Publisher’s version / Version de l'éditeur:
CANCOM: Design, Maunufacturing and Application of Composites: Proceedings Of the Canadian International Conference On Composites, 2007-08-14
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect Shearing Time and Temperature on the Dispersion of Clay in Thermoset Nanocomposites
Ngo, T.-D.; Hoa, S. V.; Ton-That, M.-T.; Wood-Adams, P. M.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=b8c7a5db-317f-4504-8119-a5353708eb8f https://publications-cnrc.canada.ca/fra/voir/objet/?id=b8c7a5db-317f-4504-8119-a5353708eb8f
Effect Shearing Time and Temperature on the Dispersion of Clay in
Thermoset Nanocomposites
T.-D. NGO1, S. V. HOA1, M.-T. TON-THAT2, P. M. WOOD-ADAMS1
1Department of Mechanical & Industrial Engineering, Concordia University
1455 De Maisonneuve Blvd., Montreal, Quebec, Canada H3G 1M8
2National Research Council Canada, Industrial Materials Institute
75 De Mortagne Blvd., Boucherville, Quebec, Canada J4B 6Y4 Concordia Center for Composites (CONCOM)
Center for applied Research on Polymers and Composites (CREPEC)
ABSTRACT
The effect of shearing time and temperature on the dispersion of organoclay in epoxy was studied. The epoxy resin was Shell EPON 828 and the organoclay was Cloisite 30B from Southern Clay Products. Epoxy and Cloisite 30B were mixed at different durations of 2, 4, 10, 20, 30, 45 and 60 minutes at different temperatures of room temperature (RT), 120oC and 180oC. The quality of dispersion and intercalation/exfoliation of clay after mixing was analyzed by X-ray diffraction (XRD) and by rheological measurement (for epoxy-clay suspensions after mixing) at room temperature. The quality of dispersion and intercalation/exfoliation of clay after curing step were characterized by the field emission gun scanning electron microscopy (FEGSEM). The results illustrate that full exfoliation of can not be achieved at mixing step, but the clay particles were broken down to smaller sizes with increase of mixing time and temperature. Although mixing time and temperature do not significant affect the intercalation/exfoliation of organoclay at mixing step, they have indirect effect on intercalation/exfoliation at curing step. Very fine dispersion and uniform distribution of clay in epoxy nanocomposites were achieved.
INTRODUCTION
Both intercalated and exfoliated nanocomposites offer some enhanced physical and mechanical properties compared to the conventional composites [1-5]. Reinforcing effect of clay in epoxy clay nanocomposites is dependent on the quality of clay dispersion, interfacial adhesion between epoxy resin and dispersed clay [6, 7] clay loading [6, 8]. Processing parameters have an important influence on the dispersion of clay in polymers. Direct stirring of organoclay and epoxy with mechanical stirring and sonication is widely used to disperse nanoclay in epoxy [5, 9, 10-12]. Although this approach is simple and practical but good dispersion of clay cannot be achieved in epoxy nanocompostes. Yasmin A. et al. [13] used a three-roll mill to disperse the clay nano-particles in an epoxy matrix in order to improve the distribution of nanoclay particles in epoxy matrix but exfoliation could not be achieved. Solution process has also been used for preparing epoxy nanocomposites [14, 15, 16, 17]. Chen C. and Tolle T. B. [14] achieved a certain exfoliated layer silicate epoxies by high-shear mixing in the presence of a high amount of acetone. Clay and acetone was passed through high pressure chamber at high velocity to break down the agglomerates of clay has been reported by Liu et al [15, 18, 19]. The
method to prepare the organoclay/epoxy nanocomposites in liquid (ethanol and water solution) without any intermediate drying step of clay has been used but full exfoliation still can not be achieved [17]. The major advantage of the solution method is that it offers the possibilities to prepare intercalated nanocomposites from polymers with low or even no polarity. However, the “solution” approach is difficult to apply in industry due to problems associated with the use of large quantities of solvent.
