KINETIC STUDIES ABOUT THE OXIDATION OF HYDROGEN
SULFIDE WITH IRON AMINOPOLYCARBOXYLATE
COMPLEXES IN PRESENCE OF DISSOLVED OXYGEN
Relevance to other reduced sulfur compounds
originating front kraft pulping
Thèse présentée
à la Faculté des études supérieures de l'Université Laval
dans le cadre du programme de doctorat en génie chimique
pour l'obtention du grade de Philosophiae Doctor (Ph. D.)
Département de génie chimique
FACULTÉ DES SCIENCES ET DE GÉNIE
UNIVERSITÉ LAVAL
QUÉBEC
OCTOBRE 2006
SUMMARY
Forty percent of North American pulping mills are exploiting the versatile kraft process to convert wood chips into high-quality pulp. Regrettably, it générâtes important amounts of chemically reduced sulfur compounds (TRS) of low molecular mass (H2S, CH3SH, (CH3)2S, (CH3)2S2) responsible for stinking odors. Although effective, current abatement technologies are employed to eradicate the TRS predicament without incentives for sulfur valorization. Hence, the current project investigates a prospective abatement approach that would convert TRS into recoverable sulfur species. It took origin from the Lo-Cat process which uses chelated iron(III) solutions to convert H2S into colloidal sulfur.
An investigation was initiated to establish the fundamentals (reaction kinetics, mechanisms, thermodynamic properties) about the HîSaq oxidation reaction with iron(III) chelates and assess if this concept could be further applied to other TRS species. The expérimental workload was carried out with the iron rrara-l,2-diaminocyclohexanetetraacetic acid (iron-cdta) chelate in alkaline solutions where H2S absorption (H2Saq - ^ HS~ +H+ ; pK° =7.1) is promoted.
Kinetic studies in anoxie solutions revealed that accumulation of polysulfide ions (Sj;~ ; n = 2-9) gained from the oligomerization of HS" with iron(HI)-cdta complexes accelerate the HS' conversion rate. Polysulfides eventually transform into colloidal sulfur (a-Sg). The kinetics is also strongly dépendant of the Fe3+cdta4" + H2O ^ Fe3+OH~cdta4~ + H+ (pK° = 9.7; 298 K) complex formation reaction which is influenced by ionic composition and température. The Fe3+cdta4" species is about 10 times more reactive than Fe3+OH~cdta4" toward the polysulfide ensemble. Accordingly, superior HS' uptake could be achieved in
the 8-9 pH range where Fe3+cdta4" and HS" species are prevailing. Dissolved oxygen was shown to disturb the polysulfide formation cycle by generating thiosulfate precursors restricting the accumulation of polysulfides that would otherwise improve the HS" conversion rates. This effect is yet conflicting with the strong re-oxidation potential of oxygen toward iron(II)-cdta keeping iron(III)-cdta concentrations constantly elevated. The following reaction settings were assessed to improve HS" conversions, and thus possibly the H2Sg absorption rates: mildly alkaline pH, high ionic strength, moderate dissolved oxygen concentrations and présence of dispersed colloïdal sulfur leading to the formation of polysulfides.
RESUME
Quarante pour cent des fabriques nord-américaines de pâte utilisent le procédé kraft afin de convertir la fibre de bois en pâte de haute qualité. Ceci engendre toutefois d'énormes quantités de composés de soufre réduit (SRT) de basse masse moléculaire (H2S, CH3SH, (CH3)2S, (01^3)282) qui sont responsables d'odeurs nauséabondes. Les techniques courantes d'abattement des SRT se focalisent exclusivement à éliminer le problème sans valoriser le soufre. Or, une approche qui convertirait les SRT en espèces récupérables serait une option valable dans le contexte papetier actuel.
L'étude prend origine du procédé Lo-Cat qui utilise des solutions de chélate de fer(III) pour convertir le sulfure d'hydrogène (H2S) en soufre colloïdal. Certains principes fondamentaux (cinétique réactionnelle, mécanisme, propriétés thermodynamiques) concernant l'oxydation de l^Saq en présence d'un chélate de fer(III) ont donc été établies afin d'évaluer l'applicabilité du présent concept pour le quatuor SRT en entier. Les travaux ont été réalisés avec l'acide trans di(aminediacétique)-l,2 cyclohexane (fer(III)-cdta) en milieu alcalin où l'espèce HS* prédomine sur H2Saq(H2Saq - ^ HS~ +H+ ; pK° = 7.1).
L'étude cinétique en milieu anoxique a permis de démontrer que les polysulfures (S^ ; n = 2-9) formés par oligomerisation de HS" avec l'aide du complexe fer(III)-cdta accélèrent la conversion de HS". La cinétique de conversion de HS" dépend également de la réaction de complexation suivante: Fe3+cdta4" + H2O ^ Fe3+OH~cdta4~ + H+ (pK° = 9.7; 298 K). En effet, l'espèce Fe +cdta " est environ 10 fois plus réactive envers les polysulfures que son analogue (Fe3+OH"cdta4"). Par conséquent, de grands taux de conversion ont été obtenus à un pH de 8-9 où les espèces Fe3+cdta4" et HS' prévalent. L'oxygène moléculaire quant à lui
perturbe le cycle de formation des polysulfures qui autrement auraient amélioré le taux de conversion de HS" par leur effet auto-catalytique. Ceci vient toutefois en contradiction avec le grand potentiel de l'oxygène pour la ré-oxydation du produit fer(H)-cdta en fer(HI)-cdta. Finalement, les conditions opératoires suivantes ont permis d'obtenir de hauts taux de conversion de HS": pH légèrement alcalin, haute force ionique, légère concentration d'oxygène dissous et la présence de soufre colloïdal permettant la formation de polysulfures ( S8 + HS~ $± S2g' + H+ ).
PREFACE
Public awareness about important environmental issues is constantly driving the chemical industries to adapt and develop processes yielding low pollution for highest product standards. This research project deals with one of thèse issues: the formation of foui odours from wood pulping processes. Although the situation is under control today, the operating cost for odour abatement is still excessive with little in return other than comply with the régulations. This thesis présents the global problem, the current solutions and their pitfalls and finally suggests a promising approach to curb the situation. The technical feasibility of this method based on strong scientific backgrounds has been explored in order to décide if it could be eventually applied in genuine conditions. This is the ultimate objective of this Ph. D. thesis.
A total of 8 research papers ail presented as chapters in this thesis were submitted for publication in chemical engineering journals. Six research papers ([1]—[4], [6], [8]) were already published at the time of thesis registration (September 2006). Another ([5]) is accepted and is pending publication soon while the last one submitted in July 2006, is still under review for publication in the Environmental Science and Technology [7] journal.
[1] Piché, S.; Grandjean, B.; Larachi, F. Equilibrium constants for the iron(III) trans-1,2-diaminocyclohexanetetraacetic acid hydroxy complexation reaction (Fe3+cdta4' /Fe3+OH'cdta4") in NaCl, Na2SO4 and LiCl aqueous solutions at 298 K. Journal of Chemical Engineering and Data 2003,48 (6), 1578-1582.
[2] Piché, S.; Larachi, F. Iron(III) trans- 1,2-diaminocyclohexanetetraacetic acid (Fe3+cdta47Fe3+OH"cdta4") in NaCl aqueous solutions: Effect of température on the
hydroxy complexation thermodynamic constant and on the activity coefficient ion-interaction parameters. Journal of Chemical Engineering and Data 2005, 50 (3), 863-868.
[3] Piché, S.; Larachi, F. Degradability of iron(III)-aminopolycarboxylate complexes in alkaline média: Statistical design and X-ray photoelectron spectroscopy studies. Industrial and Engineering Chemistry Research 2005,44 (14), 5053-5062.
[4] Piché, S.; Larachi, F. Oxidation kinetics of iron(II) complexes of fram1 -1,2-diamino-cyclohexanetetraacete (cdta) with dissolved oxygen: Reaction mechanism, parameters of activation and kinetic sait effects. Chemical Engineering Science 2006, 61 (11), 3452-3462.
[5] Piché, S.; Larachi, F. Dynamics of pH on the oxidation of HS~ with iron(HI) chelates in anoxie conditions. Chemical Engineering Science, accepted September 2006.
[6] Piché, S.; Larachi, F. Kinetic effect of electrolytes on the oligomerization of HS" into polysulfides and colloidal sulfur with iron(III) frara-l,2-diaminocyclohexanetetra-acetic acid in anoxie aqueous solutions. Chemical Engineering Science 2006, 61 (21), 7171-7176.
[7] Piché, S.; Larachi, F. Rôle of dissolved oxygen on hydrosulfide oxidation, and on iron chelate simultaneous in situ régénération. Environmental Science and Technology, submitted July 2006.
[8] Piché, S.; Ribeiro, N.; Bacaoui, A.; Larachi, F. Assessment of a redox alkaline/iron-chelate absorption process for the removal of dilute hydrogen sulfide in air émissions. Chemical Engineering Science 2005, 60, 6452-6461.
