Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
The Journal of Physical Chemistry Letters, 10, 20, pp. 6399-6408, 2019-10-08
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=d505925a-56c2-4363-a053-36aba6682df0 https://publications-cnrc.canada.ca/fra/voir/objet/?id=d505925a-56c2-4363-a053-36aba6682df0
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/acs.jpclett.9b02439
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Identifying clusters and/or small-size quantum dots in colloidal CdSe
ensembles with optical spectroscopy
Li, Lijia; Zhang, Meng; Rowell, Nelson; Kreouzis, Theo; Fan, Hongsong; Yu,
Qiyu; Huang, Wen; Chen, Xiaoqin; Yu, Kui
Identifying Clusters and/or Small-Size Quantum Dots in Colloidal
CdSe Ensembles with Optical Spectroscopy
Lijia Li,
†Meng Zhang,
∥Nelson Rowell,
‡Theo Kreouzis,
§Hongsong Fan,
†Qiyu Yu,
#,⊥Wen Huang,
∇Xiaoqin Chen,
*
,†and Kui Yu
*
,†,∥,⊥†Engineering Research Center in Biomaterials, Sichuan University, Chengdu, Sichuan 610065, People’s Republic of China ∥Institute of Atomic and Molecular Physics, Sichuan University, Chengdu, Sichuan 610065, People’s Republic of China ‡Metrology Research Centre, National Research Council Canada, Ottawa, Ontario K1A 0R6, Canada
§School of Physics and Astronomy, Queen Mary University of London, London E1 4NS, United Kingdom
#College of Materials Science and Engineering, Sichuan University of Science and Engineering, Zigong 643000, China
∇Laboratory of Ethnopharmacology, West China School of Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan
610065, People’s Republic of China
⊥State Key Laboratory of Polymer Materials Engineering, Chengdu, Sichuan 610065, People’s Republic of China
*
S Supporting InformationABSTRACT: It is well-known that optical absorption and photoluminescence (PL) provide information that is sensitive to the size and size distribution of colloidal binary semiconductor quantum dots (QDs). To explore the nature of reaction products, clusters, and/or small-size QDs, we show that it is important to perform as well photo-luminescence excitation (PLE) spectroscopy. For two non-hot-injection reactions of cadmium oleate (Cd(OA)2) and selenium (Se) in 1-octadecene (ODE), we show that sequentially extracted products displayed a similar apparent red shift in both absorption and PL with a full width at half-maximum (fwhm) of∼30 nm. We demonstrate that one reaction (with the presence of diphenyl phosphine (HPPh2)) produced multiple types of
clusters (with slightly different optical properties) in one ensemble, while the other reaction (without HPPh2) yielded primarily small-size QDs. Our findings provide
evidence for the probable existence of clusters within small-size CdSe QD products, the existence of which complicates the size determination of small-size CdSe QDs.
O
ptical absorption and photoluminescence (PL) have been shown to be practical tools for estimating the size and size distribution in colloidal semiconductor cadmium chalcogenide quantum dot (CdE QD) samples.1−16The peak positions of the bandgap absorption and emission have been correlated with the size of QDs, while the full width at half-maximum (fwhm) has been related directly to the size distribution in a QD sample. For a single CdSe QD species (with a bandgap of∼545 nm), a value of fwhm of ∼17 nm was reported, together with that of∼33 nm for the corresponding ensemble.13 For one small-size CdSe sample ensemble, a relatively sharp bandgap PL (peaking at ∼480 nm) can be accompanied by a relatively broad trap emission at relatively long wavelengths (centered at∼600 nm).7For QD samples extracted sequentially from a reaction batch, the peak positions of their bandgap absorption and emission normally red shift in a continuous fash-ion.1−6,8−12,14−16 It is noteworthy that there is another type of evolution of the peak positions, which is discontinuous and attributed to the presence of clusters instead of QDs.17−23The key difference between clusters and QDs should be their structures. Meanwhile, the PL spectra may contain features from both band-edge and trap emission. For CdSe sample
ensembles reported for small-size QDs (a) and clusters (b), a representative summary of the literature results regarding the optical properties is presented in Figure S1-1 for the spectra and inTable S1for the peak positions. Apparently, it appears somewhat challenging to distinguish whether small-size QDs or clusters are produced from a reaction, from just optical absorption and PL, especially when a continuous red-shift pattern of peak positions is not recognizable and when the fwhm is about 30 nm. To the best of our knowledge, the present study is thefirst to address this practical issue.
Here, we report our study on the differentiation of the production of CdSe clusters and/or small-size QDs via combining the results from three optical spectroscopic techniques. In addition to the aforementioned optical absorption and PL, we add photoluminescence excitation (PLE). For colloidal semiconductor CdSe nanocrystals (NCs) including QDs6,7,24,25 and clusters,6,23,24,26−29 PLE has been reported to reveal specific contribution of absorption to a
Received: August 20, 2019 Accepted: September 26, 2019 Published: October 8, 2019
Letter pubs.acs.org/JPCL
Cite This:J. Phys. Chem. Lett. 2019, 10, 6399−6408
© 2019 American Chemical Society 6399 DOI:10.1021/acs.jpclett.9b02439 J. Phys. Chem. Lett. 2019, 10, 6399−6408
spectral band of PL selected at a particular energy. PLE moderates the inhomogeneity impact of one sample ensemble while reflecting the very portion that contributes mainly to the selected PL spectral band. In the production of our colloidal nanomaterials, we performed two non-hot-injection conven-tional reactions of cadmium oleate (Cd(OA)2) and selenium
(Se) in 1-octadecene (ODE), with the feed molar ratios of 4Cd to 1Se (Batch a) and 1Cd to 1Se (Batch b). The feed Se concentration was 30 mmol/kg in ODE, and a secondary phosphine diphenyl phosphine (HPPh2) was applied for Batch
a only.Figure 1shows the optical absorption and PL spectra of one Batch a sample and of one Batch b sample. The two CdSe samples display essentially similar bandwidths in optical absorption and PL, which are comparable to that of a single conventional CdSe QD ensemble.1−16 Figure 2 suggests that Batch a produced mixtures of clusters including Clusters-429, -437, -453, and -467 (denoted in reference to their absorption peak positions in wavelength nanometers (nm)). Figure 3
indicates that Batch b produced small-size QDs mainly, together with a relatively small amount of clusters such as Cluster-467.Figure 4discusses the production of Clusters-437 and -453 from Batch a but kept at a constant temperature of 185 °C. On the basis of the cluster peak positions of PL (Figure 4) and of absorption obtained from PLE (Figure 2),
Figure 5addresses the deconvolution of the absorption and PL spectra presented in Figure 2. Table 1 summarizes the information obtained for samples from Batches a and b. The present study narrows a knowledge gap regarding the determination of CdSe clusters and/or CdSe small-size QDs for a reaction production. Our findings suggest that clusters can also be present in small-size QD ensembles (even for those exhibiting single-bandgap absorption peaks), the coexistence of which complicates the size assignment for small-size CdSe QDs,1,10−13,30and call for synthetic efforts that are aimed at
eliminating the formation of clusters in the production of small-size CdSe QDs with an enhanced particle yield.
