HAL Id: hal-02185271
https://hal-amu.archives-ouvertes.fr/hal-02185271
Submitted on 16 Jul 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Thomas Giraud, Charlotte Jeanneau, Charlotte Rombouts, Hengameh
Bakhtiar, Patrick Laurent, Imad About
To cite this version:
Thomas Giraud, Charlotte Jeanneau, Charlotte Rombouts, Hengameh Bakhtiar, Patrick Laurent, et
al.. Pulp capping materials modulate the balance between inflammation and regeneration. Dental
Materials, Elsevier, 2019, 35 (1), pp.24-35. �10.1016/j.dental.2018.09.008�. �hal-02185271�
Thomas
Giraud
a,b,
Charlotte
Jeanneau
a,
Charlotte
Rombouts
a,
Hengameh
Bakhtiar
c,
Patrick
Laurent
a,b,
Imad
About
a,∗ aAixMarseilleUniv,CNRS,ISM,InstMovementSci,Marseille,FrancebAPHM,HôpitalTimone,Serviced’Odontologie,Marseille,13005,France
cDentalMaterialResearchCenter,TehranDentalBranch,IslamicAzadUniversity,Tehran,Iran
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20July2018 Receivedinrevisedform 14September2018
Accepted16September2018
a
b
s
t
r
a
c
t
Theinterrelationsbetweeninflammationandregenerationareofparticularsignificance withinthedentalpulptissueinextensibleenvironment.Recentdatahavedemonstrated thepulpcapacitytorespondtoinsultsbyinitiatinganinflammatoryreactionanddentin pulpregeneration.Differentstudymodelshavebeendevelopedinvitroandinvivoto inves-tigatetheinitialstepsofpulpinflammationandregeneration.Theseincludeendothelial cellinteractionwithinflammatorycells,stemcellinteractionwithpulpfibroblasts, migra-tionchamberstostudycellrecruitmentandentirehumantoothculturemodel.Usingthese models,thepulphasbeenshowntopossessaninherentanti-inflammatorypotentialanda highregenerationcapacityinallteethandatallages.Thesamemodelswereusedto inves-tigatetheeffectsoftricalciumsilicate-basedpulpcappingmaterials,whichwerefound tomodulatethepulpanti-inflammatorypotentialandregenerationcapacity.Amongthese, resin-containingmaterialssuchasTheraCal®
shiftthepulpresponsetowardsthe inflamma-toryreactionwhilealteringtheregenerationprocess.Ontheopposite,resin-freematerials suchasBiodentineTMhaveananti-inflammatorypotentialandinducethepulpregeneration
capacity.Thisknowledgecontradictsthenewtendencyofdevelopingresin-basedcalcium silicatehybridmaterialsfordirectpulpcapping.Additionally,itwouldallowinvestigating themodulatoryeffectsofnewlyreleasedpulpcappingmaterialsonthebalancebetween tis-sueinflammationandregeneration.Itwouldalsosetthebasisfordevelopingfuturecapping materialstargetingtheseprocesses.
©2018TheAcademyofDentalMaterials.PublishedbyElsevierInc.Allrightsreserved.
∗ Correspondingauthorat:InstitutdesSciencesduMouvement(ISM),UMR7287CNRS&Universitéd’Aix-Marseille,Facultéd’Odontologie,
27BdJeanMoulin,MARSEILLECedex513385,France.
E-mail addresses: thomas.giraud@univ-amu.fr (T. Giraud), charlotte.jeanneau@univ-amu.fr (C. Jeanneau),
romboutscharlotte@gmail.com(C.Rombouts),hengamehbakhtiar@yahoo.com(H.Bakhtiar),patrick.laurent@univ-amu.fr(P.Laurent),
imad.about@univ-amu.fr(I.About).
https://doi.org/10.1016/j.dental.2018.09.008
Contents
1. Introduction...25
2. Tricalciumsilicatesasdirectpulpcappingmaterials...26
3. Calciumsilicatecementsbyproductspromotemineralization...28
4. Impactofdirectpulpcappingontheinitialstepsofinflammation...28
5. Pulpcappingmaterialsmodulatethepulpregenerationpotential...29
6. Pulpcappingmaterialsoutcomeinvivo...32
7. Conclusions...33 8. Perspectives...33 ConflictofInterest ... 33 Acknowledgments...33 References...33
1.
Introduction
Thedentalpulptissueislocatedwithinrigiddentinalwalls
[1].Thisuniquelocationinaterminalbloodcirculation ren-dersthistissuevulnerableunlessalocalregulationprovides protectivemechanismstothisparticulartissue.Inthisregard, severallinesofevidencesuggestthatthedentalpulphaslocal regulationmechanismsofitsinflammationandregenerative capacity.Inafirst-linedefence,odontoblaststhatlieunderthe dentinbarrierandpulpfibroblastsexpresspattern recogni-tionreceptors(PRRs).TheseincludeToll-likereceptors(TLRs)
[2,3]whichareabletodetectbacterialinvadersbyrecognizing commonmoleculesontheirsurface,thepathogen-associated molecularpatterns(PAMPs).Afterthisrecognition,thesecells initiateaninflammatorycascadebyactivatingtheNF-kB path-way, essential for the inflammatory response by initiating theproductionofpro-inflammatorycytokines[4].Thesewill establishachemotacticgradientforguidinginflammatorycell migrationtowardstheinflammationsite(Fig.1).Duringthis process,inflammatorycellsadhereonthe activated vascu-larendothelium,then migrate throughthe endothelialcell layer and reach the inflammatory site guided by the pro-inflammatorycytokines[5].Thentheywillbeactivatedinto macrophage-likecells atthe inflammatorysitewhere they eliminatepathogensandcelldebris(Fig.1).
