HAL Id: tel-03022201
https://tel.archives-ouvertes.fr/tel-03022201
Submitted on 24 Nov 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Unravelling the tangle of motor neuron diseases :
insights from neuroimaging and neurophysiology
Giorgia Querin
To cite this version:
Giorgia Querin. Unravelling the tangle of motor neuron diseases : insights from neuroimaging and neurophysiology. Neuroscience. Sorbonne Université, 2019. English. �NNT : 2019SORUS329�. �tel-03022201�
Sorbonne Université
Cerveau Cognition Comportement
Laboratoire d’Imagerie Biomédicale / Systèmes Dynamiques Anatomo-Fonctionnels chez l’Homme Thèse de Doctorat en Neurosciences
Présentée par
Giorgia QUERIN
Unravelling the tangle of motor neuron diseases: insights from neuroimaging
and neurophysiology.
Dirigée par Véronique MARCHAND-PAUVERT et
Pierre-François PRADAT
Présentée et soutenue le 24 Juin 2019 devant le jury composé de Devant un jury composé de :
M. Guillaume NICOLAS (PU-PH) Université de Versailles
Saint-Quentin-en-Yvelines Rapporteur
Mme. Virginie CALLOT (DR) Université d’Aix-Marseille Rapporteur
M. Bertrand FONTAINE (PU-PH) Sorbonne Université Examinateur
Mme. Dorothée Lulé (PU-PH) Ulm University Examinateur
Mme. Véronique Marchand-Pauvert (DR) Sorbonne Université Directeur de thèse
Contents Contents ……… i Acknowledgments ……… iii List of tables ..……….………... iv List of figures ..……….. iv Glossary ..……….. v Abstract ..………...……… ix
Published papers ..………..……….. xii
Original articles arising from this project……… xii
Literature reviews arising from this project……… xii
Additional contributions……….. xiii
Introduction………... 1
1. Background………...……… 2
1.1 Hints on spinal cord anatomy………...………..……...…………. 2
1.2 Basic principles of MRI………... 5
1.3 Amyotrophic Lateral Sclerosis.………..………..……….. 14
1.3.1 Clinical presentation.………...……….. 15
1.3.2 Extra-motor involvement in ALS.………...……….. 17
1.3.3 Diagnosis………...……… 20
1.3.4 Clinical genetics of ALS………...…………. 25
1.3.5 Pathology and pathogenesis in ALS……….. 29
1.3.6 Pathological staging of ALS……….. 32
1.3.7 Advanced neuroimaging in ALS………...……… 33
1.3.8 Brain imaging in ALS………...………. 34
1.3.9 Spinal cord imaging in ALS.………...……….. 39
1.3.10 Biomarkers in ALS………….………. 41
1.4 5q-Spinal Muscular Atrophy (SMA)………..…….…….. 43
1.4.1 Clinical presentation of adult 5q-SMA.………...……….. 43
1.4.2 Genetic background…………...……… 45
1.4.3 Diagnosis………...……… 46
1.4.4 Outcome measures.………...………. 48
1.4.5 Upcoming therapies………... 50
2. Objective of the study.……….. 52
Study 1: Spinal cord multi-parametric magnetic resonance imaging for survival
prediction in amyotrophic lateral sclerosis...………... 54
Study 2: Multi-modal spinal cord MRI offers accurate diagnostic classification in ALS………. 60
Study 3: Presymptomatic longitudinal cord pathology in c9orf72 mutation carriers: longitudinal neuroimaging study...……….. 69 Study 4: The spinal and cerebral profile of adult spinal-muscular atrophy: a multimodal imaging study...……… 84 Study 5: The motor neuron number index (MUNIX) profile of patients with adult Spinal Muscular Atrophy...………. 101 Study 6: Biomarkers definition for adult spinal muscular atrophy (SMA): experience from a longitudinal study.………. 112
4. General discussion………..………….. 119
4.1 Pathological implications of spinal cord MRI studies……….. 119
4.2 Development of diagnostic and prognostic biomarkers………... 123
4.3 Future perspectives ………... 124
Bibliography…...………... 126
Acknowledgments
I gratefully thank all the people who participated directly or indirectly to this thesis project.
Firstly, I would like to express my sincere gratitude to my advisors, Dr. Pierre-François Pradat and Véronique Marchand-Pauvert, for the continuous support of my PhD study and related research, for their patience, motivation, and immense knowledge. Their guidance helped me in all the time of research and writing of this thesis.
Besides them, I would like to thank the rest of my thesis committee and especially the reviewer of this work, Prof. Guillame Nicolas and Prof. Virginie Callot, for their insightful comments and encouragement, but also for the questions which incented me to widen my research from various perspectives.
My sincere thanks also go to Dr. Lori Bridal and Dr. Pascal Laugier, who gave me access to their laboratory and research facilities.
I thank my fellow labmates for the stimulating discussions, for all the fun we have had in the last three years and for their sincere friendship.
I gratefully thank all the patients who took part in this project and the founders of the project (AFM Téléthon, Target ALS, ANR).
Last but not the least, I would like to thank my family and all my friends for supporting me spiritually throughout writing this thesis and my life in general.
List of tables
Table 1. Diagnostic criteria for the diagnosis of ALS………20
Table 2. Differential diagnoses of ALS………...25
Table 3. Main differential diagnoses of adult onset SMA……….47
List of figures Figure 1. Anatomical features of the cervical spinal cord………2
Figure 2. Anterior anatomical view of the spinal cord……….3
Figure 3. Vertebral body and spinal cord segment location……….4
Figure 4. Transversal representation of the cervical spinal cord……….….5
Figure 5. Physics of MRI……….6
Figure 6. Example of automated spinal cord segmentation……….9
Figure 7. Representation of an automated pipeline for spinal cord data treatment………...……….10
Figure 8. Schematic representation of isotropic and anisotropic molecules diffusion………11
Figure 9. Template-based identification of the main cervical spinal cord white matter tracts………12
Figure 10. The motor system and its involvement in ALS……….15
Figure 11. Representation of MUNIX technique………22
Figure 12. Conventional MR imaging findings in patients with ALS………24
Figure 13. ALS gene discovery since 1993……….27
Figure 14. Cellular processes implicated in the pathogenesis of c9orf72 ALS-FTD……….28
Figure 15. Mechanisms of disease implicated in ALS pathogenesis……….…….29
Figure 16. Factors governing the genesis, replication, and spread of proteopathic seeds…………...32
Figure 17. Sequential progression of TDP-43 pathology in ALS………33
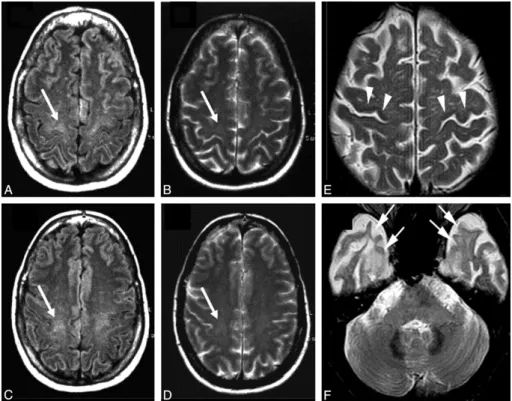
Figure 18. 18F-FDG PET imaging in ALS patients………39
Figure 19. Spinal cord MRI in ALS………39
Figure 20. Genetic and pathogenic mechanisms in SMA………46
Glossary
6MWT: 6-minute Walk Test AAV: Adeno-Associated Virus APB: Abductor Pollicis Brevis AD: Axial Diffusivity
AD: Alzheimer’s Disease AD: Autosomal Dominant ADM Abductor Digiti Minimi ALS: Amyotrophic Lateral Sclerosis ALS-bi: ALS with Behavioral Impairment ALS-ci: ALS with Cognitive Impairment
ALSFRS-R: ALS Functional Rating Scale Revised ASO: Antisense Oligonucleotide
AUC: Area Under the Curve BMI: Body Mass Index
BOLD: Blood Oxygenation Level Dependent Cho: Choline
CI: Confidence Interval CK: Creatine-kinase
CMAP: Composed Motor Action Potential CNR: Contrast-to-Noise Ratio
CNS: Central Nervous System Cr: Creatinine
CSA: Cross Sectional Area CSF: Cerebro-Spinal Fluid CST: Cortico-Spinal Tract DNA: Deoxy-Ribonucleic Acid DPR: Dipeptide-Repeat Proteins DTI: Diffusion Tensor Imaging DWI: Diffusion Weighted Imaging
ECAS: Edinburgh Cognitive and Behavioral ALS Screening EMG: Electromyography
EPI: Echo-Planar Imaging ER: Endoplasmic Reticulum
FA: Fractional Anisotropy fALS: Familiar ALS
FDG: Fluoro-Deoxy-Glucose
fMRI: Functional Magnetic Resonance Imaging FLAIR: Fluid Attenuated Inversion Recovery FDR: False-Discovery Rate
FOV: Field of View
FTD: Frontotemporal Dementia FUS: Fused-in Sarcoma gene FVC: Forced Vital Capacity GABA: γ-amino Butyric Acid GM: Grey Matter
HARDI: High-Angular Resolution Diffusion Imaging HC: Healthy Control
HFMSE: Hammersmith Functional Motor Scale Expanded HR: Hazard Ratio
ihMT: Inhomogeneous Magnetic Transfer LMN: Lower Motor Neuron
LL: Lower Limbs MD: Mean Diffusivity
MEDIC: Multi-Echo Data Image Combination MFM: Motor Function Measure
MEP: Motor Evoked Potential mI: Myo Inositol
MLPA: Multiplex Ligation-Dependent Probe Amplification MMT: Manual Muscle Testing
MN: Motor Neuron
MND: Motor neuron disease MRC: Medical Research Council MRI: Magnetic Resonance Imaging MRS: Magnetic Resonance Spectroscopy MT: Magnetization Transfer
MTR: Magnetization Transfer Ratio MU: Motor Unit
MUNE: Motor Unit Number Estimation MUNIX: Motor Unit Number Index MUSIX: Motor Unit Size Index NAA: N-Acetil-Aspartate NDI: Neurite Density Index
NGS: Next Generation Sequencing
NODDI: Neurite Orientation Dispersion and Density Imaging ODI: Orientation Dispersion Index
PET: Positron Emission Tomography PMA: Progressive Muscle Atrophy
pNH: Phosphorylated Neurofilaments Heavy PLS: Primary Lateral Sclerosis
PSIR: Phase Sensitive Inversion Recovery QBI: Q-ball Imaging
RAN: Repeated-associated non-ATG Translation RD: Radial Diffusivity
RF: Random Forest RNA: Ribo-Nucleic Acid ROI: Region of Interest sALS: Sporadic ALS SC: Spinal cord
SIP: Surface Interference Pattern SMA: Spinal Muscular Atrophy
SMAFRS: Spinal Muscular Atrophy Functional Rating Scale SMN-1: Survival Motor Neuron-1 Gene
SNR: Signal-to-Noise Ratio SVC: Slow Vital Capacity TA: Tibialis Anterior
TBSS: Tract-Based Spatial Statistics TDP-43: TAR DNA binding protein-43 TE: Echo time
TFCE: Threshold-free Cluster Enhancement TMS: Transcranial Magnetic Stimulation TSE: Turbo-spin Echo
UL: Upper Limbs
UMN: Upper Motor Neuron VBM: Voxel Based Morphometry WM: White matter
Abstract
Degenerative motor neuron diseases (MNDs) are characterized by progressive dysfunction and loss of ventral horn MNs in the spinal grey matter (GM). Beyond this common anatomical susceptibility, different MNDs such as amyotrophic lateral sclerosis (ALS) and survival motor neuron-1 gene linked spinal muscular atrophy (SMN1-linked SMA) present with specific motor and sometimes extra-motor features. Knowledge of pathogenic mechanisms and of patterns of degeneration spread is still limited both in ALS and SMA, while the establishment of possibly highly effective treatments for MNDs determines an urgent need for better patient stratification in clinical trials and for the development of reliable biomarkers of disease progression.