Each chapter is composed of one publication in its integrity. However, the introduction section for each chapter was partly modified to avoid répétition on recurring aspects. The research papers were prepared on my own and revised by my director, Prof. Faïçal Larachi. Prof. Bernard Grandjean, my co-director, also revised the first publication and was included as a co-author.
Acknowledgements
My thanks go first to the technical staff of the chemical engineering départaient at Laval University for their help during the course of this project.
I am taking the occasion to thank M. Nicolas Ribeiro (École Supérieur d'Ingénieurs de Chambery) who did most of the expérimental work related to publication [8] during his research training in summer 2004. My sincère thanks are also offered to M. Abdelaziz Bacaoui (post-doctorate, Laval University) who provided a helping hand for the development and analysis of the factorial design matrix associated to this study. M. Ribeiro and M. Bacaoui were included as co-authors.
My gratitude goes to Ms. Maude Gagnon (B.Sc. student, Chemical Engineering, Laval University) who did most of the expérimental work related to publication [3] during her research training in summer 2003. I am also thankful to M. Alain Adnot for collecting the XPS data for the same study.
My appréciation goes also to the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds Québécois de Recherche sur la Nature et les Technologies (FQRNT) for granting me scholarships during my stay at Laval University. Likewise, financial support from the NSERC for the proposed research project Nouveau procédé multifonctionnel pour l'élimination et la valorisation des SRT dans les émissions atmosphériques des fabriques papetier es led by Prof. Larachi is gratefully acknowledged.
M. André Normandin from Mésar/Environair Inc. also provided effort and documentation for this project. So I am taking the occasion to thank him for his precious help.
My co-director, Prof. Grandjean, is also acknowledged for this support.
Finally, I wish to express my eternal reconnaissance to Prof. Larachi for his support, help and leadership during my stay at Laval University leading to the Ph. D. degree.
TABLE OF CONTENT
SUMMARY i RÉSUMÉ iii PREFACE v TABLE OF CONTENT viii LIST OF FIGURES xii LIST OF TABLES xviii NOTATION xxii INTRODUCTION 1
1.1 Pulp and paper making 2 1.2 Origin of TRS in the kraft process 4 1.2.1 TRS characteristics 4 1.2.2 Kraft cycle 6 1.2.3 TRS formation 8 1.3 Actual TRS régulations 9 1.4 TRS removal stratégies 10 1.4.1 Major abatement technologies 11 1.4.2 Potential abatement methods 12 1.5 Research project objectives 14 1.6 TRS abatement via a redox reaction 15 1.6.1 H2S oxidation with the FeII1/Fe" redox couple 18
1.6.2 Fein/Fen chelates overview 21
1.6.3 H2S oxidation with iron(III) chelates 24 1.7 Scientific objectives 26 1.7.1 Iron(III)-cdta complex chemistry 26 1.7.2 Iron(III) chelates dégradation 26 1.7.3 Iron(II)-cdta + DO2 oxidation kinetics 27
1.7.4 Iron(IH)-cdta + HS" oxidation kinetics 27 1.7.5 H2S chemical absorption in a packed column 28
CHAPTER l.Equilibrium constants for the iron(III)
frvmy-l,2-diaminocyclohexane-tetraacetic acid hydroxy complexation reaction (Fe3+cdta47Fe3+OH"
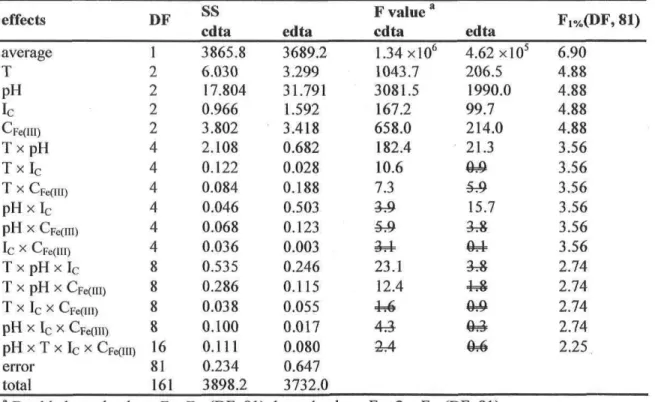
cdta4") in NaCl, Na2SO4 and LiCl aqueous solutions at 298 K 30 1.1 Introduction 32 1.2 Expérimental section 33 1.3 Expérimental results 35 1.4 Activity coefficients modeling 37 1.4.1 Hûckel équation 3 7 1.4.2 Bromley models 37 1.4.3 Scatchard expansion 38 1.4.3 Pitzermodel 39 1.5 Conclusion 41
CHAPTER 2.Iron(III) /raw,svl,2-diaminocyclohexanetetraacetic acid (Fe3+cdta4" /Fe3+OH"cdta4") in NaCl aqueous solutions: Effect of température on the hydroxy complexation thermodynamic constant and on the activity coefficient ion-interaction parameters 43 2.1 Introduction 45 2.2 Expérimental section 46 2.3 Equlibrium reaction product 48 2.4 Activity coefficients modeling 48
2.5 K° and y± normalization 52
2.6 Conclusion 54
CHAPTER 3.Degradability of iron(III)-aminopolycarboxylate complexes in
alkaline média: Statistical design and X-ray photoelectron
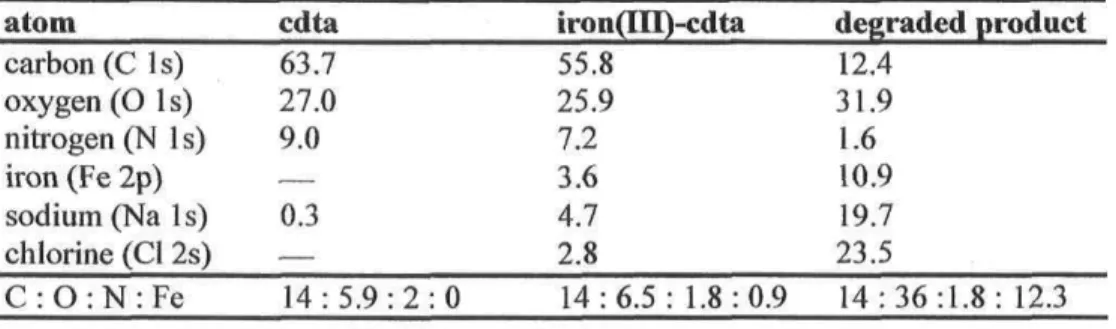
spectroscopy studies 56 3.1 Introduction 58 3.2 Factorial design and analysis 59 3.3 Expérimental procédure 60 3.4 Data analysis 61 3.5 Factorial analysis 64 3.6 Characterization of the dégradation product 67 3.6.1 Survey scans 68 3.6.2 XPS characterization of cdta 69 3.6.3 XPS characterization of iron(III)-cdta 71 3.6.4 XPS characterization of iron 73 3.7 Conclusion 76
CHAPTER 4.Oxidation kinetics of iron(II) complexes of
frww-l^-diamino-cyclohexanetetraacete (cdta) with dissolved oxygen: Reaction
mechanism, parameters of activation and kinetic sait effects 77 4.1 Introduction 79 4.2 Expérimental section 82 4.3 O2, iron(II)-cdta and iron(IH)-cdta quantification 83 4.4 Results and discussion 85 4.4.1 Kinetics in alkaline solutions 85 4.4.2 Activation parameters 89 4.4.3 Kinetic electrolyte effects 92 4.4.4 Kinetics in acid-to-neutral solutions 93 4.4.5 Iron-cdta dégradation 98 4.5 Conclusion 99
CHAPTER 5.Dynamics of pH on the oxidation of HS" with iron(III) chelates in
anoxie conditions 100 5.1 Introduction 102 5.2 Expérimental section 103 5.3 Preliminary observations 104 5.4 Reaction mechanism 106 5.5 Data réduction assumptions and quantification procédure 110 5.5.1 Iron(III)-cdta, iron(II)-cdta and HS" 112 5.5.2 Elemental sulfur (Sg) 113 5.5.3 Polysulfides 114 5.6 Results and discussion 115 5.6.1 Effect of pH on kinetics 115 5.6.2 pH effect on formation of a-Sg 119 5.6.3 Reaction mechanism parameters and model discrimination 120 5.7 Conclusion 123
CHAPTER ô.Kinetic effect of electrolytes on the oligomerization of HS' into
polysulfides and colloidal sulfur with iron(III)
trans-1,2-diaminocyclohexanetetraacetic acid in anoxie aqueous solutions 124 6.1 Introduction 126 6.2 Expérimental approach 127 6.3 Results and discussion 128 6.3.1 Apparent kinetic effect of electrolytes 128 6.3.2 Rate laws constants 132 6.4 Conclusion 134
CHAPTER 7. Rôle of dissolved oxygen on hydrosulfide oxidation, and on iron
7.1 Introduction 137 7.2 Expérimental section 137 7.3 Preliminary observations 138 7.4 Species quantification 140 7.5 Results and discussion 142
7.5.1 Effect of DO2 on kinetics 145
7.5.2 Reaction mechanism proposai 149 7.5.3 Sulfide profiles balance 151
CHAPTER 8.Assessment of a redox alkaline/iron-chelate absorption process for the
removal of dilute hydrogen sulfide in air émissions 155 8.1 Introduction 157 8.2 Factorial design approach 158 8.3 Expérimental section 159 8.4 Factorial analysis , 162 8.4.1 Plackett-Burman matrix 162 8.4.2 Factorial model and optimisation 165 8.5 Influence of iron(III)-cdta on H2S scrubbing 168 8.6 Conclusion 172
CONCLUDING REMARKS 173
C l Results overview 174 C.2 Results significance 177 C. 3 Future work recommendations 181
LITERATURE CITED 184 APPENDIX A. UV spectra calibration measurements 199 APPENDIX B. Dégradation of iron-cdta and iron-edta: Results in function of pH,
ionic strength, température and iron chelate concentration 205
APPENDIX C.Oxidation kinetics of iron(II)-cdta with DO2: Mechanism, rate law,
expérimental conditions and gênerai results 212
APPENDIX D.Oxidation kinetics of HS" with iron(III)-cdta in anoxie conditions:
Mechanism, rate law, expérimental conditions and gênerai results 222
APPENDIX E. Effect of electrolytes on the oxidation kinetics of HS' with
iron(III)-cdta in anoxie conditions: Expérimental conditions and results 234 APPENDIX F. H2S scrubbing with alkaline iron(III)-cdta solutions: Expérimental
conditions and gênerai results 238
LIST OF FIGURES
INTRODUCTION Figure 1.1Figure 1.2
Figure 1.3
Figure 1.4
Figure 1.5
Figure 1.6 CHAPTER 1 Figure 1.1 Figure 1.2 Figure 1.3 Figure 1.4 Figure 1.5 CHAPTER 2 Figure 2.1 Figure 2.2Chemical structure of TRS species with their Chemical Abstract Services (CAS) number.