Besides cytokines, the complement system is another importantactorof theinflammatory process.Complement isactivatedbytheclassical,alternative,ormannose-binding lectinpathway[6].Uponpulptissuedamageand/orinfection, the Complement provides the signals required for elimi-nating invading pathogens and altered host cells. Recent data have shown that pulp fibroblasts are the first non-immunecellscapableofproducingallcomponentsrequired forComplementactivation[7].Complementactivationbypulp fibroblastsleadstotheproductionofinflammatorymediators andrecruitmentofinflammatorycellsbyanaphylatoxinssuch asC5aandC3a[8–10].Theseanaphylatoxinsinducethe vas-cularmodificationsrequiredtoallowinflammatorycells to migratetowardstheinflammationsiteinordertoeliminate theinfectiousagents[11].Additionally,Complement activa-tionbypulpfibroblastsleadstotheformationofthecytolytic membraneattackcomplex(MAC)[12].Afterfixationon cario-genicbacteria,thiscomplexleadstotheirdirectdestruction
[13].Thus,Complementactivationappearstobeessentialin
initiatingtheinflammatoryreactionandincontrolling cario-genicbacteria.
Althoughinflammationisaprerequisiteforhealingand regeneration[1]itcanalsobedetrimentalifitpersistsgiven thefactthatthepulpisconfinedinarigidenvironment, leav-ingnoroomforswelling[2].Incaseofsevereinflammation, thismayleadtopulpdestruction.Additionally,ifthe infec-tionpersists,theresultingchronicinflammationwillhamper the regenerativeprocessesandwilleventuallyleadtopulp necrosis.
Besides controlling bacterialprogression and inflamma-tion, it is well established that the dental pulp of both primary and permanent teeth, and at all ages, is rich in stemcells[14–16].Incaseoftraumaticinjuriesand/orpulp infection,regenerationsignals,suchasgrowthfactors,induce theirproliferation,migrationanddifferentiationtoregenerate the dentin-pulp tissue [14] (Fig. 2). Indeed, after stimula-tion, these dental pulp stem cells (DPSC) migrate to the injury/inflammatorysiteanddifferentiateintoodontoblast-like
cells.Upondifferentiation, theyexpressspecificmarkersof odontoblastssuchasthe intermediatefilament Nestinand DentinSialoProtein(DSP)whichisknownforitsimplication inthemineralizationprocess.Indeed,cellsexpressingNestin andDSPwereseenincontactandwithinmineralizedfociin thedentalpulpclosetotheinjurysite[17,18].
DPSCrecruitmenttotheinjury/inflammationsiterequires the presence ofactivemolecules to directtheir migration. RecentworksonComplementactivationhavedemonstrated thatpulpfibroblastComplementactivation isalsoinvolved indental-pulpregenerationbyprovidingComplementactive fragmentssuchasC5aandC3a.Indeed,ithasbeenshown thatDPSCrecruitment,isselectivelyguidedbyaC5agradient
[19].Anotherfragment,C3a,alsopromotestheregenerative processes byincreasingDPSCand pulpfibroblast prolifera-tion,mobilizingDPSCandguidingpulpfibroblastmigration to theComplement activation site [20]. Thisindicates that in addition to its role in initiatingthe inflammatory reac-tion,Complementactivationbypulpfibroblastsproducethe regenerationsignalsrequiredfortheregenerationprocess par-ticularlyinguidingDPSCmigrationtotheinjurysite.Thus, Complement activation provides the missing linkbetween inflammationandregeneration[21].
Fig.1–Schematicrepresentationoftheinitialstepsofpulpinflammation.
Followingacariouslesion,theinflammatoryreactionimpliessecretionofpro-inflammatorycytokines(1)byresidentcells, suchaspulpfibroblasts.Circulatinginflammatorycellsadhereontheactivatedvascularendothelium(2),thenmigrateand reachtheinjuredsite(3)tobefinallyactivatedasmacrophage-likecells(4).
2.
Tricalcium
silicates
as
direct
pulp
capping
materials
Calcium-silicatebased cements(CSC) havebeen developed more than 20years ago with Mineral Trioxide Aggregate (MTA®)being the mostwell-known and most widely used formulation [22]. It is a Portland Cement (PC) based for-mulationcontainingmainlytricalcium(C3S) and dicalcium silicates (C2S) [23] which sets and develops its properties in the presence ofmoisture. CSC were initially developed asendodontic repair and root-endfilling materials[22–24]. Given their biocompatible properties, their clinical usage rapidlyexpandedtowardsdirectand indirectpulpcapping.
Nevertheless,therearesomedisadvantagesassociatedwith theseclassicMTAformulationsincludingalongsettingtime, difficulthandling,poormechanicalpropertiesandtooth dis-coloration [24–26]. Researchers have thus been working to improve CSC’sphysico–mechanicaland handlingproperties andmanynewproductshavebeenintroducedonthemarket, eachhavingtheirspecificsetofcomponents(setting modu-lators,radiopacifyingagentsanddrugs).However,besidesthe requiredphysico-mechanicalproperties,pulp-capping mate-rials should have suitable biological properties given their directcontactwithvitalpulptissue.
Takingintoconsiderationthehandlingandbiological prop-erties,twoCSCmaterialshavebeendevelopedwithenhanced mechanical properties: 1.) BiodentineTM (Septodont,
Fig.2–Schematicrepresentationoftheinitialstepsofdentin-pulpregeneration.
Followingacariouslesion,dentalpulpcellssuchasfibroblastssecretegrowthfactors(1).Thesegrowthfactorscreatea gradientleadingtoperivascularstemcellproliferation(2)andtheirmigrationtotheinjuredsite(3).Finally,migratingstem cellsdifferentiateintoodontoblasts-likecellsandsecretemineralizedmatrixtoprotectthepulptissue(4).
Saint-Maur-des-Fossés, France) is resin-free and mainly composedofpuretricalciumsilicatesandcalciumchlorideas asettingaccelerator.Itispresentedaspowderandliquidtobe preparedbymixingbothcomponentswithanamalgamator. It sets in 12min which is much shorter than MTA® that setsafter2h45min[27].2.)TheraCal® (Bisco,Schaumburg, IL,USA), iscomposed ofPC and contains 43%of resins.It is presented as a ready-to-use material in a syringe and sets by photopolymerization (20s per 1mm increment) in a hydrophobic environment. The complete composition of thesematerialshasbeenreported[28].