In general, there are several different possible levels of dispersion of organoclays in epoxy nanocomposites including 1) exfoliated or delaminated clay layer (single platelet); 2) non intercalated stacks (multiple layers not intercalated by the matrix) or intercalated stacks (multiple layers intercalated either uniformly or not uniformly by the matrix); 3) multiple stack aggregates (multiple stacks to form aggregates, which are in the micro-scale) and the macro-aggregates (combination of many multiple stacks aggregates, which are in the macro-scale). Most research focused on intercalation, exfoliation of the clay in epoxy, but not many studies have been done on how to disperse the clay uniform distribution in this thermoset polymer. The dispersion, intercalation and exfoliation are controlled by the diffusion of epoxy resin (both epoxy and hardener) into the clay galleries, which is governed by thermodynamic forces. While breaking down the macro-aggregate to the multiple stack macro-aggregates or further down to the single stacks can be effectively made using mechanical forces, such as shear, impact, etc. Vaia et al. [3] suggested that the degree of exfoliation could be improved through the aid of conventional shear devices. They assumed that the individual plates peel apart through a combination of shear and diffusion of polymer chains in the organoclay gallery. Although the shear force is an important processing parameter for the synthesis of polymer-clay nanocomposite, the study of the shear effect on thermoset nanocomposites has been very limited due to their low viscosity. This paper will present the effect of shearing time and temperature at mixing step on the dispersion, intercalation/exfoliation of clay in both mixing and curing steps.
EXPERIMENTAL Materials
The resin selected for this study was EPON™ 828, from Resolution Performance Products LLC (Houston, TX, USA). The hardener was amine-terminated polyoxypropylene Jeffamine® D-230, from Huntsman Corp. An organo-nanoclay recommended for use with amine-cured epoxy systems was used, namely Cloisite® 30B (montmorillonite treated with methyl tallow bis-(2-hydroxyethyl) quaternary ammonium) from Southern Clay Products Inc. (Gonzales, TX, USA). Henceforth the hardener and clay will be designated in shortened form as D230 and C30B, respectively.
Sample preparation and measurements
Samples were prepared by high speed processing in the mixer at 24000 rpm. The clay and epoxy were mixed at room temperature (RT), 120°C and 180°C for different mixing
times of 2, 4, 10, 20, 30, 45 and 60 minutes. Samples were cured either at room temperature for 2 days or at 120°C for 2 hours, with subsequent post cure at 140°C for 2 hours in both cases. Rheological measurement at room temperature for epoxy-clay suspensions after mixing was performed on a Brookfield CAP2000+ viscometer for the epoxy and the epoxy-organoclay suspensions using cone and plate geometry at room temperature. To evaluate the intercalation/exfoliation of the nanoclay in the polymer matrix, X-ray diffraction (XRD) patterns were obtained from the surface of the samples with a Bruker Discover 8 powder X-ray diffractometer with CuKα radiation. A Hitachi-S4700 FEGSEM was used to observe the dispersion of clay in the epoxy matrix at the micro-level.