InFigure 1, we present the optical absorption (gray traces) and PL (blue traces) spectra of two samples (a and b) extracted from two synthetic batches with the Se concen-trations of 30 mmol/kg in ODE. The sample from Batch a was extracted from the reaction of Cd(OA)2+ Se + HPPh2, with a
feed molar ratio of 4Cd to 1Se to 1HPPh2. The sample from
Batch b was taken from the reaction of Cd(OA)2+ Se, with a feed molar ratio of 1Cd to 1Se. In steps of 20°C, the reaction temperature was increased from 120 to 220°C (Batch a) or from 220 to 260°C (Batch b). After the reaction temperature at each step was held for 10 min for Batch a and for 10 and 30 min for Batch b, samples were taken. For each of the six as-synthesized samples from Batche a or b, an aliquot with a respective volume of 25 or 20μL was dispersed in 3.0 mL of Tol. The optical properties of the third samples from both Batch a (160°C/10 min) and Batch b (240 °C/10 min) are presented in Parts a and b ofFigure 1, respectively.
The sample from Batch a exhibits well-defined absorption peaking at 436 nm (2.84 eV) with a blue-side shoulder at 398 nm (3.12 eV); the energy difference between the two peaks is 38 nm (0.28 eV). Also, this sample displays a relatively narrow PL peak at 462 nm (2.68 eV) (upon excitation at 400 nm), together with a relatively broad PL peak centered at 593 nm (2.09 eV). The former PL peak has a fwhm of∼30 nm (0.18 eV). Thus, the apparent Stokes shift is 26 nm (0.16 eV). The sample from Batch b exhibits similar distinctive absorption peaking at 515 nm (2.41 eV), with a blue-side shoulder peaking at 464 nm (2.67 eV). Thus, the energy difference between the two absorption peaks is 51 nm (0.26 eV). Also, the sample displays a relatively narrow PL peak at 530 nm (2.34 eV) (upon excitation at 400 nm). The PL peak has a fwhm of ∼26 nm (0.11 eV). The apparent Stokes shift is therefore 15 nm (0.07 eV).
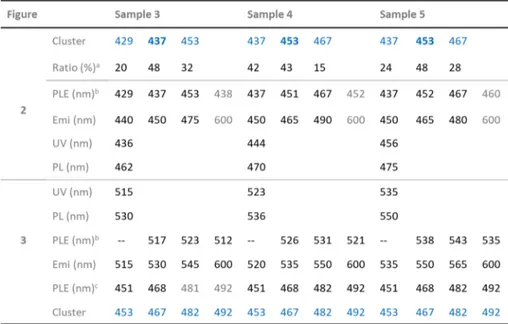
Table 1. Summary of the Samples Presented inFigures 2and3, Which Contain Mainly Clusters and QDs, Respectively, With Their Optical Peak Positions of PLE, Absorption (UV), and PL
aThe absorption peak area of each cluster based on the deconvolution shown inFigure 5. The clusters bolded dominate in the sample.b
Lowest-energy peak positions.cRelatively high energy peak positions. For the latter (presented in dark color), they were obtained with emission at the four positions indicated. For the 481 nm PLE peak position in gray color, it was monitored with emission at 545 and 600 nm; the 492 nm position in gray color was obtained with emission at 600 nm.
The two CdSe samples present essentially similar bandwidths of optical absorption and PL, the feature of which is comparable to that shown by single conventional CdSe QD ensembles.1−16 This similarity is not sufficient to support the conclusion that the two CdSe samples contain small-size QDs free of clusters. It is well-known that to characterize the size and size distribution of one ensemble of clusters or QDs the approach should be quite different. The present study does not address this challenging issue but focuses on the optical properties that allow us to distinguish clusters from small-size QDs in one ensemble.
For the six samples extracted from the two batches, their optical properties are presented in Parts a (top) and b (bottom) of Figure S1-2, with Part 1 (left) for optical absorption spectra (offset) and Part 2 (right) for PL spectra (excited at 400 nm and offset). For the samples from Batch a, the absorption peaks red-shifted from 420 nm (Sample 1 120 °C/10 min) to 475 nm (Sample 6 220 °C/10 min) along with the temperature increase. Also, the relatively narrow PL peaks red-shifted from 446 nm (Sample 1) to 493 nm (Sample 6). From Samples 1 to 6, the continuous weakening of the relatively broad trap emission at the relatively long wavelength region indicates the probable disappearance of traps. For the
samples from Batch b, similarly, the absorption peaks red-shifted from 493 (Sample 1 220 °C/10 min) to 541 nm (Sample 6 260°C/30 min), and the emission peaks red-shifted from 510 (Sample 1) to 554 nm (Sample 6).
Regarding the red shifts of the absorption and emission peaks, as well as the values of the fwhm, the samples from Batches a and b are similar, and these features are comparable to those of QD samples that were taken from a single CdSe reaction batch that underwent a growth in size.1−4,6,8−12,14−16 The presence and near-absence of trap emission for the samples from Batches a and b, respectively, are worthy of notice. The nature of the surface ligand for the two batch samples should be identical,31,32while the Cd and Se reactivity in Batch a is much higher than that in Batch b due to the presence of HPPh2.31−34SeeFigure S1-3for results pertaining to Batch a but without the use of HPPh2. It is known that the
nature of surface ligands affects the electronic structures of QDs and thus the optical absorption and emission properties of QDs.35−42
Some sample ensembles from Batch a (shown inFigure S1-2a) have somewhat unusual optical properties. Samples 1−3 (160 °C/10 min) each exhibited a single absorption peak, while Samples 4 (180°C/10 min) and 6 did not as their peaks are broader and appear to consist of multiple peaks centered at 444 and 468 nm, respectively. The lowest-energy peak for Sample 5 (200 °C/10 min) is at 456 nm; close observation shows that the second-lowest-energy peak of Sample 5 is at 430 nm and seems to correspond to the lowest-energy peak of Samples 2 (140°C/10 min) and 3. The increase in strength of the peak at 456 nm correlated with the broad peaks observed for both Samples 4 and 6. Also, the PL peaks of Samples 4−6 are obviously asymmetric, with significant blue-side shoulders. Therefore, each of the six samples from Batch a would appear to contain multiple types of clusters and/or small-size QDs.