Simplifiée! diagram representing the kraft process cycle.
Chemical structure of tetraanionic ethylenediaminetetraacetic acid (edta4") complexed with iron(III) (Fe3+edta4" and Fe3+OH'edta4"). Chemical structure of hexa- and hepta-coordinated iron(III)
trans-1,2,-diaminocyclohexanetetraacetic acid (Fe3+cdta4'; Fe3+OH"cdta4'). Relative concentration distribution in the pH domain for iron(II)-cdta, hydrated iron(II) ions, cdta4"(H+)4, cdta4"(H+)3 and cdta4'(H+)2 in demineralised water at T = 25 °C.
Simplified Lo-Cat process flow chart.
UV light spectra (225-325 nm) for individual Fe3+cdta4' and Fe3+OH"cdta4' species with an example of combinatory spectra. Stirred cell reactor with appendices.
Typical concentration distribution of Fe3+cdta4" and Fe3+OH"cdta4' as a fonction of pH at T = 298 K and Im = 0.5 mol NaCl/kg. log(Kc) values vs the ionic strength for NaCl solutions (Im < 0.1 mol/kg).
log(Kc) values vs the ionic strength for NaCl, Na2SÛ4 and LiCl solutions according to Hûckel, Bromley, Scatchard and Pitzer activity coefficient models.
Stirred cell reactor with appendices.
Expérimental log(Km) as a fonction of ionic strength (Im) for NaCl solutions maintained at 280 K, 288 K, 298 K, 305 K, 313 K and 323 K. 4 7 22 24 24 25 34 35 36 38 41 47 51
Figure Figure 2.3 2.4 CHAPTER3 Figure Figure Figure Figure Figure Figure Figure Figure Figure 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 CHAPTER 4 Figure Figure 4.1 4.2 Figure 4.3 Figure 4.4 Figure 4.5
ln(K°) against 1000/T (K"1) schematic plot based on averaged K° values obtained from ail four activity coefficient models fitting. Evolution of Pitzer's empirical ion-interaction summations (ZP±,
S P±, Psalt ) with température.
Evolution of iron(III) chelates daily dégradation with pH and température.
Evolution of iron(III) chelates daily dégradation with ionic strength and iron(III) concentration.
Quality-of-fit portrayal (parity diagram, residual plot, normal quantile-quantile plot of the residuals) of the optimized ANOVA models.
Speculated cdta oligomerization through carbonyl and aminé condensations.
High-resolution XP C ls spectra for samples cdta, iron(III)-cdta and dégradation product along with the fitted elemental spectra for cdta.
High-resolution XP N 1 s spectra for samples cdta and iron(III)-cdta along with the fitted elemental spectra for cdta.
High-resolution XP O ls spectra for samples cdta, iron(HI)-cdta and dégradation product along with the fitted elemental spectra for cdta.
High-resolution XP Fe 2p spectra for the iron(III)-cdta sample with fitted elemental spectra.
High-resolution XP Fe 2p spectra for the dégradation product sample with the fitted elemental spectra.
Stirred cell reactor with appendices.
Concentration profiles for dissolved O2, iron(II)-cdta and
(Fe3+cdta4" + Fe3+OH"cdta4") products during a kinetic trial at T = 297.6 K, le = 0.05 mol/L NaCl, pH = 9.55, C ^ =61.9 umol/L and
C°Fe(II) =212nmol/L.
UV absorbance spectra at room température for individual Fe2+cdta4\ Fe3+cdta4' and Fe3+OH'cdta4" species.
Fe2+cdta4" concentration profiles for reactions operated at pH = 8.05 (fFe3+ = 98%) and pH = 10.55 (fFe3+ = 15%) with best fitting of a second-order rate law.
Eyring plot with kiiapp in mVmol-s.
52 54 63 63
66
68 70 71 72 74 75 84 85 87 91Figure 4.6 Figure 4.7 Figure 4.8 Figure 4.9 CHAPTER 5 Figure 5.1 Figure 5.2 Figure 5.3 Figure 5.4 Figure 5.5 Figure 5.6 Figure 5.7 Figure 5.8 Figure 5.9 Figure 5.10 Figure 5.11 CHAPTER 6 Figure 6.1 Figure 6.2 Figure 6.3
Linearization of (ln(ki>apph/kBT) + 92600/(RT))/ln(10)) with respect to the molar concentration of NaCl, LiCl and Na2SC<4 with ki>app
[m3/mol-s].
Relative concentration distribution of iron(II) and superoxide species in acidic conditions.
Average kijapp values based on eq 4.29 for each pH set.
UV absorbance spectra of a non-oxidized iron(II)-cdta solution for a wide pH range.
Evolution of UV absorbance during an oxidation experiment in function of wavelength and reaction time at À, = 260 nm.
Evolution of the solution's turbidity in contrast with the solution's absorbance at X = 260 nm.
Typical évolution of species concentrations in the reaction enclosure.
Polysulfide anions (S,~ — S^") absorbance approximation for C 2. = 125/n umol/L based on Licht et al. [173] numerical analysis.
Diagram representing the final HS" molar conversion (mmol/m3) with the final turbidity measurement (NTU) for 50+ experiments. HS" consumption rate profiles for solutions at pH = 9, pH = 9.5, pH = 10,pH = 1 0 . 5 a n d p H = l l .
Dependence of the HS" half-life with respect to the fraction of the non-hydroxylated iron(III)-cdta species ( fFe3+ ).
HS" consumption rate profiles for solutions containing iron(III)-edta at pH = 9, pH = 9.5 and pH = 10.
Iron(III)-cdta reaction rate in the time domain for solutions at pH = 9, pH = 6 and pH = 4.
HS" molar conversion when turbidity first appeared in the solution
HS
f u n c t i o n o f f
3
re
Optimized kinetic parameter experiments.
for 5 0 + experiments. in function of ffe3+ for 50+
Schematic diagram representing the oligomerization of HS" into polysulfides and colloidal sulfur assisted by the FeIIIcdta/Fe11 cdta redox System.
Evolution of HS" conversion, iron(IH)-cdta conversion and turbidity in the time domain for NaCl solutions of 0 to 0.2 mol/L and pH approaching 10.5.
Effect of NaCl concentration on the autocatalytic induction period, HS" half-life moment and 80% HS" conversion moment.
93 95 97 98 105 106 111 112 114 116 117 118 119 120 121 127 129 130
Figure 6.4 Effect of electrolyte concentration on the HS" half-life for différent fFe,+ values (15-70%) and différent electrolytes (NaCl, LiCl,
Na2SO4). 131
Figure 6.5 Logarithmic linearization of the computed k4;app values in function
of the cation concentration. 133
CHAPTER 7 Figure 7.1 Figure 7.2 Figure 7.3 Figure 7.4 Figure 7.5 Figure 7.6 Figure 7.7 Figure 7.8 Figure 7.9 CHAPTER 8 Figure 8.1 Figure 8.2 Figure 8.3 Figure 8.4 Figure 8.5 Figure 8.6
Evolution of the solution's absorbance spectrum at 1 minute intervais for a kinetic run.
Electrophoregram confirming the présence of HS', SO^", Cl", S2C>3~ and polysulfides prior and 60 minutes after injection of iron(IH)-cdta.
Iron(IH)-cdta, HS", S2Oj~ and DO2 concentration profiles
computed from raw spectral measurements depicted in Figure 7.1. Species concentration profiles for two trials with similar initial conditions.