Previously published works have already reported that resin-based materials cannot be recommended for direct
pulpcapping[29].However,therecentdevelopmentof resin-containing hybrid materials for direct and indirect pulp capping, stating their improved mechanical and handling properties,raisesquestionsabouttheirconsequencestothe pulphealingpotential.Forinstance,thebyproductformation fromcalciumsilicateinthesehybridmaterialsonsettingis differentfromthoseobservedinresin-freeCSCmaterials.In addition,thedevelopmentofthesematerialsrepresentsarisk tothe pulpvitality duetothe resin componentsand their potentialtoxicity.
In this review we willfocus on the effect ofthese two recently developed CSC, the resin-free BiodentineTM and
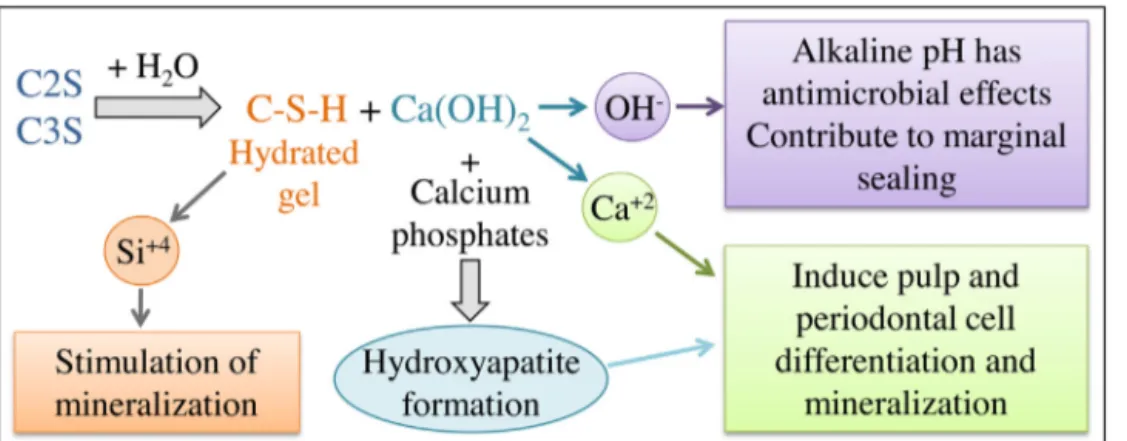
Fig.3–Silicate-basedmaterialhydrationbyproductsonsettingandtheirbiologicaleffects.
Schematicrepresentationofthesettinghydrationreactionofsilicate-based(C2S:dicalciumsilicate,C3S:tricalciumsilicates) materials.Thisreactionleadstobyproductsformation:OH−,Ca2+andSi4+.ThereleasedhydroxylionsincreasethepHin
theunderlyingtissueleadingtoanti-microbialeffect.Calciumionsareinvolvedindentin-bridgeformationasthey stimulateDPSCdifferentiation.Siliconionsalsopromotemineralization.Calciumhydroxideinducesdentinbridge formation.
crucial biological processes which determine the suc-cess/failure of the clinical outcome: inflammation and regeneration.
3.
Calcium
silicate
cements
byproducts
promote
mineralization
Calciumsilicatesettingreactionishydration.Duringthis reac-tion,hydrationbyproductscanform/bereleased(Fig.3).Most CSC lead to calcium hydroxide formation, and leaching of hydroxylionsandcalciumionsasdemonstratedforMTA®and BiodentineTM,amongstothers[30–32].Thereleasedhydroxyl
ionsuponhydrationwillincreasethepHintheunderlying tis-sueleadingtoathinnecroticlayerbetweentheremainingvital tissueandthepulpcappingagent[33,34].Thepresenceofthis necroticzoneprotectstheunderlyingvitalpulpcellsfromthe material’salkalinepH.Furthermore,itallowstheunderlying pulpcells tocarry out the healingand regeneration func-tions[35].ThealkalinepHalsoensuresanti-microbialactivity
[27].Subsequentcalcificationofthissuperficialnecroticlayer followed bytertiary dentinformation from stimulated and differentiateddentalpulpstemcellsgiverisetoaprotective dentin-bridge[36].Calciumionscontributetothisprotective dentin-bridgeformationastheystimulateDPSC differentia-tionandincreasetheformationofmineralizedmatrixnodules
[37,38]. Interestingly,TheraCal® has been shown torelease lesscalciumionscomparedtoBiodentineTMandnocalcium
hydroxideformationwasseenwhenstudiedbyX-ray diffrac-tion analysis. Thismay bedue to the lackof moisture to allowproperhydrationofthetricalciumsilicateelementsin TheraCal®,whichexplainstheabsenceofcalciumhydroxide formation[32].
Besides the release of these ions involved in dentin-bridgeformation,a“bioactive”surfaceisformedduetothe nucleation of calcium phosphates and subsequent apatite formation, in a moist environment. This apatite layer is suggestedtostimulatecelldifferentiation,tissuerepair,
osteo-genesis and cementogenesis [22]. Silicon ions are another elementthatmayplayaroleindentin-bridgeformation.Their releasehasbeenknowntostimulateyoungboneformation by stimulatingosteoblasts [39]. In caseof directpulp cap-ping,it isbelieved thatthepresenceofsiliconionsinCSC, such as BiodentineTM, also promote mineralization. An ex
vivotoothculturemodelshowedthatafterpulpcappingwith BiodentineTM,smallCSCparticleswereentrappedinthe
min-eralized noduleswhich suggests that the materialitself is involvedinodontoblasticdifferentiationandmineralization
[17,18].
4.