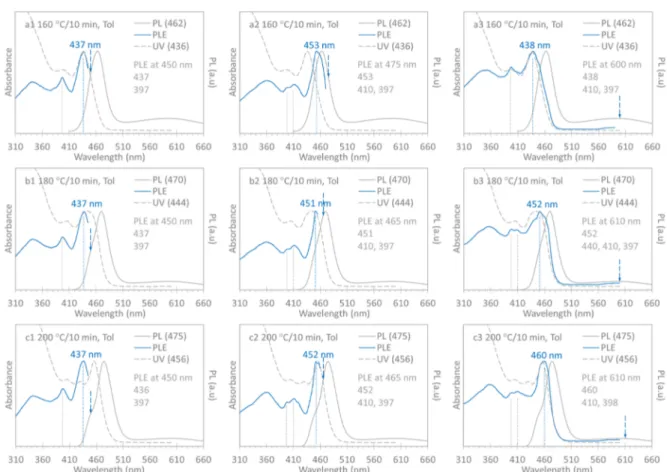
To differentiate clusters from small-size QDs, we performed PLE measurements with excitation wavelengths set around the blue and red sides of the relatively narrow PL peaks and at the center of the broad trap peaks detected. InFigure 2, we show the normalized absorption (dashed gray traces), PL (gray traces), and PLE (blue traces) spectra of Samples 3 (a 160°C/ 10 min, top panel), 4 (b 180°C/10 min, middle panel), and 5 (c 200°C/10 min, bottom panel) of Batch a. Together with each PLE spectrum, corresponding absorption and PL spectra are presented to identify the species that possibly contribute to the absorption and PL peaks observed. Part 1 (left panel) shows the PLE spectra obtained with the emission wavelength set at 450 nm. Part 2 (middle panel) illustrates the PLE spectra collected with the emission wavelengths set near the relatively narrow PL peaks, which are on the red side for Sample 3, on the peak for Sample 4, and on the blue side for Sample 5. In the samefigure, Part 3 (right panel) displays the PLE spectra collected with the emission wavelength set to the center of the relatively broad trap PL peaks. The emission wavelength settings for the PLE spectra are indicated by the dashed downward blue arrows. The positions of the main PLE peaks are highlighted by dashed vertical lines. The relevant wavelengths in nm are indicated, including those of the absorption, PL, and PLE peak positions, which are also summarized inTable 1. For each of the three samples, there was one additional PLE spectrum collected, which is presented inFigure S2-1(with the same presentation format).
For Sample 3 (160 °C/10 min), the PLE spectra had the lowest energy peaks at 429, 437, 453, and 438 nm when the Figure 1.Optical absorption (gray traces, left y axis) and PL (blue
traces, excited at 400 nm, right y axis) properties for two CdSe samples extracted from two synthetic batches. The reaction for (a) was Cd(OA)2+ Se + HPPh2, and that for (b) was Cd(OA)2+ Se. Both reactions had a Se concentration of 30 mmol/kg in ODE and a total weight of 5.0 g. The feed ratios were 4Cd(OA)2(0.60 mmol) to 1Se (0.15 mmol) to 1HPPh2 (0.15 mmol) for (a) and 1Cd(OA)2 (0.15 mmol) to 1Se (0.15 mmol) for (b). Aliquots of the two samples from Batch a (25μL, 160 °C/10 min) and Batch b (20 μL, 240 °C/ 10 min) were each dispersed in 3.0 mL of toluene (Tol). The optical spectra of the two samples are similar and resemble those of a conventional CdSe QD ensemble, particularly with regard to the fwhm.
The Journal of Physical Chemistry Letters Letter
DOI:10.1021/acs.jpclett.9b02439 J. Phys. Chem. Lett. 2019, 10, 6399−6408
emission was set at 440 (Figure S2-1a), 450 (Figure 2a1), 475 (Figure 2a2), and 600 nm (Figure 2a3), respectively. Interestingly, when the emission was set at both 450 (Figure 2a1) and 600 nm (Figure 2a3), the lowest-energy PLE and absorption peaks seemed to overlap with each other quite well. These PLE observations suggest that Sample 3 contains Clusters-429, -437, and -453. It seems that Cluster-437 is the majority that dominates the absorption to peak at 436 nm and the trap emission to center at 600 nm. The presence of the other two clusters results in a fwhm of∼30 nm. We have noted that CdSe Cluster-437 was produced together with QDs (with a bandgap of∼550 nm), from one quite different reaction of cadmium octadecylphosphonate (Cd-ODPA) and tri-n-octyl-phosphine selenide (SeTOP) in tri-n-octyltri-n-octyl-phosphine oxide (TOPO) at 370 °C for ∼10 s.6 Table S2 summarizes the comparison of Cluster-437 reported in the previous6 and present study (which are respectively passivated by phospho-nate and oleate ligands). Accordingly, it seems reasonable that for the two cases it is the core structure of Cluster-437 rather than the nature of their surface ligands that dictates the electronic structure of Cluster-437, at least the lowest-energy electronic transition.
For Sample 4 (180 °C/10 min), with the emission at 450 (Figure 2b1), 465 (Figure 2b2), and 490 nm (Figure S2-1b), the PLE spectra had their lowest-energy peaks at 437, 451, and 467 nm, respectively. For the trap emission at 610 nm (Figure 2b3), the PLE spectrum showed a peak that was similar to the
corresponding absorption peak. These results indicate that Sample 4 contains Clusters-437, -453, and -467, thefirst two of which were also presented in Sample 3. Cluster-453 increased in population, which led to the relativelyflat absorption with similar strengths at 437 and 451 nm, the positions of which were the lowest-energy PLE peaks when emission was respectively set at 450 and 465 nm. Cluster-467 developed and contributed to the emission peak at 470 nm, and became asymmetric with a blue-side shoulder at∼450 nm.
For Sample 5 (200°C/10 min), when the emission was set at 450 (Figure 2c1), 465 (Figure 2c2), 480 (Figure S2-1c), and 610 nm (Figure 2a3), the lowest-energy peaks of the PLE spectra were at 436, 452, 467, and 460 nm, respectively. Again, when the emission was set at 465 (Figure 2a1) and 610 (Figure 2a3) nm, the lowest-energy PLE and absorption peaks overlapped well with each other. Thus, the same three types of Clusters-437, -453, and -467 were present in Sample 5, with a larger population of Cluster-453 that caused the absorption to peak at 456 nm (with the second-lowest-energy peak at 436 nm). Cluster-467 evolved further, and thus, the PL became more asymmetric and red-shifted to peak at 475 nm. For further evidence for Cluster-467, we performed another synthesis of Batch a but with samples taken at higher temperatures (with a preponderance for Cluster-467 and more red-side ones, such as Clusters-482 and -492 demonstrated inFigure S2-2).