Magnetic resonance imaging (MRI) has emerged as the most powerful approach at the brain and spinal cord (SC) level to extract quantitative data on degeneration. At the same time, neurophysiological techniques including motor unit number index (MUNIX) could represent a useful tool to map MN loss over time. Recent statistical and informatic approaches, such as those related to artificial intelligence, could support clinical and instrumental techniques in the prediction of prognosis and disease progression.
The objective of the present project was to use a multi-modal approach combining SC and brain MRI and MUNIX with new data analysis approaches to better characterize GM and WM degeneration in MNDs with the ambition of improving the knowledge about the pathology underlying the clinical presentation as well as of identifying possible markers of disease progression over time.
In ALS patients, we showed that SC MRI parameters can be analyzed through dedicated models to improve diagnostic and prognostic ability. Secondly, we longitudinally analyzed a wide population of pre-symptomatic carriers of the c9orf72 mutation, detecting early and progressive cervical WM degeneration, thus improving the knowledge on degeneration patterns.
Finally, we considered a cohort of SMN1-related adult SMA patients who underwent a SC and brain MRI protocol combined with MUNIX. We demonstrated that isolated GM atrophy is present at the cervical level and that it is not associated nor with brain neither with WM pathology. After 24 months observation time, significant MUNIX modifications were demonstrated, suggesting that neurophysiological techniques could be the most effective tool in the monitoring of such slow-progressive disease.
Keywords: Motor neuron disease, Amyotrophic Lateral Sclerosis, Spinal Muscular Atrophy, Spinal Cord, MRI, MUNIX.
Résumé
Les maladies du motoneurone sont caractérisées par une atteinte progressive des motoneurones au niveau de la corne antérieure de la moelle épinière. Au-delà de cette susceptibilité anatomique commune, chaque maladie du motoneurone, et notamment la Sclérose Latérale Amyotrophique (SLA) et l’Amyotrophie Spinale liée aux mutations du gène SMN1 (SMA), se caractérise pour un tableau clinique spécifique. Malgré une amélioration significative des connaissances, les mécanismes à l’origine de la neurodégénérescence dans les différentes maladies du motoneurone ne sont pas complètement élucidés. De même, il est nécessaire d’identifier des biomarqueurs efficaces pour améliorer la stratification des patients pour les essais thérapeutiques futurs, ainsi que pour orienter les prédictions pronostiques.
L’imagerie par résonance magnétique (IRM) est désormais reconnue comme l’approche la plus performante à l’étage cérébral et spinal, permettant d’extraire des indices quantitatifs de dégénérescence. En même temps, les explorations par des techniques de neurophysiologie pourraient être des biomarqueurs sensibles pour détecter la progression de la perte des neurones. Récemment, des nouveaux modelés statistiques et des techniques d’intelligence artificielle ont été développés, qui pourraient soutenir et améliorer la capacité de prédiction liée aux approches d’imagerie et de neurophysiologie.
L’objectif de cette thèse a été d’utiliser une approche multimodale en associant l’imagerie par IRM de la moelle épinière et du cerveau à des techniques d’évaluation neurophysiologique pour analyser l’atteinte centrale et périphérique dans la SLA et la SMA, ainsi que leur évolution au cours du temps. Nous avons montré que, dans la SLA, les paramètres d’IRM de la moelle épinière sont prédicteurs de la survie et qu’ils peuvent être utilisés pour modéliser des algorithmes diagnostiques et de prédiction de la progression. De plus, nous avons analysé par IRM de la moelle épinière une population de sujets pre-symptomatiques porteurs de la mutation c9orf72 et nous avons mis en évidence une atteinte précoce de la substance blanche.
Nous avons également exploré une cohorte de patients adultes atteints de SMA, qui ont été étudiés avec un protocole utilisant l’IRM de la moelle épinière cervicale et du cerveau combiné à des techniques d’exploration neurophysiologique visant à calculer un index de la perte d’unités motrices (MUNIX). Nous avons montré que, dans la SMA, on peut détecter une atrophie isolée de la substance grise au niveau cervical. De plus, après 24 mois d’observation, nous avons révélé des modifications significatives des valeurs du MUNIX qui semblent être le paramètre le plus sensible pour décrire la progression de la maladie.
Mots clés : Maladies du Motoneurone, Sclérose Latérale Amyotrophique, Amyotrophie Spinale, Moelle épinière, Imagerie par résonance magnétique, MUNIX.
Published papers
Original articles arising from this project
1. Querin G, El Mendili MM, Lenglet T, Delphine S, Marchand-Pauvert V, Benali H, Pradat PF. Spinal cord multi-parametric magnetic resonance imaging for survival prediction in
amyotrophic lateral sclerosis. Eur J Neurol 2017; 24(8):1040-1046. doi: 10.1111/ene.13329.
2. Querin G, El Mendili MM, Bede P, Delphine S, Lenglet T, Marchand-Pauvert V, Pradat PF.
Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J Neurol
Neurosurg Psychiatry. 2018 ;89(11):1220-1221. doi: 10.1136/jnnp-2017-317214.
3. Querin G, El Mendili MM, Lenglet T, Behin A, Stojkovic T, Salachas F, Devos D, Le Forestier N, Del Mar Amador M, Debs R, Lacomblez L, Meninger V, Bruneteau G, Cohen-Adad J, Lehéricy S, Laforêt P, Blancho S, Benali H, Catala M, Li M, Marchand-Pauvert V, Hogrel JY, Bede P, Pradat PF. The spinal and cerebral profile of adult spinal-muscular
atrophy: A multimodal imaging study. Neuroimage Clin. 2019; 21:101618. doi:
10.1016/j.nicl.2018.101618.
4. Querin G, Lenglet T, Debs R, Stojkovic T, Behin A, Salachas F, Le Forestier N, Amador MDM, Lacomblez L, Meininger V, Bruneteau G, Laforêt P, Blancho S, Marchand-Pauvert V, Bede P, Hogrel JY, Pradat PF. The motor unit number index (MUNIX) profile of
patients with adult spinal muscular atrophy. Clin Neurophysiol. 2018;129(11):2333-2340.
doi: 10.1016/j.clinph.2018.08.025.
5. Querin G, Bede P, El Mendili MM, LI M, Pélégrini-Issac M, Rinaldi D, Catala M, Saracino D, Salachas F, CAmuzat A, Marchand-Pauvert V, Cohen-Adad J, Colliot O, Le Ber I, Pradat PF for The Predict to Prevent Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis (PREV-DEMALS) Study Group. Presymptomatic longitudinal cord pathology
in c9orf72 mutation carriers: longitudinal neuroimaging study. Submitted to Annals of
Neurology, in revision.
Reviews arising from this project
1. Querin G, Bede P, Marchand-Pauvert V, Pradat PF. Biomarkers of Spinal and Bulbar
Muscle Atrophy (SBMA): A Comprehensive Review. Front Neurol. 2018; 9:844. doi:
10.3389/fneur.2018.00844.
2. Querin G, Sorarù G, Pradat PF. Kennedy disease (X-linked recessive bulbospinal neuronopathy): A comprehensive review from pathophysiology to therapy. Rev Neurol (Paris). 2017;173(5):326-337. doi: 10.1016/j.neurol.2017.03.019.
3. Grollemund V, Pradat PF, Querin G, Delbot F, Le Chat G, Pradat-Peyre JF, Bede P. Machine
Learning in Amyotrophic Lateral Sclerosis: Achievements, Pitfalls, and Future Directions. Front Neurosci. 2019; 13:135. doi: 10.3389/fnins.2019.00135
4. El Mendili MM, Querin G, Bede P, Pradat PF. Spinal cord imaging in Amyotrophic lateral
sclerosis: old concepts – new techniques. Front Neurol. 2019. doi: 10.3389/fneur.2019.00350.
5. Bede P, Querin G, Pradat PF. The changing landscape of motor neuron disease imaging:
the transition from descriptive studies to precision clinical tools. Curr Opin Neurol.
2018;31(4):431-438. doi: 10.1097/WCO.0000000000000569.
Additional contributions
1. Marchand-Pauvert V, Peyre I, Lackmy-Valée A, Querin G, Bede P, Lacomblez L, Debs R, Pradat PF. Absence of Hyperexcitability of spinal motoneurons in patients with ALS. Submitted to Journal of Physiology, in revision.
