harbor) and K10 protein by incubation with 1:250 dilution of the DEK10 mouse monoclonal
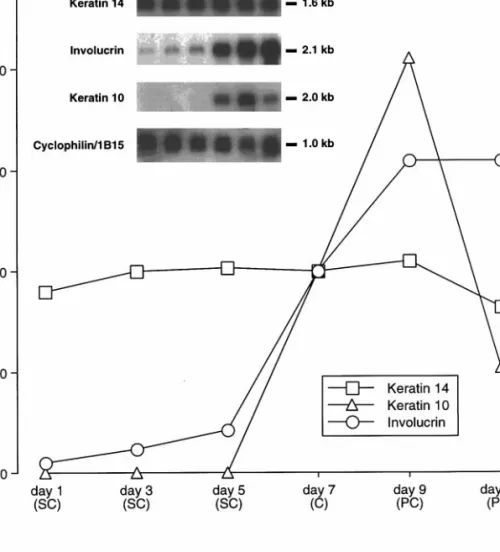
anti-FIG. 2. Modulation of epidermal differentiation-related RNA expression by cell density in autocrine cultures of human keratinocytes. Poly(A)RNA was extracted from keratinocytes cultured in autocrine conditions for 1 (lane 1), 3 (lane 2), 5 (lane 3), 7 (lane 4), 9 (lane 5), and 11 (lane 6) days. Subconfluence (SC), confluence (C), and postconfluence (PC) of the culture were determined by direct phase contrast microscopic observation. Relative gene expression was calculated as the ratio of the keratin 10, keratin 14, or involucrin gene expression to the expression of the cyclophilin/1B15 gene, using densitometric measurements of the respective Northern blot hybridizations. The results are expressed as percentages of the relative gene expression calculated on the first day of culture confluence (day 7).
body (Cappel). Immunoreactivity was detected with 1:1000 dilutions of horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibodies (Dako), and visualized using the Supersignal West Pico chemi-luminescent substrate (Pierce).
RESULTS
Cell Density Regulates Expression of K10 and Involucrin in Autocrine Culture Conditions
Subconfluent keratinocytes grown under autocrine culture conditions demonstrated mitoses in each repli-cating colony (Fig. 1A). At culture confluence, keratin-ocyte growth-arrest, as reported previously (7, 11), is documented by the absence of mitotic figures and the typical quiescent appearance (Fig. 1B). Four days after culture confluence, at postconfluence, cellular stratifi-cation is clearly evident in many areas of the culture (Fig. 1C). Northern blot analysis of epidermal gene expression at increasing cell densities shows that, fol-lowing the onset of terminal differentiation at culture
confluence (7), K10 and involucrin are differentially controlled during the postconfluent period of culture (Fig. 2). Indeed, whereas K10 is strongly induced by confluence, we demonstrate that K10 is downregulated during postconfluent culture. Similar results are ob-served for K1 mRNA expression (data not shown). However, downregulation of the K1 and K10 tran-scripts is accompanied by cellular accumulation of both cytoskeletal keratin proteins (7, data not shown). Un-like suprabasal keratins, involucrin is faintly ex-pressed by subconfluent keratinocytes. However, in-volucrin expression is enhanced respectively 8.3 and 12.8 fold at confluence (day 7) and postconfluence (day 11), in comparison with expression of subconfluent cul-tures analyzed on the third day (day 3) of autocrine conditions (Fig. 2). This observation is in accordance with late expression of involucrin in the upper layers of normal epidermis. On the other hand, basal K14 gene expression is not markedly regulated by cell density of autocrine cultures, suggesting that keratinocytes ex-hibiting the basal phenotype persist under these
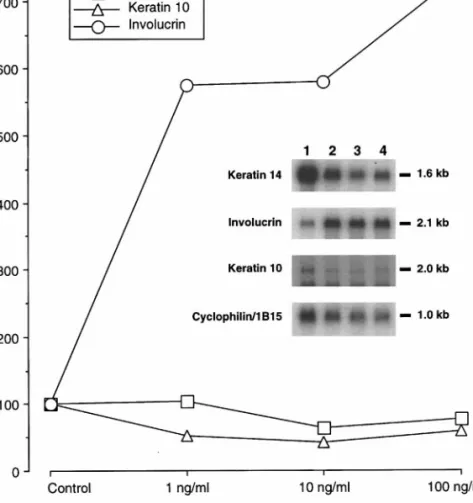
dif-FIG. 3. Modulation of epidermal differentiation-related RNA expression by TPA in subconfluent autocrine keratinocyte cultures. Poly(A)RNA was extracted from keratinocytes cultured in autocrine conditions at subconfluence after treatment for 18 h with DMSO 0.01% (Control, lane 1), or with 1 ng/ml (lane 2), 10 ng/ml (lane 3), or 100 ng/ml (lane 4) of TPA. Relative gene expression was calculated as in Fig. 2. Results are expressed as percentages of the relative gene expression calculated in the control culture.
