©
by
Oldenbourg Verlag,
-0044-3336/89
Effect of
Hydrogen
on
the
Sintering
of
Pt-Black
Catalyst
and
on
its
Activity
in
Skeletal
Hydrocarbon
Reactions*
Zoltan
Paal,
Helga
ZimmerInstitute of
Isotopes
of theHungarian Academy
ofSciences,
H-1525Budapest,
P.O.Box77,
Hungary
and John R. Giinter
Institute for
Inorganic Chemistry, University
ofZurich,
Winterthurer-strasse
190,
CH-8057Zurich,
SwitzerlandPt-black
samples
were sintered inhydrogen
at 473 and 633 K. Their surfacecomposition
and electronmierographic
images
are discussedtogether
with theircatalytic
activity
in reactions of n-hexane andmethylcyclopentane.
Pt-473 is more active in n-hexane reactions thanPt-633;
also a differenthydrogen
pressuresensitivity
is observed.Methylcyclopentane
transformations show much lessdifferences;
thus these two reactants exhibit different structure sensitivities. Hydro-carbons andhydrogen
compete
for activesites,
theavailability
andhyd-rogen content of which may be lower on the
-more carbonized
-Pt-633.
Introduction
Group
VIII metals
-in
particular
Pt
-are
typical
catalysts
ofhydrocarbon
transformationsIII.
Hydrogen
is a stoichiometricpartner
in
hydrogenation
reactions; however,
the presence ofhydrogen
is also necessary fordehydrogenation
reactions,
e.g. innaphtha
refor-ming
where alkanes andcycloalkanes
give
aromatics. ThePt-containing
catalysts
(supported
alone ortogether
with other metallic additives on* Presented
as anInvitedLectureatthe International
Symposium
onMetal—
Hydrogen
AlgOg)
loserapidly
theiractivity
withouthydrogen 121.
Several suchnonstoichiometric effects of
hydrogen
in metalcatalyzed
reactions have beenrecognized;
they
were summarizedrecently /3,4/.
Their commoncharacteristic is that
hydrogen
actively participates
in the formation of thecatalytically
activeentity.
Inaddition,
hydrogen
present
during
catalytic
processes canconsiderably
influence
the actualcatalytic
properties.
The main
problem
in thesehydrogen
effects is the determination ofhow much
hydrogen
ispresent
onand/or
in thecatalyst
in variousstages
of itslife,
where it is situated and what is thelikely
form ofits
binding
to the metal. No accurate answer can begiven
to theseproblems
so far.Temperature-programmed
desorption
(TPD)
studiesdetected three to five
types
of adsorbedhydrogen
on Pt-black andPt/SiC^
/5-8/.
It is also debated what is thetemperature
at which all desorbablehydrogen
leaves thecatalyst.
The moststrongly
bondedtype
ofhydrogen
may be situated in subsurfacelayers
as demonstra-ted for Pt/9/
and Pd-blacks/107;
on Pt thishydrogen
may beexchanged
withhydrogen
atoms ofhydrocarbon
reactants/9/.
Maybe
thepenetration
ofhydrogen
into subsurfacelayers
is the reasonwhy
hydrogen
dramatically
accelerates the reconstruction of ahigh-surface
freshly
reduced Pt-black when heated inhydrogen atmosphere
between 470 and 750 K/ll/.
A"quasi-liquid hydrogen-platinum
surfaceover-layer"
has beenproposed
during sintering
process/12/.
When
hydrocarbons
are reacted in the presence ofhydrogen
onPt-catalysts,
it is more correct toregard
thecatalyst
as a "Pt-Hsystem".
Presintering
inhydrogen
will determine thecrystallite
size/ll/,
the surfacemorphology
ofPt,
this latterbeing
decisive inde-termining
the character of active sites as shownby
single-crystal
studies
/13/.
Presently
weattempt
atcomparing
twosamples,
presin-tered at 473 and 633 K. Earlier studies reveal that the formersample
has smallercrystallites /ll/,
less surface carbon andoxygen
impurity
/14/.
The chemical state of carboncorresponds
to individual C-atomson Pt-473 while a more
polymeric
carbon ispresent
on Pt-633/15/.
The formersample
contains morehydrogen
/16/.
Surfaceanalysis,
electron
microscopy
andcatalytic activity
in model reactions as abe
reported.
Experimental
The
catalysts
were reduced from aqueous solution of^PtClg
by
HCHO in the presence ofKOH,
between 273 and 278 K/17/.
They
were sintered
according
to a "standard thermalcycle"
as describedby
Baird et al.
/II/,
at 473 and 633 K(Pt-433
andPt-633,
respectively).
Secondary
Ion MassSpectrometry
(SIMS)
was carried out in a+ -5 -2
Balzers
instrument,
using
Arprimary
ions(3 keV,
1.2x10 A cm);
for more
detail,
see ref./16/.