2. Spinelli EG, Agosta F, Ferraro PM, Querin G, Riva N, Bertolin C, Martinelli I, Lunetta C, Fontana A, Sorarù G, Filippi M. Brain MRI shows white matter sparing in Kennedy's
disease and slow-progressing lower motor neuron disease. Hum Brain Mapp 2019. doi:
10.1002/hbm.24583.
3. Querin G, Battel I, Mometto L, Martinelli I, Bertolin C, Pegoraro E, Sorarù G. Preliminary
design and validation of the “6-K-scale” for bulbar symptoms evaluation in SBMA.
Neurol Sci 2019. doi: 10.1007/s10072-019-03850-2NEUS-D-18-00259.1.
4. Francini-Pesenti F, Querin G, Martini C, Mareso S, Sacerdoti D. Prevalence of metabolic
syndrome and non-alcoholic fatty liver disease in a cohort of Italian patients with spinal-bulbar muscular atrophy. Acta Myol 2018;37(3):204-209.
5. Lombardi V, Querin G, Ziff OJ, Zampedri L, Martinelli I, Heller C, Foiani M, Bertolin C, Lu CH, Malik B, Allen K, Rinaldi C, Zetterberg H, Heslegrave A, Greensmith L, Hanna M, Sorarù G, Malaspina A, Fratta P. Muscle and not neuronal biomarkers correlate with
severity in spinal and bulbar muscular atrophy. Neurology 2019;92(11): e1205-e1211.
doi: 10.1212/WNL.0000000000007097.
6. Riva N, Mora G, Sorarù G, Lunetta C, Ferraro OE, Falzone Y, Leocani L, Fazio R, Comola M, Comi G; CANALS Study Group. Safety and efficacy of nabiximols on spasticity
symptoms in patients with motor neuron disease (CANALS): a multicenter, double-blind, randomized, placebo-controlled, phase 2 trial. Lancet Neurol 2018. pii:
S1474-4422(18)30406-X. doi: 10.1016/S1474-S1474-4422(18)30406-X.
7. Grunseich C, Miller R, Swan T, Glass DJ, Mouelhi ME, Fornaro M, Petricoul O, Vostiar I, Roubenoff R, Meriggioli MN, Kokkinis A, Guber RD, Budron MS, Vissing J, Sorarù G, Mozaffar T, Ludolph A, Kissel JT, Fischbeck KH; BVS857 study group. Safety,
tolerability, and preliminary efficacy of an IGF-1 mimetic in patients with spinal and bulbar muscular atrophy: a randomised, placebo-controlled trial. Lancet Neurol 2018;
8. Querin G, Bertolin C, Martinelli I, Pennuto M, Pegoraro E, Sorarù G. Insights into the
genetic epidemiology of SBMA: prevalence estimation and multiple founder haplotypes in the Veneto Italian region. Eur J Neurol. 2019;26(3):519-524. doi: 10.1111/ene.13850.
9. Marcato S, Kleinbub JR, Querin G, Pick E, Martinelli I, Bertolin C, Cipolletta S, Pegoraro E, Sorarù G, Palmieri A. Unimpaired Neuropsychological Performance and Enhanced
Memory Recall in Patients with SBMA: A Large Sample Comparative Study. Sci Rep
2018;8(1):13627. doi: 10.1038/s41598-018-32062-5.
10. Feron M, Couillandre A, Mseddi E, Termoz N, Abidi M, Bardinet E, Delgadillo D, Lenglet T, Querin G, Welter ML, Le Forestier N, Salachas F, Bruneteau G, Del Mar Amador M, Debs R, Lacomblez L, Meininger V, Pélégrini-Issac M, Bede P, Pradat PF, de Marco G.
Extrapyramidal deficits in ALS: a combined biomechanical and neuroimaging study. J
Neurol. 2018; 265(9):2125-2136. doi: 10.1007/s00415-018-8964-y.
11. Nicolas A, Kenna KP, Renton AE, et al. Genome-wide Analyses Identify KIF5A as a Novel
ALS Gene. Neuron 2018; 97(6):1268-1283.e6. doi: 10.1016/j.neuron.2018.02.027.
12. Mandrioli J, Ferri L, Fasano A, Zucchi E, Fini N, Moglia C, Lunetta C, Marinou K, Ticozzi N, Drago Ferrante G, Scialo C, Sorarù G, Trojsi F, Conte A, Falzone YM, Tortelli R, Russo M, Sansone VA, Mora G, Silani V, Volanti P, Caponnetto C, Querin G, Monsurrò MR, Sabatelli M, Chiò A, Riva N, Logroscino G, Messina S, Calvo A. Cardiovascular diseases
may play a negative role in the prognosis of amyotrophic lateral sclerosis. Eur J Neurol.
2018;25(6):861-868. doi: 10.1111/ene.13620.
13. Querin G, Bertolin C, Bozzoni V, Martinelli I, De Bortoli M, Rampazzo A, Gellera C, Pegoraro E, Soraru G. New FIG4 gene mutations causing aggressive ALS. Eur J Neurol. 2018; 25(3): e41-e42. doi: 10.1111/ene.13559.
14. Trojsi F, Siciliano M, Femiano C, Santangelo G, Lunetta C, Calvo A, Moglia C, Marinou K, Ticozzi N, Drago Ferrante G, Scialò C, Sorarù G, Conte A, Falzone YM, Tortelli R, Russo M, Sansone VA, Chiò A, Mora G, Poletti B, Volanti P, Caponnetto C, Querin G, Sabatelli M, Riva N, Logroscino G, Messina S, Fasano A, Monsurrò MR, Tedeschi G, Mandrioli J.
Comorbidity of dementia with amyotrophic lateral sclerosis (ALS): insights from a large multicenter Italian cohort. J Neurol. 2017; 264(11):2224-2231. doi:
10.1007/s00415-017-8619-4.
15. Querin G, Corcia P, Lenglet T, Stojkovic T, Leguern E, Cazeneuve C, Pradat PF. Motor
neuron disease of very long disease duration or Charcot-Marie-Tooth disease? A novel phenotype related to the SOD1 p.E22G variant. Rev Neurol (Paris).
2017;173(10):671-673. doi: 10.1016/j.neurol.2017.05.008.
16. Querin G, Martinelli I, Bertolin C, Pegoraro E, Pennuto M, Sorarù G. The role of AR polyQ
tract in male breast carcinoma: lesson from a SBMA case. Ann Oncol. 2017; 28(5):
1160-1161. doi: 10.1093/annonc/mdx0.38.
17. Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E, Sorarù G. Diagnostic and Prognostic Biomarkers in Amyotrophic
Lateral Scerosis: Neurofilament Light Chain levels in definite subtypes of disease. JAMA
18. Calvo A, Moglia C, Lunetta C, Marinou K, Ticozzi N, Ferrante GD, Scialo C, Sorarù G, Trojsi F, Conte A, Falzone YM, Tortelli R, Russo M, Chiò A, Sansone VA, Mora G, Silani V, Volanti P, Caponnetto C, Querin G, Monsurrò MR, Sabatelli M, Riva N, Logroscino G, Messina S, Fini N, Mandrioli J. Factors predicting survival in ALS: a multicenter Italian
study. J Neurol. 2017; 264(1): 54-63. doi: 10.1007/s00415-016-8313-y.
19. Kenna KP, van Doormaal PT, Dekker AM, Ticozzi N, Kenna BJ, Diekstra FP, van Rheenen W, van Eijk KR, Jones AR, Keagle P, Shatunov A, Sproviero W, Smith BN, van Es MA, Topp SD, Kenna A, Miller JW, Fallini C, Tiloca C, McLaughlin RL, Vance C, Troakes C, Colombrita C, Mora G, Calvo A, Verde F, Al-Sarraj S, King A, Calini D, de Belleroche J, Baas F, van der Kooi AJ, de Visser M, Ten Asbroek AL, Sapp PC, McKenna-Yasek D, Polak M, Asress S, Muñoz-Blanco JL, Strom TM, Meitinger T, Morrison KE; SLAGEN
Consortium, Lauria G, Williams KL, Leigh PN, Nicholson GA, Blair IP, Leblond CS, Dion
PA, Rouleau GA, Pall H, Shaw PJ, Turner MR, Talbot K, Taroni F, Boylan KB, Van Blitterswijk M, Rademakers R, Esteban-Pérez J, García-Redondo A, Van Damme P, Robberecht W, Chio A, Gellera C, Drepper C, Sendtner M, Ratti A, Glass JD, Mora JS, Basak NA, Hardiman O, Ludolph AC, Andersen PM, Weishaupt JH, Brown RH Jr, Al-Chalabi A, Silani V, Shaw CE, van den Berg LH, Veldink JH, Landers JE. NEK1 variants confer
susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2016;48(9):1037-42. doi:
10.1038/ng.3626.
20. Querin G, DaRe E, Martinelli I, Bello L, Bertolin C, Pareyson D, Mariotti C, Pegoraro E, Sorarù G. Validation of the Italian version of the SBMA Functional Rating Scale as
outcome measure. Neurol Sci. 2016;37(11):1815-1821.
21. Bertolin C, Querin G, Da Re E, Sagnelli A, Bello L, Cao M, Pennuto M, Ermani M, Pegoraro E, Mariotti C, Gellera C, Hanna GM, Pareyson D, Fratta P, Sorarù G. No effect of AR polyG
polymorphism on Spinal and Bulbar Muscular Atrophy phenotype. Eur J Neurol. 2016;
23(6):1134-6. doi: 10.1111/ene.13001.
22. Querin G, Bertolin G, Da Re E, Volpe M, Zara G, Pegoraro E, Caretta N, Foresta C, Silvano M, Corrado D, Iafrate M, Angelini L, Sartori L, Pennuto M, Gaiani A, Bello L, Semplicini C, Pareyson D, Silani V, Ermani M, Ferlin A, Sorarù G; on behalf of the Italian Study Group on Kennedy’s disease. Non-neural phenotype of Spinal and Bulbar Muscular Atrophy:
results from a large cohort of Italian patients. J Neurol Neurosurg Psychiatry.
2015-311305.