Figure 2.Normalized PLE (blue traces, with corresponding emission wavelengths indicated by dashed blue arrows), optical absorption (dashed gray traces), and PL (gray traces excited at 400 nm) spectra collected from Sample 160°C/10 min (top panel), Sample 180 °C/10 min (middle panel), and Sample 200°C/10 min (bottom panel) from Batch a presented in Part a ofFigure S1-2. It is reasonable that the three samples contained multiple clusters including Cluster-437 and Cluster-453; Cluster-437 displays more trap emission than Cluster-453 does. The population ratio of Cluster-437 to Cluster-453 decreased from Sample 160°C/10 min to Sample 200 °C/10 min, which resulted in a flat spectral shape in the absorption for Sample 180°C/10 min and a single peak in the absorption of Sample 200 °C/10 min.
Accordingly, to distinguish clusters from small-size QDs, it is necessary to explore several sequential samples. With the collective characterization of optical absorption, PL, and PLE, it is critical to collect PLE spectra with emission set at several wavelengths that are around the relatively sharp band-edge emission, in addition to the center of the relatively broad trap emission. For example, the PLE spectra of Samples 160°C/10 min to 200°C/10 min shown inFigure 2retained their lowest-energy peak positions at 437 (left panel) and 453 nm (middle panel). Thus, it appears that the three samples contained Clusters-437 and -453 rather than small-size QDs. Due to the relative reduction in magnitude of the trap emission from Samples 160 °C/10 min to 200 °C/10 min, it appears that Cluster-437 contributes more to the trap emission than Cluster-453 does. This is reasonable because Cluster-437 may have relatively more surface atoms than Cluster-453 due to a possible increase of volume17,23 and/or morphological change to more spherical like along the reaction. Further diminishing of trap emission was apparent when Clusters-467, -482, and -492 were produced from a Batch a reaction at higher temperatures (Figure S2-2). In general, to obtain meaningful results from PLE for one sample ensemble exhibiting both relatively narrow emission on the blue side of a spectrum and relatively broad trap emission on the red side, it is necessary to set emission around the former and at the center of the latter. For the three sequential samples studied, in addition to the qualitative identification of the clusters, we are able to draw a semiquantitative conclusion that Clusters-437 and -453 are the majority in Samples 160°C/10
min and 200°C/10 min, respectively. Captivatingly, the result of deconvolution (shown inFigure 5) summarized inTable 1
validates this semiquantitative conclusion.
In Figure 3, we demonstrate the absorption, PL, and PLE spectra of three samples sequentially extracted from Batch b (shown inFigure S1-2). The three samples are Sample 3 (240 °C/10 min, top Panel a), Sample 4 (240 °C/30 min, middle Panel b), and Sample 5 (260°C/10 min, bottom Panel c). In
Figure 3, we use the same presentation format as that inFigure 2. The left panel (Part 1) shows the PLE spectra obtained with the emission wavelength set at the center of the PL peaks. The middle panel (Part 2) illustrates the PLE spectra collected with the emission wavelengths set on the red side of the PL peaks (about 15 nm away). The right panel (Part 3) displays the PLE spectra collected with the emission set on the red side tail of the PL (at 600 nm). For each of the three samples, an additional PLE spectrum was collected (with the emission placed on the blue side of the PL peaks), which is presented in
Figure S3-1with the same presentation format.
When the emission wavelength was set on the blue side of the PL peaks (Figure S3-1), the PLE spectra of Samples 3−5 overlapped well with the corresponding blue side of the absorption peaks of the lowest energies. When the emission was set at the center of the PL peaks (left panel ofFigure 3), the PLE spectra had the lowest-energy peaks at 517, 526, and 538 nm, which are slightly to the red side of the lowest-energy peaks in absorption at 515, 523, and 535 nm, respectively. Thus, it seems reasonable that each of the three samples contained QDs, as indicated by the red-shift feature of the Figure 3.Normalized PLE (blue traces, with corresponding emission wavelengths indicated by dashed blue arrows), optical absorption (dashed gray traces), and PL (gray traces excited at 400 nm) spectra collected from Sample 240°C/10 min (top panel), Sample 240 °C/30 min (middle panel), and Sample 260°C/10 min (bottom panel) from Batch b presented in Part b ofFigure S1-2. When emission was set at the PL peak positions (left panel) and on the red side about 15 nm away from the PL peak (middle panel), the lowest-energy PLE peak red-shifted. Thus, the three samples contained QDs, which dictate the optical absorption and PL. Meanwhile, they had clusters, Clusters-453, -467, -482, and -492, as indicated by PLE.
The Journal of Physical Chemistry Letters Letter
DOI:10.1021/acs.jpclett.9b02439 J. Phys. Chem. Lett. 2019, 10, 6399−6408
lowest-energy peaks of PLE. From Samples 3 to 5, these QDs grew in size, as indicated by red shifts of the absorption, PL, and PLE peaks. When the emission of PLE was set on the red side of the PL peaks (middle panel of Figure 3), the lowest-energy peaks of the three PLE spectra were at 523, 531, and 543 nm, located between the corresponding absorption and PL peaks. This is reasonable because it is the relatively large QDs in one ensemble (with a certain size distribution) that contribute to the PL on the red side of the PL band. When the PLE emission was positioned at 600 nm (right panel of
Figure 3), the lowest-energy PLE peaks of Samples 3 and 4 were at 512 and 521 nm, the positions of which were slightly to the blue side of the lowest-energy absorption peaks at 515 and 523, respectively. For Sample 6, the lowest-energy PLE and absorption peaks were both at 535 nm.
Importantly, there is one significant difference between the PLE spectra shown inFigures 2andS2-1and those inFigures 3 and S3-1. For the three sequential samples from Batch a containing clusters (Figures 2 and S2-1), the lowest-energy peak positions of the PLE spectra remained constant when emission was set at one wavelength (or within about a range of 10 nm) around the band-edge PL peaks. For example, with emission set at 450 nm, the PLE position was at 437 nm. With emission set at 465−475 nm, the PLE position was at 452 ± 1 nm. With emission set at 480−490 nm, the PLE position was at 467 nm. For the sequential samples from Batch b containing QDs (Figures 3andS3-1), the lowest-energy peaks of the PLE spectra displayed red shifting (with emission set near the bandgap PL peaks within about a range of 5 nm). Such a red-shift feature is quite similar to that of the corresponding absorption and PL. For example, for the two 240°C samples with growth periods of 10 (Sample 3) and 30 min (Sample 4), with emission set at 530 and 535 nm, the lowest-energy PLE peaks were respectively at 517 and 526 nm; with emission set
at 545 and 550 nm, the lowest-energy PLE peaks were at 523 and 531 nm, respectively.