HS" and corresponding iron(III)-cdta conversion profiles for trials having différent initial iron(III)-cdta concentration.
HS" and corresponding iron(III)-cdta conversion profiles for trials at pH = 10.2 ±0.1 having différent initial DO2 concentration. HS" and corresponding iron(III)-cdta conversion profiles for trials at pH = 9.9 ±0.1 having différent initial DO2 concentration. HS" and corresponding iron(III)-cdta conversion profiles for trials at pH = 9.3 ± 0.1 having différent initial DO2 concentration. Proposed mechanism regarding the simultaneous formation of colloidal sulfur (Sg), thiosulfate (S2O32" ) and sulfate (SO," ) from aqueous solutions of HS", O2 and iron chelate catalyst.
Laboratory packed column.
Screening coefficients based on the Plackett-Burman design matrix for the six factors plus the mean conversion value.
Parity plot for the factorial corrélation.
Linear, quadratic and first-order interaction coefficients distribution.
Surface response diagrams in function of pH, L and C^ s.
Influence of iron(III)-cdta for the removal of H2S at pH = 9.5 and pH = 10.5 based on the average H2S conversion in function of iron(III)-cdta concentration and the enhancement factor in function of Cpe(IU) square root.
139 140 142 144 145 146 147 148 150 160 163 166 166 168 171
CONCLUDING REMARKS
Figure C. 1 Fraction of HS' multiplied by the fraction of the non-hydroxylated
iron(IH) chelate in the pH domain. 178 Figure C.2 An HS" oxidation trial in anoxie, alkaline (pH =10) solution in
terms of HS" reaction rates and turbidity units. 179 Figure C.3 Evolution of the solution's UV absorbance spectrum at 1 minute
intervais for a preliminary test between dissolved CHsSNa and
iron(III)-cdta in function of wavelength and reaction time. 182 Figure C.4 Preliminary test between iron(III)-cdta and aqueous dimethyl
sulfide. 183 Figure C.5 Preliminary test between iron(III)-cdta and aqueous dimethyl
disulfide. 183
APPENDIX A
Figure A. 1 Spectra for individual Fe3+cdta4", Fe3+OH"cdta4", Fe2+cdta4" and HS" species having a concentration of 150 umol/L. 200 Figure A.2 Spectra for individual Fe3+edta4" and Fe3+OH"edta4" species having
a concentration of 150 umol/L. 200 Figure A.3 Absorbance at specified wavelength of Fe3+cdta4" solutions for
various concentrations at pH = 4. 201 Figure A.4 Absorbance at specified wavelength of Fe +OH"cdta " solutions for
various concentrations at pH = 12. 201 Figure A. 5 Absorbance at specified wavelength of Fe3+edta4" solutions for
various concentrations at pH = 4. 202 Figure A.6 Absorbance at specified wavelength of Fe3+OH'edta4" solutions for
various concentrations at pH =12. 202 Figure A.7 Absorbance at specified wavelength of Fe2+cdta4" solutions for
various concentrations at pH =10. 203 Figure A. 8 Absorbance at specified wavelength of HS" solutions for various
concentrations at pH = 10. 203 Figure A.9 Absorbance at specified wavelength of S2C>3~ solutions for various
concentrations at pH = 9. 204
APPENDIX C
Figure C. 1 Expérimental iron(II)-cdta concentration profile having the best fit
over the corresponding rate law. 221 Figure C.2 Expérimental iron(II)-cdta concentration profile having the worst
fit over the corresponding rate law. 221
APPENDIX D
Figure D. 1 Expérimental HS" and iron(III)-cdta concentration profiles
Figure D.2 Expérimental HS' and iron(III)-cdta concentration profiles showing the worst mismatch with the corresponding rate laws. 233
APPENDIX E
Figure E. 1 Expérimental HS" and iron(IH)-cdta concentration profiles
matching best the corresponding rate laws. 237 Figure E.2 Expérimental HS" and iron(HI)-cdta concentration profiles showing
LIST OF TABLES
INTRODUCTION Table 1.1 Table 1.2 Table 1.3 Table 1.4 Table 1.5 Table 1.6 Table 1.7 Table 1.8 CHAPTER1 Table 1.1 Table 1.2 CHAPTER2 Table 2.1 Table 2.2 Table 2.3Physical and chemical properties of TRS species 5 TRS safety concerns 6 Typical NCG chemical composition 9 Régulations on TRS émissions for mills exploitée in the province of Québec and the United States 10 Advantages and inconveniences for current and potential TRS abatement Systems 12 Standard potentials for common oxidizing agents in aqueous solutions at 25 °C 16 Détails of investigations about the oxidation of sulfide species in aqueous solutions of dissolved oxygen or hydrogen peroxide 17 Examples of aminopolycarboxylate and heterocyclic ligands with their corresponding formation constant (KML) with iron(II) and iron(III) 22
Average log(Kc) from 3-5 values for the Fe3+cdta47Fe3+OH"cdta4" pair in NaCl, Na2SÛ4 and LiCl solutions of various ionic strength 36 Optimized log(K°) and ion-interaction parameters for each activity coefficient model 40
Température-dépendant water dissociation constant (Kw), water
density (pw) and Debye-Huckel constant (Aj,) 46 Average log(Km) on 5-7 values for the Fe3+cdta47Fe3+OH~cdta4~
pair in NaCl aqueous solutions controlled at différent températures
and ionic strengths 48 Optimized log(K°) and activity coefficient ion-interaction
Table 2.4 Table 2.5 CHAPTER3 Table 3.1 Table 3.2 Table 3.3 Table 3.4 Table 3.5 Table 3.6 CHAPTER 4 Table 4.1 Table 4.2 Table 4.3 CHAPTER 5 Table 5.1 Table 5.2 CHAPTER 7 Table 7.1 Table 7.2
Ion-interaction parameter average and the conséquence of their application in the corresponding model on the average absolute
error (AAE) 53 Normalization of the enthalpy and entropy of formation with the
Pitzer ion-interaction summations ( Z P ° , ZP±, P'ait ) based on the
whole database 54
Average (six-value ± standard déviation) of relative dégradation
rates in function of pH, température and ionic strength 62 Sum of squares (SS) and F values for each factorial models based
on complète expérimental matrices 64 Atomic constitution (atom%) for each studied sample 69 XP spectra fit assessment for C ls, N ls, and O i s core électrons
with peak assignaient for the cdta sample (SI) 70 XP spectra fit assessment for C ls, N ls, and O ls core électrons
with peak assignaient for the iron(III)-cdta sample (S2) 73 XP spectra fit assessment for Fe 2p3/2 and Fe 2pi/2 core électrons
with peak assignaient for the iron(III)-cdta (S2) and dégradation
product (S3) samples 73
Rate law constants (kiiapp , (k.i/k2)apP) average for différent alkaline settings (T = 297.2 ± 6.5 K, Ic = 0.05 mol/L NaCl) 89 Rate law constant (kijapp) average for différent acidic-to-neutral
settings (T - 297.2 ± 0.5 K, Ic = 0.05 mol/L NaCl) 96 Average O2 consumption level for each sub-study (a = moles of O2 consumed per mole of iron(II)-cdta oxidized) 99
Molar absorptivities (r\) of individual chemical species at différent
wavelength (À,) 113 Evolution of rate laws constants along the optimization procédure
with corresponding fitting errors on HS" and iron(III)-cdta
concentration profiles 122
Molar absorptivities (r|) for individual species following the
Beer-Lambert law at différent wavelength (k) 142 Review of expérimental conditions and corresponding results 153
CHAPTER 8
Table 8.1 Packing characteristics (15-mm plastic Ralu ring) with pressure
drop measurements and liquid hold-up estimations 160 Table 8.2 Complète expérimental matrix (conditions and results) used for the
détermination of linear, quadratic and first-order interaction
coefficients on H2S conversion 164 Table 8.3 Average iron(III)-cdta concentration ratio ( C*!,,,)/Cpe,m, ) and the
final hydrosulfide (HS") content in the scrubbing liquid phase 170
APPENDIX B
Table B. 1 Iron-cdta daily dégradation in terms of pH, ionic strength and iron-cdta concentration at 25 ± 1 °C 206 Table B.2 Iron-cdta daily dégradation in terms of pH, ionic strength and
iron-cdta concentration at 40 ± 1 °C 207 Table B.3 Iron-cdta daily dégradation in terms of pH, ionic strength and
iron-cdta concentration at 55 ± 1 °C 208 Table B.4 Iron-edta daily dégradation in terms of pH, ionic strength and
iron-edta concentration at 25 ± 1 °C 209 Table B.5 Iron-edta daily dégradation in terms of pH, ionic strength and
iron-edta concentration at 40 ± 1 °C 210 Table B.6 Iron-edta daily dégradation in terms of pH, ionic strength and
iron-edta concentration at 55 ± 1 °C 211
APPENDIX C
Table C l Expérimental conditions and results for the kinetics in alkaline
solutions sub-study 215 Table C.2 Expérimental conditions and results for the activation parameters
sub-study 216 Table C.3 Expérimental conditions and results for the kinetic sait effects
sub-study 217 Table C.4 Expérimental conditions and results for the kinetic in
acid-to-neutral solutions sub-study 219
APPENDIX D
