Conservation and divergence in the transcriptional
programs of the human and mouse immune systems
The MIT Faculty has made this article openly available.
Please share
how this access benefits you. Your story matters.
Citation
Shay, T., V. Jojic, O. Zuk, K. Rothamel, D. Puyraimond-Zemmour,
T. Feng, E. Wakamatsu, C. Benoist, D. Koller, and A. Regev.
“Conservation and divergence in the transcriptional programs of the
human and mouse immune systems.” Proceedings of the National
Academy of Sciences 110, no. 8 (February 19, 2013): 2946-2951.
As Published
http://dx.doi.org/10.1073/pnas.1222738110
Publisher
National Academy of Sciences (U.S.)
Version
Final published version
Citable link
http://hdl.handle.net/1721.1/80383
Terms of Use
Article is made available in accordance with the publisher's
policy and may be subject to US copyright law. Please refer to the
publisher's site for terms of use.
Conservation and divergence in the transcriptional
programs of the human and mouse immune systems
Tal Shaya,1, Vladimir Jojicb,1, Or Zuka, Katherine Rothamelc, David Puyraimond-Zemmourc, Ting Fengc, Ei Wakamatsuc, Christophe Benoistc,2, Daphne Kollerb, Aviv Regeva,d, and the ImmGen Consortium3
aBroad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, MA 02142;bDepartment of Computer Science, Stanford University, Stanford, CA 94305;cDivision of Immunology, Department of Microbiology and Immunobiology, Harvard Medical School, Boston, MA 02115; anddHoward Hughes Medical Institute, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02142
Contributed by Christophe Benoist, January 3, 2013 (sent for review November 14, 2012)
Much of the knowledge about cell differentiation and function in the immune system has come from studies in mice, but the relevance to human immunology, diseases, and therapy has been challenged, perhaps more from anecdotal than comprehensive evidence. To this end, we compare two large compendia of transcriptional profiles of human and mouse immune cell types. Global transcription profiles are conserved between correspond-ing cell lineages. The expression patterns of most orthologous genes are conserved, particularly for lineage-specific genes. How-ever, several hundred genes show clearly divergent expression across the examined cell lineages, and among them, 169 genes did so even with highly stringent criteria. Finally, regulatory mecha-nisms—reflected by regulators’ differential expression or enriched cis-elements—are conserved between the species but to a lower degree, suggesting that distinct regulation may underlie some of the conserved transcriptional responses.
comparative transcriptomics
|
evolution|
gene expression|
microarrayT
he immune system is extensively studied in both human and mouse. Studies of human immune cells are restricted largely to in vitro or ex vivo assays, whereas studies in mice allow ma-nipulation and monitoring of the immune system in an organis-mal setting. Although the mouse is an invaluable model for studies of immune function, there are substantial differences between the two species because of both biological and experi-mental factors, and studies have repeatedly suggested caution when translatingfindings from mouse to human (1, 2).Charting the similarities and differences between immune cell lineages of human and mouse can provide a reference map that will help translate mousefindings to human and determine when (and why) the mouse immune response is likely to diverge from the human immune response. On the one hand, many ortholo-gous transcription factors play conserved roles in both human and mouse immune system differentiation. On the other hand, studies reported prevalent variation between human and mouse (3–5), but it is hard to distinguish real differences from differ-ences caused by the conditions compared, representation of cell populations per se, and their ability to interact and communicate with each other. Genome-wide profiling of mRNA levels in each lineage opens the way for such a comprehensive comparison.
Here, we compare two compendia of transcriptional profiles collected from human and mouse cell lineages during immune system differentiation. As expected, we show that the tional program is extensively conserved from global transcrip-tional profiles to lineage-specific gene expression to the underlying regulatory mechanisms. Extensive conservation is also observed even when comparing activated immune T cells. We highlight genes with different expression patterns in human and mouse that were not previously reported and validate a few of them experimentally. We provide our data and analysis in a web portal that will serve as a reference map for future immunology studies.
Results
Transcriptional Maps of the Human and Mouse Immune Systems.We compared two compendia of the human and mouse mRNA ex-pression profiles measured using microarrays from immune cells at different differentiation states. The human D-MAP compen-dium (6) consists of 38 cell types (Fig. S1A). The mouse ImmGen compendium (7) consists of 244 cell types (Fig. S1B). Both span a wide range of myeloid and lymphoid cells of the innate and adaptive immune systems. Using the Ensembl COMPARA da-tabase, we mapped 10,248 one-to-one orthologs between the two species that were measured in both studies. Although many of the major cell lineages are common to both compendia (Fig. 1A), there are several important distinctions in the design of the two studies in the measured cell types, which were morefinely parsed in the mouse data, and the tissue source (only blood for human; all lymphoid organs for mouse) (Dataset S1, Note 1). In addition, different samples of the same cell type in human were typically from unrelated healthy donors, whereas mouse data were almost exclusively derived from inbred C57BL/6 (B6) mice raised in a barrier facility.
