HAL Id: hal-02715246
https://hal.inrae.fr/hal-02715246
Submitted on 1 Jun 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Detection of geminiviruses from tropical countries by a
double monoclonal antibody elisa using antibodies to
african cassava mosaic virus
Léonie Givord, Denis Fargette, B. Kounounguissa, J.C. Thouvenel, Bernard
Walter, M.H.V. van Regenmortel
To cite this version:
Léonie Givord, Denis Fargette, B. Kounounguissa, J.C. Thouvenel, Bernard Walter, et al.. Detection
of geminiviruses from tropical countries by a double monoclonal antibody elisa using antibodies to
african cassava mosaic virus. Agronomie, EDP Sciences, 1994, 14 (5), pp.327-333. �hal-02715246�
Plant
pathology
Detection of
geminiviruses
from
tropical
countries
by
a
double monoclonal
antibody
ELISA
using
antibodies
to
African
cassava
mosaic virus
L
Givord
D
Fargette
2
B
Kounounguissa
3
JC
Thouvenel
4
B
Walter
MHV
Van
Regenmortel
1 Immunochimie, Institut de
Biologie
Moléculaire et Cellulaire du CNRS(IBMC),
15, rue René-Descartes, F67084Strasbourg
cedex;2
LPRC-ORSTOM,
CIRAD, BP 5035, F34032Montpellier
cedex 3INRA, BP 507, F68021Colmar, France;
4ORSTOM,
13, midan Ibn Afan, Dokki Cairo,
Egypte
(Received
7 December 1993;accepted
6May 1994)
Summary — Using
doubleantibody
sandwichenzyme-linked
immunosorbent assay(DAS-ELISA)
tests,
monoclonal antibodies(mAbs),
which wereprepared against
particles
of aNigerian
isolate of African cassava mosaic virus(ACMV),
differentiated Africangeminiviruses
(tomato
yellow
leaf curlvirus,
TYLCV;
ACMV from West and EastAfrica;
and okra leaf curl
virus)
from thoseoriginating
in other countries of the world(euphorbia
mosaicvirus;
Indian cassavamosaic
virus;
and TYLCV(Indian isolate)).
All these virusesbelong
to thewhitefly-transmitted geminivirus subgroup
III.One mAb reacted with all 7 tested viruses and isolates. For
detecting
TYLCV fromSenegal,
itproved
necessary to use areducing
agent
in extraction buffer. Adiagnostic
DAS-ELISA wasdeveloped.
This reliesonly
on the use of mAbs andis useful for
large-scale
fieldscreening.
geminivirus
III / monoclonalantibody
/ ELISA / African cassava mosaic virus / tomatoyellow
leaf curl virusRésumé — Détection de
geminivirus
de paystropicaux
par DAS-ELISA utilisant desanticorps dirigés
contre levirus de la
mosaïque
africaine du manioc. Desanticorps
monoclonaux(AcMc) dirigés
contre le virus de lamosaïque
africaine du manioc(«ACMV») (isolat
duNigéria)
et utilisés dans un testDAS-ELISA («double
antibody
sandwich-ELISA»)
permettent
dedistinguer (à
l’intérieur du sous-groupe III desgeminivirus),
lesgeminivirus
origi-nairesdAfrique (ACMV
souche Ouest etEst ;
virus de l’enroulementjaunissant
de la tomate :«TYLCV» ;
virus de l’enroulement dugombo)
de ceuxprovenant
des autres continents du monde(virus
de lamosaïque
del’euphorbe ;
virus indien de lamosaïque
dumanioc ;
TYLCV del’Inde).
L’un de ces AcMcréagit
avec les 7geminivirus
testés.Néanmoins,
la détection du TYLCV(isolat
duSénégal)
nécessite untampon
d’extractionspécifique,
contenant unagent
réducteur. Un test dediagnostic
ou de détection utilisant seulement desAcMc,
et parconséquent applicable
àgrande
échelle,
a été mis aupoint.
geminivirus III / anticorps
monoclonaux / ELISA / virus de lamosaïque
africaine du manioc / virus del’enroule-ment jaunissant
de la tomate*
INTRODUCTION
Cassava
mosaic disease(Warburg,
1894)
isthe
most
important
factor
limiting
cassavayields
in
many
parts
of Africa
(Storey
and
Nichols,
1938)
and
canbe observed almost
everywhere
onthe
continent where
cassavais grown
(Fauquet
and
Fargette, 1990).