ferentiating culture conditions and are in homeostatic balance with terminal differentiating cells.
TPA Regulates Epidermal Differentiation under Autocrine Culture Conditions
TPA induces terminal differentiation of keratino-cytes in vivo (15). Subconfluent cultures were treated with TPA to assess whether this active phorbol ester is able to trigger differentiation in epidermal keratino-cytes cultured in autocrine conditions. Involucrin mRNA expression is clearly induced by TPA (Fig. 3) similarly to previous reports on keratinocytes cultured in different models (16, 17). However, no induction of the suprabasal K10 expression could be found. On the contrary, the weak K10 expression seen by prolonged exposure of the Northern blot was inhibited by TPA (Fig. 3), an effect already reported for K1 and K10 expression in mouse cells (18). Interestingly, the coor-dinate inhibition of K14 mRNA expression by TPA in human keratinocytes has not been observed for mouse
cells, further suggesting distinctive regulation of epi-dermal keratin expression between the two species (7, 19). Diminution of K14 mRNA expression by exposure of human keratinocyte to TPA indicates progressive loss of the basal cell phenotype that parallels the loss of clonogenic, proliferative potential of TPA-treated hu-man keratinocytes (20).
Retinoic Acid Differentially Regulates K10 and Involucrin Expression in Differentiating Autocrine Cultures
RA is known to exert a wide range of effects on keratinocytes during epidermal differentiation. In these sets of experiments, all-trans retinoic acid was added to the medium of autocrine keratinocyte cul-tures to determine whether this retinoid also regulates differentiation under these conditions. When 1028-1026 M RA were added to subconfluent, growing autocrine cultures of keratinocytes, no effect on K14, K10 or involucrin mRNA expression was found (data not
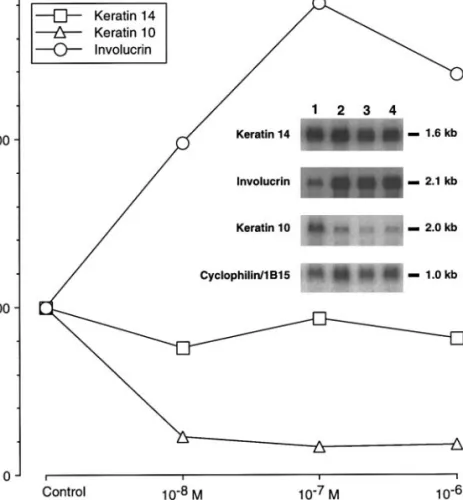
FIG. 4. Modulation of epidermal differentiation-related RNA expression by RA in confluent autocrine keratinocyte cultures. Poly(A)RNA was extracted from keratinocytes cultured in autocrine conditions at confluence after treatment for 18 h with DMSO 0.01% (Control, lane 1), or with 1028M (lane 2), 1027M (lane 3), or 1026M (lane 4) all-trans retinoic acid. Relative gene expression was calculated as in Fig. 2, and expressed as in Fig. 3.