Because the differences between the compendia may con-found subsequent comparisons, wefirst identified matching cell types in the two studies. We reasoned that broad comparisons are likely to be more robust, because individual cell types are harder to map between the species, and there are many more cell types in the mouse compendium. We, therefore, focused on comparing at the level of seven broad cell groups: hematopoietic stem and progenitor cells (HSPCs), granulocytes (GNs), mono-cytes (MOs), dendritic cells (DCs), B cells, natural killer cells (NKs), and T cells (Fig. 1A, filled rectangles). Using other groupings, such as separating CD8 and CD4 naïve and memory T cells, adding progenitors, or combining all myeloid cells into one group, did not qualitatively change the results. Within each group, we selected the specific cell types to include based on known biology (Dataset S2). For example, we excluded one human mature NK cell population (CD56− CD16+ CD3− cells), which is more similar to human monocytes than to NK cells, and mouse plasma cells, whose equivalent is missing from the human compendium. Many of the relevant cell surface markers are similarly expressed in the corresponding groups in both species, confirming their comparability (Fig. S2A). Overall, we compared seven groups of cell types, consisting of 80 human and 137 mouse samples.
Author contributions: T.S., V.J., C.B., D.K., and A.R. conceived the study; T.S. and D.P.-Z. analyzed the data; V.J. and O.Z. developed Ontogenet and motif-related methods, re-spectively; T.F. and E.W. generated the T-cell activation data; K.R. performedflow cytom-etry and sorted human spleen samples; and T.S., C.B., and A.R. wrote the paper. The authors declare no conflict of interest.
1T.S. and V.J. contributed equally to this work.
2To whom correspondence should be addressed. E-mail: cb@hms.harvard.edu. 3A complete list of the ImmGen Consortium can be found inSI Materials and Methods.
This article contains supporting information online atwww.pnas.org/lookup/suppl/doi:10. 1073/pnas.1222738110/-/DCSupplemental.
Global Expression Profiles and Lineage-Specific Signatures Are Conserved.The global expression profiles of matching lineages
are similar between the two species for both individual cell types and average group profiles (Fig. 1B, dotted rectangles; andFig. S2B, thick black rectangles). The correlation between a pair of mouse/human profiles from the same lineage group (mean cor-relation = 0.2) is lower than the correlation between two cell types of the same lineage within a species (mean correlation in human= 0.51, mouse = 0.58) or between profiles of the exact same cell type in different human donors (mean correlation= 0.58) or different inbred mouse strains (mean correlation = 0.79). Finally, the correlation between matching immune cell
groups is comparable with the correlation between two matching solid tissues (8) (Fig. S2C).
Similarly, signatures of lineage-specific gene expression are conserved between the species. For each lineage in each species, we defined two signatures (induced and repressed) as the set of genes with significantly high or low expression, respectively (Bonferroni-corrected t test < 0.05), in this lineage compared with the other lineages. For all lineages, there was a significant overlap in corresponding gene signatures between species (Fig. 1C and Dataset S2). Overall, of 5,588 orthologous gene pairs that belong to a signature in at least one species, 22% (1,227 orthologs) also belong to the corresponding signature in the other species (i.e., induced or repressed in the same lineage). Because the signatures were defined strictly, the 22% estimation is most likely an underestimate of the level of conservation.
Majority of Genes Have Conserved Expression.More broadly, there is a significant similarity in the expression of individual pairs of orthologous genes between the species. To test this similarity, we first defined, for each gene in each species, an immune expres-sion profile based on its median expresexpres-sion in each of the seven groups. We then calculated a conservation of expression (COE) measure as the correlation between the immune expression profiles of each pair of one-to-one human and mouse orthologs in our set (Fig. 2A). COE values are significantly higher com-pared with a null distribution [P< 10−10, Kolmogorov–Smirnov (KS) test against a permutation of ortholog assignments] (Fig. 2B). A similar COE distribution is obtained when using a tissue atlas of human and mouse gene expression (8) (Fig. 2B).
We next used the COE distributions to estimate the pro-portion of conserved genes. We assume that the observed COE distribution is a mixture of two distributions: one reflecting the conserved genes and the other reflecting the nonconserved genes. The COE distribution of nonconserved genes should be similar to the ImmGen-DMAP permutation distribution (Fig. 2B, black), whereas the COE distribution of conserved genes should be concentrated in the positive correlation region of the overall observed COE distribution. Thus, the observed total distribution of COE values for all genes, denoted Fall-genes, is
obtained as a mixture of the COE distribution of nonconserved genes F0(estimated by the permutation-based distribution) and
the (unknown) COE distribution of conserved genes Fconserved:
proB Naive CD4 T Naive CD8 T HSPC CMP GMP MEP GN MO DCs B NKMemory CD4 TMemory CD8 T
A
C
Human Mouse NK B DC HSPC GN MO T NK B DC HSPC GN MO T HSPC GN MO DC B NK Naive Memory Naive Memory HSPC GN MO DC B NK Naive Memory Naive MemoryB
NK B DC T4 HSPC GN T8 MO NK B DC T4 HSPC GN T8 MO −1 0 1 Human Mouse Human Mouse −2 0 2 T4 T8 T4 T8Fig. 1. Conserved transcriptional profiles in matched immune lineages be-tween human and mouse. (A) Simplified lineage tree for the immune system cell populations measured in both human and mouse compendia. CMP, common myeloid progenitor; GMP, granulocyte–monocyte progenitor; MEP, megakaryocyte–erythroid progenitor. Filled rectangles, cell types included in the common lineages. Colors mark those lineages in allfigures. (B) Global correlation matrix of the Pearson coefficients (yellow/purple color scale on the bottom) between every pair of human and mouse samples in the com-mon lineages. Samples are in rows and columns sorted by the lineage tree. Red lines separate species, black lines separate lineages, thick black rec-tangles mark correlations within each lineage within a species, and dotted rectangles mark correlations between samples of the same lineage between species. (C) Mean-centered expression values (red/blue color scale on the right) of the genes shared between matching lineage signatures in human (Left) and mouse (Right). Signatures were defined in each species separately as composed of genes that are expressed in a lineage-specific pattern in that species. Every pair of matching signatures was compared between the spe-cies to identify all those pairs of orthologs that were members of both sig-natures. Genes are sorted by human lineage with maximal expression level.
