Malaysian Journal of Microbiology
Published by Malaysian Society for Microbiology
(In since 2011)
Screening and characterization of plant growth promoting traits of phosphate
solubilizing bacteria isolated from wheat rhizosphere of Algerian saline soil
Chibani Hiba Rahman1*, Bouznad Ahcene1, Bellahcene Miloud2, Djibaoui Rachid1
1
Laboratory of Microbiology and Plant Biology, Faculty of Natural and Life Sciences, University of Mostaganem, Algeria.
2
University Center of Ain Temouchent, Algeria. Email: hiba.chibani@univ-mosta.dz
Received 16 August 2016; Received in revised form 10 September 2016; Accepted 7 November 2016
ABSTRACT
Aims: The capacity of some soil microorganisms to solubilize in soil is an important activity exhibited by plant growth
promoting rhizobacteria (PGPR) to increase plant performance. This study aimed at isolation and selection of phosphate solubilizing bacteria from saline soil and in vitro evaluation of their plant growth promoting traits.
Methodology and results: Phosphate solubilizing bacteria isolated from wheat rhizosphere, of saline soil in western
region of Algeria were tested for their plant growth promoting traits such us indole acetic acid (IAA), hydrogen cyanide (HCN), siderophore and ammonia production and their ability to fix nitrogen. Among 104 bacterial isolates, 41 were selected for their phosphate solubilizing activity using tricalcium phosphate (TCP) as a sole phosphorus source. IAA production was shown by almost all the bacterial isolates. Twelve isolates were recorded positive for HCN production, 32 produced siderophore and 31 were able to fix nitrogen. The most dominant phosphate solubilizing bacteria found were identified as Pseudomonas followed by Aeromomas hydrophila Bacillus sp. and Burkholderia cepacia.
Conclusion: Phosphate solubilizing bacteria that were isolated from saline soil showed a high potential in to producing
growth promoting traits and can be used as inoculants to increase the phosphorus uptake by plants.
Keywords: Phosphate solubilising bacteria, saline soil, tricalcium phosphate, plant growth promoting traits
INTRODUCTION
Phosphorus is considered to be the most important element in plant nutrition of, after nitrogen. It is an essential component in all main metabolic procedures in plants for example energy transfer, photosynthesis, signal transduction and respiration (Khan et al., 2010). Inorganic phosphorus is found in soils, mostly in insoluble mineral complexes such as tricalcium phosphate Ca3 (PO4)2, ion
phosphate FePO4 and aluminium phosphate AlPO4
(Barber, 1995), which appear after repeated applications of chemical fertilizers. Plants have not the capacity to absorb these insoluble forms besides only 0.1% of total phosphorus is in soluble form and it is available for plant nutrition (Zhou et al., 1992).
It is for this reason that the available phosphorus levels have to be supplemented in most agricultural soil by adding chemical phosphorus fertilizers. The frequent and imprudent applications of chemical phosphorus fertilizers lead to the decrease of soil fertility by perturbing microbial population thus reduce crops yield (Gyaneshwar and Naresh, 2002).
Among chemical environmental stress soil salinity is the most important stress factor for plants (Idikut et al., 2012). Salinity leads to osmotic stress and causes the
formation of reactive oxygen species (ROS) thus disturbs cellular structures which and consequently damage mitochondria and chloroplast (Mittler, 2002). Soil salinity considerably reduces absorption of mineral nutrients, particularly phosphorus for the reason that phosphate ions precipitate with calcium ions in saline soil and become inaccessible to plants (Grattan and Grieve, 1999). Utilization of phosphate solubilizing bacteria to solve this problem for raison of their ability to solubilize phosphate in soil is supported by many researchers (Khan et al., 2007). Mechanisms like organic acid production, chelation, and ion exchange reactions are implemented by these bacteria to solubilize inaccessible phosphorus forms and make them available to plants (Vessey, 2003). Phosphate solubilizing bacteria (PSB) are part of plant growth promoting rhizobacterias (PGPRs) and are capable of solubilizing inorganic phosphate from different compounds, such as dicalcium phosphate, tricalcium phosphate and rock phosphate. Moreover, PSMs may also showed plant growth promoting traits such as indole acetic acid (IAA), cytokinins, gibberellic acid and ethylene production, hydrogen cyanide (HCN) and siderophore
production, nitrogen fixation and resistance to soil pathogens etc (Cattelan et al., 1999).