23. Pensato V, Tiloca C, Corrado L, Bertolin C, Sardone V, Del Bo R, Calini D, Mandrioli J, Lauria G, Mazzini L, Querin G, Ceroni M, Cantello R, Corti S, Castellotti B, Soldà G, Duga S, Comi GP, Cereda C, Sorarù G, D'Alfonso S, Taroni F, Shaw CE, Landers JE, Ticozzi N, Ratti A, Gellera C, Silani V; SLAGEN Consortium. TUBA4A gene analysis in sporadic
amyotrophic lateral sclerosis: identification of novel mutations. J Neurol. 2015;
262:1376-8. doi: 10.1007/s00415-015-7739-y.
24. Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L, Carletti S, Giorgi C, Spera C, Domenico F, Bersano E, Petruzzelli F, Cisari C, Maglione A, Sarnelli MF, Stecco A, Querin G, Masiero S, Cantello R, Ferrari D, Zalfa C, Binda E, Visioli A, Trombetta D, Novelli A, Torres B, Bernardini L, Carriero A, Prandi P, Servo S, Cerino A, Cima V, Gaiani A, Nasuelli N, Massara M, Glass J, Sorarù G, Boulis NM, Vescovi AL. Human neural stem cell transplantation in ALS: initial results from a phase
25. Calvo V, Bianco F, Benelli E, Sambin M, Monsurrò MR, Femiano C, Querin G, Sorarù G, Palmieri A. Impact on children of a parent with ALS: a case-control study. Front Psychol. 2015; 6:288. doi: 10.3389/fpsyg.2015.00288.
26. Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu YF, Wang Q, Krueger BJ, Ren Z, Keebler J, Han Y, Levy SE, Boone BE, Wimbish JR, Waite LL, Jones AL, Carulli JP, Day-Williams AG, Staropoli JF, Xin WW, Chesi A, Raphael AR, McKenna-Yasek D, Cady J, Vianney de Jong JM, Kenna KP, Smith BN, Topp S, Miller J, Gkazi A; FALS Sequencing Consortium, Al-Chalabi A, van den Berg LH, Veldink J, Silani V, Ticozzi N, Shaw CE, Baloh RH, Appel S, Simpson E, Lagier-Tourenne C, Pulst SM, Gibson S, Trojanowski JQ, Elman L, McCluskey L, Grossman M, Shneider NA, Chung WK, Ravits JM, Glass JD, Sims KB, Van Deerlin VM, Maniatis T, Hayes SD, Ordureau A, Swarup S, Landers J, Baas F, Allen AS, Bedlack RS, Harper JW, Gitler AD, Rouleau GA, Brown R, Harms MB, Cooper GM, Harris T, Myers RM, Goldstein DB. Exome sequencing in
amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;
347:1436-41. doi: 10.1126/science.aaa3650.
27. Smith BN, Ticozzi N, Fallini C, Gkazi AS, Topp S, Kenna KP, Scotter EL, Kost J, Keagle P, Miller JW, Calini D, Vance C, Danielson EW, Troakes C, Tiloca C, Al-Sarraj S, Lewis EA, King A, Colombrita C, Pensato V, Castellotti B, de Belleroche J, Baas F, ten Asbroek AL, Sapp PC, McKenna-Yasek D, McLaughlin RL, Polak M, Asress S, Esteban-Pérez J, Muñoz-Blanco JL, Simpson M; SLAGEN Consortium, van Rheenen W, Diekstra FP, Lauria G, Duga S, Corti S, Cereda C, Corrado L, Sorarù G, Morrison KE, Williams KL, Nicholson GA, Blair IP, Dion PA, Leblond CS, Rouleau GA, Hardiman O, Veldink JH, van den Berg LH, Al-Chalabi A, Pall H, Shaw PJ, Turner MR, Talbot K, Taroni F, García- Redondo A, Wu Z, Glass JD, Gellera C, Ratti A, Brown RH Jr, Silani V, Shaw CE, Landers JE. Exome-wide rare
variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron.
2014; 84:324-31. doi: 10.1016/j.neuron.2014.09.027.
28. Di Rosa E, Sorarù G, Kleinbub JR, Calvo V, Vallesi A, Querin G, Marcato S, Grasso I, Palmieri A. Theory of mind, empathy and neuropsychological functioning in X-linked
spinal and bulbar muscular atrophy: a controlled study of 20 patients. J Neurol. 2015;
262:394-401. doi: 10.1007/s00415-014-7567-5.
29. Querin G, Bertolin C, Martinuzzi A, Bassi MT, Arnoldi A, Polo A, Pegoraro E, Sorarù G.
The blurred scenario of motor neuron disorders linked to Spatacsin mutations: a case report. Eur J Neurol. 2014; 21: 85-6. doi: 10.1111/ene.12481.
30. Palmieri A, Mento G, Calvo V, Querin G, D'Ascenzo C, Volpato C, Kleinbub JR, Bisiacchi PS, Sorarù G. Female gender doubles executive dysfunction risk in ALS: a case-control
study in 165 patients. J Neurol Neurosurg Psychiatry. 2015; 86: 574-9. doi:
10.1136/jnnp-2014-307654.
31. Bertolin C, D'Ascenzo C, Querin G, Gaiani A, Boaretto F, Salvoro C, Vazza G, Angelini C, Cagnin A, Pegoraro E, Sorarù G, Mostacciuolo ML. Improving the knowledge of
amyotrophic lateral sclerosis genetics: novel SOD1 and FUS variants. Neurobiol Aging.
2014; 35:1212. e7-1212.e10. doi: 10.1016/j.neurobiolaging.2013.10.093.
32. Lauria G, Dalla Bella E, Antonini G, Borghero G, Capasso M, Caponnetto C, Chiò A, Corbo M, Eleopra R, Fazio R, Filosto M, Giannini F, Granieri E, La Bella V, Logroscino G,
Mandrioli J, Mazzini L, Monsurrò MR, Mora G, Pietrini V, Quatrale R, Rizzi R, Salvi F, Siciliano G, Sorarù G, Volanti P, Tramacere I, Filippini G; EPOS Trial Study Group.
Erythropoietin in amyotrophic lateral sclerosis: a multicentre, randomised, double blind, placebo controlled, phase III study. J Neurol Neurosurg Psychiatry. 2015;
86:879-86. doi: 10.1136/jnnp-2014-308996.
33. Fogh I, Ratti A, Gellera C, Lin K, Tiloca C, Moskvina V, Corrado L, Sorarù G, Cereda C, Corti S, Gentilini D, Calini D, Castellotti B, Mazzini L, Querin G, Gagliardi S, Del Bo R, Conforti FL, Siciliano G, Inghilleri M, Saccà F, Bongioanni P, Penco S, Corbo M, Sorbi S, Filosto M, Ferlini A, Di Blasio AM, Signorini S, Shatunov A, Jones A, Shaw PJ, Morrison KE, Farmer AE, Van Damme P, Robberecht W, Chiò A, Traynor BJ, Sendtner M, Melki J, Meininger V, Hardiman O, Andersen PM, Leigh NP, Glass JD, Overste D, Diekstra FP, Veldink JH, van Es MA, Shaw CE, Weale ME, Lewis CM, Williams J, Brown RH, Landers JE, Ticozzi N, Ceroni M, Pegoraro E, Comi GP, D'Alfonso S, van den Berg LH, Taroni F, Al-Chalabi A, Powell J, Silani V; SLAGEN Consortium and Collaborators. A genome-wide
association meta- analysis identifies a novel locus at 17q11.2 associated with sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2014; 23:2220-31. doi:
10.1093/hmg/ddt587.
34. Querin G, Melacini P, D'Ascenzo C, Morandi L, Mazzini L, Silani V, Romito S, Mandrioli J, Raimondi M, Pegoraro E, Soraru' G. No evidence of cardiomyopathy in spinal and
bulbar muscular atrophy. Acta Neurol Scand. 2013; 128: e30-2. doi: 10.1111/ane.12140.
35. Querin G, D'Ascenzo C, Peterle E, Ermani M, Bello L, Melacini P, Morandi L, Mazzini L, Silani V, Raimondi M, Mandrioli J, Romito S, Angelini C, Pegoraro E, Sorarù G. Pilot trial
of clenbuterol in spinal and bulbar muscular atrophy. Neurology. 2013; 80:2095-8. doi:
10.1212/WNL.0b013e318295d766.
36. Malena A, Pennuto M, Tezze C, Querin G, D'Ascenzo C, Silani V, Cenacchi G, Scaramozza A, Romito S, Morandi L, Pegoraro E, Russell AP, Sorarù G, Vergani L.
Androgen-dependent impairment of myogenesis in spinal and bulbar muscular atrophy. Acta
Neuropathol. 2013; 126:109-21. doi: 10.1007/s00401-013-1122-9.
37. Bruson A, Sambataro F, Querin G, D'Ascenzo C, Palmieri A, Agostini J, Gaiani A, Angelini C, Galbiati M, Poletti A, Pennuto M, Pegoraro E, Clementi M, Sorarù G. CAG repeat length
in androgen receptor gene is not associated with amyotrophic lateral sclerosis. J Neurol.
2012; 19:1373-5. doi: 10.1111/j.1468-1331.2011.03646.x.
38. D'Ascenzo C, Cecchin D, Santelli L, Palmieri A, Gaiani A, Querin G, Cima V, Volpe M, Bello L, Bui F, Cagnin A, Angelini C, Pegoraro E, Sorarù G. Parkinson-like features in
ALS with predominant upper motor neuron involvement. Amyotroph Lateral Scler. 2012;
13: 137-43. doi: 10.3109/17482968.2011.603732.
39. Orsetti V, Pegoraro E, Cima V, D'Ascenzo C, Palmieri A, Querin G, Volpe M, Ermani M, Angelini C, Sorarù G. Genetic variation in KIFAP3 is associated with an upper motor
neuron-predominant phenotype in amyotrophic lateral sclerosis. Neurodegener Dis.
2011; 8:491-5. doi: 10.1159/000327755.
40. Sorarù G, Clementi M, Forzan M, Orsetti V, D'Ascenzo C, Querin G, Palmieri A, Ermani M, Angelini C, Pegoraro E. ALS risk but not phenotype is affected by ataxin-2 intermediate
length polyglutamine expansion. Neurology. 2011; 76:2030-31. doi: 10.1212/WNL.0b013e31821e557a.