A
1 ImmGen–DMAP ImmGen–DMAP permutation Tissue atlas GATA3 COE=0.94 Human Mouse HSPC GN MO DC B NK T TMEM176A COE=0.22 Human Mouse HSPC GN MO DC B NK T ImmGen–DMAP induced signatures membersB
–1 0 0.05 0 Genes fraction 0.15 0.1 0.25 0.2 Human–mouse correlation −2 0 2Fig. 2. COE between human–mouse orthologs. (A) COE is calculated for a pair of human–mouse orthologs as the Pearson correlation coefficient between their median expression profiles (color scale on the bottom) in each lineage. Shown are examples for (Left) highly conserved orthologs and (Right) lowly conserved orthologs. (B) Significantly high COE values in hu-man and mouse. Shown is a distribution of huhu-man and mouse COE values (blue), only lineage signature genes (light blue), a tissue atlas (8) (red), and a null distribution created by random pairings of one-to-one orthologs (black).
Shay et al. PNAS | February 19, 2013 | vol. 110 | no. 8 | 2947
IMMUNOLO
Fall-genes= α Fconserved+ (1 − α) F0, where the mixture parameter
α is the fraction of genes with conserved COE.
We next sought to estimate the mixture parameterα repre-senting the fraction of genes with conserved COE. Wefirst used a nonparametric method applicable for any distribution. Because Fall-genes(x)≥ (1 − α) F0(x) for any x, we can estimate the
max-imal possible fraction of the nonconserved genes (1 − α) by calculating the ratio between the ImmGen-DMAP and Imm-Gen-DMAP permutation distributions: Fall-genes(x)/F0(x) for any
value of x (Fig. 2B, black and dark blue curves, respectively). To avoid errors resulting from inaccurate estimates of Fall-genes(x)
and F0(x) from data that may skew our estimator for 1− α , we
took the minimal ratio Fall-genes(x)/F0(x) for values of x on the
negative region (x < 0) and excluded the left tail (x > −0.8), resulting in a minimal ratio of 1− α = 0.3 (Fig. 2B, black arrows) and indicating that at leastα = 1 − 0.3 = 70% of the genes have conserved COE scores. As an alternative strategy, we fit the COE data with a mixture of Gaussians model (Fig. S2D), where the nonconserved genes Gaussian mean and SD are taken from the permuted data. In the resulting model, the conserved genes Gaussian (black) containedα = 51% of the genes (Fig. S2D). Thus, both the parametric and nonparametric estimators for the fraction of conserved genes indicated that the majority of genes (51–70%) had conserved COE score.
Genes with high COE (Fig. S2E) share several transcriptional characteristics, including higher maximal expression (P = 2.7 × 10−9and 2.9× 10−24, human and mouse, respectively) (Dataset S1, Note 2 discusses alternative explanations of this phenomenon and an analysis of their likelihood), membership in lineage-specific induced signatures (KS test, P = 5.6 × 10−20) (Fig. 2B),
and a presence of TATA box in their promoter (t test P= 0.04). The latterfinding is in contrast to previous reports in yeast and other species of enhanced expression divergence of TATA-containing genes (9).
Known and Previously Undescribed Differences in Individual Genes.
On the backdrop of the global conservation pattern, there are differences in the expression of certain genes, some consistent with previous reports and others identified in this study. First, we found several differences already noted previously (Fig. 3A,Fig. S3A, andDataset S3), including in THY1, KLRK1, FLT3, CD38, CD2 (reviewed in ref. 10), NCAM1 (CD56) (11), and CD8A (12). Importantly, most of the human–mouse differences are also observed when using data from human monocytes and B, NK, and T cells isolated from spleen (Fig. 3A, Center andFig. S3A), ruling out the possibility that the differences are merely caused by different tissue sources in the human and mouse compendia. The few previously reported differences that we did not observe in our analysis are typically in cells or expression levels that we do not expect to detect in our data (Dataset S3).