Table D. 1 Expérimental conditions and results for the kinetic study between
HS" and iron(III)-cdta in anoxie alkaline solutions 231
APPENDIX E
Table E. 1 Expérimental conditions and results for the kinetic study between
Table E.2 Expérimental conditions and results for the kinetic study between
HS' and iron(III)-cdta in LiCl solutions 236 Table E.3 Expérimental conditions and results for the kinetic study between
HS" and iron(III)-cdta in Na2SÛ4 solutions 236
APPENDIX F
Table F. 1 Expérimental conditions and results for the effect ofiron(III)-cdta
NOTATION
Acronyms
AAE average absolute error
AARE average absolute relative error ANOVA analysis-of-variance
APC aminopolycarboxylic acid BE binding energy
CAA clean air act
cdta fr*amc-l,2-diaminocyclohexanetetraacetic acid CE capillary electrophoresis
DF degree of freedom DO2 dissolved oxygen DP dégradation product
edta ethylenediaminetetraacetic acid emf electromotive force
EPA Environmental Protection Agency EPR électron paramagnetic résonance fwhm Ml widthat half maximum GTP Gas Technology Products LLC
hedta (hydroxyethyl)-ethylenediaminetetraacetic acid HVLC high volume low concentration
LVHC low volume high concentration MSDS material safety data sheet NCG noncondensible gases
NSPS nta PCR RSA SI S2 S3 SS SSE TRS UV Vis XP XPS
Symbols
Anew source performance standards nitrilotriacetic acid
principal component régression routine sampler accessory cdta powder sample (Chapter 3) dried iron(III)-cdta sample (Chapter 3)
iron(III)-cdta dégradation product sample (Chapter 3) sum of squares
sum of squared error total reduced sulfur ultraviolet
visible
X-ray photoelectron spectra X-ray photoelectron spectroscopy
absorbance fcm"1! Bi, B2, Ba C° Ci, C2, C3 Cs DH,s " ]
Debye-Huckel parameter [kg"°5 mol"0 5] factorial linear coefficients (Chapter 8) factorial quadratic coefficients (Chapter 8)
factorial first-order interaction coefficients (Chapter 8) Bromley model empirical constants [mol kg"1, - , kg0 5moi"°5] standard state concentration [mol L"1] (Chapter 4)
Scatchard expansion empirical constants [kg mol"1, kg2 mol"2, kg mol^] a species molar concentration [mol L"1]
electrolyte molar concentration [mol L"1] H2S diffusion coefficient in solution [m2 s" ] chemical enhancement factor
standard potential [V]
fFe2+ fraction of iron(II)-cdta species as Fe2+cdta4" [%] fFe2+H+ fraction o f iron(II)-cdta species a s Fe2 +cdta4"(H+) [%] fHS_ fraction o f sulfïde species as HS" [%]
fQ._ fraction o f superoxide species as O'2~ [%]
f n2_ fraction o f converted HS" into S . O ^ [%] F Faraday constant [C mol"1]
G gas mass flow rate [kg m"2 s"1] h Planck constant [J s]
H a Hatta number
hT total bed liquid hold-up [%]
l e ionic strength based o n molar concentration [mol L"1] Im ionic strength based on molal concentration [mol kg*1]
K ° thermodynamic reaction equilibrium constant Kai iron(II)-cdta protonation constant
ka a reaction step kinetic constant [L mol"1 s"1] ke Boltzmann constant [J K"1]
Kc reaction equilibrium constant based on species molarity K D I - K D 4 A P C ligand deprotonation constants
kûaw voiumetric gas film mass transfer coefficient [s"1]
K o aw voiumetric mass transfer coefficient on the gas phase [s"1] kL liquid film mass transfer coefficient [m s"1]
kLaw voiumetric liquid film mass transfer coefficient [s"1] Km reaction equilibrium constant based on species molality KML iron chelate stability (or formation) constant
ks salting-out (Setchenow) constant [L mol"1] Ksi-Ks3 Fe(OH)3 protonation constants
Ksp solubility product [moln L"n]
Kw water dissociation constant [mol2 kg"2]
L m MN a Ci NH 2s NTU P ra R R2 t tl/2 T
liquid mass flow rate [kg m"2 s'1] thermodynamic partition coefficient a species molality [mol kg"1] NaCl mass [g]
H2S molar flux [mol L'1 s'1]
nephelometric turbidity units [NTU] pressure [Pa]
a species reaction rate [mol L"1 s"1] gas constant [J mol'1 K"1]
corrélation coefficient time [s]
half-life [s] température [K]
gas superfïcial velocity [m s"1] U L liquid superficial velocity [m s"1] VB pH buffer volume [mL]
VFe,m) volume of iron(HI) chelate stock solution [mL] VG gas volume [mL]
Xa v g expérimental average H2S conversion in the gas phase [%]
LFe(lII)
XF, , iron(III)-cdta molar conversion [mol L'1] X HS" molar conversion [mol L"1]
Xpred predicted H2S conversion in the gas phase [%] z bed length axis [m]
Z packed bed height [m] Z1-Z4 linearized factorial variables
Greek letters
a moles of O2 consumed per mole iron(II)-cdta oxidized (Chapter 4) P^alt Pitzer model electrolyte interaction constant
P ° , (^ Pitzer model individual cation/anion interactions iron chelate daily dégradation rate [umol L"1 day"1] /ACHS^ mole of DO2 consumed per mole HS" oxidized ÀCS sulfur mass balance discrepancy [%]
ÀE redox potential différence [V]
AE° standard redox potential différence [V] AG Gibbs free energy [kJ mol"1]
AG° standard Gibbs free energy [kJ mol'1] A*H°, A*H? enthalpy of activation [kJ mol'1]
ArHm complex formation reaction enthalpy [kJ mol'1] APp pressure drop in dry bed [Pa m"1]
APW pressure drop in irrigated bed [Pa m'1] A*S°, A*S: entropy of activation [J mol"1 K"1]
ArSm complex formation reaction entropy [J mol*1 K"1]
Az2 s u m of squared product charge minus the s u m of squared reactant charge s, ss Htickel équation salting-out constant [kg mol"1]
ya a species activity coefficient y± activity coefficients quotient
r)a a species molar absorptivity [L mol"1 cm"1] K Eyring équation transmission coefficient X wavelength [nm]
LJ. average absolute relative error [%] (Chapter 3) p aqueous solution density [kg L"1]
pw water density [kg L"1]
aM standard déviation on u [%] (Chapter 3)
± , ZP± Pitzer model cation/anion interaction summations
app aq Fe Fe(II) Fe(III) Fe2+ Fe31 apparent aqueous
combined iron(III) and iron(II) species combined iron(II) species
combined iron(III) species Fe +cdta4" species Fe +cdta4" species gas phase I L ref S •H
-Superscript
0 a avg c c C min N T U > 0 * intercept liquid phase référence slope activated complex initial reaction order averageend of reaction (Chapter 7) exit (Chapter 8)
corrected minimum
time when turbidity is first appearing uncorrected value
INTRODUCTION
Environmental issues play a key rôle in driving the pulp and paper industry into new directions. Through environmentally incentive and energy-saving programs, the future of the industry relies on the économie modernization of pulping, bleaching and chemical recycling processes whether they are progressive or innovative, so as to meet toughest environmental régulations and market demand. For instance, the kraft process, relying on hydroxide and sulfide as principal pulping ingrédients, générâtes important amounts of chemically reduced sulfur species (TRS) of low molecular mass (hydrogen sulfide, H2S; methyl mercaptan, CH3SH; dimethyl sulfide, (CHb^S; dimethyl disulfide, (€£[3)282) which are responsible for the distinctive foui odour neighbouring pulp mills. Thèse airborne pollutants can be perceived by humans at concentrations as low as 1 ppbv forcing the industry to deal with this problem in response to the environmentally conscious population. Accordingly, the latest review of the législative document entitled Règlement sur les fabriques de pâtes et papiers, Québec (1998) [1] covering a range of technical and environmental matters related to the pulp and paper industry in Québec imposes a strict control on TRS émissions from important process equipments. Similar régulations were adopted in the United States (US Fédéral Register 40 CFR part 63, 1998) [2].
Various TRS abatement measures are presently considered by the pulp and paper industry. The traditional approach is thermal incinération in a plant boiler or dedicated incinerator with other noncondensible gases [3, 4]. Although efficient, it remains an expensive method which can lead to potential explosion risks when highly concentrated TRS streams are loaded with air. It also créâtes another environmental concern by releasing substantial quantities of sulfur dioxide, a precursor to acid rain [5, 6]. Alkaline (pH > 9) and
aminé-based scrubbing are also convenient for H2S and CH3SH but are not suitable for the removal of (CH3)2S and (CH3)2S2. Chemical wet scrubbing with a strong oxidizer (i.e. CI2, CIO2, H2O2, KMnO4, NaOCl) is also a viable method for transforming TRS, for example, into methanesulfonic acid [7]. However, the présence of other organic compounds in flue gases causes this method to be expensive since the oxidation is non-specific to TRS.