RESULTS AND DISCUSSION
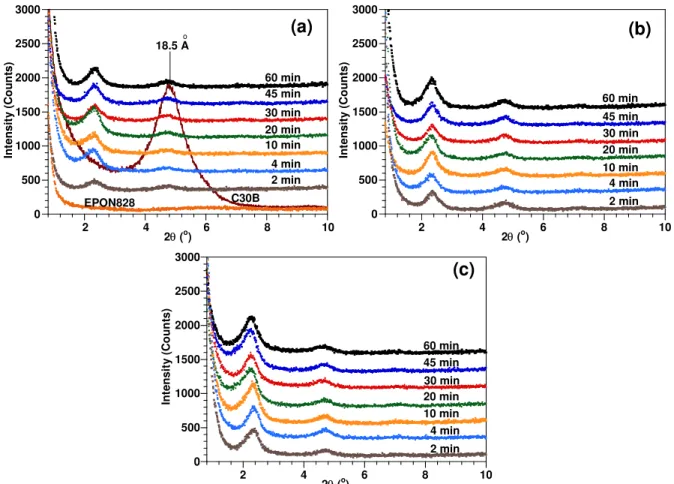
XRD curves are widely used to determine the structure of polymer nanocomposites (PNCs) due to its ease of use and results interpretation. In XRD curves, the peak location in terms of angle 2θ can indicate the intercalation degree as it relates to the gallery
distance. The intensity of the peaks or the area under the peaks can indicate the exfoliation degree or the size of cluster as it relates to these structural parameters. The influence of mixing duration on intercalation/exfoliation of clay in epoxy at mixing step was also studied. The results for liquid epoxy-clay after mixing experiments at high speed (24000 rpm) at room temperature, 120oC, and 180oC for different mixing durations are
shown in Figure 1. The summary of the first peak positions (d001) on the XRD curves of
the epoxy-clay mixtures is shown in Figure 2. C30B has one peak at 4.8o related to the d-spacing of 18.5 Å. It appears on the XRD curves that, the peak shifted to a lower angle compared to the peak of the starting clay C30B for all epoxy-clay mixtures. A shift of the peak to lower angle indicates that intercalation has been taken place in the mixing step. The clay layer separation (between 37.2 Å to 38.1 Å) is considerably higher than in the original C30B (18.5 Å). The results show that there is only a very small effect of mixing time and mixing temperature on the XRD peak positions (Figure 2). Therefore one can conclude that mixing temperature and duration do not significantly affect the intercalation/exfoliation of clay in epoxy resin at this step of fabrication (for mixing speed of 24000 rpm). The results also indicated that full exfoliation can not be achieved at the mixing step due to the presence of the peaks on XRD curves. Even the mixing time and mixing temperature do not show strong influence on intercalation/exfoliation of clay in epoxy resin at this step, but this does not mean that they do not have effect on the quality of micro-dispersion of clay in epoxy. That is the limitation of the XRD result, it can not reveal the whole picture of dispersion, intercalation/exfoliation.
0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In te n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min 0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In te n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min EPON828 C30B 18.5 A o 0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In te n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min (a) (b) (c)
Figure 1. X-ray diffraction curves of EPON828, C30B and EPON828-C30B mixtures after being mixed at (a) room temperature, (b) 120oC, and (c) 180oC.
0 10 20 30 40 50 60 RT 120oC 180oC 36.5 37.0 37.5 38.0 38.5 d001 ( A ) Time (minutes) o o d 001 for C30B = 18.5 A
Figure 2. The effect of mixing time and temperature on d001.
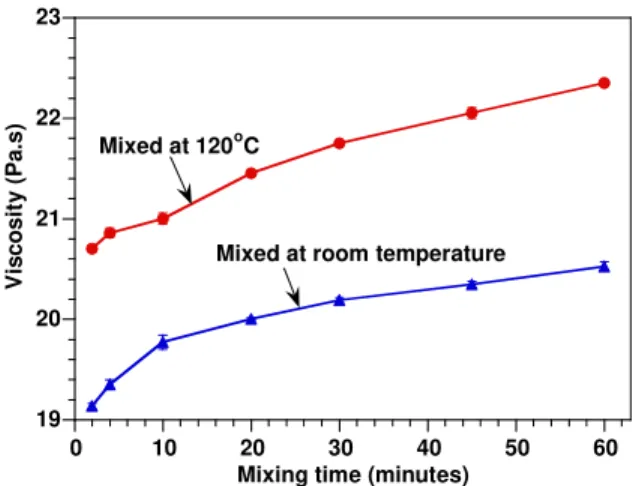