Next, we identified cell surface markers that display different expression patterns and systematically identified another 169 orthologous genes with expression that differs between human and mouse and were not previously reported (using stringent criteria of COE< 0.25 and at least a fourfold change between the lineage means in each species). These genes include IL15, ETS2, TMEM176A, TMEM176B, SNN, DPP4, NT5E, and CD27 (Fig. 3B,Fig. S3B, andDataset S3). For example, IL15, a trophic cytokine for NK cells and memory T cells (13), is expressed in human in granulocytes, monocytes, and B cells, but in mouse, it is expressed in HSPC and some myeloid cells only. Some dif-ferences do not agree with previousfindings (e.g., TLR1) (14) or may result from distinct immune activation states (e.g., JUN). Importantly, the differences are also detected comparing with cells isolated from human spleen (Fig. 3B, Center andFig. S3B). We confirmed by flow cytometry a few of the differences in cell surface markers (Fig. 3C), staining human blood cells and mouse splenocytes in parallel. CD26 (DPP4) was, indeed, expressed in
*SNN CMAH CPM GIMAP6 SDC4 *TLR1 PEA15 ATP1B1 LGALS1 PADI2 GIMAP4 S100A10 SEPT11 ST3GAL6 MR1 CD4 PDLIM1 MGST2 FAM46C TCF12 PVR RCBTB1 PPM1H DUSP10 PRKCA F2RL1 Human (DMAP) ASNS IFT57 ADK SGALNACT. PRRG4 CA2 SYNE2 TPD52 TMEM156 *IL15 NT5E C9ORF95 BNIP3 RNF144A PRR5L ALOX5AP ISG20 BMPR1A PBXIP1 SORL1 SYNE1 *ETS2 GGH *TMEM176B SLC16A10 NUCB2 HPGD *TMEM176A CLIC4 *JUN ITM2C CD8A CYP27A1 PLXDC1 CDK14 CDC14A VRK2 NR4A3 GPR126 Human spleen
C
A
T4 B CD26 (DPP4) Human Mouse T4 B NK T4Human (DMAP) Human spleen
CD27
Human Mouse HumanCD73 (NT5E)Mouse
−2 0 2 *Snn Cmah Cpm Gimap6 Sdc4 *Tlr1 Pea15a Atp1b1 Lgals1 Padi2 Gimap4 S100a10 Sept11 St3gal6 Mr1 Cd4 Pdlim1 Mgst2 Fam46c Tcf12 Pvr Rcbtb1 Ppm1h Dusp10 Prkca F2rl1 Csgalnact1 *Tmem176b *Tmem176a Asns Ift57 Adk Prrg4 Car2 Syne2 Tpd52 Tmem156 *Il15 Nt5e Bc016495 Bnip3 Rnf144a Prr5l Alox5ap Isg20 Bmpr1a Pbxip1 Sorl1 Syne1 *Ets2 Ggh Slc16a10 Nucb2 Hpgd Clic4 *Jun Itm2c Cd8a Cyp27a1 Plxdc1 Pftk1 Cdc14a Vrk2 Nr4a3 Gpr126 Mouse
B
THY1 KLRK1 FLT3 CD38 CD2 NCAM1 CD8A DPP4 NT5E CD27 Mouse NK B DC H GNMO T MO B NK T H GNMODC B NK T Thy1 Klrk1 Flt3 Cd38 Cd2 Ncam1 Cd8a Dpp4 Nt5e Cd27 NK B DC H GNMO T MO B NK T H GNMODC B NK TFig. 3. Differentially expressed genes between human and mouse. (A) Ex-pression profiles (color scale on the bottom) in (Left) the human blood cell compendium, (Center) human splenocytes, and (Right) mouse of genes that were previously reported to be differentially expressed between human and mouse and are consistently different in our datasets or different in our datasets and validated below. (B) Selected genes with different expression patterns between human (Left) blood, (Center) spleen, and (Right) mouse immune cell types that were not previously reported. Shown are mean-centered expression values of genes sorted by mouse lineage with maximal expression level. Genes discussed in the text are marked with bold and asterisks. (C) Flow cytometry analysis of predicted human/mouse differences. Several cell surface markers were chosen (based on reagent availability) among differentially expressed genes and analyzed by staining of human peripheral blood mononuclear cells (PBMCs) or B6 splenocytes. Profiles in Upper and Lower depict cell populations for which expression was predicted to be shared or divergent in both species, respectively.
mouse but not human B cells; the opposite held for CD73 (NT5E), although human B cells include a CD27lo subset that may match with the mouse; CD27 was high in mouse but not human NK cells.
Contribution of Gene Duplication to Transcriptional Divergence.The comparison above focused on one-to-one orthologs (a major portion of the genes in the human and mouse genomes), but gene duplication, loss, and appearance events were previously shown to play a major role in divergence between species in other systems (15). To explore the contribution of gene dupli-cation to divergence here, we considered 66 cases where one ortholog in one species has many paralogs in the other. There are 16 conserved cases, in which expression of all paralogs is conserved with the expression of their ortholog (COE > 0.5) (Dataset S4). For example, the human gene FTL and its two mouse orthologs Ftl1 and Ftl2 are all expressed in GNs, MOs, and DCs (Fig. S4). Another 17 cases show potential evidence for neofunctionalization, with at least one of the paralogs having COE> 0.5 and another paralog having COE < 0.5 (Dataset S4). For example, human OAS1 and its mouse orthologs Oas1a and Oas1g are expressed in myeloid cells (GNs, MOs, and DCs), whereas two other mouse orthologs, Oas1b and Oas1c, are also expressed in T cells. In another example, human SCD is induced in HSPC along with one of its mouse orthologs, Scd2. The other mouse ortholog, Scd1, is induced in B cells, suggesting neofunc-tionalization (Fig. S4). Finally, in 33 groups, all paralogs have diverged in expression compared with their one ortholog (COE≤ 0.5) (Dataset S4): in some cases, because of subfunctionalization and in others cases, because of neofunctionalization of all paral-ogs. One example of subfunctionalization is human myeloid cell nuclear differentiation antigen (MNDA; expressed in all myeloid cells) and its mouse orthologs, Ifi204 and Ifi205 (each induced in different myeloid cell subtypes) (Fig. S4).