Current abatement methods focus exclusively on the élimination of TRS without valorization of sulfur, which is unfortunate since it could hâve a positive impact on the kraft pulp industry. Indeed, application of such greener abatement technique would certainly improve the économie viability of the process considering the récent and future tightening of the régulations. It is in this state of mind this research project was initially proposed.
1.1 Pulp and paper making
The pulp and paper industry includes manufacturing activities that convert cellulose fibre (i.e. wood, cotton, linen rags) into pulps, papers and paperboards. It is today a large capital-intensive industry characterized by complex Systems for manufacturing high-quality products vital to éducation, communication, packaging and construction just to name a few. Canada ranks second to the United States in pulp and paper manufacture with its 140-180 pulp, pulp and paper, and paper mills [8]. Together, the US and Canada produce about 35% of the world pulp. Annual pulp production of over 20 million tons (40% - newsprint; 37% - paper and paperboard; 23% - packaging papers, boards and others) in the mid 1980s has been valued at $14 billion and has accounted for about 3% of the Canadian GNP. Exports of $11 billion to the US (66%), western Europe (15%), Japan (6%) and other markets (13%) hâve comprised about 9% of total Canadian exports. Needless to say, the pulp and paper industry is a comerstone of Canadian économie life being the third largest industrial employer with about 85000 factory and office jobs.
The full potential of Canadian pulp and paper industry based on the vast forest resource was realized after the discovery of groundwood chemical pulping in 1840. Indeed, pulp fibres can be extracted from almost any vascular plant found in nature. However, a high yield of fibres is necessary if the plant is to hâve économie importance. The major plant
sources are: coniferous wood, deciduous wood, canes, bamboos, leaf fibres and seed fibres [9]. In North America, wood is the most abundant and only source of papermaking fibres. More specifically, the source will corne from lumber and wood chips (21%), sawdust residues (55%) and recycled fibre (24%). Wood is composed of cellulose (-(QHioOs),,- or polymeric glucose) at ± 45% and hemicelluloses (polymeric glucose, mannose, galactose, xylose and arabinose) at ± 30%. Thèse carbohydrates are cemented together with a highly-polymerized substance called lignin which composes ± 25% of wood. Extractives (± 5%) are also présent.
Commercial puiping processes can be classified in three catégories: mechanical, chemicai and semi-chemical. Mechanical puiping (i.e. stone groundwood, refîner mechanical pulp) uses mechanical energy with little amounts of chemicai and heat to produce short impure fibres of good printing quality. In contrast, chemicai puiping (i.e. soda, sulfite, kraft) uses heat and chemicals to produce longer and stronger fibres. Semi-chemical processes (i.e. high-yield kraft) use a combination of both stratégies. The objective of puiping is to dissolve and dégrade the lignin while leaving most of the cellulose and hemicelluloses intact. Unfortunately, 40-50% of the original wood substance is degraded during chemicai puiping, which contrasts with the mechanical puiping methods. Yet, it occupies a significant position in North America because of its advantages regarding chemicai recovery, energy consumption and pulp strength.
The first important chemicai puiping process used sodium hydroxide to cook wood chips under pressure (soda process). An évolution was proposed by C. F. Dahl in 1885 in an effort to find a substitute for the expensive sodium carbonate (Na2CC>3) makeup chemicai [9]. From experiments, Dahl established that sodium sulfate (Na2S(>4) chemically reduces into sulfide salts (i.e. Na2S) in the recovery furnace. Addition of thèse salts into the cooking liquor accélérâtes delignification and produces a much stronger pulp. The new puiping method which combines sodium hydroxide and sodium sulfide as part of the cooking liquor was first implemented in Sweden in 1885. Its versatility and fiexibility makes it suitable for use with most wood species. The kraft process is actually used in about 40% of mills exploited in North America [9]. The principal drawbacks are the pulp dark color, its bleaching costs and the malodorous TRS émissions.
1.2 TRS origin in the kraft process
1.2.1 TRS charactemtics
The chemical structure of TRS species is presented in Figure 1.1. Each contains one sulfur atom except dimethyl disulfide which has 2 sulfur atoms. Ail sulfur atoms hâve an oxidation state of-2.
H H H H H
H-S-H HhC^-^-H H-C-^-S—C—H H~C—S-S-C^H
f f f
H fi fi fi H
Mydrogen sulfîde Mtttiyl mweaptan Oimethyl sutfitte Dtmethyl disulfid© CAS #7783-064 CAS #74-93-1 CAS #75-08-1 CAS «62442-0 FIGURE 1.1. Chemical structure of TRS species with their Chemical Abstract Services (CAS) number
Important physical and chemical properties of TRS are presented in Table 1.1. Most of the information was obtained from material safety data sheets (MSDS) published on Matheson Tri-Gas web site (Appendix G [10]). TRS boiling points, solubilities and acidities are of signifïcance for some abatement processes. For example, chemical wet scrubbing dépends greatly on the solubility of TRS species in aqueous solutions which unfortunately is not very high for (CH3)2S and (CH3)2S2 even with relatively high Henry's law constants (H2S: 0.08 mol/L-atm [11]; CH3SH: 0.20 mol/L-atm [12]; (CH3)2S: 0.53 mol/L-atm [13]; (CH3)2S2: 0.76 mol/L-atm [14]). The poor absorption of (CH3)2S and (CH3)2S2 is due to their neutrality compared to the weak acidity of H2S and CH3SH. Indeed, their dissociation into HS7S2" and CH3S" accélérâtes the solubility/absorption phenomenon, especially in alkaline solutions.
Protective measures are required when dealing with concentrated TRS émissions considering that they hâve the potential to create highly flammable and toxic fumes (Table 1.2). TRS compounds are highly reactive with strong oxidizing agents such as peroxides, metallic and organic oxides. Table 1.2 also reveals the high flammability and explosive nature of H2S, CH3SH and (CH3)2S while (CH3)2S2, although flammable, can be
manipulated with less risk. H2S has exceptionally wide explosion limits (i.e. 4 . 3 ^ 6 % of H2S in air) compared to others. Of course, great précautions must be taken when dealing with thèse concentrations, a situation that can be met in kraft pulp mills.
TABLE 1.1. Physical and chemical properties of TRS species
H2S C H3S H (CH3)2S (CH3)2S2
synonyms
hydrogensulfide methylmercaptan ,. .. . , ~ , u A %f • -A *u *u« 1 dimethyl sulfide hydrosuliuric acid methanethiol .. , , _ .
u- JM J - J xi methyl sulfide sulfur dihydnde mercaptomethane
dimethyl disulfide methyl disulfide molecular weight (g/mol) boiling point vapour pressure (atm at Tref)
gas density at 1 atm (kg/m3atTref) liquid density (kg/m3atTref) solubility in water solvent solubility acidity 34.1 -61 20 (25 °C) 1.40 (25 °C) 2.6% (20 °C) carbon disulfide alcohol alkali solutions yes 48.1 6 2(21 °C) 1.97 (25 °C) 2.4% (20 °C) hydrocarbons alcohol ether yes 62.1 37 1 (36 °C) 2.44 (37 °C) 800 (20 °C) slightly alcohol ether 110 94.2 116 0.04 (25 °C) 2.95(116 °C) 1046 (20 °C) insoluble ethanol ether no
Diluted TRS concentrations are considered inoffensive to the human health. However, toxicology studies hâve revealed that it could be potentially dangerous (Table 1.2). For example, H2S will cause irritation of the eyes and respiratory tract with an exposure at 20 ppmv. It will cause severe sickness at 500 ppmv and fatality at 1000 ppmv. CH3SH and (CH3)2S2 are somewhat less toxic than H2S, but produce similar effects at higher
j
TABLE 1.2. TRS odour threshold [15] safety concerns H2S 0.5-5.0 ppbv (rotten egg) CH3SH 0.3-3.0 ppbv (garlic) (CH3)2S 1-15 ppbv (vegetable sulfide) (CH3)2S2 1-20 ppbv (vegetable sulfide) incompatibilities [10] hazardous conditions [10] flash point (°Q [10] auto-ignition (°C) [4] explosion limits (vol%) [4]
strong oxidizers strong oxidizers metals métal oxides métal oxides peroxides combustibles acids
. . , , „ , . highly flammable highly flammable & .
. corrosive corrosive ., „ , , e form flammable torm explosive . x .^.
r ... . mixtures with mixtures with air
moist -82 260 4.3-45 -18 3.9-21.8 oxidizers peroxides extremely flammable form explosive mixtures with moist air -45 206 2.2-19.7 strong oxidizers flammable 24 300 1.1-16.1 toxicity (inhalation) [10] toxicity (skin)[10] highly toxic harmful (may be fatal)
cause burning irritant
moderately toxic highly toxic
(may be fatal)
irritant irritant
1.2.2 Kraft cycle
The kraft process involves three major area of opération: the wood cooking sector, the black liquor evaporation/incineration sector and the chemical recovery sector (Figure 1.2). In the first sector, wood chips or sawdust are introduced in a batch or continuous digester with the cooking liquor also known as the white liquor. Na2S hydrolyses in water to form sodium hydrosulfide and sodium hydroxide (eq I.l). As a resuit, the white liquor has a high pH (over 13) which allows a fraction of the hydrosulfide ion (HS") to be converted into the sulfide ion (S"2) since the pK for eq 1.2 approaches 14 at infinité dilution [16, 17].