The rheological properties of the epoxy-clay suspensions with C30B after being mixed at room temperature and at 120oC for different mixing durations of 2, 4, 10, 20, 30, 45 and 60 minutes were also examined at room temperature (suspensions were cooled down before measurement was done). Figure 3 shows the viscosity of the EPON828-C30B mixtures. The results indicate that the viscosity of the suspension (at room temperature)
increases with mixing duration. The increase in viscosity of the epoxy-clay suspension may be related to the level of micro-dispersion of the clay in the epoxy [17]. The clay particles (agglomerates) were broken down into smaller, higher aspect ratio particles with the duration of mixing and results in an increase in the viscosity. In addition, at the same mixing duration, the viscosity of epoxy-clay suspension prepared at 120oC is always higher than the viscosity of suspension prepared at room temperature. This indicates the better dispersion of clay in epoxy prepared at 120oC compared to room temperature
19 20 21 22 23 0 10 20 30 40 50 60 V isc o s it y ( P a. s )
Mixing time (minutes) Mixed at 120oC
Mixed at room temperature
Figure 3. Viscosity of EPON828-C30B mixtures at different mixing durations after being mixed at room temperature and 120oC (at shear rate 167 (1/s))
In order to study the effect of mixing conditions on the intercalation/exfoliation of the cured epoxy nanocomposites (ENCs), the samples were cured at room temperature and at 120oC and submitted to XRD analysis. The XRD curves for these samples are shown in Figures 4 to 6. The summary of the first peak positions (d001) of the mixtures as well as
the ∆d between the positions of these peaks compared the peak of C30B are shown in
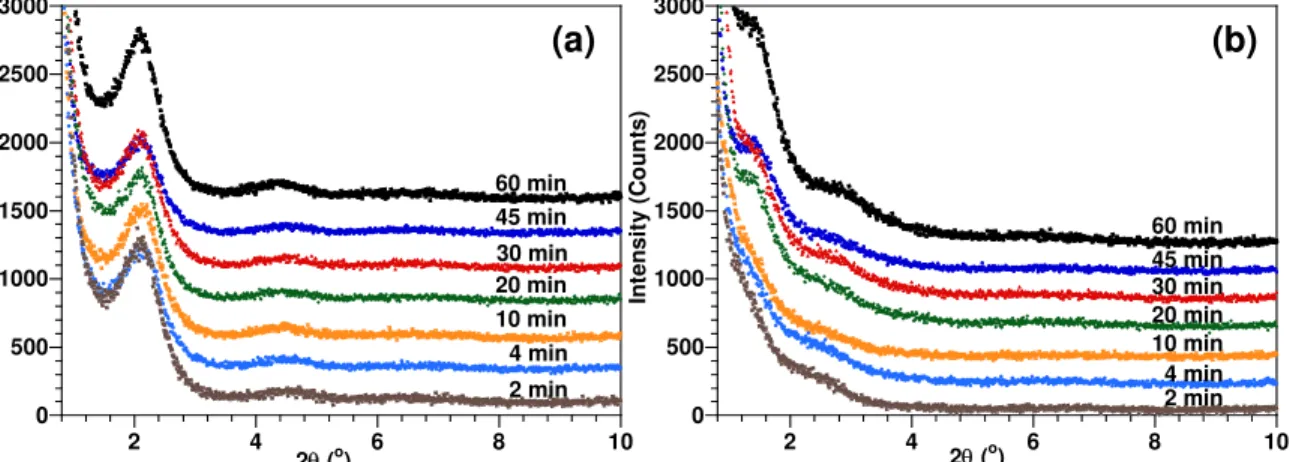
Table 1. There are two peaks at around 39.0 Å and 18.5 Å on each XRD curve for all ENCs cured at room temperature independent of mixing time and temperature (Figures 4a, 5a and 6a). However, the longer is the duration of mixing; the lower is the intensity of XRD peaks. A reduction in the peak intensity indicates that the amount of intercalated clay has decreased, or in other words, the dispersion has been improved by breakdown of clusters or even exfoliation. The improvement of dispersion and intercalation/exfoliation by time of mixing can be understood by the better diffusion of epoxy and hardener into the clay galleries. With longer time of mixing, the smaller aggregates can be achieved. Because of this, epoxy and hardener have more chance to penetrate to further expand the clay galleries at the curing step.
0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In te n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min 0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In te n s it y ( C ou nt s ) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min (a) (b)
Figure 4. X-ray diffraction curves of nanocomposite based on EPON828-D230 and 2 wt% C30B mixed at
room temperature for different durations and cured at (a) room temperature and (b) 120oC.