The causal
agent
is transmitted
by
the
whitefly
Bemisia tabaci
(Kufferath
andGhesquière,
1932)
and is maintained and carried
to new areas in
vegetatively propagated
planting
material
(stem
cuttings).
Cassava mosaic is
caused
by
ageminivirus (Bock
and
Woods,
1983)
first isolated in
Kenya
under the
namecas-sava
latent virus
(Harrison
etal,
1977;
Bock etal,
1978)
and since renamed African
cassavamosa-ic virus
(ACMV;
Bock and
Woods,
1983).
It has
been detected
inCôte d’lvoire
(Walter,
1980),
Angola
(Sequeira
and
Harrison,
1982), Nigeria
(Adejare
and
Coutts,
1982)
and several other
African countries
(Harrison
and
Robinson, 1988;
Harrison
et al,
1991 a).
ACMV
canbe transmitted
by
inoculation of
sap from
cassava toNicotiana benthamiana and
can
be detected
by
immunosorbent
electronmicroscopy
(Sequeira
and
Harrison,
1982),
enzyme-linked
immunosorbent assay
(ELISA)
(Sequeira
and
Harrison,
1982;
Fargette
etal,
1987)
with
polyclonal
antibodies,
and
by
hybridi-sation
testswith cDNA
probes (Roberts
etal,
1984;
Robinson
et al,
1984).
ACMV is
placed
in
the
geminivirus
subgroup
III,
whose members
have
bipartite
genomes and
whitefly
vectors(Francki
et al,
1991)
and
aretypically
serologi-cally
related
to one another(Roberts
etal,
1984).
These
relationships
reflect the
degree
ofamino-acid sequence
identity (about
70% ormore)
among
the coatproteins
of different
members
(Hamilton
etal,
1984;
Howarth
etal,
1985).
Among whitefly-transmitted geminiviruses,
the
particles
of ACMV
(Thomas
etal,
1986),
Indian
cassavamosaic
(ICMV;
Aiton and
Harrison,
1989)
and okra leaf curl
(OLCV;
Swanson and
Harrison,
1993)
viruses have
been used
to raisemonoclonal antibodies
(mAbs).
In
testswith
apanel
of
17mAbs
toACMV
(Harrison
and
Robinson,
1988;
Harrison
et
al,
1991a)
virus isolates from
cassavafell into
3
groups
onthe basis
of theirpatterns
of
reac-tions. These groups had different
geographical
distributions: group A
(Africa,
westof the Rift
Valley),
group B
(Africa,
eastof the
RiftValley),
and group C
(ICMV;
Indian
subcontinent).
Several of the mAbs
toACMV and ICMV
react-ed with other
whitefly-transmitted
geminiviruses
infecting
awide range of crop and weed
species
in many countries
inAfrica,
the
Americas,
Asia
and
Europe (Thomas
et al, 1986;
Harrison
et al,
1991b;
Muniyappa
et al,
1991;
Macintosh
et al,
1992;
Swanson
etal, 1992a,
1992b).
Some of
the
mAbs,
in various
combinations,
wereuseful
for
detecting
and
identifying
individual
viruses,
and others such
asSCR20 detected
nearly
allthe viruses.
The
mAbsof Thomas
etal
(1986)
werepre-pared
toACMV-JI,
anisolate derived
by Stanley
(1983)
from the
Kenyan
type
strain of ACMV. As
the
plant-breeding
program for the
improvement
of
cassavain West Africa necessitated
afield
test
for the
diagnosis,
and
hopefully,
theeradica-tion
of
ACMV,
weprepared
mAbs
toACMV
iso-late TMS
30211from
Nigeria.
For routine
detec-tion
of
ACMV,
adouble
antibody
sandwich
(DAS)
ELISA
relying only
on the useof
mAbs wasdeveloped.