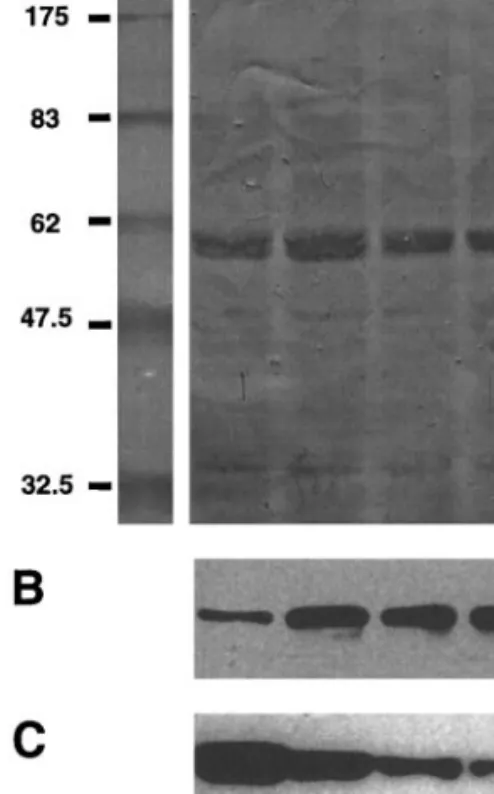
shown). However, addition of 1028-1026 M RA to the medium of confluent differentiating cultures resulted in the inhibition of K10 mRNA expression (Fig. 4), similar to previously reported results (21, 22). K14 expression was unaffected by the range of RA concen-trations tested, but by contrast, for each RA concentra-tion tested the relative gene expression of involucrin increased at least two fold for cultured keratinocytes grown in autocrine conditions at confluence (Fig. 4) and postconfluence (data not shown). We believe this is the first report of a differential in vitro pro-differentiating effect of RA. To confirm these results, we used western blot analysis to determine whether the upregulation of involucrin mRNA expression by RA is paralleled by enhanced involucrin protein expression (Fig. 5). In-deed, when identical confluent autocrine keratinocyte cultures were treated for 18 h with 1028-1026M RA, an increase in involucrin level was seen compared to trol cultures (Fig. 5B). Conversely, increasing RA con-centrations resulted in decreasing K10 protein level (Fig. 5C) (21, 22).
DISCUSSION
Application of the autocrine keratinocyte culture system to investigate the regulation of epidermal dif-ferentiation and effects of pharmacologic and other chemical agents provides the potential to totally define the culture environment of the keratinocyte. To date, these defined culture conditions have been used to examine early events of keratinocyte commitment to differentiation (7), but they are also useful for studies of later events in the keratinocyte terminal differenti-ation process. Indeed, we demonstrate here that con-tinued culture of keratinocytes after confluence is at-tained not only maintains a subpopulation of basal cells expressing K14 but also induces later differenti-ation markers. Subsequently the mRNA encoding the early K10 marker is downregulated but involucrin is further upregulated. These findings correlate well with the inherent steps of differentiation seen in epidermis and suggest that the autocrine keratinocyte culture system after confluence is a robust model for investi-gating molecular regulators of epidermal growth and differentiation (9).
The regulation of differentiation at postconfluence has been reported to be mediated by activation of pro-tein kinase C (PKC) in human keratinocytes (9). The tumor-promoter and PKC-activator TPA is well-known as a strong inducer of terminal differentiation in ker-atinocytes (1, 15, 16, 20). Indeed, we found in autocrine cultures of keratinocytes that TPA strongly upregu-lates expression of involucrin, but downreguupregu-lates ex-pression of specific keratin genes. These results are in accordance with the AP-1 activating effects of TPA and its enhancer activity on the involucrin promoter (17, 23). These observations in human cells also correlate
with inhibitory effect of TPA on the suprabasal K1/K10 expression in mouse cells (18, 24). Finally, we show that the phenotypic effects induced in low density au-tocrine cultures by the phorbol ester mimic the regu-lation of the epidermal differentiation marker expres-sion observed in postconfluent high density cultures.