Na2S + H2O -> NaOH + NaSH -> 2Na+ + OH" + HS"
HS +H2O; :S2~+H3O+
(1.1)
• • • • • i l i Wood chips • iiiiii|niiiilililtii|
V
V—
: Digester : > :j
f Brownstock washers : 2 >—,
/ * LVHC *' ^"V TDS White liquor . . . . »* HVT f * Pi '• TRS il,. / Black liquor f Evaporators \ • • Recovery furnace : Smelt • • • • • 3 White liquor clarifier > Slaker & Causticizer > F Lime Green liquor Dissolving tank Lime mud washing >f \
Lime kilnFIGURE 1.2. Simplifiée! diagram representing the kraft process cycle: (1) wood cooking; (2) black liquor evaporation/incineration; (3) chemical recovery.
The hydroxide and hydrosulfide/sulfide ions coupled with high températures (160-180 °C) chemically split the wood chips into fragments. It results in raw pulp mixed with black liquor containing soluble organic and alkali by-products. After cooking, the content is discharged into a blow tank where the softened chips are disintegrated into fibres. The pulp is then separated from the residual black liquor in brownstock washers.
Now in the second area of opération, the weak black liquor (± 15% alkali/organic solid) is relayed to the evaporators. The resulting strong black liquor (± 75% solid) is then ready to be incinerated into the recovery furnace where organic by-products (i.e. lignin, hemicelluloses, wood extractives, acetic acid, formic acid [18]) are thermally degraded into gaseous species. The furnace also reduces the oxidized sulfur compounds into sulfide. The inorganic smelt composed mostly of Na2CÛ3 and Na2S is recovered and dissolved in water to form green liquor.
The chemical recovery sector has two purposes: remove the carbonate ion ( C O ^ ) and regenerate the active alkalinity of the liquor. This is achieved by adding calcium oxide (CaO) in the green liquor forming calcium hydroxide (Ca(OH)2) via a lime slaking reaction (eq 1.3). The solubility product of Ca(OH)2 (KSP = 5.02 x 10"6 mol3/L3) is much superior to
the solubility product of CaCÛ3 (KSP = 3.36 x 10' mol /L ) meaning that most of the calcium will precipitate with carbonate to form a lime mud while releasing two hydroxides (eq 1.4). The washed lime mud is then conveyed to kilns where CaCC>3 is calcined at high températures (800 °C) to restore the active CaO (eq 1.5). Meanwhile, dissolved Na2S and NaOH are extracted from clarifiers and stored into white liquor mixing tanks. The kraft cycle is now complète.
2O - > C a ( O H )2+ h e a t (1.3)
Ca(OH)2 + Na2CO3 -> CaCO3 + 2NaOH (1.4)
CaCO3 + heat - • CaO + CO2 (1.5)
1.2.3 TRSformation
The TRS predicament originates essentially from the reactions between the methoxyl groups of lignin and the hydrosulfide/sulfide ion in the cooking liquor. Equations 1.6—1.9 are examples of TRS formation in the digester [15]. The majority is collected with other noncondensible gases (NCG) at the top of the vessel with a pressure control relief valve. Still, a percentage of TRS remains dissolved in the pulp/black liquor mixture and will be released eventually from brownstock washers, black liquor storage tanks and evaporators. Ail of the above point sources generate low volume high concentration (LVHC) émissions which are usually put under the NCG banner along with turpentine recovery Systems and continuous digester flash steam condensers. Typical composition of NCG is given in Table 1.3. Of course, it will vary from System to System.
2 2 (1.6)
lignin - OCH3 + HS" - • lignin - O~ + CH3SH (1.7) lignin - OCH3 + CH3S~ ->• lignin - O" + (CH3 )2 S (1.8) 2CH3S" + 0.5O2 + H2O -> (CH3 )2 S2 + 2OH" (1.9)
TABLE 1.3. Typical NCG chemical composition [19] compound hydrogen sulfide, H2S methyl mercaptan, CH3SH dimethyl sulfide, (CH3)2S dimethyl disulfide (CH3)2S2 turpentine, CioH16 methanol, CH3OH water, H2O nitrogen, N2 oxygen, O2 %vol 1.7 2.1 2.1 1.7 0.1 0.2 6.0 77.2 8.9
Poor combustion in the recovery furnace of sulfured organic by-products can lead to the formation of TRS instead of SO2. With high volume discharge rates, the resulting TRS concentration from the recovery furnace exhaust usually approaches 200 ppmv before treatment which makes it a high volume low concentration (HVLC) point source. The persistence of sulfur impurities in the recovery furnace smelt will spread the TRS nuisance to the chemical recovery section as well (i.e. dissolving tank, lime kiln, white liquor clarifiers).
1.3 Actual TRS régulations
The alleviation of foui odours nearby kraft pulp mills inevitably proceeds with the implementation of strict régulations. In Canada, the provinces hâve jurisdiction over environmental matters. In Québec, a document legislating technical and environmental aspects of pulp and paper production was first published in 1981 and further amended in 1994 [1]. A summary of TRS discharge régulations relevant to the kraft process is given in Table 1.4. As mentioned previously, the recovery furnace releases in average 200 ppmv of TRS at high discharge rates (8000-25000 Sm3/h), which is at least 10 times the regulated concentration. The variability of LVHC process equipment (i.e. brownstock washers) makes it impossible to forecast the TRS émissions from corresponding point sources. However, it could be expected to reach concentrations larger than 1000 ppmv [20] making the LVHC régulation equivalently steep. No régulation modifications are expected in the
province of Québec for now. In the United States, current Environmental Protection Agency (EPA) air émission control programs are based upon a 1970 version of the original Clean Air Act (CAA), which was passed in 1963. The 1970 CAA version is also referred as the 1990 CAA since the law was only enacted in 1990. As a sub-category of the CAA, the new source performance standards (NSPS) promulgated in 1976 régulâtes the TRS
émissions (Table 1.4).
TABLE 1.4. Régulations on TRS émissions for mills exploited in the province of Québec and the United States
point _ .. US régulationsc
source p r 0 C e S S Q u e b e C for equipment installed
category « n " p m e » t r é g u l a t i o n s recovery furnace 20 ppmv (5 ppmvb) 5 ppmv HVLC 20 ppmv lime kiln 10 ppmv 8 ppmv digester / NCG émission LVHC brownstock washer 10 ppmv 5 ppmv 5 ppmv evaporator System
a Concentrations are reported on dry basis and corrected for 8 vol% oxygen. b Régulation if the recovery furnace has started opération after 1992.
c Concentrations are reported on dry basis and corrected for 10 vol% oxygen.
1.4 TRS removal stratégies
Substantial advances in émission control technôlogy, for instance installation of efficient NCG collection and treatment Systems, hâve reduced pulp mills' sulfur émission considerably. For example, the Finnish pulp industry has contracted its TRS émissions by 70% between 1993 and 1998, despite a 10% increase in pulp production [21]. A similar trend was noticed by Pinkerton [22, 23] estimating a décline in TRS émissions by 85% over the past 25 years while increasing the pulp production by at least 80%. The récent progress in this field can be justified by the surge of public pressure on environmental issues which led to the investigation of original abatement schemes [20, 24-27]. Still, most industrial TRS abatement Systems are restricted to incinération and wet scrubbing, probably due to the limited confidence in new technologies.
/. 4.1 Major abatement technologies
Thermal incinération is particularly efficient for low discharge gas streams [3, 15]. As a resuit, NCGs from cooking vessels, brownstock washers and evaporators are relatively easy to handle by incinération with a proper setup (températures > 650 °C, excess oxygen and suffïcient résidence tirne). In such conditions, TRS are usually oxidized into sulfur oxides (i.e. eql.10).
(CH3 )2 S2 + XY2 O2 -> 2SO2 + 2CO2 + 3H2O (1.10)
With high operating costs due to important energy requirements, incinération is recognized as an efficient but uneconomical way to clear reduced sulfur molécules from gas streams. Besides, the formation of sulfur dioxide creating acid rain conditions in the atmosphère shifts the problem to another level [6] requiring now installation of a SO2 scrubber downstream. The economical improvement of gas incinération essentially rests on the use of on-site process equipment (i.e. recovery furnace, lime kiln, bark boiler) that could accept NCGs without disturbing the process itself [3, 21, 26]. It allows maximum recovery of sulfide in the kraft cycle as sulfur atoms from oxidized TRS (i.e. SO2) are captured into the recovery furnace smelt or lime kiln mud [28]. Apparently, it improves the white liquor sulfidity by 1%, which ultimately reduces the makeup Na2SÛ4 requirements. Key advantages and inconveniences of TRS incinération are summarized in Table 1.5.