MATERIALS AND METHODS
Viruses
The viruses were maintained at Scottish
Crop
Research Institute(SCRI,
Dundee,
UK)
at ca 25°C inan
insect-proof
glasshouse
under licence from theDepartment
ofAgriculture
and Fisheries for Scotland.The
following
hosts were used: N benthamiana forACMV from West Africa
(Nigeria) (ACMV-W)
andICMV from
India;
Manihot esculentus for ACMV from East Africa(Malawi)
(ACMV-E); Lycopersicon
esculen-tum from tomato
yellow
leaf curl virus isolates fromSenegal (TYLCV-S)
and India(TYLCV-I);
Abelmoschus esculentus for OLCV from Africa(Côte
d’lvoire);
and N benthamiana foreuphorbia
mosaic virus(EMV)
from America(USA).
ACMV-E was main-tained incuttings,
TYLCV-S and TYLCV-Iby grafting
and OLCVby whitefly
transmission.At the INRA research station of Colmar
(France)
ACMV,
isolate TMS 30211(kindly provided by
the International Institute forTropical
Agriculture,
Ibadan,
Nigeria)
and other isolates of ACMV(Kounounguissa
et al,
1989)
were maintained inmanually
inoculated N benthamiana in aninsect-proof glasshouse
undercon-trolled conditions
(18-27°C,
16 hlight/day).
Particles of ACMV TMS 30211 were
purified
according
toKounounguissa
et al (1989).
Thepurified
preparations
were stored frozen at -20°C after addi-tion of 0.03%NaN
3
.
Preparation
of rabbit
immunoglobulins
The methods described
by Kounounguissa et al (1989)
were used to prepare rabbit
immunoglobulins.
Hybridoma production
For the first fusion
experiment,
3-6-week-old BALB/c and B 10 Brown mice were immunizedby
3intraperi-toneal
injections
of ACMV(TMS
30211 virusisolate).
Each animals wasinjected
with 75 μgpurified
virus for the 2 firstinjections
and 100 μg for the third one. The virus was emulsified with anequal
volume of Freund’sincomplete adjuvant
for the firstinjection
andcomplete
adjuvant
for the second and thirdinjections.
The micewere rested for 10
weeks,
and then 2 mice(1
B10Brown and 1
BALB/c)
weregiven
3intraperitoneal
boosterinjections
with 50 μg virus in saline each ondays
5,
4 and 3 before fusion. For the secondfusion,
3-6-week-old BALB/c and B 10 Brown mice were
immunized
by
4intraperitoneal
injections
at 2 weeksinterval for the first 3 and then 1 month interval for the
last
injection
(60,
45,
50 and 35 μg,respectively).
Sixweeks
later,
the mice weregiven
1 intravenous and 1intraperitoneal
boosterinjection
of 34 and 80 μg virusin
saline,
respectively.
Threedays
after the second boosterinjection,
thespleens
were excised. Cell fusionwas
performed
as describedby
Al Moudallal et al(1982) using
the PAImyeloma
cell line(Stocker
etal,
1982).
Selectedpositive
cultures were clonedby
limit-ing
dilution,
using
10
7
thymocytes/ml
as feeder cells. All clones were derived from BALB/c mice cells.The clones were
multiplied
inpristane-primed
BALB/c miceby injection
0.5 x 107hybridoma
cells in 0.5 ml Dulbecco’s modifiedEagle’s
medium into theperi-toneal
cavity.
The ascitic fluid was harvestedby
tap-ping
theperitoneum
with a19-gauge
syringe
needle,
and the cells and debris were removed
by
centrifuga-tion at 750
g for
30 min in a Jouan E96centrifuge.
Immunoassay
used
for
screening hybridomas
Wells
containing growing hybridoma
clones werescreened for the presence of
specific
antibodies toACMV TMS 30211 in both DAS and
antigen-coated
plates (ACP)
indirect ELISA. In ACP-ELISAprocedure
the microtitre
plates (Falcon,
Oxnard,
CA;
Ref3912)
were coated with a
purified preparation
of ACMV(0.35
μg/ml)
in 0.05 M carbonate bufferpH
9.6(coating
buffer)
for 2 h. The DAS-ELISA tests were carried outusing
rabbitIgG
raisedagainst
ACMV as a firstanti-body,
at 1μg/ml (2 h),
incoating
buffer,
followedby
incubation with theantigen
at 0.35μg/ml
inphosphate-buffered
saline,
pH
7.4,
containing
0.05% Tween 20(PBS-T) overnight
at 4°C. The rabbitIgG
used in thistest has been described
previously (Kounounguissa
etal, 1989).