As indicated by the results shown inFigures 3andS3, there are four higher-energy PLE peaks, which are at constant positions of 492, 482, 468, and 451 nm. These positions match well, respectively, those of Clusters-492, -482, -467, and -453, which are illustrated in Figures 2 andS2. Accordingly, these peaks should be attributed to the presence of the four types of clusters and not to higher-order electronic transitions of the QDs contained in the three samples. Thus, the three samples from Batch b contained both QDs and clusters. The information for the samples shown inFigure 3are summarized as well inTable 1. By a side note, for the literature-reported CdSe ensemble (summarized in Part a ofFigure S1-1b and in Table S2),6which evidently consisted of both small-size QDs and clusters such as Cluster-437, the PLE peak at∼440 nm might be assigned to Cluster-437 (when the emission was set at 550 nm). Again, with and without the use of HPPh2,
respectively, the same four types of clusters were produced in both the Batches a and b reactions, the results of which are presented inFigure S1-2.
For a further qualitative validation regarding the presence of clusters instead of QDs in the Batch a reaction as presented in
Figure S1-2, we repeated the reaction at a constant temperature of 185 °C with 20 samples taken consecutively during a period from 10 s to 180 min. Again, an aliquot with a volume of 25μL of each sample was dispersed in 3.0 mL of Tol for optical absorption and PL (excited at 400 nm).Figure S4-1shows all of the spectra collected in the top and bottom panels without (left panel) and with offset (right panels). Unambiguously, a quantized growth pattern is evident. In the relatively early stage of the reaction, Cluster-437 evolved and emitted at 451 nm. In the relatively late stage of the reaction, Cluster-453 developed and emitted at 472 nm.
Figure 4.Evolution of absorption (left axis) and emission (right axis, excited at 400 nm) of six representative samples from theFigure S1-2Batch a reaction but at a constant temperature of 185°C. There were a total of 20 samples extracted during the period of 10 s to 180 min. The reaction periods for the six samples were 10 s (a), 10 min (b), 20 min (c), 45 min (d), 75 min (e), and 150 min (f). An aliquot (25μL) of each sample was dispersed in toluene (3.0 mL) for the optical measurements. During the period of 20 (c) to 75 min (e), Cluster-437 seemed to be relatively stable, exhibiting distinguishable band-edge PL peaking at 451 nm; also, Cluster-453 developed emitting at 472 nm. Interestingly, the 10 s sample (a) exhibited one absorption peak at 435 nm and one PL peak at 462 nm, while the 45 min sample (d) displayed two absorption peaks at 433 and 456 nm and two PL peaks at 451 and 472 nm.
InFigure 4, we follow the optical absorption (dashed traces, left axis) and emission (solid traces, right axis) properties of six representative samples to understand the development of the clusters. The growth periods of the samples were 10 s (a, Sample 1 inFigure S4-1), 10 (b, Sample 6), 20 (c, Sample 8), 45 (d, Sample 12), 75 (e, Sample 14), and 150 min (f, Sample 19). For the growth period from 10 s (a) to 10 min (b), the absorption peak broadened with a red-side shoulder develop-ing at around∼454 nm. As well, the PL broadened with a blue-side shoulder peaking at∼451 nm. From 20 (c) to 75 min (e), the absorption seems to have two recognizable peaks at∼433 and∼456 nm, and the PL also has two distinct peaks at ∼451 nm and ∼472 nm. During this period, Cluster-437 was relatively stable, emitting at 451 nm, while Cluster-453 emitting at∼472 nm kept evolving.
After 90 min (Sample 15 in Figure S4-1), Cluster-437 disappeared, as indicated by the relative strength of the blue-side absorption decreasing and the absorption peaking at∼457 nm at 150 min (f). Meanwhile, the 451 nm PL peak
diminished; a red-side shoulder of the 475 nm PL peak developed, which indicates the presence of the next cluster, Cluster-467. To promote Cluster-437 and to suppress the red-side clusters, we performed Batch a reactions at constant temperatures of 175 (Figure S4-2) and 195°C (Figure S4-3). Similar quantized growth behaviors were detected, and the presence of Cluster-437 lasted at least 117 min at 175°C, less than 90 min at 185°C, and about 10 min at 195 °C. Clearly, Cluster-467 was suppressed at the constant reaction temper-ature of 175°C.
Also, we performed the Batch b reaction (illustrated in
Figure S1-2) at a constant temperature of 230°C (Figure S4-4) and extracted 13 samples during the period from 10 s to 60 min. A continuous red shift of the absorption peaks of the 13 samples was monitored from 490 (Sample 1) to 514 nm (Sample 13). Meanwhile, the PL peaks red-shifted from 505 (Sample 1) to 530 nm (Sample 13). Such a red-shift behavior, together with the symmetric features of the absorption and PL peaks, is indicative of the production of QDs. This temporal Figure 5.Illustration of the deconvolution of the optical absorption and emission spectra shown inFigure 2for Sample 160 °C/10 min (top panel), Sample 180°C/10 min (middle panel), and Sample 200 °C/10 min (bottom panel). Baseline subtraction was performed prior to the deconvolution for the absorption spectra (left panel, black traces) and is discussed withFigure S5-1. The deconvolution was carried out with a least-squaresfitting to four Gaussian peaks. Each of the red traces shows the superimposed result of the four Gaussian peaks, which overlaps well with each of the black traces. For the four types of clusters, Clusters-429 (blue traces), -437 (cyan traces), -453 (green traces), and -467 (orange traces), the deconvolution suggests that Sample 160°C contains the first three types, while Sample 180 °C and Sample 200 °C contain the last three types, and that Cluster-429 and Cluster-467 emit at 440 and 490 nm, respectively.
The Journal of Physical Chemistry Letters Letter
DOI:10.1021/acs.jpclett.9b02439 J. Phys. Chem. Lett. 2019, 10, 6399−6408
evolution pattern of the optical properties of QDs is significantly different from that of the clusters shown in
Figures S4-1 and S4-2.
For a semiquantitative corroboration of the presence of the various clusters in the three samples presented inFigure 2, we investigated deconvolution (for the optical absorption and PL spectra presented inFigure 2). Figure 5 contains the optical absorption spectra after baseline subtraction (left panel, black traces) and PL spectra (right panel, black traces) of Sample 160°C/10 min (top panel), Sample 180 °C/10 min (middle panel), and Sample 200°C/10 min (bottom panel).Figure S5-1 illustrates the baseline subtraction, which was performed prior to deconvolution. For each of the absorption and PL spectra of the three samples, the deconvolution was carried out with a least-squares fitting to four Gaussian peaks, and their superposition is represented by a red trace (Figure 5). For the four Gaussian peaks from the deconvolution, three of them reflect the three types of the four clusters, Clusters-429 (blue traces), -437 (cyan traces), -453 (green traces), and -467 (orange traces).