0 500 1000 1500 2000 2500 3000 2 4 6 8 10 Int e n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min 0 500 1000 1500 2000 2500 3000 2 4 6 8 10 Int e n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min (a) (b)
Figure 5. X-ray diffraction curves of nanocomposite based on EPON828-D230 and 2 wt% C30B mixed at 120oC for different durations and cured at (a) room temperature and (b) 120oC.
0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In te n s it y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min 0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In ten sit y ( C o u n ts) 2θ (o) 2 min 4 min 10 min 20 min 30 min 45 min 60 min (a) (b)
Figure 6. X-ray diffraction curves of nanocomposite based on EPON828-D230 and 2 wt% C30B mixed at 180oC for different durations and cured at (a) room temperature and (b) 120oC.
When ENCs were cured at 120oC after being mixed at room temperature, the two peaks on the XRD curves still remain at 39 Å and 18.5 Å (Figure 4b) for mixing times up to 1 hour. However, only one peak at 39 Å can be seen clearly for the ENCs which were mixed for 10 to 30 minutes at 120oC (Figure 5b). The second peak at 18.5 Å has almost disappeared. In addition, the two peaks appear again but at 67 Å and 39 Å for those samples mixed at the longer durations of 45 to 60 minutes. For samples mixed at 180oC, there are two peaks on the XRD curves at 67 Å and 39 Å (Figure 6b) for all cases of mixing duration. Note that curing at 120oC results in better intercalation/exfoliation than curing at room temperature. This can be explained by the fact when the temperature increases, the mobility of epoxy and hardener molecules increase and because of this, they can diffuse more easily into the clay galleries and further intercalate or exfoliate the clay [8, 20].
Table 1. Summary XRD results of epoxy nanocomposites with 2 wt% C30B
Gallery distance (Å)
Cured at RT Cured at 120oC Duration of
mixing
(min) d001 ∆d d001 ∆d
Mixing at room temperature
2 37.9 19.4 38.0 19.5 4 38.0 19.5 38.1 19.6 10 38.5 20.0 38.5 20.0 20 38.6 20.1 38.5 20.0 30 39.0 20.5 38.8 20.3 45 39.3 20.8 39.4 20.9 60 39.1 20.6 39.5 21.0 Mixing at 120oC 2 38.1 19.6 38.1 19.6 4 38.1 19.6 38.1 19.6 10 38.7 20.2 38.8 20.3 20 38.6 20.1 38.8 20.3 30 38.8 20.3 39.2 20.7 45 39.4 20.9 40.2-66.7 21.7-48.2 60 39.5 21.0 40.3-66.9 21.8-48.4 Mixing at 180oC 2 41.8 23.3 64.3 45.8 4 41.8 23.3 64.9 46.4 10 42.1 23.6 64.9 46.4 20 42.3 23.8 65.2 46.7 30 42.0 23.5 65.5 47.0 45 42.1 23.6 65.6 47.1 60 42.6 24.1 66.2 47.7
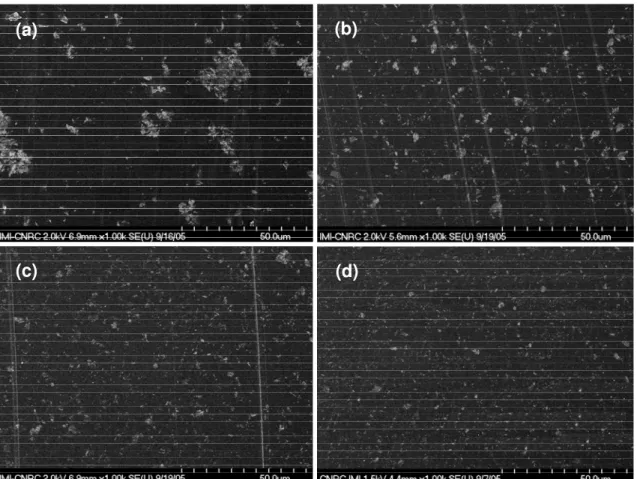
The effect of stirring duration on the dispersion of C30B in epoxy matrix was also observed by FEGSEM. FEGSEM photos of the epoxy nanocomposites based on
EPON828-D230 with 2 wt% of C30B for different mixing durations are shown in Figure 7. The bright spots on the backscattered images correspond to clay aggregates. Apparently, clay aggregates have been broken down to smaller sizes with increase in the stirring duration. It proves that the size of aggregates is smaller with stirring duration and because of this, it can help to improve the level of intercalation and exfoliation of epoxy nanocomposites during the curing step. Again, this confirms that the mixing time has an effect on the size reduction.