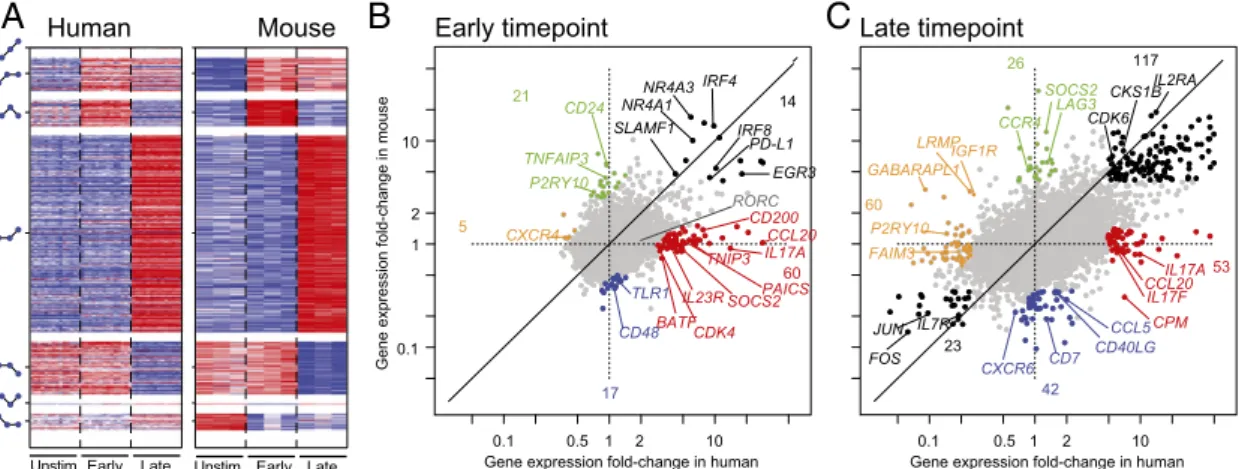
Conservation and Divergence in T-Cell Activation. The two com-pendia analyzed above primarily profiled resting cell states, and it was of interest to ask whether the balance of substantial con-servation and limited divergence would shift during cell activa-tion, especially because immune responses are under strong evolutionary selection. To test this hypothesis, we compared
expression profiles generated under parallel conditions at early (1–4 h) and late (20–48 h) times of activation of purified CD4+ T cells by anti-CD3/CD28. We found that the human and mouse responses are substantially conserved by several measures to an extent comparable with or higher than the conservation observed for the resting cell populations in the main compendia. COE measures calculated based on the three time points are signifi-cantly higher than observed for random permutations (KS test, P< 10−300) (Fig. S5A) and the COE levels calculated based on any three lineages chosen from the seven lineages analyzed above (KS test, P < 2.7 × 10−71) (Fig. S5A). Using our non-parameteric approach, we estimate that at least 64% of genes have conserved COE in this response. Finally, there is a sub-stantial overlap between the response patterns in the two species when partitioning the differentially expressed genes into distinct patterns in one species and examining their expression in the other species (Fig. 4A andFig. S5B).
A close examination of the response patterns showed that some segments of the responses were quantitatively or qualitatively different between the two species (Fig. 4 B and C andDataset S3). Strikingly, these segments include much of the canonical Th17 differentiation signature (IL17A,F, IL23R, RORC, BATF, and CCL20) because of either an inherently higher respon-siveness of the Th17 module in human or perhaps, a higher proportion of quickly reactive memory T cells in the human cells tested. Conversely, activation of CD24 or Lag3 seemed unique to mouse cells under these conditions. Some of the expression changes likely reflect functional differences: the NF-κB inhibitor TNIP3 is induced in human but not mouse, consistent with the fact that the mouse ortholog is nonfunctional because of lack of an essential homology domain (16). Most differences reflect lack of activation in one species, whereas in a few others, genes are either already fully expressed in the prestimulated state in mouse (e.g., CDK4 and PAICS) (Fig. 4B, red) or induced earlier in hu-man than in mouse (e.g., SOCS2) (Fig. 4 B, red and C, green). Some respond in opposite ways in each species (e.g., LRMP, IGF1R, and GABARAPL1) (Fig. 4C, orange).
Comparison of Regulatory Mechanisms in Immune Cells. We next asked how conservation and/or changes in regulatory mecha-nisms relate to expression patterns between human and mouse.
A
Human MouseUnstim. Early Late Unstim. Early Late
C
CKS1B CKS1B CDK6 Late timepoint 53 CCL20IL17A IL17F CPM 60 P2RY10 26 LAG3 CCR4 42 CD7 CCL5 CXCR6 CD40LG 117 IL2RA 23 FOS IL7R JUN 0.1 0.5 1 2 10 Gene expression fold-change in humanSOCS2 FAIM3 GABARAPL1LRMPIGF1R
B
0.1 0.5 1 2 10 0.1 1 2 10 Early timepointGene expression fold-change in human
Gene expression fold-change in mouse
60 IL17ACCL20 BATF TNIP3 IL23R 5 CXCR4 21 CD24 TNFAIP3 P2RY10 17 CD48 TLR1 14 EGR3 IRF4 NR4A1 IRF8 NR4A3 RORC PD-L1 SLAMF1 SOCS2 CD200 CDK4 PAICS
Fig. 4. Transcriptional conservation in human- and mouse-activated CD4+ T cells. (A) Conservation of activation patterns. (Right) The significantly differ-entially expressed mouse genes were sorted by their expression patterns (schematically showing change over time on the left of the heat maps) and (Left) the expression of their orthologous genes in human-activated T cells. (B and C) Comparison of differential expression. Shown are scatter plots comparing the change in expression in (B) early and (C) later time points relative to prestimulation in human (x axis) and mouse (y axis) CD4+ T cells. The global off-diagonal skew reflects the slightly weaker activation of mouse CD4+ T cells under these conditions. The black points denote transcripts that are induced in both species or repressed in both species. The colored points denote transcripts that are affected in only one species (red, unchanged in mouse and induced in human; orange, unchanged in mouse and repressed in human; green, induced in mouse and unchanged in human; blue, repressed in mouse and unchanged in human) (Dataset S3).