The main objectives of this study were the isolation and screening of phosphate solubilizing bacteria from wheat rhizosphere of salt affected soil and in vitro assessment of their plant growth promoting activities.
MATERIALS AND METHODS Soil sampling
Saline soil samples were collected randomly from the rhizosphere regions of wheat plants growing at different sites at Relizane (western Algeria). All samples were kept in plastic bags and transported to the laboratory and stored at 4 °C. Total of fifteen soil samples were air-dried, ground and passed through 2 mm sieve before chemical analyses pH, soil moisture and conductivity of the soil samples were measured.
Isolation of total phosphate solubilizing bacteria
Isolation of PBS bacteria was carried out by serial dilution method. Ten grams of rhizosphere soil was dissolved in 90 mL of saline phosphate buffer, then shook for 30 min. One mL of rhizosphere soil suspension was added to 9 mL of sterile saline water to obtain a suspension with a 10
-2
dilution. Subsequently 0.1 mL of the suspension diluted to 10-5 was grown on Pikovskaya (Pikovskaya 1948) PVK agar supplemented by 5 g of tricalcium phosphate (TCP) as a sole phosphorus source. Inoculated plates were incubated for 7 days at 28± 2 °C. Appearance of clear halo zone on Pikovskaya’s agar plates indicates positive phosphate solubilization ability. Bacterial colonies surrounded by a transparent halo on PVK agar were picked off, checked for purity and classified as putative P-solubilizers.
Quantitative estimation of phosphate solubilization in culture broth
The isolated bacteria presenting halo zones on solid PVK medium were used to measure phosphate solubilization in liquid medium. The in vitro phosphate solubilizing capacity of each strain was determined on National Botanical Research Institute’s Phosphate growth medium (NBRIP) (Nautiyal, 1999) supplemented by 5 g of TCP as sole phosphorus source. The flasks containing 50 mL of NBRIP medium was inoculated with 1 mL of bacterial suspension (2×109 CFU/mL) in triplicates and incubated at 28±2 °C on a rotary shaker 180 rpm for 7 days. The cultures were harvested by centrifugation at 6000 rpm for 30 min. The phosphorus in supernatant was estimated by vanado-molybdate-yellow colour method (Jackson, 1973). The total soluble phosphorus was calculated from the regression equation of standard curve. The values of soluble phosphate liberated were expressed as µg/ml over control. The pH of culture supernatants were also measured using a pH Meter.
Production of indole acetic acid (IAA)
Production of auxin indole-3-acetic acid (IAA) by bacteria was tested using Lauria Bertani (LB) and Salkowski reagent. Fifty milliliter of Lauria Bertani (LB) containing (0.1 g/L of L-tryptophan was inoculated with 1 mL of bacterial suspension (approximately 109 CFU/mL) in triplicates and incubated in incubator Shaker at 28± 2 °C and 180 rpm for 4 days in dark. The bacterial cultures were centrifuged at 6,000 rpm for 30 min. Estimation of indole-3-acetic acid (IAA) in the supernatants was done using colorimetric assay (Brick et al., 1991). From a standard curve prepared with known concentration of IAA, the quantity in the culture was determined and expressed as µg/mL.
Hydrogen cyanide production
Screening of bacterial isolates for hydrogen cyanide (HCN) production was carried out following the method described by Bakker and Schippers (1987). Bacterial cultures were streaked on nutrient agar medium containing 4.4 g/L of glycine. A Whatman filter paper No. 1 soaked in 0.5% picric acid solution (in 2% sodium carbonate) was placed inside the plate lid. Plates were sealed with parafilm and incubated at 28±2 °C for 4 days. HCN production was evaluated according to the colour change, ranging from yellow to orange.
Siderophore production
Siderophore production by rhizobacterial isolates was detected according to Schwyn and Neilands (1987). Autoclaved CAS agar medium was poured in each Petri dish. The bacterial inoculum was placed in the centre of a Petri dish. The plates were incubated in the dark at 28± 2 °C for 5 days. The change of CAS agar colour from blue to orange around bacterial colony was considered as positive production of siderophore.