Treatment of differentiating autocrine cultures with RA produced unexpected observations related to in-volucrin expression. It is well established that RA reg-ulates epidermal differentiation. However, the main disparity concerning the keratinocyte response to RA is the effect of the compound in vitro or in vivo. Basically, RA has consistently inhibited epidermal differentia-tion in previous studies performed on cultured keratin-ocytes (1, 21, 25–31), but with in vivo application of the compound, no significant alteration of K1 and K10 has been found, and an increased number of cell layers expressing filaggrin and involucrin are routinely de-tected (10). Also, loricrin expression was reduced in
vivo only after acute treatment with RA, whereas an
increased number of cell layers expressing loricrin is observed after chronic treatment (10). In confluent au-tocrine keratinocyte cultures, we find here inhibition of K10 expression at both mRNA and protein levels,
con-FIG. 5. Western blot analysis of involucrin and K10 protein expression in confluent autocrine keratinocyte cultures treated with RA. Cellular protein extracts were prepared from keratinocytes cul-tured in autocrine conditions at confluence after treatment for 18 h with DMSO 0.01% (lane 1), or with 1028M (lane 2), 1027M (lane 3), or 1026M (lane 4) all-trans retinoic acid. Amido black total protein staining of the membrane (A). Immunodetection of involucrin (B) or K10 (C) was performed on the blot shown on A. Position of molecular weight markers expressed in kDa are shown on the left of A.
firming previous in vitro studies (21, 22, 28), but, of significance, we observe that involucrin is upregulated by RA in autocrine culture conditions. This result is surprising initially, because several previous reports have demonstrated inhibition of involucrin expression by RA in cultured keratinocytes (32, 33). However, these results were observed in malignant keratino-cytes isolated from human squamous cell carcinomas (SCC). Further scrutiny of investigations performed on normal human cultured keratinocytes (22, 26) raises doubt as to whether RA has a clear suppressing effect on involucrin expression. Together, these previous re-sults suggest that the regulation of involucrin expres-sion by RA might differ between normal and SCC ker-atinocytes. The difference may be explained by altered RA receptor (RAR) expression in SCC (34). However,
by analyzing the 59 regulatory sequences of the human
involucrin gene using the transcription element search software (TESS) package (URL: www.cbil.upenn.edu, May 20, 1999) to localize potential RA responsive ele-ments, several imperfect (20% of mismatch allowed) sequences are identified. These sequences may poten-tially bind the predominant RA receptors in human
skin, RAR-g and RAR-a (35).
In conclusion, the present study demonstrates that autocrine culture conditions for keratinocytes which we have previously developed provide an adequate model for future investigations, in a defined environ-ment, of epidermal differentiation. We suggest that this model should be utilized when discrepancies exist with other in vitro models and the in vivo state. In particular, using this autocrine in vitro model, we have identified for the first time that involucrin expression is upregulated as a pro-differentiating effect of RA in epidermal keratinocytes similar to its effect in vivo. ACKNOWLEDGMENTS
The authors thank Dr. B. Bienfait (Clinique Saint-Luc, Bouge) for providing skin samples, and Drs. D. R. Roop (Houston) and R. L. Eckert (Cleveland) for their gift of keratins and involucrin cDNA probes. I. Y. De Potter holds a fellowship from the Fonds pour la Formation a` la Recherche dans l’Industrie et l’Agriculture (FRIA).
REFERENCES
1. Eckert, R. L., Crish, J. F., and Robinson, N. A. (1997) Physiol. Rev. 77, 397– 424.
2. Pittelkow, M. R. (1994) in Handbook of Mouse Mutations with Skin and Hair Abnormalities (Sundberg, J. P., Ed.), pp. 117–127, CRC Press, Boca Raton.
3. Watt, F. M. (1989) Curr. Opin. Cell Biol. 1, 1107–1115. 4. Poumay, Y., and Leclercq-Smekens, M. (1998) Folia Medica
(Plovdiv) 40, 5–12.
5. Cook, P. W., Pittelkow, M. R., and Shipley, G. D. (1991) J. Cell. Physiol. 146, 277–289.
6. Pittelkow, M. R., Cook, P. W., Shipley, G. D., Derynck, R., and Coffey, R. J., Jr. (1993) Cell Growth & Differentiation 4, 513–521. 7. Poumay, Y., and Pittelkow, M. R. (1995) J. Invest. Dermatol. 104,
271–276.