Specimen preparation
for electronmicroscopy
was carried outby
dispersing
the metalpowder
samples
in water or methanol; afterultra-sonic treatment,
they
were dried on carbon coatedgrids
in air. A JEOL 200 CXmicroscope operated
at 200kV,
equipped
with anultra-high
resolutionobjective pole piece
was used.Catalytic
runs were carried out in a static-circulation reactor/18/.
n-Hexane(nH)
andmethylcyclopentane
(MCP)
were used as feeds at a pressure of 10 Torr(1
Torr = 0.133kPa).
Feedpurity
was betterthan 99.5%.
Hydrogen
pressure varied between 50 and 500 Torr.Cata-lytic
activities areexpressed
as "turnovernumbers",
meaning
the amount ofproduct formed,
molecules per surface Pt-atom per second.Results
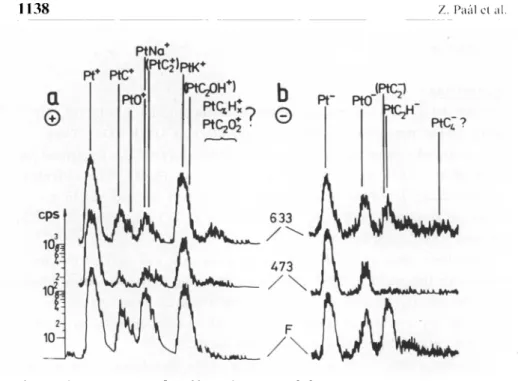
In
agreement
withprevious
data/14-16/,
sintering
at 473 K resulted in somecleaning
of the "as reduced" surfaces.Sintering
at633 K
gives
more surface clusterions,
presumably, owing
to thepoly-merization of surface carbon which is bound more
firmly
to the metal.This is illustrated
by
Fig.
1. It has to be remarked that the residualhydrogen
content of thecatalysts
decreased in the order Pt-fresh> > Pt-473 > Pt-633/16/.
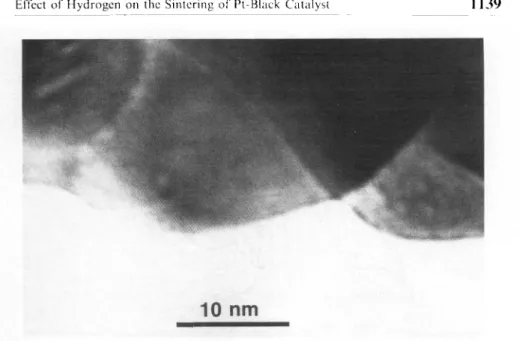
Electron
micrographs
show agradual
increase ofparticle
sizes(Figure
2)
as thetemperature
ofsintering
increases. These wereestimated to be 5-10 nm for
Pt-fresh,
20-30 nm for Pt-473 and around 100 nm for Pt-633. This latter value ishigher
than thatreported
earlier/ll,19/.
Thehigh-resolution
electronmicrograph
of Pt-473Figure
1.Secondary
Ion MassSpectra
of fresh and two sintered Pt-blacksamples
in the cluster ion range.Figure
2. Electronmicrographs
of Pt-blacksamples.
From left to10
nm
Figure
3.High
resolution electronmicrograph
of Pt-473141.
fringes
(Figure
3).
Pt-633 was nottransparent
for the electron beam;the rounded character of the
particles
can be seen inFig.
2.Various reactions of n-hexane and
methylcyclopentane require
surface intermediates of variousdegree
ofdehydrogenation.
The amount of surfacehydrogen
available controls the extent ofdehydro-genation
ofhydrocarbons
bound to the active sitesand,
by
doing
so,determines which reaction will
prevail
/3,4/.
That is the reasonwhy
maxima are observed in the
yields
of individualproduct
classes/18,20,21/.
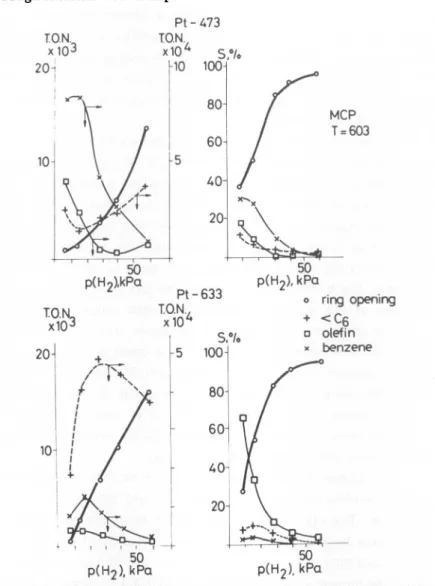
These maxima are not well-defined with Pt-473(Figure
4)
and appearclearly
with Pt-633(Figure
5).