41. Palmieri A, Naccarato M, Abrahams S, Bonato M, D'Ascenzo C, Balestreri S, Cima V,
Querin G, Dal Borgo R, Barachino L, Volpato C, Semenza C, Pegoraro E, Angelini C, Sorarù
G. Right hemisphere dysfunction and emotional processing in ALS: an fMRI study. J Neurol. 2010; 257:1970-78. doi: 10.1007/s00415-010-5640-2.
42. Palmieri A, Sorarù G, Albertini E, Semenza C, Vottero-Ris F, D'Ascenzo C, Querin G, Zennaro A, Pegoraro E, Angelini C. Psychopathological features and suicidal ideation in
amyotrophic lateral sclerosis patients. Neurol Sci. 2010; 31:735-40. doi:
10.1007/s10072-010-0332-3.
Abstracts presented at international meetings (oral communications)
1. Querin G, Bede P, El Mendili MM, et al. Presymptomatic spinal cord degeneration in
C9orf72 hexanucleotide carriers: a longitudinal neuroimaging study. Oral presentation at
the 2019 ENCALS meeting (Tours, France).
2. Querin G, Bede P, El Mendili MM, et al. Presymptomatic spinal cord degeneration in
C9orf72 hexanucleotide carriers: a longitudinal neuroimaging study. Oral presentation at
the NiSALS meeting (Neuroimaging Society for ALS) (Edinburgh, UK, 2018).
3. Querin G, El Mendili MM, Bede P, et al. Spinal cord and brain alterations in adult
spinal-muscular atrophy: from motor cortex to anterior horns. Oral presentation at the French
annual meeting on ALS (Journées de la recherché sur SLA) (Paris, France, 2018).
4. Querin G, El Mendili MM, et al. Unravelling the tangle of MNDs: insights from advanced
spinal cord MRI. Oral presentation at the NiSALS meeting (Neuroimaging Society for ALS)
(Boston, USA, 2017).
5. Querin G, El Mendili MM, et al. Spinal cord MRI: is it an effective classification tool for
the diagnosis of motor neuron disease conditions? Oral presentation at the French annual
meeting on ALS (Journées de la recherche SLA) (Paris, France, 2017).
6. Querin G, El Mendili MM, et al. Spinal cord multi-modal MRI for survival prediction in
ALS. Oral presentation at the French annual meeting on MNDs (Journées de la recherché
sur la SLA) (Paris, France, 2016).
7. Querin G, Martinelli I, Sorarù G. Role of biomarkers of disease progression in SBMA.
Oral presentation at the II Italian meeting on Kennedy’s disease (Milan, Italy, 2016).
8. Querin G, Martinelli I, Bertolin C, et al. Spinal and Bulbar Muscular atrophy (SBMA)
outcome measures and the role of creatinine. Oral presentation at the XLVI annual meeting
of the Italian Society of Neurology (Venice, Italy, 2016).
9. Querin G. Palliative care in ALS. Oral presentation at the annual meeting on ALS of the University of Padova (Padova, Italy, 2015).
10. Querin G et al. A pilot trial of Clenbuterol in SBMA. Neurology 2013; Vol 80: Meeting Abstracts 1 S36.003. Oral presentation at the 65th meeting of the American Academy of Neurology (San Diego, USA, 2013).
11. Querin G et al. A pilot trial with Clenbuterol in SBMA. Neurological Sciences 2012, Vol 33, S 33. Oral presentation at the annual meeting of the Italian Society of Neurology (Rimini, Italy, 2012).
12. Querin G et al. Clinical presentation of ALS. Oral presentation at the annual meeting on ALS of the University of Padova (Padova, Italy, 2011).
13. Querin G, El Mendili MM, Pradat PF. Unravelling the tangle of MNDs: insights from
advanced spinal cord MRI. Oral presentation at the NiSALS meeting 2017 (Boston, USA,
2017).
14. Querin G, El Mendili MM, Delphine S, et al. Spinal cord MRI: is it an effective
classification tool for the diagnosis of motor neuron disease conditions? Oral presentation
at 2017 Journées de la recherché SLA. (Paris, France, 2017).
15. Querin G, Martinelli I, Da Re E, et al. Disease progression in SBMA: is creatinine a new
reliable biomarker? Oral presentation at the XLVII Congress of the Italian Society of
Neurology (Venice, Italy, 2016).
16. Querin G, Da Re E, Martinelli I, et al. Spinal and Bulbar Muscular atrophy (SBMA)
outcome measures and the role of creatinine. Oral presentation at the XLVI Congress of
the Italian Society of Neurology (Genova, Italy, 2015).
17. Querin G, D’Ascenzo C, Peterle E, et al. A Pilot Trial of Clenbuterol in Spinal and Bulbar
Muscular Atrophy. Neurology 2013; 80: Meeting Abstracts 1 S36.003. Oral presentation at
the 65th Meeting of the American Academy of Neurology (San Diego, USA, 2013).
18. Querin G, D’Ascenzo C, Morandi L, et al. A pilot trial with Clenbuterol in SBMA. Oral presentation at the XLIII Congress of the Italian Society of Neurology (Rimini, Italy, 2012).
Abstracts presented at international meetings (posters)
1. Pradat PF, Rolland AS, Kuchinski G, Lopes R, Bertouni N, Pruvo JP, Querin G, Chupine M, Gay D, El Mendili M, Djobeir S, Devos D for the PULSE study group. A prospective
multicenter French study of brain and spinal cord imaging in ALS patients. Poster
presentation at the 2019 ENCALS meeting (Tours, France). 2.
3. Querin G, Hogrel JY, Debs R, Pradat PF, Lenglet T. Étude MUNIX chez des patients
atteints de formes modérées d’amyotrophie spinale SMN1. Poster flash at the Journées de
Neurologie de Langue Française (JNLF 2019, Lille, France).
4. Querin G, Lenglet T, Debs R, Marchand-Pauvert V, Hogrel JY, Bede P, Pradat PF.
Improvements in the definition of biomarkers for Spinal Muscular Atrophy (SMA) type III and IV: a multimodal neuroimaging and neurophysiology study. Abstract presentation
5. Querin G, El Mendili M, Lenglet T, Debs R, Marchand-Pauvert V, Hogrel JY, Bede P, Pradat PF. The spinal and cerebral profile of adult spinal-muscular atrophy: a multimodal
imaging study. Amyotroph Lateral Scler Frontotemporal Degener. 2018; 19: 85-87
(Abstracts from the 29th International Symposium on ALS/MND; Glasgow, UK, 2018). 6. Querin G, Hogrel JY, Debs R, Marchand-Pauvert V, Bede P, Pradat PF, Lenglet T. The
Motor Unit Number Index (MUNIX) profile of adult forms of Spinal Muscular Atrophy (SMA). Amyotroph Lateral Scler Frontotemporal Degener. 2018; 19: 85-87 (Abstracts from
the 29th International Symposium on ALS/MND; Glasgow, UK, 2018).
7. Querin G, Hogrel JY, Debs R, Pradat PF, Lenglet T. Étude MUNIX chez des patients
atteints de formes modérées d’amyotrophie spinale SMN1. Poster presentation at the 21th
Journées Francophone d’ElectroNeuroMyoGraphie, Paris, France, 2018.
8. Querin G, El Mendili M, Bede, Marchand-Pauvert V, Pradat PF. The radiological spectrum
of motor-neuron diseases: a multimodal spinal cord study. Poster presentation at the 2018
ENCALS Meeting, Oxford, UK, 2018.
9. Querin G, El Mendili MM, Lenglet T, Marchand-Pauvert V, Benali H, Pradat PF. Spinal
cord multi-parametric MRI for survival prediction in ALS. Poster presentation at the 2017
ENCALS Meeting, Ljubljana, Slovenia, 2017.
10. Querin G, Bozzoni V, Bertolin C, Martinelli I, Gellera C, Sorarù G. New FIG4 gene
mutation causing fast progressing ALS phenotype: a case report. Poster presentation at
the 2017 ENCALS Meeting, Ljubljana, Solvenia, 2017.
11. Querin G, Da Re E, Martinelli I, Bello L, Bertolin C, Pareyson D, Mariotti C, Pegoraro E, Sorarù G. Validation of the Italian version of the SBMA Functional Rating Scale as
outcome measure. Poster presentation at the XLVII Congress of the Italian Society of
Neurology, Venice, Italy, 2016.
12. Martinelli I, Da Re E, Querin G, Bertolin C, Pennuto M, Guarineri V, Pegoraro E, Sorarù G.
Breast cancer suscepitibility in patients with spinal and bulbar musculare atrophy. A case report. Eur J Neurol 2016; 23 Suppl.1: 947-955 (Abstracts from the 2nd EAN meeting,
2016, Copenhagen, Denmark, 2016).
13. Querin G, Bello L, Da Re E, Martinelli I, Bertolin C Gaiani A, Pennuto M, Pareyson D, Pegoraro E and Sorarù G. Spinal and Bulbar Muscular Atrophy (SBMA) outcome
measures: is creatinine a new reliable biomarker? Eur J Neurol 2016; 23 Suppl.1: 947-955
(Abstracts from the 2nd EAN meeting, 2016, Copenhagen, Denmark, 2016).
14. Martinelli I, Nguyen AA, Bertolin C, Malena A, Querin G, Pegoraro E, Sorarù G. The
Nogo-A protein is not a biomarker in amyotrophic lateral sclerosis (Nogo-ALS). Nogo-Amyotroph Lateral
Scler Frontotemporal Degener. 2017; 18: 325-332 (Abstracts from the 27th Symposium on ALS/MND, Dublin, Ireland, 2016).
15. Querin G, Da Re E, Martinelli I, Bello L, Bertolin C, Pareyson D, Mariotti C, Pegoraro E, Sorarù G. Disease Progression in SBMA: is serum creatinine a reliable biomarker? Amyotroph Lateral Scler Frontotemporal Degener. 2017; 18: 325-332 (Abstracts from the 27th Symposium on ALS/MND, Dublin, Ireland, 2016).