Impact
of
direct
pulp
capping
on
the
initial
steps
of
inflammation
Upon cariousand/orphysicalinjury ofthe dental-pulp,an inflammatoryreactionisinitiatedintheremaininghealthy pulp tissue [40]. While mild or moderate inflammation is required to stimulate the regenerative process, severe and/orchronicinflammationwillbedetrimentaltothepulp. Significant advances in investigating the initial steps of inflammationusingdifferentcellcultureandco-culture mod-elsinvitroclearlyestablishedthatpulpfibroblastsplayamajor roleintheinitialstepsofinflammatoryprocess.Secreted pro-inflammatorycytokinessuchasVascularEndothelialGrowth Factor (VEGF), Interleukine 6 (IL-6) and Complement frag-ments such asC3a and C5ainduce vascular modifications to initiate inflammatorycell recruitment to the inflamma-tionsite[41–44].Theseincludeadhesionofinflammatorycells toactivated vascularendothelium, theirmigrationthrough theendothelialcelllayerandsubsequentrecruitmenttothe inflammationsitewheretheyareactivated(Fig.1).
In order to evaluate in vitro inflammatory mediators secreted indamagedandinflamed pulptissue,pulp fibrob-lastshavebeeninjuredand/orstimulatedwithGrampositive lipoteichoicacid(LTA)and/orGramnegative lipopolysaccha-ride (LPS). Quantification ofthe pro-inflammatory released
Fig.4–Pulpcappingmaterialeffectsontheinitialstepsof inflammationinvitro.
(A)Effectsofmaterialsoncytokinesecretion.IL-6andVEGF secretionbypulpfibroblastssignificantlyincreasedwith bothBiodentineTMandTheraCal®
conditionedmediaafter 48hbuttoalesserextentwithBiodentineTMascompared
tothecontrolasdescribed[46].(B)Effectsofmaterialson inflammatorycell(THP-1)recruitmentsequence.
BiodentineTMsignificantlydecreasesTHP-1adhesionto
endothelialcells,migrationandactivation[46].(*) correspondstosignificantdifferenceascomparedtothe control,(**)representssignificantdifferencesbetweenthe twobiomaterials(p-value<0.05).
mediatorsshowedthat IL-6, IL-8, IL1␣ andTumor Necrosis Factor-␣(TNF-␣)secretionincreasedinLPS-stimulatedhuman DPSC[45].ApplicationofCSChasbeenshowntomodulate pro-inflammatorymediatorsecretion.Forinstance, applica-tionofCSCextracts(TheraCal®andBiodentineTM)oninjured
and LTA-stimulatedpulp fibroblasts showed increasedIL-6 andVEGFsecretion(Fig.4A),whichwassignificantlyhigher forTheraCal®[46].IL-8secretionbypulpfibroblastswasalso significantlyhigherwithTheraCal®comparedtoBiodentineTM [18].ThismodulationofIL-8secretionbypulpcapping materi-alsisofinterestasIL-8isapotentchemokineandplaysarole incontrollingthedurationoftheinflammatoryprocess.MTA® hasalsobeenshowntoincreaseIL-8secretionbyhuman neu-trophils,thefirstinflammatorycellstoreachthedamagedsite
[47].
or BiodentineTM demonstrated thatpulpcappingmaterials
modulate the inflammatory response. Inflammatory THP-1 cells adhesion to endothelial cells and their activation werereducedbyBiodentineTMandTheraCal®
.However,their migrationdecreasedonlywithBiodentineTM[46](Fig.4B).
Given that severe pulp inflammation is detrimental to clinical outcome, pulp capping materials that reduce the inflammatoryprocessareofparticularinterest.Several stud-ies haveshown favourable clinicaloutcome withmaterials suchasMTA®andBiodentineTM,whichismostlikelyrelated
toanattenuatedinflammatoryresponse.Forinstance,Kang etal.demonstratedthat,afterdirectpulpcappingfor8weeks, acalcifieddentinbarrierwasformedwithProRoot®MTAand Ortho® MTAwhereas dentin barrierformationwas incom-plete with Endocem® MTA. Thelatterwas associatedwith aninflammatoryreaction[48]. Anotherstudy alsosuggests thatmildinflammationisassociatedwiththickerandmore continuousdentinebridgeformation.Thiswasobservedafter 45daysforBiodentineTMasopposedtoDycal®
[49].
Variousinvitrostudieshaveprovidedinsightinthe under-lyingprocesses bywhichparticularpulpcappingmaterials shift thebalance from inflammationtowards regeneration. Forinstance,TNF-␣,animportantpro-inflammatorycytokine releasedduringpulpinflammation,hasbeenshowntoinduce Transient Receptor PotentialAnkyrin 1 (TRPA1)expression, which plays a role in nociception and neurogenic inflam-mation.Interestingly,BiodentineTMisabletoattenuatethis
TNF-␣-inducedTRPA1expressionandtoreduceitsfunctional activity[50].
5.
Pulp
capping
materials
modulate
the
pulp
regeneration
potential
Inagreementwiththe aboveinflammation-reducingeffect, BiodentineTM isabletopromoteregenerativeprocesses.For
instance,BiodentineTMincreasesTransforminggrowthFactor
1 (TGF-1) and Fibroblast Growth Factor 2 (FGF-2) secre-tion by injured pulp fibroblasts, as opposed to TheraCal® (Fig.5A)[17].TGF-1growthfactorhasbeenshownto stimu-lateodontoblasticdifferentiation[51]contributingassuchto dentin-bridgeformation.Recently,theinterestofthisgrowth factorrelease hasbeeninvestigatedbyencapsulatingthese growth factors in PLGA microspheres allowing their grad-ualrelease.Thisinvivostudydemonstratedthatthegradual releaseofFGF-2inducesfibroblastandstemcellproliferation whileTGF-1guidesDPSCrecruitment[52],odontoblastic dif-ferentiationandtertiarydentinformation[53].
Whenthepulpinjurywassimulatedinthescratchassay, an experimentalmodel which simulates a physical tissue injury,pulpfibroblastmigrationtocolonizetheinjurysitewas significantlyhigherwithBiodentineTM thanwithTheraCal®
[46](Fig.5B).InvestigatingDPSCmigrationinBoyden cham-ber also demonstrated a significantly higher migration of thesecellsinthepresenceofBiodentineTM ascomparedto
TheraCal®[54](Fig.5B).