(a) (b)
(d) (c)
Figure 7. SEM photos of nanocomposite based on EPON828-D230 and 2 wt% C30B were mixed at 120oC
for different times: (a) 0 minute, (b) 2 minutes, (c) 10 minutes, and (c) 60 minutes.
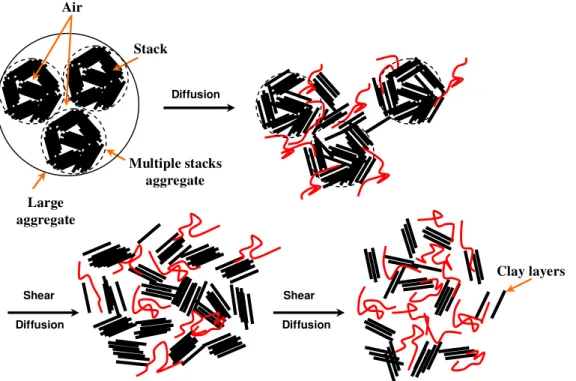
Generally speaking, with the studied scale, the duration of mixing does not bring to the exfoliation of clay in epoxy. However, they have influences on the dispersion of organoclay in epoxy resin at the micro level, and thus facilitate the diffusion of epoxy and hardener at curing step. The mechanism for dispersion of clay in epoxy resin is proposed in Figure 8. Before mixing with epoxy, the clays intend to form large aggregates (many layers of clay form the stack, multiple stacks form a multiple stack aggregates and several multiple stack aggregates stay together in large aggregates). That is very impotant to break down the large aggregates to achieve a good and uniform dispersion of the clay in epoxy. The bouderies of these large aggregates and the air pockets within them are the weakness points in the materials. When clay was mixed with epoxy, the epoxy can diffuse into between the stacks and the between outer clay layers of stack with the help of temperature. With sufficient shear by high speed mixing, the clay aggreagtes can be
broken down to smaller sizes (stacks) and lead to very fine and uniform dispersion with mixing time.
The reduction of size of aggregates will have an indirect effect on the intercalation/exfoliation of clay at curing step. Curing temperature facilitates the diffusion of epoxy into the clay galleries.
Shear Diffusion Shear Diffusion Diffusion Multiple stacks aggregate Stack Clay layers Large aggregate Air
Figure 8. Mechanism of dispersion at stirring step
CONCLUSION
The effects of shearing time and temperature on the fabrication of nanocomposites on dispersion and intercalation/exfoliation of clay have been studied. Although shearing time and temperature do not significantly affect the intercalation/exfoliation of organoclay at the mixing step, they have a positive influence on the viscosity of the epoxy-clay suspension and the dispersion of nanoclay in epoxy. Shearing breaks down clay aggregates to smaller ones and indirectly affects the intercalation/exfoliation of the clay at the curing step especially at high curing temperature. Temperature facilitates the diffusion of epoxy into the lay galleries at mixing step. Temperature also facilitates the diffusion of epoxy and hardener at curing step. Very fine dispersion and uniform distribution of clay in epoxy nanocomposites were achieved with high speed and high temperature mixing.
ACKNOWLEDGEMENTS
We acknowledge the Vietnamese Government for a scholarship to T.-D. Ngo. We also would like to thank the Natural Sciences and Engineering Research Council of Canada
for funding for this project (grant N00784 - Development of Polymer Nanocomposites, and grant N00004 - Analysis and Vibration of Elastic and Viscoelastic systems).
REFERENCES
[1] Pinnavaia T. J. and Beall G. W., Polymer-clay nanocomposites, John Wiley & Sons, New York (2000).
[2] Xu W.-B., Bao S.-P., He and P.-S., Intercalation and exfoliation behavior of epoxy resin/curing agent/montmorillonite nanocomposite, J. Appl. Polym. Sci. , 84, 842-849 (2002).