For Sample 160 °C/10 min, the first three clusters are addressed. For Sample 180°C/10 min and Sample 200 °C/10 min, the last three clusters are dealt with. For all of the clusters, a fwhm value of 20 nm was used. Furthermore, for each of the baseline-subtracted absorption spectra (a−c), the peak positions of the three clusters are from the PLE study addressed inFigures 2andS2-1, and the fourth Gaussian peak is the most blue-side peak (around 405−410 nm, dashed gray traces); the present study does not address its origin. For each of the PL spectra (a−c), the PL peak positions at 451 and 472 nm are respectively used for Clusters-437 and -453 only, which come from the PL study in Figure 4, and the fourth PL Gaussian peak is the most red-side peak (centered at∼600 nm, dashed gray traces) and is attributed to trap emission.Table S3
displays thefixed and free parameters for the deconvolution. The deconvolution suggests that Clusters-429 and -467 emit at 440 and 490 nm, respectively. Also, the returned values for the free parameters of the absorption strength for the four clusters (as shown inFigure S5-2and summarized inTable 1) are in agreement with our above arguments (Figure 2), suggesting that Cluster-437 is in the majority for Sample 160°C/10 min and Cluster-453 increases in population in Sample 180°C/10 min and dominates in Sample 200°C/10 min.
Accordingly, it seems reasonable that there are two categories of CdSe clusters. One category exhibits one sharp absorption doublet and readily self-assembles into two-dimensional nanoplatelets and even helical nanostructures; extraordinarily, the clusters and their assembled 2D nanoma-terials have similar optical properties.22,30,43−45 The other category mainly displays one sharp absorption sin-glet,17−23,26−29,45,46 which includes these discussed in the present study. Unfortunately, it appears technically impossible at present to obtain the composition of the CdSe clusters. Recently, a two-pathway model (Yu) was proposed, explaining the prenucleation stage of colloidal QDs.47Starting from metal (M) and chalcogenide (E) precursors, one pathway leads to precursor compounds (PCs) of magic-size clusters (MSCs, such as CdSe MSC-415 with a fwhm of∼12 nm).29The other pathway involves monomers and fragments that result in nucleation and growth of QDs. The interplay between the two pathways has been demonstrated by the growth of QDs or MSCs accompanied by the disappearance of MSCs or QDs.29,44,47Remarkably, the two-pathway model is apparently
consistent with the reported coexistence of InP intermediates (with a constant mass of∼10 kDa) and InP QDs (from 60 to 90 kDa) and with the observation that the size growth of the latter was accompanied by the consumption of the former.48 For the present study, we show that one CdSe reaction product, which exhibits one optical absorption peak with a fwhm from 25 to 30 nm, may contain clusters with slightly different band-edge energies or small-size QDs and a relatively small population of clusters. While the growth relationship of these clusters in the present study is unknown at this stage, it is notable that various CdSe clusters have been increasingly revealing,17−23,26−30,43−46 similar to fullerene family com-pounds.49 At the same time, the details of identification of the clusters in the present study suggest the probable existence of an additional pathway in the prenucleation stage, yet to be elucidated.
In conclusion, we have systematically studied the nature of the products from two non-hot-injection reactions of Cd-(OA)2) and Se in ODE, with HPPh2in one reaction (Batch a)
and not in the other reaction (Batch b). We performed a comprehensive characterization using a number of optical spectroscopic methods, which included absorption, PL, and PLE. On the basis of the optical measurement results, we conclude that the Batch a reaction (Figure S1-2afrom 120 to 220°C) produced clusters, while that of Batch b (Figure S1-2b
from 220 to 260 °C) yielded essentially small-size QDs, together with a relatively small amount of clusters. With regard to the spectral fwhm (Figure 1) as well as the peak red-shift behavior in optical absorption and PL, the sequential samples from Batch a (Figure S1-2a) would seem to be similar to those from Batch b (Figure S1-2b). However, PLE tells a different story, which strongly suggests that the nature of the products from the two batches is different. This conclusion was reached from a sequence of samples studied by PLE with the emission wavelengths set near the relatively sharp band-edge or bandgap emission and at the center of the relatively broad trap emission (Figures 2and3). When emission was set on the red side of the relatively sharp emission, the lowest-energy PLE peak wavelength was constant for the samples from Batch a (Figure 2) indicating the presence of Clusters-429, -437, -453, and -467, while it continuously red-shifted for the samples from Batch b (Figure 3). Furthermore, for the samples from Batch b, their higher-energy PLE peaks were at constant positions in agreement with the presence of Clusters-492, -482, -467, and -453. The identification of clusters is supported by other Batch a reactions performed at constant temperatures of 185 (Figures 4andS4-1), 175 (Figure S4-2), and 195°C (Figure S4-3), and that of small-size QDs is supported by a Batch b reaction carried out at a constant temperature of 230°C (Figure S4-4). For further information, we performed spectral deconvolution (Figure 5) by curve fitting on the absorption and emission spectra in Figure 2. The deconvolutions were based on the absorption peak positions of Clusters-429, -437, -453, and -467 from the PLE spectra ofFigures 2andS2-1and the PL peak positions of Clusters-437 and -453 respectively at 451 and 472 nm from the PL spectra ofFigure 4. The deconvolution results are consistent with our qualitative validation and semi-quantitative identification of the clusters and returned the PL peak positions of Clusters-429 and -467 as 440 and 490 nm, respectively. The present study demonstrates that it is probable for a range of clusters (with slightly different band-edge energies) to be simultaneously present in a single CdSe sample ensemble and thus to have optical absorption and PL spectra
that are similar to those of a single CdSe QD ensemble. Also, the peak positions in optical absorption and PL depend on the relative amounts of the coexisting clusters.17−22,27−29,45,46For a small-size QD ensemble that displays one sharp bandgap absorption peak, it is possible that clusters are also present. When both the bandgap and trap emission are observed, the coexisting clusters may contribute mainly to the trap emission, with more from the blue-side ones (than the red-side ones). The development of a synthetic method that minimizes the presence of clusters in small-size QD products would result in an enhanced QD production yield.
■
ASSOCIATED CONTENT*
S Supporting InformationThe Supporting Information is available free of charge on the
ACS Publications website at DOI:
10.1021/acs.jp-clett.9b02439.
Experimental details of synthesis and characterization and deconvolution together with a literature summary and additional optical spectra (PDF)
■
AUTHOR INFORMATIONCorresponding Authors
*E-mail:xqchen@scu.edu.cn(X.C.). *E-mail:kuiyu@scu.edu.cn (K.Y.). ORCID Meng Zhang:0000-0002-2852-2527 Nelson Rowell:0000-0001-7616-9396 Theo Kreouzis:0000-0003-2326-5338 Hongsong Fan:0000-0003-3812-9208 Qiyu Yu:0000-0001-9570-1755 Wen Huang:0000-0002-9772-9492 Kui Yu: 0000-0003-0349-2680 Notes
The authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSK.Y. is grateful to the National Natural Science Foundation of China (NSFC) 21773162 and 21573155 and the State Key Laboratory of Supramolecular Structures and Materials of Jilin University for SKLSSM 201935. K.Y. and Q.Y. thank the State Key Laboratory of Polymer Materials Engineering of Sichuan University for Grant No. sklpme2018-2-08 and Grant No. sklpme2019-4-38, respectively. H.F. and W.H. thank the National Major Scientific and Technological Special Project for“Significant New Drugs Development” (2018ZX09201009-005-002 and 2018ZX09201009-005-001).