Thesedataclearlyshowthatpulpcappingmaterialsalso modulatetheinitialstepsofpulpregeneration[46].Moreover, whilecellviabilitywasmaintainedwithBiodentineTM,a
sig-Fig.5–Pulpcappingmaterialeffectsontheinitialstepsofpulpregenerationinvitro.
(A)Effectsofmaterialsongrowthfactorsecretion.PulpfibroblastssecretedsignificantlymoreTGF-1andFGF2after24hof incubationwithBiodentineTMthanwithTheraCal®
asdescribed[46].(B)Effectsofmaterialsonstemcellmigrationand fibroblastcolonization.(a)Representativepicturesand(b)quantificationofpulpfibroblastsscratchwoundhealingassayand DPSCsBoydenchambermigrationassaywithBiodentineTMandTheraCal®
asdescribed[46,54].BiodentineTMsignificantly
inducedfibroblastcolonizationwhileTheraCal®significantlydecreasedDPSCsmigration.(*)correspondstosignificant differenceascomparedtothecontrol,(**)representssignificantdifferencesbetweenthetwobiomaterials(p-value<0.05). Scalebars:200mforscratchwoundhealingassaysand50mforBoydenchamberassays.
nificantdecreaseinpulpcellproliferationwasreportedwith TheraCal®(Fig.6A).Investigatingthepulpcelldifferentiation potentialwiththematerialsdemonstratedahigher expres-sionofodontoblasticmarkerssuchasNestinandDSPwith BiodentineTM than withTheraCal®
(Fig. 6B). When applied
as direct pulp capping material in entire human tooth cultures,asignificantnumber ofmineralizedfoci wasseen underBiodentineTMinanintactpulptissuewhilea
disorga-nizedpulptissuewasobservedunderTheraCal® withsmall andadispersedmineralization(Fig.6C)[18].Thisresultwas
Fig.6–Effectsofsilicate-basedpulpcappingmaterialsonpulpcellproliferationandpulpmineralizationinvitroandinvivo. (A)EffectofBiodentineTMandTheraCal®
onhumanpulpfibroblastproliferation[18].Asignificantdecreaseinpulp fibroblastproliferationwasobservedwithTheraCal®conditionedmediaforallincubationperiods.(*)correspondsto significantdifferenceascomparedtothecontrolmedium(p-value<0.05).(B)EffectofthecappingbiomaterialsonDSPand NestinexpressionbyDPSCsasdescribed[18].Anincreaseofbothmarkerswasobservedbyimmunofluorescencewhen DPSCswereculturedincontactwithBiodentineTM.Scalebars:50m.(C)Histologyresultsafterdirectpulpcappinginvitro usingthehumantoothculturemodel(15days)asdescribed[18]andinvivoaftertoothextraction(8weeks)asdescribed[55]
withBiodentineTMandTheraCal®
.EntiretoothculturehistologyshowedahighermineralizationwithBiodentineTMwhile
significanttissuedisorganizationwasobservedwithTheraCal®.Afterpartialpulpotomyinhumanteethacompletedentin bridgeformedwithBiodentineTMwhileonlydispersedmineralizationsinadisorganizedpulptissuewereobservedwith
Fig.7–Biologicaleffectsofsilicate-basedmaterialsonthedentalpulp.
confirmed on partialpulpotomies in humanthird molars. Indeed,whileacompletebridgeunderBiodentineTMformed
inanintactpulpafter8weeks,onlyadispersedmineralization inadisorganizedpulpwasobservedunderTheraCal®(Fig.6C)
[55].
6.
Pulp
capping
materials
outcome
in
vivo
Clinical successofpulpcappingproceduresinvolves many aspectsand isdifficultto mimicin vitro. In vivoand clini-calstudies are thus an essential researchpart to evaluate not only dentin-pulp regeneration, but also inflammation and overall pulp response. CSC such as BiodentineTM and
MTA®showedgoodclinicaloutcomeswithhighsuccessrates whereasTheraCal®waslesssuccessful.Forinstance,aclinical trialonpulpotomyinprimaryteethshowedahighrateof clin-icalandradiographicsuccessforMTA®andBiodentineTMafter 12months(92%and97%,respectively)[56].Anotherstudyin permanentteethalsoshowedfavourableoutcomesforpulp cappingwithBiodentineTMandMTA®
.Theirhistological anal-ysisshowedthatthemajorityofteethhadcompletedentinal bridgeformationandnoinflammationafter6weekswithboth BiodentineTMandMTA®
[57].Asimilarstudycomparedpulp cappingwithcalciumhydroxide,MTA®,BiodentineTMand
Sin-gleBondUniversalinhumanteeth.After6weeks,thedentin bridges formedin BiodentineTM group showed the highest
averagemineralizationandmaximumvolumeswhileSingle BondUniversalthelowest[58].Indogpartialpulpotomy,itwas observedthatTheraCal®cappingledtoextensive inflamma-tionandincompletecalcifiedbarrierformation.Ontheother hand,completedentin-bridgeformationwithno inflamma-tion was observed with ProRoot® MTA [59]. A clinical trial
in adults showed complete dentin-bridge formation in all BiodentineTMcaseswhereasthisratewasonly11%and56%in
TheraCal®andProRoot®MTAgroups.IntheTheraCal®group, twopatientshadseverepainanddiscomfortafteroneweek
[55].ThiscanbeexplainedbytheobservedTheraCal® toxi-cityininvitrostudies[18,60].Indeed,leachingofmonomers duetoincompletehydrationofTheraCal®leadstotoxicityof theunderlyingpulpcellsandinduceassuchaninflammatory response. Thisisparticularly disadvantageousinthe tooth confinedenvironmentwheresevereinflammationwillleadto painandsubsequentclinicalfailure.Ithasalsobeenshown thatnontoxicconcentrationsofthesemonomersinhibitthe secretionofdentinsialoproteinsandosteonectin,whichare involvedinthemineralizationprocess[61].