In the former case, theyields
ofisomers,
fragments
increasemonotonically
withp(H^)
whereasminima are seen in MCP and benzene
yields.
No olefin is detected onPt-633 while its amount decreases with
p(B^)
over Pt-473. The overallactivity
ofsamples
shows drastic differences: Pt-473 is more activeby
about an order of
magnitude.
Thecomparison
of selectivities reveal thatfragmentation
is mostimportant
over Pt-633 whereas Pt-473 is abetter
catalyst
fornondegradative
reactions,
first ofall,
isomerization.Pt-473
50
P(H2),
kPaFigure
4.Activity
(in
terms of turnovernumber,
T.O.N.)
andselec-tivity,
S%
of Pt-473 in n-hexane transformations , as a function ofhyd-18 rogen pressure,
p(H2).
T = 603K,
0.1 gcatalyst,
4.9x10 surface Pt-atom(Pt ).
sp(H2).
kPup(H2),kPcT
Figure
5.Activity
andselectivity
of Pt-633 in n-hexanetransforma-18
tions. T = 603
K,
catalyst:
0.1 g;Ptg:
2.25x10 atom. Data weredecreases at
higher
pf^I^).
Again,
a minimum is seen in the latter value onPt-473;
the absolute values are alsohigher
on thatcatalyst.
No differences in overall
activity
were observed in MCP reactions(Figure
6).
Ring opening
reactionprevailed
over bothPt-samples,
enormously
increasing
withpiH^).
At lowp(H^),
olefins were also ofimportance
over Pt-633 and
-like with n-hexane
-benzene over
Pt-473.
Fragmentation
wasunimportant
in both cases.Pt-
473
p(H2),
kPap(H2).
kPa
Figure
6.Activity
andselectivity
of
Pt-473 and Pt-633 inmethylcyclo-pentane
transformations.
T = 603 K.Discussion
Surface
geometry, hydrogen availability
and the eventual presenceof
impurities
determine the actualcatalytic
properties
of Pt.Single
crystals
withhexagonal
symmetry
-(111), (322)
and(10,8,7)
faces-were more active in nH aromatization than those with
tetragonal
-(100)
-symmetry
/13/.
Five-coordinatedB,.
sites were claimed to be active inhydrogenolysis
/23/.
Such structures alsorepresent
peculiar
hydrogen adsorption
sites/247.
No suchstructure-sensitivity
wasfound for MCP reactions
/23/.
Small ensembles of 1-3 Pt-atoms werethe active sites of aromatization
/25/.
Hydrogen participates
in theac-tive ensemble of
Cj--cyclic
reactions/28/;
its intermediates may desorbas MCP or may
give
isohexanes withoutdesorption
/26/.
Morehydrogen
favours the latter process
/4/.
Our
samples
show a roundedmorphology
(Figures
2 and3)
notexposing
flat low indexcrystal
faces/20/.
Wang
et al.suggested
/27/
that such roundedcrystal shapes
indisperse
systems
may ariseowing
to their contaminated surfaces. This is inagreement
with surfaceana-lysis
datareported
inFigure
1 and also with earlier studies/14-16,19/.
Sintering
inhydrogen
creates, thus,disperse
Pt-catalysts
with ill—-defined
crystal
plane shapes
and with varioushydrogen
content.Changing
thehydrogen
pressure in the gasphase
brings
about various response of thecatalytic activity
on the twosamples
studied.Our
catalytic
data confirmed the lowerstructure-sensitivity
of MCP reactions. The dramatic increase ofring opening yields
athigher
p(H^)
is in
agreement
with oursuggestions
/18.20/
that this reactionrequires
two
neighbouring
metal atoms which also holdhydrogen
during
the reaction. Such an ensemble can be found on anycrystal
plane
and-
judging
from these results
-the amount of eventual carbon on the
surface does not affect these sites at all.
The
higher
activity
of
Pt-473 related to unit surface indicatesthat its surface atoms are more
exposed
and moreeasily
available for reactions. Thehigher
isomerization/MCP
formationselectivity
overPt-473 may be due to the
higher
hydrogen
content of thiscatalyst
/16/.
Isomer and MCPyields
areparallel
over Pt-633 butdistinctly
moreisomer is formed on Pt-473 at
higher
p(H2>
(cf.
Figs.
4 and5),
thatThis can be attributed to
hydrogen
retained and thefacility
of its interaction with thehydrocarbon
reactant.Both benzene and
fragments
require
deeply dehydrogenated
surfaceintermediates.
Obviously,
aromatization is favoured over Pt-473. Withincreasing
p(Hg),
thecompetition
betweenhydrogen
andhydrocarbons
first decreases aromatization
activity;
thereafter, however,
astrong
increase is seen
(Fig.