8. Poumay, Y., Jolivet, G., Pittelkow, M. R., Herphelin, F., De Potter, I. Y., Mitev, V., and Houdebine, L.-M. (1999) Arch. Bio-chem. Biophys. 364, 247–253.
9. Lee, Y.-S., Yuspa, S. H., and Dlugosz, A. A. (1998) J. Invest. Dermatol. 111, 762–766.
10. Rosenthal, D. S., Griffiths, C. E. M., Yuspa, S. H., Roop, D. R., and Voorhees, J. J. (1992) J. Invest. Dermatol. 98, 343–350. 11. Wille, J. J., Jr., Pittelkow, M. R., Shipley, G. D., and Scott, R. E.
(1984) J. Cell. Physiol. 121, 31– 44.
12. Roop, D. R., Krieg, T. M., Mehrel, T., Cheng, C. K., and Yuspa, S. H. (1988) Cancer Res. 48, 3245–3252.
13. Eckert, R. L., and Green, H. (1986) Cell 46, 583–589.
14. Danielson, P. E., Forss-Petter, M., Brow, M. A., Calavetta, L., Douglass, J., Milner, R. J., and Sutcliffe, J. G. (1988) DNA 7, 261–267.
15. Reiners, J. J., Jr., and Slaga, T. J. (1983) Cell 32, 247–255. 16. Yaar, M., Gilani, A., DiBenedetto, P. J., Harkness, D. D., and
Gilchrest, B. A. (1993) Exp. Cell Res. 206, 235–243.
17. Takahashi, H., and Iizuka, H. (1993) J. Invest. Dermatol. 100, 10 –15.
18. Dlugosz, A. A., and Yuspa, S. H. (1993) J. Cell Biol. 120, 217– 225.
19. Drozdoff, V., and Pledger, W. J. (1993) J. Cell Biol. 123, 909 – 919.
20. Wille, J. J. Jr., Pittelkow, M. R., and Scott, R. E. (1985) Carci-nogenesis 6, 1181–1187.
21. Kopan, R., Traska, G., and Fuchs E. (1987) J. Cell Biol. 105, 427– 440.
22. Brown, L. J., Geesin, J. C., Rothnagel, J. A., Roop, D. R., and Gordon, J. S. (1994) J. Invest. Dermatol. 102, 886 – 890. 23. Welter, J. F., Crish, J. F., Agarwal, C., and Eckert, R. L. (1995)
J. Biol. Chem. 270, 12614 –12622.
24. Toftgard, R., Yuspa, S. H., and Roop, D. R. (1985) Cancer Res. 45, 5845–5850.
25. Fuchs, E., and Green, H. (1981) Cell 25, 617– 625.
26. Green, H., and Watt, F. M. (1982) Mol. Cell. Biol. 2, 1115–1117. 27. Yaar, M., Stanley, J. R., and Katz, S. I. (1981) J. Invest.
Derma-tol. 76, 363–366.
28. Floyd, E. E., and Jetten, A. M. (1989) Mol. Cell. Biol. 9, 4846 – 4851.
29. Rosdy, M., Bertino, B., Butet, V., Gibbs, S., Ponec, M., and Darmon, M. (1997) In Vitro Toxicol. 10, 39 – 47.
30. Hohl, D., Lichti, U., Breitkreutz, D., Steinert, P. M., and Roop, D. R. (1991) J. Invest. Dermatol. 96, 414 – 418.
31. Magnaldo, T., Bernerd, F., Asselineau, D., and Darmon, M. (1992) Differentiation 49, 39 – 46.
32. Cline, P. R., and Rice, R. H. (1983) Cancer Res. 43, 3203–3207. 33. Monzon, R. I., LaPres, J. J., and Hudson, L. G. (1996) Cell
Growth & Differentiation 7, 1751–1759.
34. Monzon, R. I., Fillmore, C., and Hudson, L. G. (1997) Mol. Phar-macol. 51, 377–382.
35. Fisher, G. J., Talwar, H. S., Xiao, J. H., Datta, S. C., Reddy, A. P., Gaub, M. P., Rochette-Egly, C., Chambon, P., and Voorhees, J. J. (1994) J. Biol. Chem. 269, 20629 –20635.