In both methods theremaining
binding
siteson the
plastic plates
were then saturatedby
incubation with 1% bovine serum albumin(BSA)
in PBS-T. PBS-Twas used as the
diluting
buffer in thesubsequent
steps.
Hybridoma
culturesupernatant
fluids weredi-luted 1/3 or 1/5 and an alkaline
phosphatase-conjugat-ed
affinipure
sheep
anti-mouseIgG (H
+L) (Jackson
ImmunoResearch
Laboratories,
WestGrove,
PA)
di-luted 1/3 000 was used as the
detecting antibody.
Thebound
conjugate
was detectedby
addition of thesub-strate,
p-Nitrophenylphosphate
in 0.1 Mdiethanolamine buffer
pH
9.8.Optical density (405 nm)
values were measured with a Titertek Multiskanpho-tometer
(Flow
Laboratories).
Absorbance values wereconsidered
positive
ifthey
exceeded that of the buffercontrol
by
a factor of 3.Labelling
of
reagent
For
biotinylating
mAbs,
N-hydroxysuccinimidobiotin
(Sigma) (biotin)
dissolved in distilleddimethylform-amide
(0.2 mg/ml)
was added to mAbs(ascitic
fluid)
diluted at around 1.5mg/ml,
in a 1:5 biotin/mAbs ratio(w/w);
afterincubating
the mixture at 25°C for 2h,
the reaction wasstopped by
addition of 1 MNH
4
Cl
(Zrein
et al,
1986).
Alkalinephosphatase-conjugated
strepta-vidin was from Jackson Immuno Research.
Crude
extractpreparation
Plant extracts
prepared
at SCRI wereground
in mortarand
pestle
in 0.05 M Tris/HClbuffer,
pH
8.0,
2%polyvinylpyrrolidone (PVP),
0.5% Tween 20(1
g tissuein 10 ml
buffer)
containing
0.005 M EDTA. Aspecific
bufferfor
TYLCV was devisedthrough
ELISA testsby
optimising
successivesteps
including
bufferion,
pH,
molarity
and additives: 0.05 MTris/HCl,
pH 8.5,
60 mM sodium sulfite.Extracts
prepared
at IBMC(Strasbourg, France)
and the ORSTOMlaboratory (Côte d’Ivoire)
weremade with cassava
tip
leavesrapidly
frozen inliquid
nitrogen
in a chilled mortar, andground
with PBS-T(1
g tissue to 3 mlbuffer)
and sterilizedquartz
sand. Thenitrogen step proved
essential as otherwise novirus could be detected
by
ELISA test in the extract.For
clarification,
the crude extract wascentrifuged
at3 000 g for 20 min in Jouan E96
centrifuge.
ELISA
testsused
for
diagnosis
MAbs were used in indirect DAS-ELISA
(Procedure 1)
as described in
Muniyappa
et al(1991).
Microtiterplates
were coated with 1μg/ml γ-globulin
frompoly-clonal antiserum to
ACMV-W;
infectious sap waspre-pared
as describedabove;
bound viralantigen
wasexposed
to diluted ascitic fluid at a concentrationhigh
enough
togive
astrong
reaction with thehomologous
virus
(dilution
between10
-4
to10
-5
),
followedby
2 h incubation at roomtemperature
with rabbit anti-mouseOptical density (OD) readings (A
405nm)
were takenafter incubation with substrate for 1 or 2 h at room
tem-perature
andagain
afterovernight
incubation at 5°C.Procedure 2
corresponds
to direct DAS-ELISA. The mAb was diluted(ascitic
fluiddilution
1:1000)
incoat-ing
buffer and incubated in microtiter wells for 3 h atroom
temperature.
The viralantigens (crude
sap)
werediluted in 0.05 M Tris/HCl
buffer,
atpH
8.0 2%PVP,
0.5% Tween
containing
0.005 MEDTA,
and incubatedover
night
at 5°C.Biotinylated
mAb(dilution 1:10 000)
was used to detect
captured
antigen (2
h at roomtem-perature)
and was revealedusing streptavidin
conju-gated
to alkalinephosphatase,
diluted 1:1 000 in PBS-Tcontaining
1% BSA and 2% PVP(2
h at roomtem-perature) following
by
the substratep-nitrophenyl
phosphate.