Itisworthnotingthatrecentdatahavedemonstratedthat usingbioactivematerialssuchasBiodentineTMandMTA®
in partialorfullpulpotomytotreatirreversiblepulpitisleadsto pulpfunctionrestorationanddentinbridgeformation.This hasbeenreportednotonlyinimmaturebut alsoinmature teeth[62–65].Theseresults,whichrepresentaparadigmshift inirreversiblepulpitistreatment,appeartobeduetomultiple factors:
1) Thelocalregulationofpulpinflammationandregeneration
[66]
2) Thepresence ofstem cells and the inherenthigh pulp regenerationcapacity[51,67]
3) Theanti-inflammatoryactivityofbioactivematerialssuch asBiodentineTM[46,50]
4) Thematerialbyproductsonsettingwhichinducestemcell differentiationanddentinbridgeformation[28]
subsequentrelease offactors suchasFGF-2 andTGF-1 involvedinpulptissueregeneration[52]
7.
Conclusions
Overall,eveniftheinitialinflammationisapre-requisitefor healing,arapidresolutionofinflammationwouldfavourthe regenerativeprocesswhichiskeyforasuccessfulclinical out-come [68]. Choosingan appropriate pulpcapping material iscrucialgiventhat theycanmodulatethecourseofthese events.ItappearsclearlythatthepresenceofresinsinCSC pulpcappingmaterialssuchasTheraCal®shiftsthebalance towards inflammation. Their incomplete photopolymeriza-tionleadstofreemonomersrelease.Whenthesemonomers reachtheunderlyingpulp,theyexerttheirtoxicityas demon-stratedbydecreasedcellviability,releaseofpro-inflammatory cytokinesand recruitment ofinflammatory cells.Itcan be assumedthatthiscreatesaninflammatorystatewhich com-promisestheregenerativeprocess.
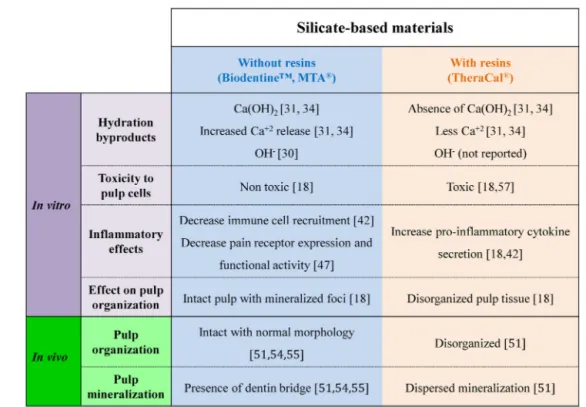
Inaddition,theincompleteTheraCal®hydrationduetothe presenceofahighpercentageofresin,leadstoareduced Cal-ciumionreleaseandtheabsenceofCa(OH)2,bothofwhich
are known for their positive impact on the mineralization process (Fig. 7). Thus,pulpcappingwithTheraCal® can be heldresponsibleforadisorganizedpulptissuewithoutdentin bridgeformation[18,55].Basedonthesescientificfindings,it canbeconcludedthatevencombinedwithCalciumsilicates, resin-containingmaterialsarenotcompatiblewiththespirit ofdirectpulpcapping.
On the opposite, CSC pulp capping materials such as BiodentineTM and MTA®
shift the balance towards regen-eration. Indeed, recent investigations demonstrated that BiodentineTMhasananti-inflammatoryactivitybycontrolling
pro-inflammatoryfactorssecretionanddecreasing inflamma-torycells recruitment [46]. Atthe same time, the material hydration is complete leading to the formation/release of byproductswhichshiftsthepulpresponsetowards regenera-tionasdemonstratedthroughincreasedexpressionoffactors involvedintheregenerationprocesssuchasFGF-2and TGF-1,andtheinductionofdentinbridgeformationwhilekeeping anintactpulp(Fig.7).
8.
Perspectives
Currentknowledgeofpulpanti-inflammatoryand regenera-tionpotentialwouldpavethewayfordevelopmentoffuture therapeuticagentsthatcantargetnotonlytheregeneration but atthe same timethe pulpinflammation.Indeed, both inflammationandregenerationarerequiredforasuccessful clinicaloutcomewithinthepulpinextensibleenvironment. Thefactthattissuelysisanddestructionmayresultfroma severeinflammationsuggeststhat,beyondthepulp,thiswill alsosetthebasisfordevelopmentofbioactivematerialsinthe treatmentofothertissueslocatedinaterminalcirculation.
Conflict
of
Interest
Pr.Imad ABOUTreportsfinancialsupportofresearchfrom SeptodontduringthedevelopmentofBiodentineTM.
Acknowledgments
Theoriginalworksreportedinthispaperweresupportedby Aix-MarseilleUniversityandCNRS.
r
e
f
e
r
e
n
c
e
s
[1] GoldbergM,NjehA,UzunogluE.Ispulpinflammationa prerequisiteforpulphealingandregeneration?Mediators Inflammation2015;2015:347649.
[2] CooperPR,HolderMJ,SmithAJ.Inflammationand regenerationinthedentin-pulpcomplex:adouble-edged sword.JEndod2014;40:S46–51.
[3] daRosaWLO,PivaE,daSilvaAF.Disclosingthephysiologyof pulptissueforvitalpulptherapy.IntEndodJ2018;51:829–46.
[4] LawrenceT.ThenuclearfactorNF-Bpathwayin inflammation.ColdSpringHarbPerspectBiol2009;1, a001651.
[5] LeyK,LaudannaC,CybulskyMI,NoursharghS.Gettingto thesiteofinflammation:theleukocyteadhesioncascade updated.NatRevImmunol2007;7:678–89.
[6] MurphyK,TraversP,WalportM,JanewayC.Janeway’s immunobiology.8thed.NewYork:GarlandScience;2012.
[7] ChmilewskyF,JeanneauC,LaurentP,AboutI.Pulp fibroblastssynthesizefunctionalcomplementproteins involvedininitiatingdentin-pulpregeneration.AmJPathol 2014;184:1991–2000.