16. Querin G, El Menili MM, Lenglet T, Marchand-Pauvert V, Benali H, Pradat PF. Spinal cord
multi-modal MRI for survival prediction in ALS. Amyotroph Lateral Scler Frontotemporal
Degener. 2017; 18: 325-332 (Abstracts from the 27th Symposium on ALS/MND, Dublin, Ireland, 2016).
17. Querin G, Corcia P, Lenglet T, Stojkovic T, Leguern E, Cazeneuve C, Pradat PF. Motor
neuron disease with very long disease duration or CMT? Poster presentation at the 2016
ENCALS meeting, Milano, Italy, 2016.
18. Querin G. et al. Spinal and Bulbar Muscular Atrophy (SBMA) Outcome Measures and
the Role of Creatinine. Neurology 2016; 86 (Suppl16). (Abstracts from the 68th Meeting of
the American Academy of Neurology, Vancouver, USA, 2016).
19. Ferraro PM, Agosta F, Querin G, Riva N, Bertolin C, Da Re E, Copetti M, Comi G, Falini A, Sorarù G, Filippi M. Structural Brain MRI Abnormalities in Kennedy's Disease. Neurology 2016; 86 (Suppl16). (Abstracts from the 68th Meeting of the American Academy of Neurology, Vancouver, USA, 2016).
20. Sorarù G, Bertolin C, Querin G, Fratta P, Pareyson D, Mariotti C, Gellera C. The role of
androgen receptor gene variants on SBMA phenotype. J Neurol Sci 2015; 357: e215–
e234(Abstracts from the 1st World Congress of Neurology, Santiago, Chile, 2015).
21. Querin G, Da Re E, Martinelli I, Bertolini C, Gaiani A, Pennuto M, Ferlini A, Pareyson D, Pegoraro E, Sorarù G. Spinal and Bulbar Muscular atrophy (SBMA) outcome measures
and the role of creatinine. Oral presentation at the XLVI Congress of the Italian Society of
Neurology, Genova, Italy, 2015.
22. Ferraro PM, Agosta F, Querin G, Riva N, Bertolin C, Da Re E, Copetti M, Comi G, Falini A, Sorarù G, Filippi M. Brain MRI abnornalities in Kennedy’s disease. Poster presentation at the XLVI Congress of the Italian Society of Neurology, Genova, Italy, 2015.
23. Cao M, Querin G, Gaiani A, Barp A, Bello L, Semplicini C, Sorarù G, Angelini C, Pegoraro E. FSHD: a clinical follow-up. Poster presentation at the XLVI Congress of the Italian Society of Neurology, Genova, Italy, 2015.
24. Mazzini L, Gelati M, Sorarù G, Profico D, Projetti Pensi M, Muzi G, Ricciolini C, Giorgi S, Carletti C, Spera C, Frondizzi D, Stecco A, Bersano E, Servo S, Querin G, Cantello R, Vescovi A. Human neural stem cells transplantation in ALS: results of a phase I clinical
trial. Oral presentation at the XLVI Congress of the Italian Society of Neurology, Genova,
Italy, 2015.
25. Volpe M, Zara G, Querin G, Bertolin C, Zoccarato F, Da Re E, Volpato E, Pareyson D, Ermani M, Sorarù G. A neurophysiological investigation of a large population of patients
affected with Kennedy’s disease (SBMA) confirms diffuse neurological involvement. Eur
J Neurol 2015; 22. (Abstracts from the 1st European Academy of Neurology, Berlin, Deutschland, 2015).
26. Querin G, Bertolin C, Da Re E, Volpe M, Zara G, Ferlin A, Caretta N, Foresta C, Silvano M, Corrado D, Iafrate M, Pennuto M, Bello L, Semplicini C, Pareyson D, Ermani M, Pegoraro E, Sorarù G. The wide clinical spectrum of Kennedy’s disease. Eur J Neurol 2015; 22. (Abstracts from the 1st European Academy of Neurology, Berlin, Deutschland, 2015).
27. Querin G, Bertolin C, Volpe M, Zara G, Da Re E, Zoccarato F, Caretta N, Foresta C, Marcato S, Iafrate M, Corrado D, Silvano M, Pegoraro E; Pareyson D, Ferlin A, Sorarù G. The wide
clinical phenotype of Kennedy’s disease. Neurology 2015; 84(14) Supplement P7.055.
(Abstracts from the 67th Meeting of the American Academy of Neurology, Washington, USA, 2014).
28. Peterle E, Querin G, Bruni A, D’Ascenzo C, Gaiani A, Barp A, Bertolin C, Angelini C, Pegoraro E, Sorarù G. Takotsubo cardiomyopathy as possible cause of sudden death in
ALS patients. Neurological Sci. 2013; 31: 291 (Abstracts from the XLIV Congress of the
Italian Society of Neurology, Milano, Italy, 2013).
29. Querin G, D’Ascenzo C, Peterle E, Martinuzzi A, Mostacciuolo M, Bertolin C, Bartolomei L, Pegoraro E, Sorarù G. Novel SPG11 mutation in a patient with hereditary spastic
paraplegia with thin corpus callosum: a case report. Neurological Sci. 2013; 34; 287
(Abstracts from the XLIV Congress of the Italian Society of Neurology, Milano, Italy, 2013). 30. Bertolin C, Querin G, Peterle E, Zoccarato F, Angelini C, Pegoraro E, Sorarù G. The missing
factors influencing spinal and bulbar muscular atrophy phenotype: evaluation of genetic polymorphisms. Neurological Sci. 2013; 3: 294 (Abstracts from the XLIV Congress of the
Italian Society of Neurology, Milano, Italy, 2013).
31. Querin G, Bertolin C, Martinuzzi A, Mostacciuolo ML, Polo A, Pegoraro E, Sorarù G. Novel
SPG11 mutation in a case of HSP/ALS overlap phenotype. Poster presentation at the 14thr
Congress of the Italian Myology Association, Sirmione, Italy, 2012).
32. Querin G, Melacini P, Morandi L, Mazzini L, Silani V, Gaiani A, D’Ascenzo C, Pegoraro E, Sorarù G. No heart involvement in SBMA patients. Eur J Neurology 2012; 19 (Suppl. 1): 90–457. (Abstracts from the 16th Congress of the EFNS, Stochkolm, Sweden, 2012).
33. Querin G, D’Ascenzo C, Morandi L, Mazzini L, Silani V, Gaiani A, Romito S, Melacini P, Pegoraro E, Sorarù G. No heart involvement in SBMA patients. Poster presentation at the XLVIII Congress of the Italian Association of Neuropathology and Clinical Neurobiology (AINP&NC, Roma, Italy 2012).
34. Sorarù G, Malena A, Agostini J, Loro E, Querin G, Cenacchi G, Scaramozza A, Morandi L, Mora M, Silani V, D’Ascenzo C, Mazzini L, Pennuto M, Romito S, Angelini C, Pegoraro E, Vergani L. Androgen-dependent impairment of skeletal muscle maturation in Spinal and
Bulbar Muscular Atrophy. Neurological Sci. 2012; 33: 165. (Abstracts from the XLIII
Congress of the Italian Society of Neurology, Rimini, Italy, 2012).
35. Volpe M, Bello L, Querin G, Cima V, Palmieri A, Sorarù G, Pegoraro E, Angelini C. A case
of “Never-experienced-before” headache in a young man. Neurological Sci. 2010; V31:
316 (Abstracts from the XLI Congress of the Italian Society of Neurology, Catania, Italy, 2010).
36. D’Ascenzo C, Cecchin D, Santelli L, Palmieri A, Cima V, Volpe M, Querin G, Gaiani A, Bello L, Pegoraro E, Angelini C, Sorarù G. Extrapiramidal involvement in upper motor
neuron-dominant ALS. Neurological Sci. 2010; 31: 108 (Abstracts from the XLI Congress
37. Cima V, Vianello A, Palmieri A, Querin G, Pilotto A, Pegoraro E, Angelini C, Sorarù G. An
Italian case of Brown-Vialetto-Van Laere syndrome. Neurological Sci. 2009; 30: 384
(Abstracts from the XL Congress of the Italian Society of Neurology, Padova, Italy, 2009). 38. Loro E, Querin G, Rinaldi F, Masiero V, Romeo V, Sorarù G, AngeliniC, Sandri M, Botta
A, Vergani L. Increased autophagy-apoptosis in differentiated myotonic dystrophy type
1 muscle cells. Neurological Sci. 2009: 337 (Abstracts from the XL Congress of the Italian
Society of Neurology, Padova, Italy, 2009).
39. Sorarù G, Orsetti V, Palmieri A, D’Ascenzo C, Buratti E, Mostacciuolo M, Volpe M, Querin
G, Pegoraro E, Angelini C. TARDBP gene analysis in ALS patients. Neurological Sci.
2009; 30: 284 (Abstracts from the XL Congress of the Italian Society of Neurology, Padova, Italy, 2009).
Introduction
Motor neuron diseases (MNDs) are a group of disorders characterized by selective degeneration of motor neurons in the brain, brainstem and in the ventral horn of the spinal cord (SC) (Dharmadasa et al., 2018). Even if they share a common anatomical substrate, MNDs are extremely heterogeneous from a genetic, clinical and pathophysiological point of view. The understanding of shared and disease specific pathogenic mechanisms is of primary relevance for the development of effective biomarkers and therapies and for a better and personalized clinical management of the patients. MNDs are characterized by degeneration of the cortical and spinal motor neurons. In this perspective, the SC fulfils a fundamental role integrating the entry of sensitive information from peripheral stimuli and the output of motor commands from the brain to the spinal motor neurons, which finally innervate skeletal muscles in the limbs and the trunk.
SC imaging studies have been limited for a long time by relevant technical difficulties. Nevertheless, recent methodological improvements have made possible the acquisition of quantitative data on the integrity of white matter (WM) tracts and grey matter (GM) layers and the analysis of sensory-motor degeneration of the SC in neurodegenerative diseases (Pradat and El Mendili, 2014; Bede et al., 2018b). At the same time, new and actively improving neurophysiological techniques, such as the calculation of the MUNIX index, have demonstrated high accuracy in describing motor neuron loss (Ahn et al., 2010).
The objective of the present project was to apply existing imaging and neurophysiological techniques and to develop new methodological approaches to study the role of SC pathology in different MNDs and to accurately describe disease progression with the ambition of identifying truly effective biomarkers.