■
REFERENCES(1) Murray, C. B.; Norris, D. J.; Bawendi, M. G. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706− 8715.
(2) Peng, Z. A.; Peng, X. Formation of High-Quality CdTe, CdSe, and CdS Nanocrystals Using CdO as Precursor. J. Am. Chem. Soc. 2001, 123, 183−184.
(3) Yu, K.; Singh, S.; Patrito, N.; Chu, V. Effect of Reaction Media on the Growth and Photoluminescence of Colloidal CdSe Nano-crystals. Langmuir 2004, 20, 11161−11168.
(4) Yang, Y. A.; Wu, H.; Williams, K. R.; Cao, Y. C. Synthesis of CdSe and CdTe Nanocrystals without Precursor Injection. Angew. Chem., Int. Ed. 2005, 44, 6712−6715.
(5) Li, J.; Wang, H.; Lin, L.; Fang, Q.; Peng, X. Quantitative Identification of Basic Growth Channels for Formation of Monodisperse Nanocrystals. J. Am. Chem. Soc. 2018, 140, 5474−5484. (6) Kirkwood, N.; Boldt, K. Protic Additives Determine the Pathway of CdSe Nanocrystal Growth. Nanoscale 2018, 10, 18238−18248.
(7) Luo, H.; Kebede, B. A.; McLaurin, E. J.; Chikan, V. Rapid Induction and Microwave Heat-Up Syntheses of CdSe Quantum Dots. ACS Omega 2018, 3, 5399−5405.
(8) Ekimov, A. I.; Hache, F.; Schanne-Klein, M. C.; Ricard, D.; Flytzanis, C.; Kudryavtsev, I. A.; Yazeva, T. V.; Rodina, A. V.; Efros, A. L. Absorption and Intensity-Dependent Photoluminescence Measure-ments on CdSe Quantum Dots: Assignment of the First Electronic Transitions. J. Opt. Soc. Am. B 1993, 10, 100−107.
(9) Peng, X.; Wickham, J.; Alivisatos, A. P. Kinetics of II-VI and III-V Colloidal Semiconductor Nanocrystal Growth:“Focusing” of Size Distributions. J. Am. Chem. Soc. 1998, 120, 5343−5344.
(10) Empedocles, S. A.; Neuhauser, R.; Shimizu, K.; Bawendi, M. G. Photoluminescence from Single Semiconductor Nanostructures. Adv. Mater. 1999, 11, 1243−1256.
(11) Yu, W. W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854−2860.
(12) Jasieniak, J.; Smith, L.; van Embden, J., van; Mulvaney, P.; Califano, M. Re-examination of the Size-Dependent Absorption Properties of CdSe Quantum Dots. J. Phys. Chem. C 2009, 113, 19468−19474.
(13) Cui, J.; Beyler, r A. P.; Marshall, L. F.; Chen, O.; Harris, D. K.; Wanger, D. D.; Brokmann, X.; Bawendi, M. G. Direct Probe of Spectral Inhomogeneity Reveals Synthetic Tunability of Single-Nanocrystal Spectral Linewidths. Nat. Chem. 2013, 5, 602−606.
(14) Gellen, T. A.; Lem, J.; Turner, D. B. Probing Homogeneous Line Broadening in CdSe Nanocrystals Using Multidimensional Electronic Spectroscopy. Nano Lett. 2017, 17, 2809−2815.
(15) Lim, S. J.; Schleife, A.; Smith, A. M. Optical Determination of Crystal Phase in Semiconductor Nanocrystals. Nat. Commun. 2017, 8, 14849.
(16) Slejko, E. A.; Lughi, V. Size Control at Maximum Yield and Growth Kinetics of Colloidal II−VI Semiconductor Nanocrystals. J. Phys. Chem. C 2019, 123, 1421−1428.
(17) Ptatschek, V.; Schmidt, T.; Lerch, M.; Müller, G.; Spanhel, L.; Emmerling, A.; Fricke, J.; Foitzik, A. H.; Langer, E. Quantized Aggregation Phenomena in II-VI-Semiconductor Colloids. Phys. Chem. Chem. Phys. 1998, 102, 85−95.
(18) Kudera, S.; Zanella, M.; Giannini, C.; Rizzo, A.; Li, Y.; Gigli, G.; Cingolani, R.; Ciccarella, G.; Spahl, W.; Parak, W. J.; Manna, L. Sequential Growth of Magic-Size CdSe Nanocrystals. Adv. Mater. 2007, 19, 548−552.
(19) Sun, M.; Yang, X. Phosphine-Free Synthesis of High-Quality CdSe Nanocrystals in Noncoordination Solvents:“Activating Agent” and“Nucleating Agent” Controlled Nucleation and Growth. J. Phys. Chem. C 2009, 113, 8701−8709.
(20) Cossairt, B. M.; Owen, J. S. CdSe Clusters: At the Interface of Small Molecules and Quantum Dots. Chem. Mater. 2011, 23, 3114− 3119.
(21) Evans, C. M.; Love, A. M.; Weiss, E. A. Surfactant-Controlled Polymerization of Semiconductor Clusters to Quantum Dots through Competing Step-Growth and Living Chain-Growth Mechanisms. J. Am. Chem. Soc. 2012, 134, 17298−17305.
(22) Yu, K. CdSe Magic-Sized Nuclei, Magic-Sized Nanoclusters and Regular Nanocrystals: Monomer Effects on Nucleation and Growth. Adv. Mater. 2012, 24, 1123−1132.
(23) Beecher, A. N.; Yang, X.; Palmer, J. H.; LaGrassa, A. L.; Juhas, P.; Billinge, S. J. L.; Owen, J. S. Atomic Structures and Gram Scale Synthesis of Three Tetrahedral Quantum Dots. J. Am. Chem. Soc. 2014, 136, 10645−10653.
(24) Norris, D. J.; Bawendi, M. G. Measurement and Assignment of the Size-Dependent Optical Spectrum in CdSe Quantum Dots. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 53, 16338−16346.
The Journal of Physical Chemistry Letters Letter
DOI:10.1021/acs.jpclett.9b02439 J. Phys. Chem. Lett. 2019, 10, 6399−6408
(25) Norris, D. J.; Efros, Al. L.; Rosen, M.; Bawendi, M. G. Size Dependence of Exciton Fine Structure in CdSe Quantum Dots. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 53, 16347−16354.