Nitrogen fixing activity
The visual detection of nitrogen-fixing activities of the selected isolates were observed by using nitrogen-free malate-mannitol medium (NF-MM) (Herman et al., 1994), containing bromothymol blue (BTB) as an indicator. The medium was inoculated by the bacterial isolates and incubated at 28± 2 °C for 24 h. The blue coloured zone producing isolates were marked as nitrogen fixers in the solid culture conditions. The colouring zone was calculated by deducting the colony diameter from the colouring zone diameter (Dobereiner and Day, 1975).
Ammonia production
All the bacterial isolates were tested for the production of ammonia as described by Cappuccino and Sherman (1992). Overnight grown bacterial cultures were inoculated in 10 mL of peptone broth and incubated at 28± 2 °C for 48 h on a shaker. After incubation 0.5 mL of
Nessler’s reagent was added. The development of faint yellow to dark brown colour indicated the production of ammonia.
Biochemical identification of bacterial isolates
The bacterial isolates were further characterized by Gram staining, catalase, oxidase, starch hydrolysis activity and motility followed by biochemical identification using API 20NE kit (BioMérieux, France). API 20 NE is a standardized system for the identification of non-fastidious, non-enteric Gram-negative rods combining 8 conventional tests, 12 assimilation tests. The results were interpreted with the API WebTM software (version 7.0).
Statistical analysis
The data obtained in this study was subjected to analysis of variance (ANOVA) and comparisons of means were performed by Newman and Keuls test at p ≤ 0.05 using statbox. The correlation between phosphate solubilization and pH was explored by Excel.
RESULTS
The pH of soil samples ranged from 7.82 to 8.46, moisture content from 10.52 to 18.6% and electrical conductivity from 6.1 to 13.3 ds/m. A total of 104 phosphate solubilizing isolates were obtained from different saline soil sites at Relizane (western Algeria). Out of these isolates 41 were selected for their phosphate solubilizing activity. These were screened for their plant growth promoting activities such as indole acetic acid (IAA), Siderophore production, HCN production, nitrogen fixing ability and ammonia production.
Phosphate solubilization ability is marked by the formation of transparent halos around bacterial colony on solid Pikovskaya medium supplemented with tricalcium phosphate (Ca3(PO4)2) as a only source of phosphorus
(Figure 1). All the 104 isolates were able to produce transparent halo around the colony with different diameter which indicates a positive phosphate solubilisation ability and they were also capable to solubilize inorganic phosphorus (Ca3(PO4)2) in liquid medium. The results
showed that the phosphate solubilizing ability in NBRIP liquid medium of test isolates varied from 24.29 to 738.57 µg/mL using TCP as a source of insoluble P (data of all PSB are not given). The PSB showing the highest amount of solubilized phosphorus are chosen to be tested for their plant growth promoting traits (41 isolates). The isolate that showed the best capability in solubilizing phosphate was PSB91 (738.57 µg/mL). pH values of the bacterial cultures decreased from initial value of 7.0 to values ranged from 3.9 to 4.61. A highly significant negative (r = -0, 86) correlation was observed between the amounts of solubilized P and pH values (Table 1).