Shay et al. PNAS | February 19, 2013 | vol. 110 | no. 8 | 2949
IMMUNOLO
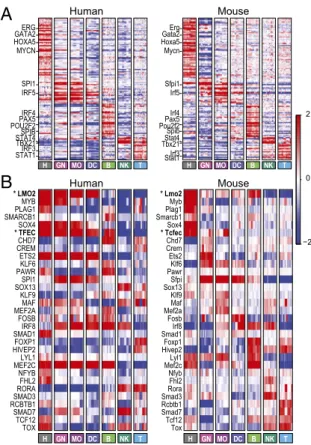
As expected, there is a significant overlap in the cis-regulatory elements enriched in the set of all lineage-specific genes in the two species (187 motifs, hypergeometric P< 10−10) (Dataset S2) and the motifs enriched in a pair of matching human and mouse signatures (Dataset S2). This overlap suggests that similar reg-ulatory elements (and cognate transcription factors) are often used to control immune system differentiation and immune cells in both species. Furthermore, the expression patterns of genes encoding known transcriptional regulators, including known master regulators, were partially conserved between mouse and human, parallel to the genome-wide conservation of expression. The 353 regulators that are expressed in each species in at least one lineage have significantly higher COE values than the COE values of the rest of the one-to-one filtered orthologs set (KS test, P = 5.27 × 10−12); 131 of them (37%) are maximally expressed in the same lineage in both species (Fig. 5A) (e.g., GATA2 and HOXA5 in HSPCs and PAX5 and SPIB in B cells). However, some of the annotated regulators expressed in both species show divergent expression patterns (Fig. 5B), and other regulators are expressed only in human lineages and not others. Intriguing examples of divergent expression include transcription factor EC (TFEC) [human: HSPCs, myeloid cells, and B cells; mouse (Tcfec): HSPCs and some myeloid cells but not B cells] and LIM domain only 2 (LMO2) (human: HSPCs and myeloid cells; mouse: HSPCs and B cells). Furthermore, 16 regulators are
expressed in human but not mouse common samples (3 regu-lators not expressed in any mouse sample) (Dataset S3). For example, MYBL1, not mentioned previously in immune context, is expressed in human NK and T cells but not expressed in mouse common lineages. LDB2, not mentioned previously in immune context, is expressed in human HSPCs and NK cells but not expressed in mouse common lineage. Similarly, there are 76 regulators expressed in mouse but not human common lineages (37 regulators not expressed in any human sample) (Dataset S3). For example, Nfix is expressed in mouse HSPCs and monocytes but not the human common lineages, and Prdm1 is expressed in mouse monocytes and NK cells but not the common human lineages. These differences must be interpreted with caution, because some may reflect technical probeset issues and not real biological differences.
Finally, we identified nine instances of putative divergent regulation based on cis-regulatory elements enriched in one species’ signature but not its counterpart signature in the other species (Dataset S3), and no corresponding change in expression pattern was found. Among those instances are ZFP161 (only enriched in the mouse NK signature), PAX3 (only enriched in human T-cell signature), and HIF1 (only enriched in mouse re-pressed HSPC signature).
Discussion
The laboratory mouse has been an important model from which many current paradigms of immunology are derived. Despite the many features conserved between the human and mouse sys-tems, there are important known differences between them (10). Systematically determining these differences is critical for translating findings from mouse immunology to the human setting. Here, we have begun the development of such a refer-ence map by comparing two extensive transcriptional compendia (6, 7).
Wefind an extensive conservation of the transcriptional pro-gram at several levels—global profiles, individual genes, and lineage-specific gene signatures. Gene and signature conserva-tion is often reflected by concomitant conservaconserva-tion of the asso-ciated regulatory mechanisms with conservation of most regulators and cis-regulatory elements. Nevertheless, in many cases, lineage-specific genes are associated with different cis-regulatory elements. Although some of these discrepancies are likely related to the lower reliability of cis-regulatory predictions, other cases may reflect real divergence, as observed elsewhere (17–19).
Recent findings that the rate of gene expression evolution varies between mammalian organs, lineages, and chromosomes (20) may create the expectation that such rates will be particu-larly high in the immune system, because it is demonstrably under strong evolutionary pressure (as observed at the sequence level) (21–25). Our analysis suggests, however, that, for both steady expression states and during CD4 T-cell activation, overall gene expression conservation level in our compendia is comparable with the conservation in a solid tissue atlas (8), al-though different genes have highly conserved expression in each context. One intriguing possibility is that some of the transcrip-tional innovation in the immune system is mediated through divergence after gene duplication and not the change in regu-lation of one-to-one orthologs.