As in Procedure1,
readings
were takenafter incubation with substrate for 1 or 2 h at room
tem-perature
andagain
afterovernight
incubation at 5°C.The same mAb was used for
coating
theplate
assec-ond
biotinylated
antibody.
To detect ACMV in extracts from infected leaves of
N
benthamiana,
mAbs were used at variousconcen-trations: ascitic fluids were diluted from
10
-3
to10
-4
for mAb 7 and from 5 x10
-4
to 10-5for mAb 11.Immunoblotting
Western blots tests were carried out as described
by
Harlow and Lane(1988). Samples
of ACMV and TYLCV were extracted from infected leaves and mixed withsample
buffer made of 60 mMTris/glycine,
2%SDS,
100 mM dithiothreitol and 0.01 %bromophenol
blue.
RESULTS
Characterisation of the mAbs
Only
one clone wasobtained from
thefirst fusion
experiment,
the mAb
41,
which
reactsonly
inACP - ELISA with
purified
ACMV. Two clones
were
obtained from the second
one,mAb
7and
mAb
11,
which
reactin ACP and
DAS -
ELISA.
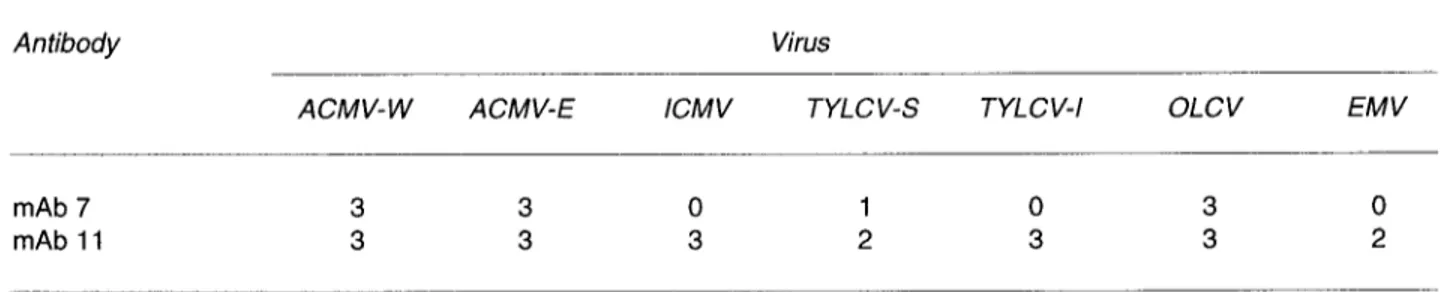
Table I indicates the reactions obtained in indirect
DAS-ELISA when mAbs
7and
11 weretested
against
arange of well-characterised
geminivirus-es
obtained from various
geographical
areasand
various host
plants (see
list inMaterials and
methods).
The
pattern
of reactions
wasdifferent
for the
2antibodies. mAb
11reacted
strongly
with
all tested
geminiviruses. By
contrast,
mAb
7reacted
only
with
geminiviruses
from Africa.
In Western-blot
tests,
mAbs
7and
11gave
noreaction with ACMV in infected leaves of N
ben-thamiana.
Immunoassays
The results
intable
I wereobtained with virus
material
extracted in PBS with 0.5% Tween-20
and 2%
PVP,
pH
7.5.In order
toestablish if
other
extraction
buffers gave
betterresults,
leaves
infected with TYLCV
wereextracted
invarious
buffers. Results obtained
indirect DAS-ELISA
with mAb
11conjugated
with biotin
areshown in
figure
1.Similar results
wereobtained
inindirect
DAS-ELISA.
Lowvalues
were reachedwhen
standard
buffers used for many viruses
(Converse
and
Martin,
1990)
and
geminiviruses
(Harrison
etal,
1991 b)
wereapplied.
Aspecific
extractionbuffer
containing
sodiumsulfite
(see
Materials and
methods)
allowed much
higher
readings
with TYLCV isolates from
Senegal,
Egypt,
Nigeria,
Burkina-Faso and
Sicily.