[3] Vaia R. A., Jandt K. D., Kramer E. J. and Giannelis E. P., Microstructural evolution of melt intercalated polymer-organically modified layered silicates nanocomposites, Chem. Mater., 8, 2628-2635 (1996).
[4] Utracki L. A., Clay-containing polymeric nanocomposites, Rapra Technology (2004).
[5] Messersmith P. B. and Giannelis E. P., Synthesis and characterisation of layered silicate-epoxy nanocomposites, Chem. Mater., 6, 1719-1725 (1994).
[6] Massam J. and Pinnavaia T. J., Clay nanolayer reinforcement of glassy epoxy polymer, Mater. Res. Soc. Symp. Proc. Spring Meeting, San Francisco, CA (US), Vol. 520, 223-232 (1998).
[7] Gilman J. W., Kashiwagi T., Nyden M., Brown J. E. T., Jackson C. L., Lomakin S., Giammelis E. P. and Manias E., Flammability studies of polymer layered silicate nanocomposites: polyolefin, epoxy and vinyl ester resins, Chapter 14 in Al-Malaika S., Golovoy A. and Wilkie C. A. (eds)- Chemistry and technology of Polymer Additives, Blackwell Science, Oxford, UK (1999).
[8] Kornmann X., Linberg H. and Berglund L. A., Synthesis of epoxy-clay nanocomposites- Infuence of the nature of the curing agent on structure, Polymer, 42, 4493-4499 (2001).
[9] Lan T. and Pinnavaia T. J., Clay-reinforced epoxy nanocomposites, Chem. Mater., 6, 2216-2219 (1994).
[10] Wang M. S. and Pinnavaia T. J., Clay-Polymer Nanocomposites formed from acidic derivatives of montmorillonite and an epoxy resin, Chem. Mater., Vol. 6, No. 4, 468-474 (1994).
[11] Becker O., Varley R. J. and Simon G. P., Thermal stability and water uptake of high performance epoxy layered silicate nanocomposites, Eur. Polym. J., Vol. 40, No. 1, 187-195 (2004).
[12] Kornmann X., Lindberg H. and Berglund L. A., Synthesis of epoxy–clay nanocomposites: influence of the nature of the clay on structure, Polymer, Vol. 42, No. 4, 1303-1310 (2001).
[13] Yasmin A., Abot J. L. and Daniel I. M., Processing of clay/epoxy nanocomposites by shear mixing, Scripta Materialia, Vol. 49, No. 1, 81-86 (2003).
[14] Chen C. and Tolle T. B., Fully exfoliated layered silicate epoxy nanocomposites, J. Polym. Sci. Part B: Polym. Phys., Vol. 42, No. 21, 3981-3986 (2004).
[15] Liu W. P., Hoa S. V. and Pugh M., Morphology and performance of epoxy nanocomposites modified with organoclay and rubber, Polym. Eng. Sci., Vol. 44, No. 6, 1178-1186 (2004).
[16] Chen C. and Curliss D., Resin matrix composites: organoclay-aerospace epoxy Nanocomposites Part II, Sample Journal, Vol. 37, No. 5, 11-18 (2001).
[17] Wang H., Hoa S. V. and Wood-Adams P. M., New method for the synthesis of clay/epoxy nanocomposites, Journal of Applied Polymer Science, Vol. 100, 4286–4296 (2006).
[18] Liu W. P., Hoa S. V. and Pugh M., Organoclay-modified high performance epoxy nanocomposites, Compos. Sci. Technol., Vol. 65, 307-316 (2004).
[19] Liu W. P., Hoa S. V. and Pugh M., Fracture toughness and water uptake of high- performance epoxy/nanoclay nanocomposites, Compos. Sci. Technol., Vol. 65, 2364-2373 (2005).
[20] Ngo T.-D., Ton-That M.-T., Hoa S. V. and Cole K. C., Curing kinetics and mechanical properties of epoxy nanocomposites based on different organoclays, in press, Polymer Engineering and Science, Vol. 47, No. 5 (2007).