On the overall background of conservation, we identified cell surface markers, regulators, and 169 one-to-one orthologs with highly distinct expression patterns between human and mouse, more than tripling the number of previously reported differences (10). We tested and validated a few of these differences at the protein level. However, these results must be interpreted with some caution, because some distinctions may stem from probeset design, differences in the sampled cell types, or population and
A
B
ERG GATA2 HOXA5 MYCN SPI1 IRF5 IRF4 PAX5 POU2F2 SPIB STAT4 TBX21 IRF3 STAT1 Human Mouse −2 0 2 NK B DC H GN MO T NK B DC H GN MO T Erg Gata2 Hoxa5 Mycn Sfpi1 Irf5 Irf4 Pax5 Pou2f2 Spib Stat4 Tbx21 Irf3 Stat1 Human Mouse * Lmo2 Myb Plag1 Smarcb1 Sox4 * Tcfec Chd7 Crem Ets2 Klf6 Pawr Sfpi Sox13 Klf9 Maf Mef2a Fosb Irf8 Smad1 Foxp1 Hivep2 Lyl1 Mef2c Nfyb Fhl2 Rora Smad3 Rcbtb1 Smad7 Tcf12 Tox NK B DC H GN MO T * LMO2 MYB PLAG1 SMARCB1 SOX4 * TFEC CHD7 CREM ETS2 KLF6 PAWR SPI1 SOX13 KLF9 MAF MEF2A FOSB IRF8 SMAD1 FOXP1 HIVEP2 LYL1 MEF2C NFYB FHL2 RORA SMAD3 RCBTB1 SMAD7 TCF12 TOX NK B DC H GN MO TFig. 5. Regulation of human and mouse immune system differentiation is largely conserved. (A) Conserved lineage-specific expression of regulators. Shown are mean-centered expression values (red/blue color bar on the right) of regulators with maximal expression in the same lineage in (Left) human and (Right) mouse. Genes are sorted by mouse lineage with maximal ex-pression. Selected gene names are highlighted on the left. (B) Divergent expression of regulators. Shown are mean-centered expression values of selected regulators with coherent expression patterns within each species that differ between the two species (Left, human; Right, mouse). Genes are sorted by mouse lineage with maximal expression. Genes mentioned in the text are marked with bold and asterisks.
environmental variation in the human samples, but they should serve as aflag for future analyses.
Our analysis provides a valuable resource for future comparison of the human and mouse immune systems and studies of evolution of gene regulation. To facilitate this comparison and other uses, we provide all of our data, analyses, and results on a web portal (http:// rstats.immgen.org/comparative/comparative_search.php). These results should help to identify when and where the mouse can, in fact, be considered predictive of human immunology.
Materials and Methods
Datasets. Gene expression in mouse cell types from the ImmGen March 2011 release robust multichip average (RMA)-normalized data was used, including 802 arrays (Affymetrix MoGeneST1.0) of 244 cell types, essentially all in triplicate (7). Gene expression in human cell types was measured on 211 Affymetrix array U133A from 38 cell types, with four to seven replicates from different donors. Data were normalized and batch-corrected as described (6). Profiles from human-activated CD4+ T cells and splenocytes on the HuGene 1.0ST platform were obtained from the ImmVar project: 14 replicates from dif-ferent human subjects per time points. Profiles from mouse-activated T cells from Wakamatsu et al. (26) were three replicates per time point from different pools of mice. Human and mouse normal tissue datasets (32 comparable tissues) were downloaded fromhttp://biogps.org/downloads/,files U133AGNF1B.gcrma. avg_ann and GNF1M_plus_macrophage_small.bioGPS_ann.
We used Ensembl COMPARA rel 63 to map orthologs between mouse and human ENSEMBL gene IDs, yielding 10,248 one-to-one ortholog pairs mea-sured in both compendia. Only 5,841 genes with expression levels above 120 (recommended ImmGen threshold) in more than three cell types of lineages common to both species were included in thefiltered one-to-one orthologs set. When a given gene was represented by more than one probeset, the probeset with highest mean expression was used.
Mapping Human–Mouse Samples. For each of the seven common lineages, comparable cell types were selected based on markers used for sorting and expert knowledge in the ImmGen Consortium. For 18 human selected cell types, we removed 23 outlier human samples (of 103 samples of those cell types) compared with all the samples from the same cell type. This removal resulted in 80 human samples of 18 cell types and 137 mouse samples of 44 cell types (Dataset S2).
Global Samples Correlation. Expression values of all the genes in thefiltered set of one-to-one orthologs were standardized per species, and Pearson correlation coefficient was calculated between the samples.
Differentially Expressed Gene Signatures. Using the same procedure described in ref. 6, we defined for each of the seven lineages (HSPC, GN, MO, DC, NK, T cell, and B cell) in each species signatures of significantly induced and re-pressed genes in that lineage (14 signatures per species) (Dataset S2) based on a t test between a gene’s expression level in a particular lineage’s samples and the samples from all other lineages [Bonferroni corrected P< 0.05 and the mean of the lineage-specific expression is higher (lower) than the global mean]. We test the significance of the overlap of two signatures using a hypergeometric test (cutoff false discovery rate< 10%).
COE. The COE of a gene is a measure of agreement of its expression in comparable lineages between two species. For each gene in each species, we first computed its median expression in a lineage. We then calculated the COE of the gene as the Pearson correlation coefficient between these group-level summaries. The significance of the COE was estimated by a KS test compared with a computed null distribution of COE (computing COE after permuting ortholog assignments, 1,000 random permutations). Matlab function Mix-tureOfGaussiansGivenInit (O.Z.;http://www.broadinstitute.org/~orzuk/matlab/ libs/stats/mog/MixtureOfGaussiansGivenInit.m) was used to estimate the mix-ture of Gaussians.