There
was a
linear
relationship
between OD
(405 nm)
values and the dilution of the
extract(in
alog/log
representation;
regression
coefficient
R
2
=0.98).
This allowed titration
andcomparisons
of virus
concentration
inleaf
extracts.When mAb
11 wasconjugated
with biotin and
used in direct DAS-ELISA
(see
Materials and
methods),
ACMV-W
wasreadily
detected
atdilu-tions of
1:1000 and
TYLCV-S up
to 1:25of
thecrude
extracts(table
II).
However,
the
sensitivity
of direct DAS-ELISA
tests waslower than that of
indirect DAS-ELISA
tests,
by
afactor of
10for
ACMV and
1:25for TYLCV.
Coating
the
plates
with mAbs gave values
comparable
and
some-times
higher
than those obtained
whencoating
with
polyclonal
serum.For
instance,
in
one setof
experiments
with
TYLCV-containing
extracts indirect
DAS-ELISA,
values
were 1.65with the
mabs and 0.95 with the
polyclonal
antibodies,
whereas
background
reactions with
healthy
sap
were
similar
(0.12
and
0.13,
respectively).
In both
tests
however,
the
background
values
werehigh-er
than those obtained in indirect DAS-ELISA
(0.12 vs 0.07).
Field
testsIndirect DAS-ELISA
using
mAb
11has been
successfully
used for
screening
cassavaplants
for the presence of ACMV. The ORSTOM field
cassava
collection in Côte d’Ivoire and the
glasshouse
collection
atthe INRA research
sta-tion of
Colmar,
which include varieties from
sev-eral
countries,
wereboth
tested inthis way.
Fourty-one
isolates weretested and
yielded
OD
(corrected
for
background) ranging
from
0.6 to2.8
(Côte
d’Ivoire:
13isolates;
Kenya:
8;
Nigeria:
8;
South American Continent:
4;
India:
3;
Madagascar:
3;
Togo:
1;
Central African
Republic: 1).
This
testhas also been
successfully
used
(OD
>1.5)
with the
sodium-sulfite-contain-ing
buffer
todetect
geminiviruses
in tomatoleaf
samples
from
Senegal, Egypt,
Nigeria,
Burkina-Faso and
Sicily,
inlaboratories located in these
countries
orin laboratories
inEurope
that had
received
samples
from there.
InEgypt,
these
mAbs have also been
successfully
used
toassess
the presence of
asuspected
whitefly-transmitted
geminivirus
inthe ornamental
plant
Althea
rosea(Malvaceae)
which exhibited
symp-toms
close
toOLCV and which
might
be
aDISCUSSION
The ELISA
results indicatethat the
2mAbs
(11
and
7)
that
wehave
prepared
with
ACMV-W,
recognize
2different
conformational
epitopes,
which
are notpreserved
when the viralprotein
is
denatured
by
treatment withSDS and
dithiothre-itol
inWestern blot
tests.They
also indicate that
mAb 11
is directed
against
anepitope
shared
by
7
viruses
orisolates from various hosts and
loca-tions,
whereas the
epitope recognized
by
mAb
7is restricted
tothe
4viruses
orisolates from
Africa. The
contrasting patterns
of reactions
between the
2mAbs
suggest
ageographical
variation of the
epitope profile,
afeature
already
reported
for other mAbs directed towards
gemi-niviruses
(Harrison
and
Robinson,
1988)
and
viruses from other
groups
(Matthews,
1990).
Any
large
epidemiological,
sanitation
orresis-tance
program
in adeveloping country implies
alarge
number
of
testsand
requires
unrestricted
access to
inexpensive immunological
reagents.
Using
monoclonal instead of
polyclonal
antibod-ies in the
coating
stages
of the DAS-ELISA
tests will overcomethe
difficulty
linked
tolimited
amounts
of
polyclonal
antibodies. Direct
DAS-ELISA
using
mAbs
ascaptured
antibodies
and second
biotinylated
antibodies is
sufficiently
sensitive for the routine detection of ACMV and
TYLCV.
However,
a moresensitive detection of
the viruses
canbe achieved
by
indirect
DAS-ELISA. The
natureof the extraction buffer
is also critical for
optimal
virus detection and
must
be
adjusted
for each
geminivirus
studied.