[8] EhrengruberMU,GeiserT,DeranleauDA.Activationof humanneutrophilsbyC3aandC5AComparisonofthe effectsonshapechanges,chemotaxis,secretion,and respiratoryburst.FEBSLett1994;346:181–4.
[9] HartmannK,HenzBM,Krüger-KrasagakesS,KöhlJ,Burger R,GuhlS,etal.C3aandC5astimulatechemotaxisofhuman mastcells.Blood1997;89:2863–70.
[10] NatafS,DavoustN,AmesRS,BarnumSR.HumanTcells expresstheC5areceptorandarechemoattractedtoC5a.J ImmunolBaltimMd1999;1950(162):4018–23.
[11] RicklinD,HajishengallisG,YangK,LambrisJD.
Complement:akeysystemforimmunesurveillanceand homeostasis.NatImmunol2010;11:785–97.
[12] TomlinsonS.Complementdefensemechanisms.CurrOpin Immunol1993;5:83–9.
[13] JeanneauC,RufasP,RomboutsC,GiraudT,DejouJ,AboutI. Canpulpfibroblastskillcariogenicbacteria?Roleof complementactivation.JDentRes2015;94:1765–72.
[14] HuangGT-J,GronthosS,ShiS.Mesenchymalstemcells derivedfromdentaltissuesvs.thosefromothersources.J DentRes2009;88:792–806.
[15] MiuraM,GronthosS,ZhaoM,LuB,FisherLW,RobeyPG, etal.SHED:stemcellsfromhumanexfoliateddeciduous teeth.ProcNatlAcadSciUSA2003;100:5807–12.
[16] SonoyamaW,LiuY,FangD,YamazaT,SeoB-M,ZhangC, etal.Mesenchymalstemcell-mediatedfunctionaltooth regenerationinswine.PloSOne2006;1:e79.
[17] LaurentP,CampsJ,AboutI.Biodentine(TM)inducesTGF-1 releasefromhumanpulpcellsandearlydentalpulp mineralization.IntEndodJ2012;45:439–48.
biologicalperspectivesandclinicalapplications.DentMater OffPublAcadDentMater2015;31:351–70.
[23] CamilleriJ,MontesinFE,BradyK,SweeneyR,CurtisRV,Ford TRP.Theconstitutionofmineraltrioxideaggregate.Dent MaterOffPublAcadDentMater2005;21:297–303.
[24] DawoodAE,ParashosP,WongRHK,ReynoldsEC,MantonDJ. Calciumsilicate-basedcements:composition,properties, andclinicalapplications.JInvestigClinDent2017;8:12195.
[25] VallésM,MercadéM,Duran-SindreuF,BourdelandeJL,Roig M.Colorstabilityofwhitemineraltrioxideaggregate.Clin OralInvestig2013;17:1155–9.
[26] VallésM,RoigM,Duran-SindreuF,MartínezS,MercadéM. Colorstabilityofteethrestoredwithbiodentine:a6-month invitrostudy.JEndod2015;41:1157–60.
[27] TorabinejadM,HongCU,McDonaldF,PittFordTR.Physical andchemicalpropertiesofanewroot-endfillingmaterial.J Endod1995;21:349–53.
[28] AboutI.RecentTrendsinTricalciumSilicatesforVitalPulp Therapy.CurrOralHealthRep2018;5:178–85.
[29] AboutI.Cytotoxicity:mechanismsandinvivostudies.In: Biocompatibilityorcytotoxiceffectsofdentalcomposites. Oxford,UK:CoxmoorPublishingCompany;2009.p.91–110.
[30] CamilleriJ,SorrentinoF,DamidotD.Investigationofthe hydrationandbioactivityofradiopacifiedtricalciumsilicate cement,BiodentineandMTAAngelus.DentMaterOffPubl AcadDentMater2013;29:580–93.
[31] NataleLC,RodriguesMC,XavierTA,SimõesA,deSouzaDN, BragaRR.Ionreleaseandmechanicalpropertiesofcalcium silicateandcalciumhydroxidematerialsusedforpulp capping.IntEndodJ2015;48:89–94.
[32] CamilleriJ.Hydrationcharacteristicsofbiodentineand theracalusedaspulpcappingmaterials.DentMaterOff PublAcadDentMater2014;30:709–15.
[33] AeinehchiM,EslamiB,GhanbarihaM,SaffarAS.Mineral trioxideaggregate(MTA)andcalciumhydroxideas pulp-cappingagentsinhumanteeth:apreliminaryreport. IntEndodJ2003;36:225–35.
[34] TéclèsO,LaurentP,AubutV,AboutI.Humantoothculture:a studymodelforreparativedentinogenesisanddirectpulp cappingmaterialsbiocompatibility.JBiomedMaterResB ApplBiomater2008;85:180–7.
[35] SchröderU.Effectsofcalciumhydroxide-containing pulp-cappingagentsonpulpcellmigration,proliferation, anddifferentiation.JDentRes1985;64:541–8.
[36] SchröderU,SundströmB.Transmissionelectronmicroscopy oftissuechangesfollowingexperimentalpulpotomyof intacthumanteethandcappingwithcalciumhydroxide. OdontolRevy1974;25:57–68.
[37] CamilleriJ,LaurentP,AboutI.Hydrationofbiodentine, theracalLC,andaprototypetricalciumsilicate-baseddentin replacementmaterialafterpulpcappinginentiretooth cultures.JEndod2014;40:1846–54.
[38] AnS,GaoY,LingJ,WeiX,XiaoY.Calciumionspromote osteogenicdifferentiationandmineralizationofhuman
2001;276:7614–20.
[42] HippenstielS,KrüllM,IkemannA,RisauW,ClaussM, SuttorpN.VEGFinduceshyperpermeabilitybyadirect actiononendothelialcells.AmJPhysiol1998;274:678–84.
[43] HeinrichPC,CastellJV,AndusT.Interleukin-6andtheacute phaseresponse.BiochemJ1990;265:621–36.