Wet scrubbing is a standard TRS control procédure for HVLC point sources. Alkaline (i.e. white liquor) and alkanolamine (RNH2; i.e. monoethanolamine, diethanolamine) [29-31] solutions procure good solubility conditions for acidic exhaust gases such as SO2, NOx, CO2, H2S and CH3SH. The Quaker Chemical company has also developed an amine-based solution that is inert toward CO2, a clear improvement compared to common aqueous solutions. Still, they remain quite ineffective on (CH3)2S and (CH3)2S2 due to their poor reactivity with aminés and hydroxides. Only cooled scrubbing solutions could improve their physical absorption. Wet scrubbing with a strong oxidizer (i.e. KMnC>4, H2O2, NaOCl) has been proven effective [7, 32] for TRS scavenging. Bowman [33] confirmed the potential of hypochlorite (OCl") for the oxidation of TRS (16.2 ppmv) in a pilot scrubber
obtaining 99% conversion for H2S, CH3SH and (CH3)2S and 80% conversion for (CH3)2S2. However, the high cost of hypochlorite or any other strong oxidizer for that matter excludes their use on a continuous basis. Some authors hâve prospected the oxidative potential of unscrubbed bleached plant stack gas as a solution to this financial burden. The combination of residual bleaching agents with TRS flue gases upstream an alkaline scrubber was successfully implemented on-site of kraft mills (i.e. weak black liquor storage tank [24], brownstock washers and other vents [25]).
TABLE 1.5. Advantages and inconveniences for current and potential TRS abatement
Systems
method suitability inconveniences
incinération
alkaline/amine wet scrubbing strong oxidizer wet scrubbing
suitable for ail TRS molécules efficient for LVHC émissions
suitable of H2S and CH3SH efficient for LVHC & HVLC very good TRS conversion oxidizer may be already available on-site
residual bleach , „ „ „
... ., .. very good TRS conversion oxidizer + alkaline ~J. ,c . , „ „ ,
. . . efficient for NCG streams scrubbing
high energy requirement
inappropriate for HVLC émissions SO2 formation -> acid rain
explosion risk
unsuitable for (CH3)2S and (CH3)2S2 parasite reactions
non-specific oxidation -» added cost high oxidizer/TRS ratio
halogenated sub-products
non-specific oxidation —> added cost high oxidizer/TRS ratio
halogenated sub-products on-site bleach plant is required activated carbon adsorption catalytic wet scrubbing aérobic biofiltration
suitable for low flow rate and diluted TRS concentrations good overall conversion produce non-odorous sulfur products
suitable for H2S and CH3SH low cost
activated carbon régénération can not deal with kraft émissions catalytic tower capital cost catalysis fouling (i.e. hard water) increased oxidizer requirements slower dégradation for (CH3)2S and (CH3)2S2
low productivity high pressure drop
1.4.2 Potential abatement methods
Some original and possibly cheaper abatement techniques are still being evaluated today. It comprehends topics like activated carbon adsorption, green liquor dregs adsorption/ oxidation, catalytic wet scrubbing and aérobic biofiltration (Table 1.5).
During the 1970's, Paprican reported fundamental results about the removal of TRS in alkaline suspensions of activated carbon. Under the best operating conditions, they could only achieve conversions of 99% for H2S, 98% for CH3SH, 50% for (CH3)2S and ca. 0% for (CH3)2S2 [34]. Obviously, activated carbon slurries would not be practical for the actual problem since its low adsorption capacity would not be compatible with the elevated TRS concentrations.
Catalytic oxidation also emerged as an attractive approach to remove TRS species but its ramifications remain relatively unknown to engineers dealing with the problem. Recently, a catalytically enhanced technology (Odorgard™) involving the use of a proprietary nickel catalyst was tested, developed and commercialized [20, 35]. The concept relies first on the chemical absorption of TRS in a solution of NaOH (pH = 9.5-10.5) and NaOCl (500-750 ppm). The resulting mixture is then relayed to a catalytic reactor where the rate and extent of TRS oxidation are said to improve. For example, the catalytic removal of methyl mercaptan leads to the formation of non-odorous sulphonic acid (eq 1.11) instead of the odorous (CH3)2S2 as it would be the case without the catalyst (eq 1.12). Naturally, it procures superior conversion numbers (-90%) for (CHb^S and (0^3)282 compared to the 20% abatement observed without the catalyst [20].
CH3SH + 3NaOCl -> CH3SO3H + 3NaCl (1.11) 2CH3SH + NaOCl -> (CH3 )2 S2 + NaCl + H2O (1.12)
Recently, dried green liquor dregs were tested as a catalytic adsorbent in a laboratory-size tubular reactor [36]. Physical characterisation of the crushed solid showed it was mainly composed of CaCO3 (± 50%) and Na2CO3 (± 15%) but also contained ferrie oxide (Fe2O3; ± 1%), manganèse oxide (MnCh; ± 1%), silicon oxide (SiÛ2; ± 0.6%) and métal sulfides (± 5%). The oxidation performance of individual chemical species found in the green liquor dregs was investigated at 180 °C with initial TRS species concentrations approaching 10 ppm each. Results showed that CaCO3 is consistently reliable for the oxidation of ail TRS species at 180 °C. Other species like activated carbon, Fe2O3, Fe3O4, MnCh and FeS were proven more or less efficient (65-95%) on H2S and CH3SH in the initial phase of the experiment. Eventually, the conversion decreased dramatically below 50%. For
and (CH3)2S2, the conversion never exceeded 60% and for some oxides (i.e. Fe2Û3,
no reaction was recorded. The potential of green liquor dregs packed 25 mm high in a column was then evaluated using the same method at 180 °C. The results mirrored the performance obtained with CaCÛ3 suggesting it is the active component in green liquor dregs.
Aérobic biofiltration using sulfide sélective microorganisms attached to surfaces of stationary matrices (i.e. soil, peat, compost, wood bark, synthetic material) excels in removing TRS molécules and has been widely documented from laboratory-scale Systems [37-45]. The efficiency of microorganisms supported by granular activated carbon was also studied [46]. When provided with an oxygen source, bacteria consume ionic sulfide species and oxidize them into non-odorous sulfur species. Of course, biofiltration is attractive from a cost perspective but requires large média volume to obtain long gas résidence time essential for the dissolution and oxidation of TRS from high discharge gas streams. Hence, the use of biofiltration for the kraft pulp émissions is not recommendable.
1.5 Research project objectives
Nowadays, the pulp and paper industry focuses exclusively on removing TRS émissions with current abatement technologies instead of preventing it. Of course, new pulping technologies if researched appropriately could résolve several environmental predicaments but the capital cost for implementing such process renders this solution improbable, especially for the actual pulp market. Another solution would be to reduce significantly the capital and operating costs of actual abatement processes without creating other concerns. Incinération is efficient but expensive. Wet scrubbing is not appropriate for (CH3)2S and (CH3)2S2 unless strong halogenated oxidizers are added to the mix, which makes the process even more expensive. Also, there is little mention about the end-products of such oxidation process. It could easily lead to the formation of organo-halogenated species [47], some of which are harmful to the human health.
The idéal solution should involve in our opinion an economical method that would retrieve sulfur from TRS émissions for re-use or trade without creating other environmental
setbacks. This is very challenging but it would certainly improve the économie viability of the industry. Such endeavour could eventually be achieved by oxidizing the TRS into recoverable sulfur species while consuming little amounts of the oxidizing agent. Theoretically, this could be done if the oxidizing agent being reduced by TRS species is regenerated in-situ to its oxidized (reactive) state by other physical or chemical means. The intent of this project is to assess scientifically the potential of one such oxidation-reduction reaction able to résolve the problem in hand. The project will strictly focus on the oxidation of H2S being the most prominent species of the TRS grouping. If the method is proven to work for H2S, then the other TRS molécules will be tested eventually.
1.6 TRS abatement via a redox reaction
Oxidation-reduction (redox) reactions implying an électron exchange between an oxidizing agent (i.e. électron captor) and a reducing agent (i.e. électron donor) are employed in a wide array of industrial processes (i.e. bleaching, electrolysis, electroplating and bioremediation). They were conceived by studying natural redox phenomena like the oxidation of carbon by oxygen, the réduction of carbon by hydrogen, the oxidation of sugar in the human body, photosynthesis and métal corrosion. Of course, implementation of a redox process for the valorisation of TRS must involve first a powerful oxidizing environment (Table 1.6) characterized by a positive redox potential différence (AE). It describes the energy released due to the movement of charged particles and can be related to the Gibbs free energy (AG) in terms of electric work (eq 1.13).
AG = -nFAE (1.13)
n represents the number of électrons involved in the redox reaction and F is the Faraday constant. The combination of the electromotive force (emf = AE) with the Gibbs free energy (eq 1.14) based on equilibrium reaction constituents gives the gênerai form of the Nernst équation (eq 1.15). It illustrâtes how a redox System is conditioned (positive AE = oxidative environment; négative AE = reductive environment). Good oxidizing agents are characterized by high standard potential différence (AE°) or high standard potential (E°) of the oxidation half-reaction. For example, the most powerful oxidizer is fluorine with E° =


![TABLE 1.6. Standard potentials for common oxidizing agents in aqueous solutions at 25 °C [48]](https://thumb-eu.123doks.com/thumbv2/123doknet/6414360.169924/45.882.129.748.414.839/table-standard-potentials-common-oxidizing-agents-aqueous-solutions.webp)