For
extracting
TYLCV-S,
for
instance,
it is
neces-sary
to usethe
reducing
agent
sodium
sulfite,
which
presumably
stabilizes the virus
structure andmaintains
theantigenic reactivity
in theELISA
test.Similar results have been described
for tobacco leaf
curl virus(Macintosh
et al,
1992).
The
2 mAbsdescribed
in thisarticle
allowedthe detection of
whitefly-transmitted
gemini-viruses from various
plants
and from various
geo-graphical origins. They
have also been
success-fully
used
to assessvirus concentrations in
plants
and
tosearch for natural reservoirs of the
virus-es.
Furthermore,
using
mAb
11in
immunoblot-ting,
oneof
us hasrecently
detected
gemini-viruses
inthe
following
hostplants
inEgypt:
tomato,
okra, cucumber,
Datura
stramonium,
Cucurbita
pepo,
and Althea rosea(Gosselin
andThouvenel,
unpublished results).
These results
confirm the
ability
of mAbs
11in the detection of
geminiviruses
in various
plant species.
However,
these
tests mustbe
complemented by
other
information such
assymptomatology,
transmis-sion and host
range
when full
identification of the
virus is needed.
ACKNOWLEDGMENTS
Part of this work was made
during
thestay
of thesec-ond author at SCRI.
Special
thanks are due to BDHarrison for
helpful
discussions. This research wasfinanced in
part
by
a collaborative program of CNRSand ORSTOM.
REFERENCES
Aiton
MM,
Harrison BD(1989)
Monoclonal antibodiesto Indian cassava mosaic
geminivirus (ICMV). Rep
ScottCrop
Res Inst1988,
175Al Moudallal
Z,
BriandJP,
VanRegenmortel
MHV(1982)
Monoclonal antibodies asprobes
of theanti-genic
structure of tobacco mosaic virus. EMBO J1,
1005-1010
Adejare
GO,
Coutts RHA(1982)
The isolation and characterisation of a virus fromNigerian
cassavaplants
affectedby
the cassava mosaic disease andattempted
transmission of the disease.Phytopathol
Z 103, 198-210
Bock
KR,
Woods RD(1983)
Etiology
of African cassa-va mosaic disease. Plant Dis67, 994-995
Bock
KR,
GuthrieEJ,
Meredith G(1978)
Distribution,
host range,
properties
andpurification
of cassavalatent
virus,
ageminivirus.
AnnAppl
Biol90,
361-367
Converse
R,
Martin R(1990) Enzyme-linked
immunosorbent assay(ELISA).
In:Serological
Methods for Detection and Identification of Viral and
Bacterial Plant
Pathogens (R Hampton,
EBall,
S deBoer,
eds),
APSPress,
SaintPaul, MN, USA,
179-196Fargette
D,
ThouvenelJC,
Fauquet
C(1987)
Virus content of leaves of cassava infectedby
Africancassava mosaic virus. Ann
Appl
Biol 110,
65-73Fauquet
C,
Fargette
D(1990)
African cassava mosaic virus:etiology, epidemiology,
and control. Plant Dis74, 404-411
Francki
RIB, Fauquet C,
KnudsonDL,
Brown F(1991)
Classification and nomenclature of
viruses;
fifthreport
of the international committee ontaxonomy
of viruses. Arch
Virol Suppl
2,
1-450Hamilton
WDO,
SteinVE,
CouttsRHA,
Buck KW(1984) Complete
nucleotide sequence of the infec-tious cloned DNAcomponents
of tomatogolden
mosaic virus:
potential coding regions
andregu-latory
sequences. EMBOJ 3,
2197-2205Harlow
E,
Lane D(1988)
Antibodies. ALaboratory
Manual. ColdSpring
HarborLaboratory,
NewYork,
Harrison
BD,
Robinson DJ(1988)
Molecular variation in vector-borneplant
viruses:epidemiological
signif-icance. Philos Trans R Soc Lond B Biol Sci321,
447-462
Harrison
BD,
BarkerH,
BockKR,
GuthrieEJ,
Meredith
G,
Atkinson M(1977)
Plant viruses withcircular
single-stranded
DNA. Nature(Lond)
270,
760-762
Harrison
BD,
SwansonMM,
McGrathP,
Fargette
D(1991a)
Patterns ofantigenic
variation inwhitefly-transmitted
geminiviruses.