The manuscript is organized as follows: In Chapter 1, a literature review will present including: i) spinal cord MRI with a special focus on advanced MRI techniques that are commonly used in the pathological context of MNDs; ii) motor neuron diseases with a specific focus on ALS and SMA. The objectives of this thesis will be presented in Chapter 2. Five published articles and one longitudinal ongoing study, which constitute the core of the present thesis, will be presented in Chapter 3. Finally, Chapter 4 will discuss our findings and future directions.
1. Background
1.1 Hints of spinal cord anatomy
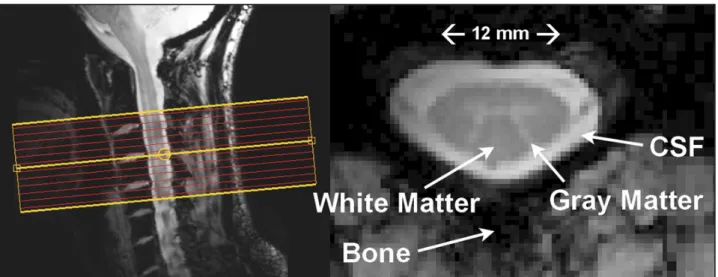
The SC lies within the spinal canal surrounded by the cerebrospinal fluid (CSF) and by the bone and cartilaginous discs between the vertebral bodies. It has the geometry of a bent elliptic cylinder with 0.8-1.4 cm transverse diameter, 0.5-0.9 cm anterior-posterior diameter and 43-45 cm length (Fradet et al., 2014) (figure 1). The CSF flows in the head–foot direction with each heart-beat with an amplitude that diminishes with greater distance from the head, thus inducing concomitant movements of the SC itself (Figley et al., 2008).
Figure 1: 7T cervical spinal cord MRI: sagittal T2-weighted image and T2*-weighted image (on the right side) of the C3-C4 axial slice with identification of main anatomical components (Barry et al., 2014).
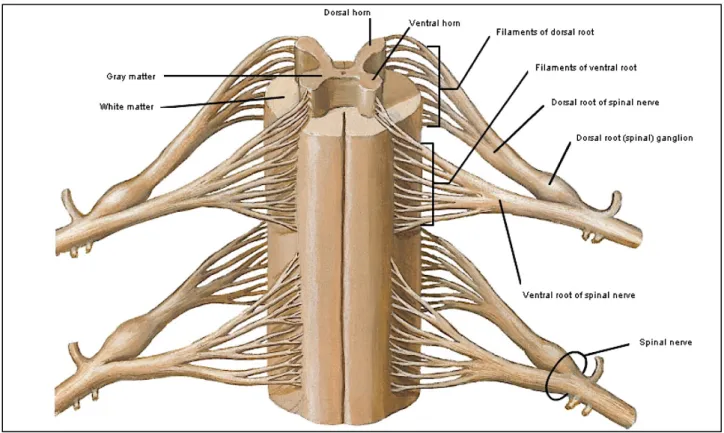
The spinal cord has 31 segments (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal), each of which (except the first cervical segment, which has only a ventral root) has a pair of dorsal and ventral roots and a pair of spinal nerves. There are no sharp boundaries between the segments within the cord, but the cervical and lumbar enlargements which give rise to nerve roots for arms and legs, respectively, are clearly apparent. Each dorsal and ventral root join in the intervertebral foramina to form a spinal nerve (figure 2).
Figure 2: Anterior view of the cord showing dorsal and ventral roots and formation of the spinal
nerves. Drawing by Frank Netter, MD. (Netter illustration from www.netterimages.com. Elsevier, Inc. All rights reserved).
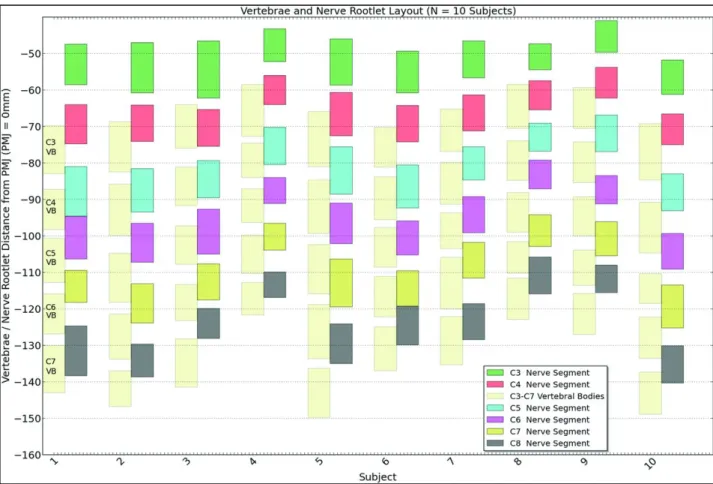
SC and vertebral column levels do not correspond (Cadotte et al., 2015) (figure 3): at the upper cervical level, the cord segment corresponds to the like-numbered vertebral body. From C5 to C8 the SC level is 1 level higher than the corresponding vertebral body. In the upper thoracic region, the vertebral spinal process is 2 segments above the corresponding cord segment. In the lower thoracic and upper lumbar regions, the difference between the vertebral and cord level is 2 or 3 segments while sacral cord levels correspond to vertebral T12-L1 levels (Bican et al., 2013).
Figure 3: Vertebral body and spinal cord segment location across 10 subjects. Vertebral bodies are
represented for each subject by light-shaded bars, whereas spinal cord segments are represented by colored bars (Cadotte et al., 2015).
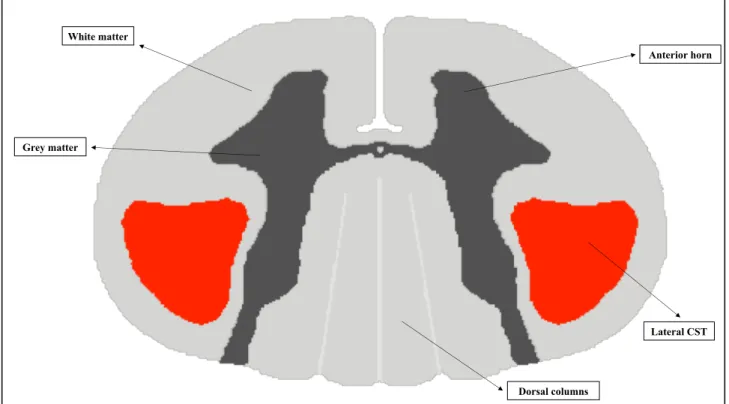
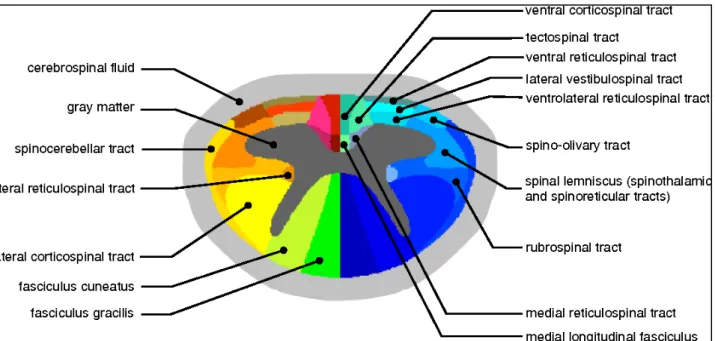
On the transversal plane, the SC is incompletely divided into two halves by a deep anterior median fissure and a posterior median sulcus.The GM of the SC is an H-shaped structure with 2 symmetric halves connected by a narrow bridge or commissure. The ratio between WM and GM is variable along the SC, with bigger GM surfaces observed at the cervical and lumbar enlargements. An imaginary coronal line through the central canal divides the GM into anterior and posterior horns. Alpha and gamma motor neurons are located in the anterior horn.
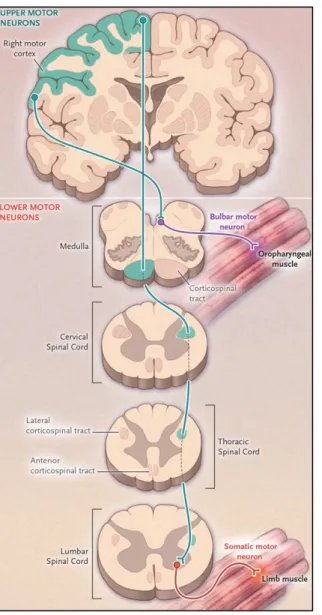
The central GM is surrounded by WM layers including descending and ascending fibers. The corticospinal tract (CST), arising from the precentral motor cortex, is the largest and most significant descending tract of the human SC. Almost 90% of the corticospinal tract fibers decussate in the lower medulla to form the lateral CST, whereas 8% of the non-decussating descending fibers form the anterior CST and 2% of them generate the uncrossed lateral CST.
On the other side, all afferent axons have their primary neurons in the dorsal root ganglia. The level of decussation varies among ascending systems. The dorsal column tract is responsible for the transmission of sensations of vibration, proprioception (position sense), and 2-point discrimination from the skin and joints (figure 4).
Figure 4: Transversal representation of the cervical SC. In dark grey: GM of anterior and posterior
horns. The lateral corticospinal tract (CST) is showed in red.