(26) Soloviev, V. N.; Eichhöfer, A.; Fenske, D.; Banin, U. Size-Dependent Optical Spectroscopy of a Homologous Series of CdSe Cluster Molecules. J. Am. Chem. Soc. 2001, 123, 2354−2364.
(27) Kucur, E.; Ziegler, J.; Nann, T. Synthesis and Spectroscopic Characterization of Fluorescent Blue-Emitting Ultrastable CdSe Clusters. Small 2008, 4, 883−887.
(28) Riehle, F. S.; Bienert, R.; Thomann, R.; Urban, G. A.; Krüger, M. Blue Luminescence and Superstructures from Magic Size Clusters of CdSe. Nano Lett. 2009, 9, 514−518.
(29) Zhu, D.; Hui, J.; Rowell, N.; Liu, Y.; Chen, Q. Y.; Steegemans, T.; Fan, H.; Zhang, M.; Yu, K. Interpreting the Ultraviolet Absorption in the Spectrum of 415 nm-Bandgap CdSe Magic-Size Clusters. J. Phys. Chem. Lett. 2018, 9, 2818−2824.
(30) Liu, Y.; Zhang, B.; Fan, H.; Rowell, N.; Willis, M.; Zheng, X.; Che, R.; Han, S.; Yu, K. Colloidal CdSe 0-Dimension Nanocrystals and Their Self-Assembled 2-Dimension Structures. Chem. Mater. 2018, 30, 1575−1584.
(31) Yu, K.; Liu, X.; Zeng, Q.; Leek, D. M.; Ouyang, J.; Whitmore, K. M.; Ripmeester, J. A.; Tao, Y.; Yang, M. Effect of Tertiary and Secondary Phosphines on Low-Temperature Formation of Quantum Dots. Angew. Chem., Int. Ed. 2013, 52, 4823−4828.
(32) Yu, K.; Liu, X.; Qi, T.; Yang, H.; Whitfield, D. M.; Chen, Q. Y.; Huisman, E. J. C.; Hu, C. General Low-Temperature Reaction Pathway from Precursors to Monomers before Nucleation of Compound Semiconductor Nanocrystals. Nat. Commun. 2016, 7, 12223.
(33) Steckel, J. S.; Yen, B. K. H.; Oertel, D. C.; Bawendi, M. G. On the Mechanism of Lead Chalcogenide Nanocrystal Formation. J. Am. Chem. Soc. 2006, 128, 13032−13033.
(34) Evans, C. M.; Evans, M. E.; Krauss, T. D. Mysteries of TOPSe Revealed: Insights into Quantum Dot Nucleation. J. Am. Chem. Soc. 2010, 132, 10973−10975.
(35) Boles, M. A.; Ling, D.; Hyeon, T.; Talapin, D. V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 141−153.
(36) Azpiroz, J. M.; De Angelis, F. Ligand Induced Spectral Changes in CdSe Quantum Dots. ACS Appl. Mater. Interfaces 2015, 7, 19736− 19745.
(37) Harris, R. D.; Amin, V. A.; Lau, B.; Weiss, E. A. Role of Interligand Coupling in Determining the Interfacial Electronic Structure of Colloidal CdS Quantum Dots. ACS Nano 2016, 10, 1395−1403.
(38) Voznyy, O.; Thon, S. M.; Ip, A. H.; Sargent, E. H. Dynamic Trap Formation and Elimination in Colloidal Quantum Dots. J. Phys. Chem. Lett. 2013, 4, 987−992.
(39) Giansante, C.; Infante, I. Surface Traps in Colloidal Quantum Dots: A Combined Experimental and Theoretical Perspective. J. Phys. Chem. Lett. 2017, 8, 5209−5215.
(40) Houtepen, A. J.; Hens, Z.; Owen, J. S.; Infante, I. On the Origin of Surface Traps in Colloidal II−VI Semiconductor Nanocrystals. Chem. Mater. 2017, 29, 752−761.
(41) Moscheni, D.; Bertolotti, F.; Piveteau, L.; Protesescu, L.; Dirin, D. N.; Kovalenko, M. V.; Cervellino, A.; Pedersen, J. S.; Masciocchi, N.; Guagliardi, A. Size-Dependent Fault-Driven Relaxation and Faceting in Zincblende CdSe Colloidal Quantum Dots. ACS Nano 2018, 12, 12558−12570.
(42) Kirkwood, N.; Monchen, J. O. V.; Crisp, R. W.; Grimaldi, G.; Bergstein, H. A. C.; du Fossé, I.; van der Stam, W.; Infante, I.; Houtepen, A. J. Finding and Fixing Traps in II−VI and III−V Colloidal Quantum Dots: The Importance of Z-Type Ligand Passivation. J. Am. Chem. Soc. 2018, 140, 15712−15723.
(43) Liu, Y.; Rowell, N.; Willis, M.; Zhang, M.; Wang, S.; Fan, H.; Huang, W.; Chen, X.; Yu, K. Photoluminescent Colloidal Nanohelices Self-Assembled from CdSe Magic-Size Clusters via Nanoplatelets. J. Phys. Chem. Lett. 2019, 10, 2794−2801.
(44) Liu, Y.; Willis, M.; Rowell, N.; Luo, W.; Fan, H.; Han, S.; Yu, K. Effect of Small Molecule Additives in the Prenucleation Stage of
Semiconductor CdSe Quantum Dots. J. Phys. Chem. Lett. 2018, 9, 6356−6363.
(45) Dukes, A. D.; McBride, J. R.; Rosenthal, S. J. Synthesis of Magic-Sized CdSe and CdTe Nanocrystals with Diisooctylphosphinic Acid. Chem. Mater. 2010, 22, 6402−6408.
(46) Hsieh, T.-E.; Yang, T.-W.; Hsieh, C.-Y.; Huang, S.-J.; Yeh, Y.-Q.; Chen, C.-H.; Li, E. Y.; Liu, Y.-H. Unraveling the Structure of Magic-Size (CdSe)13Cluster Pairs. Chem. Mater. 2018, 30, 5468− 5477.
(47) Zhang, J.; Hao, X.; Rowell, N.; Kreouzis, T.; Han, S.; Fan, H.; Zhang, C.; Hu, C.; Zhang, M.; Yu, K. Individual Pathways in the Formation of Magic-Size Clusters and Conventional Quantum Dots. J. Phys. Chem. Lett. 2018, 9, 3660−3666.
(48) Xie, L.; Shen, Y.; Franke, D.; Sebastián, V.; Bawendi, M. G.; Jensen, K. F. Characterization of Indium Phosphide Quantum Dot Growth Intermediates Using MALDI-TOF Mass Spectrometry. J. Am. Chem. Soc. 2016, 138, 13469−13472.
(49) Kroto, H. W. The Stability of the Fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature 1987, 329, 529−531.