Figure 1: Phosphate solubilisation by bacterial isolate. Table 1: Phosphate solubilization by selected bacterial
isolates. Isolate Concentration of phosphate (µg/ml) Final pH of P solubilization PSB3 605.34±15.91d 4.11±0.15 PSB5 525.60±14.73j 4.35±0.26 PSB8 652.83±25.28b 3.90±0.56 PSB11 515.90±12.78j 4.38±0.89 PSB13 584.17±13.78e 4.27±0.21 PSB16 553.62±24.17h 4.32±0.72 PSB18 555.86±16.60gh 4.31±0.41 PSB22 642.49±22.74c 3.92±0.46 PSB24 587.05±19.65e 4.18±0.27 PSB27 566.17±17.14fg 3.97±0.82 PSB29 562.90±21.92fgh 4.18±0.63 PSB31 410.89±16.62p 4.35±0.51 PSB34 453.28±16.95n 4.26±0.32 PSB36 564.74±11.5fg 4.26±0.65 PSB38 402.58±15.71pq 4.47±0.12 PSB41 610.96±13.87d 4.12±0.43 PSB43 571.22±18.70f 4.22±0.74 PSB44 541.59±23.67i 4.32±0.75 PSB45 601.85±22.60d 4.11±0.95 PSB49 561.33±12.03fgh 4.35±0.31 PSB52 370.04±10.61r 4.53±0.45 PSB56 570.63±16.51f 4.22±0.15 PSB58 398.00±11.76q 4.57±0.61 PSB64 487.71±15.35l 4.44±0.34 PSB66 502.25±14.83k 4.46±0.85 PSB67 481.71±16.49l 4.42±0.22 PSB70 568.05±25.89f 4.25±0.12 PSB71 447.20±17.94n 4.33±0.41 PSB72 466.94±18.60m 4.34±0.33 PSB74 435.52±20.87o 4.50±0.23 PSB78 348.05±15.24s 4.55±0.29 PSB82 327.50±16.50t 4.61±0.57 PSB85 398.70±12.42q 4.52±0.45 PSB87 538.37±12.70i 4.23±0.11 PSB89 521.9±14.66j 4.30±0.15 PSB91 738.56±16.07a 4.01±0.77 PSB93 455.13±13.18n 4.35±0.67 PSB95 410.35±22.16p 4.42±0.81 PSB97 521.77±23.66j 4.27±0.36 PSB99 407.67±15.57pq 4.47± 0.37 PSB102 604.37±17.85d 4.14±0.41 r -0.86
Phosphate solubilizing bacteria were assayed for their capacity to produce IAA in LB medium supplemented with L-tryptophan as precursor (1%) by the appearance of pink colour after addition of Salkowski reagent to the culture (Figure 2). IAA production was revealed by almost all the bacterial isolates and IAA quantities of estimated ranged from 10.90 to 63.35 μg/mL as shown in Table 2. A relatively higher content of IAA was found in the culture of bacterial isolate PSB45 followed by PSB85 and PSB91 with 37.43 and 32.85 μg/mL respectively compared to other isolates.
Figure 2: IAA production by bacterial isolates.
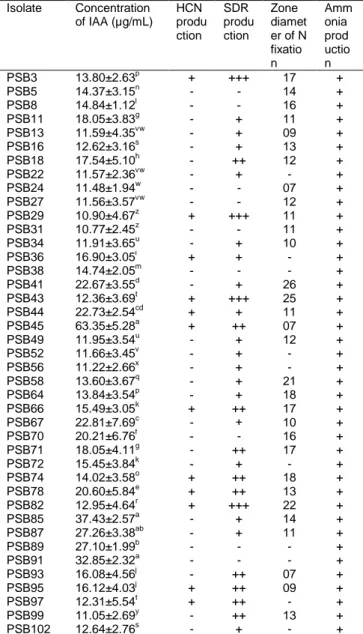
Among the 41 isolates, only twelve between them were recorded positive for HCN production by changing filter paper from yellow to orange (Table 2, Figure 3). The ability of the tested bacterial isolates to produce siderophores in vitro was assessed qualitatively using the CAS-agar plate assay. Of the 41 bacterial isolates tested, 32 strains produced siderophores. Siderophore production capacity of the isolates was evaluated according to the diameter of the orange halo around bacterial colonies as shown in Figure 5, as weak (4-10 mm), moderate (11- 20 mm) and strong (higher than 21mm). Amongst the siderophore producing isolates, 18 were weak siderophore producers, 10 isolates were moderate siderophore producers while 7 isolates were strong siderophore producers (Table 2).
Figure 3: HCN production by isolate.
Phosphate solubilizing bacteria were also tested for their ability to fix nitrogen on nitrogen free medium (NF-MM) supplemented by BTB used as colour indicator to detect the release of ammonium in the culture as shown in Figure 5. Among the 41 bacterial isolates 31 were able to fix nitrogen by changing medium colour from green to blue colour. The diameter of coloration zone ranged from 7 to 26 mm. The highest value of diameter of coloration zone was obtained with the isolate PSB41 (Table 2). All our
selected isolates were capable to produce ammonia (Table 2).
Table 2: Characterization of bacterial isolates for multiple
plant growth promoting traits.