[44] GuoR-F,WardPA.RoleofC5aininflammatoryresponses. AnnuRevImmunol2005;23:821–52.
[45] BindalP,RamasamyTS,KasimNHA,GnanasegaranN,Chai WL.Immuneresponsesofhumandentalpulpstemcellsin lipopolysaccharide-inducedmicroenvironment.CellBiolInt 2018;42:832–40.
[46] GiraudT,JeanneauC,BergmannM,LaurentP,AboutI. Tricalciumsilicatecappingmaterialsmodulatepulphealing andinflammatoryactivityinvitro.JEndod2018,
http://dx.doi.org/10.1016/j.joen.2018.06.009,0.
[47] CavalcantiBN,RodedeSM,Franc¸aCM,MarquesMM.Pulp cappingmaterialsexertaneffectonthesecretionofIL-1 andIL-8bymigratinghumanneutrophils.BrazOralRes 2011;25:13–8.
[48] KangC-M,HwangJ,SongJS,LeeJ-H,ChoiH-J,ShinY.Effects ofthreecalciumsilicatecementsoninflammatoryresponse andmineralization-inducingpotentialsinadogpulpotomy model.MaterBaselSwitz2018;11,
http://dx.doi.org/10.3390/ma11060899.
[49] JalanAL,WarhadpandeMM,DakshindasDM.Acomparison ofhumandentalpulpresponsetocalciumhydroxideand biodentineasdirectpulp-cappingagents.JConservDent JCD2017;20:129–33.
[50] ElKarimIA,McCruddenMTC,McGahonMK,CurtisTM, JeanneauC,GiraudT,etal.Biodentinereducestumor necrosisfactoralpha-inducedtrpa1expressionin odontoblastlikecells.JEndod2016;42:589–95.
[51] AboutI.Dentin–pulpregeneration:theprimordialroleofthe microenvironmentanditsmodificationbytraumatic injuriesandbioactivematerials.EndodTop2013:28,
http://dx.doi.org/10.1111/etp.12038.
[52] MathieuS,JeanneauC,Sheibat-OthmanN,KalajiN,FessiH, AboutI.Usefulnessofcontrolledreleaseofgrowthfactorsin investigatingtheearlyeventsofdentin-pulpregeneration.J Endod2013;39:228–35.
[53] ZhangW,WalboomersXF,JansenJA.Theformationof tertiarydentinafterpulpcappingwithacalciumphosphate cement,loadedwithPLGAmicroparticlescontaining TGF-beta1.JBiomedMaterResA2008;85:439–44.
[54] GiraudT,RufasP,ChmilewskyF,RomboutsC,DejouJ, JeanneauC,etal.Complementactivationbypulpcapping materialsplaysasignificantroleinbothinflammatoryand pulpstemcells’recruitment.JEndod2017;43:1104–10.
[55] BakhtiarH,NekoofarMH,AminishakibP,AbediF,Naghi MoosaviF,EsnaashariE,etal.Humanpulpresponsesto partialpulpotomytreatmentwiththeracalascompared withbiodentineandProRootMTA:aclinicaltrial.JEndod 2017;43:1786–91.
S,García-BinimelisJ,AboutI,MercadéM.Short-term treatmentoutcomeofpulpotomiesinprimarymolarsusing mineraltrioxideaggregateandBiodentine:arandomized clinicaltrial.ClinOralInvestig2016;20:1639–45.
[57] NowickaA,LipskiM,ParafiniukM,Sporniak-TutakK, LichotaD,KosierkiewiczA,etal.Responseofhumandental pulpcappedwithbiodentineandmineraltrioxideaggregate. JEndod2013;39:743–7.
[58] NowickaA,WilkG,LipskiM,KołeckiJ,
Buczkowska-Radli ´nskaJ.Tomographicevaluationof reparativedentinformationafterdirectpulpcappingwith Ca(OH)2,mta,biodentine,anddentinbondingsystemin
humanteeth.JEndod2015;41:1234–40.
[59] LeeH,ShinY,KimS-O,LeeH-S,ChoiH-J,SongJS. Comparativestudyofpulpalresponsestopulpotomywith ProRootMTA,RetroMTA,andTheraCalinDogs’teeth.J Endod2015;41:1317–24.
[60] HeblingJ,LessaFCR,NogueiraI,CarvalhoRM,CostaCAS. Cytotoxicityofresin-basedlight-curedliners.AmJDent 2009;22:137–42.
[61] DiamantiE,MathieuS,JeanneauC,KitrakiE,PanopoulosP, SpyrouG,etal.Endoplasmicreticulumstressand
mineralizationinhibitionmechanismbytheresinous monomerHEMA.IntEndodJ2013;46:160–8.
Biodentineinadultpatientswithsymptomsindicativeof irreversiblepulpitis.IntEndodJ2018;51:819–28.
[63] TahaNA,AbdulkhaderSZ.Fullpulpotomywithbiodentine insymptomaticyoungpermanentteethwithcarious exposure.JEndod2018;44:932–7.
[64] TahaNA,KhazaliMA.Partialpulpotomyinmature
permanentteethwithclinicalsignsindicativeofirreversible pulpitis:arandomizedclinicaltrial.JEndod2017;43:1417–21.
[65] AsgaryS,EghbalMJ,BaghebanAA.Long-termoutcomesof pulpotomyinpermanentteethwithirreversiblepulpitis:A multi-centerrandomizedcontrolledtrial.AmJDent 2017;30:151–5.
[66] JeanneauC,LundyFT,ElKarimIA,AboutI.Potential therapeuticstrategyoftargetingpulpfibroblastsin dentin-pulpregeneration.JEndod2017;43:S17–24.
[67] NakashimaM,IoharaK.Regenerationofdentalpulpby stemcells.AdvDentRes2011;23:313–9.
[68] FargesJ-C,Alliot-LichtB,RenardE,DucretM,GaudinA, SmithAJ,etal.Dentalpulpdefenceandrepairmechanisms indentalcaries.MediatorsInflamm2015;2015:230251.