AnnuRep
ScottCrop
Res Inst1990,
88-90Harrison
BD,
Muniyappa
V,
SwansonMM,
RobertsIM,
Robinson DJ
(1991b) Recognition
anddifferentia-tion of seven
whitefly-transmitted geminiviruses
from
India,
and theirrelationships
to African cassa-va mosaic and Thailand mung beanyellow
mosaicviruses. Ann
Appl
Biol 118,
299-308Howarth
AJ,
CatonJ,
BossertM,
Goodman RM(1985)
Nucleotide sequence of beangolden
mosaic virus and a model for generegulation
ingeminiviruses.
Proc Natl Acad Sci USA82,
3572-3576Kounounguissa
BR,
GivordL,
Walter B(1989)
Africancassava mosaic virus
(ACMV): stability
ofpurified
virus andimproved
conditions for its detection incassava leaves
by
ELISA. JPhytopathol 127,
29-41Kufferath
H,
Ghesquière
J(1932)
Lamosaïque
du manioc. CR Soc BiolBelge
109,
1146-1148Matthews REF
(1990)
PlantVirology.
Third Edition. AcademicPress,
LondonMacintosh
S,
RobinsonDJ,
Harrison BD(1992)
Detection of threewhitefly-transmitted
gemini-viruses
occurring
inEurope by
tests with heterolo-gous monoclonal antibodies. AnnAppl
Biol121,
297-303
Muniyappa
V,
SwansonMM,
DuncanGH,
Harrison BD(1991)
Particlepurification,
properties
andepitope
variability
of Indian tomato leaf curlgeminivirus.
AnnAppl Biol 118,
595-604Roberts
IM,
RobinsonDJ,
Harrison BD(1984)
Serological relationships
and genomehomologies
among
geminiviruses.
J GenVirol 65,
1723-1730Robinson
DJ,
HarrisonBD,
Sequeira
JC,
Duncan GH(1984)
Detection of strains of African cassavamosaic virus
by
nucleic acidhybridisation
and someeffects of
temperature
on theirmultiplication.
AnnAppl Biol 105,
485-493Sequeira
JC,
Harrison BD(1982) Serological
studieson cassava latent virus. Ann
Appl
Biol 101,
33-42Stanley
J(1983) Infectivity
of the clonedgeminivirus
genome
requires
sequences from both DNAs. Nature(Lond)
305,
643-645Stocker
JW,
FosterHK,
Miggiano
V et al(1982)
Generation of two newmyeloma
cell lines "PAI" and"PAI-O" for
hybridoma
production.
Res Disclos217,
155-157
Storey
HH,
Nichols RFW(1938)
Studies of the mosaic disease of cassava. AnnAppl Biol 25,
790-806 SwansonMM,
Harrison BD(1993) Serological
relation-ships
andepitope profiles
of isolates of okra leafcurl
geminivirus
from Africa and the Middle East. Biochimie75,
707-711Swanson
MM,
VarmaA,
Muniyappa
V,
Harrison BD(1992a) Comparative epitope profiles
of theparticle
proteins
ofwhitefly-transmitted geminiviruses
from nine croplegumes
in India. AnnAppl
Biol 120,
425-433Swanson
MM,
BrownJK,
PoulosBT,
Harrison BD(1992b)
Genome affinities andepitope
profiles
ofwhitefly-transmitted geminiviruses
from the Americas. AnnAppl
Biol 121,
285-296Thomas
JE,
MassalskiPR,
Harrison BD(1986)
Production of monoclonal antibodies to African cas-sava mosaic virus and differences in theirreactivity
with otherwhitefly-transmitted
geminiviruses.
J GenVirol 67,
2739-2748Walter B
(1980)
Isolation andpurification
of a virus transmitted from mosaic-diseased cassava in theIvory
Coast.Plant Dis 64,
1040-1042Warburg
O(1894)
DieKulturpflanzen
Usambaras. Mitt dtschSchutzgeb 7,
131-198Zrein
M,
BurckardJ,
VanRegenmortel
MHV(1986)
Use of the biotin-avidinsystem
fordetecting
a broadrange of