Isolate Concentration of IAA (µg/mL) HCN produ ction SDR produ ction Zone diamet er of N fixatio n Amm onia prod uctio n PSB3 13.80±2.63p + +++ 17 + PSB5 14.37±3.15n - - 14 + PSB8 14.84±1.12l - - 16 + PSB11 18.05±3.83g - + 11 + PSB13 11.59±4.35vw - + 09 + PSB16 12.62±3.16s - + 13 + PSB18 17.54±5.10h - ++ 12 + PSB22 11.57±2.36vw - + - + PSB24 11.48±1.94w - - 07 + PSB27 11.56±3.57vw - - 12 + PSB29 10.90±4.67z + +++ 11 + PSB31 10.77±2.45z - - 11 + PSB34 11.91±3.65u - + 10 + PSB36 16.90±3.05i + + - + PSB38 14.74±2.05m - - - + PSB41 22.67±3.55d - + 26 + PSB43 12.36±3.69t + +++ 25 + PSB44 22.73±2.54cd + + 11 + PSB45 63.35±5.28a + ++ 07 + PSB49 11.95±3.54u - + 12 + PSB52 11.66±3.45v - + - + PSB56 11.22±2.66x - + - + PSB58 13.60±3.67q - + 21 + PSB64 13.84±3.54p - + 18 + PSB66 15.49±3.05k + ++ 17 + PSB67 22.81±7.69c - + 10 + PSB70 20.21±6.76f - - 16 + PSB71 18.05±4.11g - ++ 17 + PSB72 15.45±3.84k - + - + PSB74 14.02±3.58o + ++ 18 + PSB78 20.60±5.84e + ++ 13 + PSB82 12.95±4.64r + +++ 22 + PSB85 37.43±2.57a - + 14 + PSB87 27.26±3.38ab - + 11 + PSB89 27.10±1.99b - - - + PSB91 32.85±2.32a - - - + PSB93 16.08±4.56j - ++ 07 + PSB95 16.12±4.03j + ++ 09 + PSB97 12.31±5.54t + ++ - + PSB99 11.05±2.69y - ++ 13 + PSB102 12.64±2.76s - + - +
+, positive; ++, moderately positive; +++, strongly positive; -, negative
A total of forty one isolates of rhizobacteria obtained were biochemically identified by several tests. 20 bacterial isolates were identified as genus Pseudomonas which is the most dominant isolate found including Gram-negative, rod shaped Pseudomonas luteola and P. fluorescens, followed by Aeromomas hydrophila (10 isolates). Some of these are Gram-positive spore forming bacteria identified as Bacillus sp. (8 isolates). Only 3 isolates were Gram-negative and rod shaped identified as B. cepacia (Table 3).
Figure 4: Siderophore production by bacteria.
In this study the highest amounts of released phosphorus were solubilized by Pseudomonas with 24.39% followed by Bacillus sp. (12.19%), Aeromomas (9.75%) and finally Burkholderia with 0.41%. Bacteria producing the highest concentration of IAA were isolates belonging to the genus Pseudomonas (24.39%) followed
by Aeromonas (9.75%), Bacillus (8.33%) and
Burkholderia with 0.41%. For HCN production Aeromonas
was the higher producer (14.63%) followed by
Pseudomonas (12.19%) Burkholderia (0.41%), and finally Bacillus with 0%. Isolates belonging to Pseudomonas
were able to produce siderophore with 48. 78% followed by Aeromonas (14.63%), Burkholderia (7.31%) and
Bacillus with 0%. The majority of isolates belonging to
genus Pseudomonas were able to fix nitrogen with
Table 3: Identification of selected bacterial isolates.