Activation of T cells. Naive CD4+ T cells from 14 human donor blood or pooled spleen and lymph nodes of male C57BL/6foxp3-gfpmice were enriched by negative selection and stimulated in vitro with anti-CD3 and -CD28– conjugated beads; human total CD4+ T cells were then purified using pos-itive magnetic selection, and mouse CD4+ GFP− T cells were flow sorted. Microarray profiling was performed on unstimulated cells and at early (4 h for human cells and pooled RNA at 1 and 4 h for mouse cells) and late (48 h for human cells and pooled 24 and 48 h for mouse cells) time points. Multiple Comparison Control. The Benjamini Hochberg False Discovery Rate (27) procedure was used to control the false discovery rate at 5% or 10% whenever stated.
ACKNOWLEDGMENTS. We thank the members of the ImmGen Consortium for discussions and eBioscience, Affymetrix, and Expression Analysis for support of the ImmGen Project. We thank L. Lanier, R. Gazit, P. A. Monach, N. Novershtern, B. L. Ebert, and M. Kowalczyk for helpful discussions. We also thank L. Gaffney and S. Hart for help with graphics. Work was supported by National Institutes of Health Grants R24 AI072073, RC2-GM093080, U54-CA149145, and 149644.0103 (to V.J. and D.K.), National Science Foun-dation Grant DBI-0345474, the Howard Hughes Medical Institute, and the Merkin Foundation for Stem Cell Research at the Broad Institute (A.R.).
1. Payne KJ, Crooks GM (2007) Immune-cell lineage commitment: Translation from mice to humans. Immunity 26(6):674–677.
2. Davis MM (2008) A prescription for human immunology. Immunity 29(6):835–838. 3. Dermitzakis ET, Clark AG (2002) Evolution of transcription factor binding sites in
Mammalian gene regulatory regions: Conservation and turnover. Mol Biol Evol 19(7): 1114–1121.
4. Odom DT, et al. (2007) Tissue-specific transcriptional regulation has diverged signif-icantly between human and mouse. Nat Genet 39(6):730–732.
5. Ravasi T, et al. (2010) An atlas of combinatorial transcriptional regulation in mouse and man. Cell 140(5):744–752.
6. Novershtern N, et al. (2011) Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144(2):296–309.
7. Heng TSP, Painter MW; Immunological Genome Project Consortium (2008) The Im-munological Genome Project: Networks of gene expression in immune cells. Nat Immunol 9(10):1091–1094.
8. Su AI, et al. (2004) A gene atlas of the mouse and human protein-encoding tran-scriptomes. Proc Natl Acad Sci USA 101(16):6062–6067.
9. Tirosh I, Weinberger A, Carmi M, Barkai N (2006) A genetic signature of interspecies variations in gene expression. Nat Genet 38(7):830–834.
10. Mestas J, Hughes CCW (2004) Of mice and not men: Differences between mouse and human immunology. J Immunol 172(5):2731–2738.
11. Lanier LL, Hemperly JJ (1995) NK3 CD56 and CD57 cluster workshop report. Leucocyte Typing V: White Cell Differentiation Antigens, eds Schlossman SF, et al. (Oxford Univ Press, Oxford), Vol 2, pp 1398–1400.
12. Kieffer LJ, et al. (1996) Human CD8α expression in NK cells but not cytotoxic T cells of transgenic mice. Int Immunol 8(10):1617–1626.
13. Ma A, Koka R, Burkett P (2006) Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol 24(1):657–679.
14. Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H (2005) Expression of toll-like receptors on B lymphocytes. Cell Immunol 236(1–2):140–145.
15. Wapinski I, Pfeffer A, Friedman N, Regev A (2007) Natural history and evolutionary principles of gene duplication in fungi. Nature 449(7158):54–61.
16. Weaver BK, Bohn E, Judd BA, Gil MP, Schreiber RD (2007) ABIN-3: A molecular basis for species divergence in interleukin-10-induced anti-inflammatory actions. Mol Cell Biol 27(13):4603–4616.
17. Tanay A, Regev A, Shamir R (2005) Conservation and evolvability in regulatory net-works: The evolution of ribosomal regulation in yeast. Proc Natl Acad Sci USA 102(20): 7203–7208.
18. Tuch BB, Galgoczy DJ, Hernday AD, Li H, Johnson AD (2008) The evolution of com-binatorial gene regulation in fungi. PLoS Biol 6(2):e38.
19. Hogues H, et al. (2008) Transcription factor substitution during the evolution of fungal ribosome regulation. Mol Cell 29(5):552–562.
20. Brawand D, et al. (2011) The evolution of gene expression levels in mammalian or-gans. Nature 478(7369):343–348.
21. Schlenke TA, Begun DJ (2003) Natural selection drives Drosophila immune system evolution. Genetics 164(4):1471–1480.
22. McTaggart SJ, Obbard DJ, Conlon C, Little TJ (2012) Immune genes undergo more adaptive evolution than non-immune system genes in Daphnia pulex. BMC Evol Biol 12(1):63.
23. Fumagalli M, et al. (2011) Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet 7(11):e1002355.
24. Nielsen R, et al. (2005) A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol 3(6):e170.
25. Downing T, Cormican P, O’Farrelly C, Bradley DG, Lloyd AT (2009) Evidence of the adaptive evolution of immune genes in chicken. BMC Res Notes 2(1):254. 26. Wakamatsu E, Mathis D, Benoist C (2012) Convergent and divergent effects of
cos-timulatory molecules in conventional and regulatory CD4+T cells. Proc Natl Acad Sci USA 110(3):1023–1028.
27. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B 57(1):289–300.
Shay et al. PNAS | February 19, 2013 | vol. 110 | no. 8 | 2951
IMMUNOLO