5).
Hydrogen
must either be able to remove in-dividual carbonaceousspecies
blocking
active sites or there must be apossibility
that under these conditions "H-Pt-C" sites are created whichare favourable for nondestructive reactions
/28/.
Ptsingle
crystal
faces with rather
large
carbon coverages have also been found to beactive,
thehydrogen
attached to Cbeing
able to interact with hydro-carbon reactants/29/.
A more
prominent competition
betweenhydrogen
andhydrocarbons
is observed over Pt-633. Here the
partly polymerized
carbonaceousoverlayer
leaves free a fraction of the surfaceonly
forcatalysis.
Ofall
reactions,
fragmentation
seems to be least affected: itsselecti-vity
remainshigh,
like in the case ofpartly
cokedPt/Si02
catalyst
/21/
andPt(lll)
single
crystal
face/29/.
The presence of extraB,.--sites is not confirmed
by
our results.Acknowledgement
The authors thank Dr. D. Marton for the SIMS measurements. The
financial
support
wassupplied
by
Grant OTKA 1309.References
1. Bond
G.C.,
Catalysis
by
Metals,
NewYork,
Acad.Press,
1962. 2. PinesH.,
TheChemistry
ofCatalytic
Hydrocarbon
Conversions,
New
York,
Acad.Press,
1981.3. Paal Z. and Menon
P.G.,
Catal. Rev.-Sci.Eng.
25,
229(1983).
4. Paal Z. and Menon P.G.
(Editors),
Hydrogen
Effect inCatalysis,
New
York,
MarcelDekker,
1988.5.
Tsuchiya
S.,
Amenomiya
A. and CvetanovicR.,
J. Catal.19,
245(1970).
6.
Moger
D.,
Besenyei
G. andNagy
F.,
Magy.
Kern.Folyoirat
81,
284(1975).
7.
Popova
N.M.,
Babenkova L.V. andSavelyeva
G.A.,
Adsorption
and Interaction of the
Simplest
Gases withGroup
VIII Metals(in
Russian) Alma-Ata,
Nauka Kaz.SSR,
1979.8. Frennet A. and Wells
P.B.,
Appl.
Catal.18,
243(1985).
9. Paal Z. and Thomson
S.J.,
J. Catal.30,
96(1973).
10.
Eley
D.D. and PearsonJ.F.,
J. Chem. Soc.Faraday
TransI,
74,
223(1978).
11.
BairdT.,
Paal Z. and ThomsonS.J.,
J. Chem. Soc.Faraday
Trans.
69,
50(1973); 69,
1237(1973).
12.
Manninger
I.,
Paal Z. andTetenyi
P.,
Z.phys.
Chem. NeueFolge,
132,
193(1982).
13. Davis
S.M.,
Zaera F. andSomorjai
G.A.,
J. Catal.85,
206(1985);
ZaeraF.,
Godbey
D. andSomorjai
G.A.,
J. Catal.101,
73(1986).
14. PaalZ.,
Tetenyi
P.,Prigge
D.,
Wang
X.Zh. and Ertl G.,Appl.
Surface Sci.
14,
307(1982-83).
15. Paal Z.,
Tetenyi
P.,
KerteszL.,
Szasz A. andKojnok
J.,
Appl.
Surface Sci.
14,
101(1982-83).
16. Paal Z. and Marton
D.,
Appl.
Surface Sci.26,
161(1986).
17.
Tetenyi
P. and BabernicsL.,
Acta Chim. Acad. Sci.Hung.
35,
419(1963).
18. Zimmer H., Paal Z. and
Tetenyi
P., Acta Chim. Acad. Sci.Hung.
Ill,
513(1982).
19. Barna
A.,
BarnaP.B.,
Toth L., Paal Z. andTetenyi
P.,
Appl.
Surface Sci.
14,
85(1982-83).
20. Zimmer
H.,
Dobrovolszky
M.,
Tetenyi
P. and PaalZ.,
J.Phys.
Chem.90,
4758(1986).
21. Paal
Z.,
Zimmer H. andTetenyi
P.,
J. Mol. Catal.25,
99(1984).
22. Paal
Z.,
Groeneweg
H. and ZimmerH.,
Catalysis Today,
in press.23. Dauscher
A.,
Garin F. and Maire G. , J. Catal.105,
233(1987).
24. Baro A.M. and Ibach
H.,
Surf. Sci.92,
237(1980).
25. Biloen
P.,
HelleJ.N.,
VerbeekH.,
Dautzenberg
F.M. andSachtler
W.M.H.,
J. Catal.63,
112(1980).
26. GaultF.G.,
Adv. Catal.30,
1(1980).
27.
Wang
T.,
Lee C. and SchmidtL.D., Surf.
Sci.163,
181(1985).
28.