1.2 Basic principles of spinal cord MRI
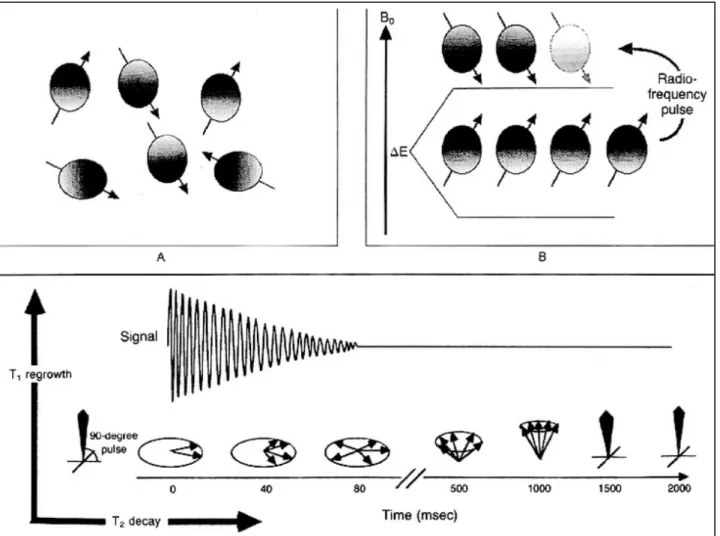
MRI makes use of the magnetic properties of certain atom nuclei present in body tissues, as for example the hydrogen one. By applying a strong and uniform magnetic field, the net magnetic moment (M) of hydrogen nuclei is induced to align (Andrew, 1992). This alignment is next perturbed by the introduction of brief radiofrequency pulses that tilt M, inducing its decay and return to the resting alignment through various relaxation processes, thus generating a current within the receiving coil. The decay between the excitation and the return to equilibrium of M is exploited to generate a contrast difference between tissues (Bitar et al., 2006). T1- and T2-weighted sequences characterized by different combinations of relaxation times can be used to give details about tissues specific features. The T1-time (longitudinal relaxation time) is the time constant which determines the rate at which excited protons return to equilibrium. The T2-time (transverse relaxation time) is the time constant which determines the rate at which excited protons reach equilibrium or go out of phase with each other (Edelman and Warach, 1993) (figure 5).
Lateral CST
Dorsal columns
Anterior horn White matter
Figure 5: Physics of MRI. In the absence of a magnetic field, the magnetic axes of a group of protons
are randomly oriented. (A) In the presence of a strong magnetic field, protons align both with and against the field. (B) A radiofrequency pulse applied at the resonance frequency will cause the protons to flip and align with the higher energy state, from which they relax back to their original alignment at a rate determined by T1 and T2 relaxation times (Edelman and Warach, 1993).
In the Central Nervous System (CNS), the T1-weighted sequence produces a high contrast difference between WM and GM, while the T2-weighted sequence is more sensitive to tissues’ water content and generates an excellent contrast between the CSF and the WM. T2*-weighted sequences are derived from T2 decay using gradient echoes. They are useful to accentuate local magnetic homogeneity effects generating high contrast difference between CSF, WM and GM (Martin et al., 2017).
Despite relevant technical improvements, MR imaging of the SC remains challenging and is frequently focused only on its cervical portion. The major imaging difficulties come from the fact that the SC has tiny cross-sectional dimensions in the axial plane, is long in the sagittal/coronal planes, is surrounded by tissues that have different magnetic susceptibilities and by organs that are prone to motion (Stroman et al., 2014, El Mendili et al., 2019).
Small cross-sectional dimensions: Considered the small dimensions of the SC, in order to effectively depict its anatomical details, high spatial resolution (at least 1 mm × 1 mm) and relatively thin imaging slices (1–2 mm) are required. Positioning the slices transverse to the SC anatomy is favorable to obtain the highest spatial resolution in the plane of the SC cross-section, where the anatomy is more varied, and the greatest resolution is needed (El Mendili et al., 2019). An unavoidable disadvantage of axial slices is that a large number of images is needed to view a sufficiently extended rostral–caudal part of the cord, thus prolonging the acquisition time (Stroman et al., 2014). Moreover, positioning slices transverse to the SC can increase the frequency of artifacts from surrounding tissues and organs (aliasing) whenever the field-of-view (FOV) is not extended enough to cover the whole SC cross-section. In this case, widening the FOV and the application of suppression pulses will be needed to improve the quality of the image (Hakky et al., 2013). An alternative is also the acquisition of sagittal slices to take advantage of the low curvature of the SC and of the smaller anterior-posterior dimensions of the chest.
Partial volume effect: Partial volume refers to the situation when different tissues contribute to the same voxel. In the SC, this occurs when a voxel is at the CSF/WM, WM/GM, and eventually CSF/WM/blood vessels interfaces, resulting in indistinct tissue-boundaries. Partial volume effects can be reduced by increasing the spatial resolution, but this in turn results in lower signal-to–noise and contrast-to-noise ratios (SNR and CNR). Higher magnet field strength (3T or 7T), higher number of phased-array coils with parallel imaging and multi-channel image acquisition (20, 32 or 64 channels) can improve spatial resolution, SNR and CNR reducing this kind of artefact (Zhao et al., 2014; Massire et al., 2018).
Inhomogeneous magnetic field: The spinal canal is surrounded by bones, ligaments, disks, arteries, and venous plexi. Its proximity to the esophagus, mediastinum and the lungs, each containing various amounts of air, create a challenging scanning environment. Adipose tissue, bone and air have different magnetic susceptibility profiles, which contribute to the inhomogeneity of the magnetic field around the SC, resulting in geometric distortions and signal intensity loss. To some extent, these artefacts can be counteracted with ‘shimming’. Shimming aims at compensating for field inhomogeneities by creating an auxiliary magnetic field via shim coils (Roméo and Hoult, 1984). While shimming improves overall field homogeneity, it is limited to smooth variations across large regions and cannot fully compensate for small and localized field inhomogeneities, such as those observed at cartilaginous discs between the vertebral bodies. Echo planar imaging sequences, such as diffusion tensor imaging (DTI), are particularly sensitive to geometric distortion around vertebral
disks (Cohen-Adad et al., 2011; Rasoanandrianina et al., 2017). In addition to shimming, parallel imaging and careful slices positioning (i.e. slices centered in the middle of each vertebral body and perpendicular to the SC) may reduce magnetic field inhomogeneity. The image quality can be further optimized by a suitable choice of the pulse sequence. With few exceptions, MRI methods are either based on a gradient-echo or a spin echo pulse sequence. As echo time (TE) increases, the sequences become progressively T2*- and T2-weighted respectively. The key difference between them is that the spin-echo employs a refocusing pulse in order to reverse the effects of static field inhomogeneity for a brief instant of time. The MRI signal at the peak of the spin-echo is effectively free of the negative effects of the inhomogeneous magnetic field, and thus spin-echo imaging provides significant advantages to obtain high quality images of the SC (Stroman et al., 2014, El Mendili et al. 2019).
Physiological motions: Due to its proximity to the lungs and the heart, almost the entire SC undergoes periodical movements due to respiratory and cardiac pulsation (Kharbanda et al., 2006; Morozov et al., 2018). Spinal imaging is also affected by CSF flow and dynamic movements and is susceptible to movement artefacts from swallowing (Verma and Cohen-Adad, 2014). By ‘gating’ the acquisition, i.e. synchronizing it with the respiratory and cardiac cycles, the effect of periodical movements can be significantly reduced (Summers et al., 2006; Cohen-Adad et al., 2011; Massire et al., 2018). Motion artefacts can also be reduced using ‘saturation bands’ that cover the esophagus, chest and abdomen, attenuating signals from moving structures. Velocity compensating gradient sequences and signal averaging across multiple phases of motion can also be applied to minimize motion artefacts. Reducing acquisition time by using fast sequences and parallel imaging increasing acquisition speed effectively reduce both physiological and subject motion effects (Jaermann et al., 2004; Noebauer-Huhmann et al., 2007).
SC MRI has been extensively applied in the study of spinal injury, inflammatory and neurodegenerative diseases. Standard analyses focus both on morphological images through the study of SC atrophy and on WM structure through the use of diffusion sequences (Wheeler-Kingshott et al., 2014).
Cord morphometry: Gross axonal, WM and GM loss have traditionally been estimated by measuring SC cross-sectional areas at specific levels and interpreted as a proxy of atrophy in the context of reference normative values (Cohen-Adad et al., 2013a; Branco et al., 2014; El Mendili et al., 2015b; Paquin et al., 2018; El Mendili et al.; 2019). The ‘cross-sectional approach’ consists of estimating a
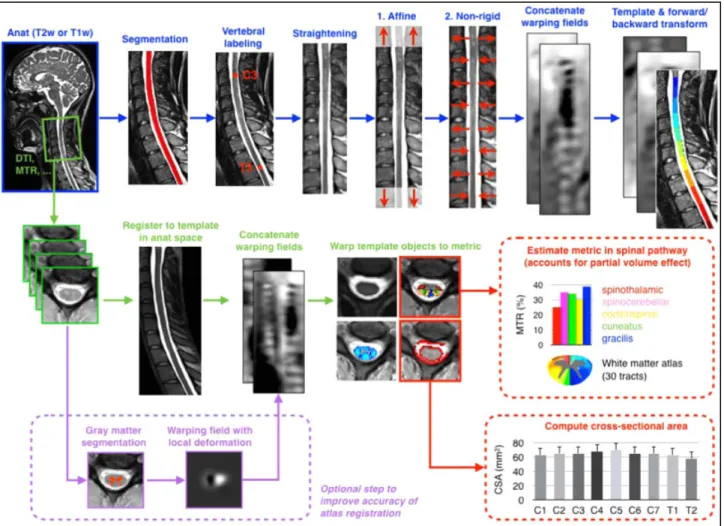
mean cord cross-sectional area over a representative number of slices at a given vertebral level, which can be relatively easily calculated from conventional anatomical MRI sequences such as T1- or T2-weighted images. Segmentation of the SC is often performed in the cervical tract and has been mostly used to quantify SC atrophy in multiple sclerosis. Nevertheless, it can also be applied for co-registration and spatial normalization to a common coordinate space (i.e., template), which can be used to quantify morphometric changes or to perform atlas-based quantitative multiparametric analyses to investigate the structural and functional integrity of the SC (De Leener et al., 2016). Segmentation can be performed on 2D or 3D images and can be done entirely manually, semi-automatically (requiring only a few manual interventions) or fully semi-automatically (El Mendili et al., 2015a; El Mendili et al., 2015b; Taso et al., 2015; De Leener et al., 2017; Dupont et al., 2017; Gros et al., 2018; Papinutto and Henry, 2019; Gros et al., 2019) (figure 6) with high reliability and precision. A variety of indexes, such as anterior-posterior dimension, left-right width, cord eccentricity and radial distance can be derived from different SC segmentation approaches. These measures reflect on different aspects of pathology, such as global versus regional, lateral versus anterior tissue loss, and are often related to predominant motor or sensory involvement (Lundell et al., 2011).
Figure 6: Example of automated cervical SC segmentation with identification of the global surface