Isolate Gram’s staining Shape Catalase Oxydase Amylase Motility ID
PSB3 - rod + - + + P. luteola PSB5 + rod + - + + Bacillus sp. PSB8 + rod + + + + Bacillus sp. PSB11 - coccibacilli + - - + A. hydrophila PSB13 - rod + - + + P. fluorescens PSB16 - rod + - + + P. luteola PSB18 - rod + - - + P. luteola PSB22 - rod + + + + B. cepacia PSB24 + rod + - + + Bacillus sp. PSB27 + rod + + + + Bacillus sp. PSB29 - coccibacilli + + - + A. hydrophila PSB31 + rod + - + + Bacillus sp. PSB34 - rod + - + + P. luteola PSB36 - rod + - + + P. luteola PSB38 + rod + + + + Bacillus sp. PSB41 - rod + - + + P. luteola PSB43 - coccibacilli + + - + A. hydrophila PSB44 - coccibacilli + + - + A.hydrophila PSB45 - rod + - + + P. luteola PSB49 - rod + - + + P. luteola PSB52 - rod + - + + P. luteola PSB56 - rod + - + + P. luteola PSB58 - rod + - + + P. luteola PSB64 - rod + - + + B. cepacia PSB66 - rod + + + + P. fluorescens PSB67 - rod + - + + P. luteola PSB70 + rod + - + + Bacillus sp. PSB71 - rod + - + + P. luteola PSB72 - coccibacilli + + - + A. hydrophila PSB74 - Rod + + + + B. cepacia PSB78 - coccibacilli + + - + A.hydrophila PSB82 - rod + + - + A.hydrophila PSB85 - rod + - + + P. luteola PSB87 - rod + - + + P. luteola PSB89 - coccibacilli + + - + A. hydrophila PSB91 + Rod + - + + Bacillus sp. PSB93 - rod + - + + P. luteola PSB95 - rod + - + + P. fluorescens PSB97 - coccibacilli + + - + A. hydrophila PSB99 - rod + + - + A.hydrophila PSB102 - rod + - + + P. luteola
19.51% followed by Aeromonas and Bacillus (19.51% and 12.19% respectively) and finally Burkholderia with 4.87%
Figure 5: Nitrogen fixation by bacterial isolates. DISCUSSION
Plant growth promoting rhizobacteria (PGPR) are a group containing a large number of bacteria number found in the rhizosphere, at root exteriors and in association with them, which can enhance directly and/or indirectly yield and growth of agricultural crops. The capability of some rhizospheric microorganisms to solubilize soil phosphate and make it available for plants by converting it into accessible forms like orthophosphate, is an important activity in a PGPB for improving plant growth (Chen et
al., 2006). In our study 104 phosphate solubilizing
bacterial isolates were isolated from wheat plant rhizosphere of salt affected soil; these bacteria are more effective in phosphorus solubilization than fungi as reported by Alam et al. (2002). Our rhizobacterial isolates were biochemically identified as Pseudomonas, A.
hydrophila, Bacillus sp. and B. cepacia. Srinivasan et al.
(2012) also isolated Pseudomonas and Bacillus as phosphate solubilizers from salt affected soil. Moreover A.
hydrophila and Bacillus sp. were isolated from the
rhizospheric soil of wheat grown in saline soil by Ashraf et
al. (2004). In quantitative estimation, all isolates showed
diverse levels of phosphate solubilizing activity. The higher concentration of released phosphorus in cultures was exhibited by genus Pseudomonas followed by
Bacillus. The same results were described by Ahmad et al. (2008) who reported that solubilization of phosphate
was commonly detected in the isolates of Bacillus and
Pseudomonas. Prior reports defined some Burkholderia
strains as competent phosphate solubilizers (Peix et al., 2001; Caballero-Mellado et al., 2007). pH of bacterial cultures dropped significantly compared to the control. Similar results were observed by Mardad et al. (2013) and Alam et al. (2002). A negative correlation (r = −0.86) was detected between the amounts of solubilized phosphorus of bacterial cultures and their pH values. The same negative correlation was reported by Xiao et al. (2009). Mainly, the mode of action of phosphate solubilising bacteria in soil is by the secreting of acids into the medium (Khan et al., 2014; Oteino et al., 2015). Hence, various genus of bacteria including Bacillus,
Pseudomonas, Enterobacter, Serratia and Azotobacter
sp. employ many solubilization reactions, such as acidification, exchange reactions, chelation and production of acids to release phosphorus from mineral complexes (Pandey and Maheshwari, 2007).
Recently many researchers have studied production of phytohormones by PGPR (Rajkumar and Freitas, 2008; Ahmad and Khan, 2012). The most important plant growth regulators produced by phosphate solubilizing microorganisms are auxins (Oves et al., 2013). Approximately 75% of bacteria isolated from saline soil have the capacity to produce IAA which concluded that saline soil is a rich source of IAA producing bacteria. A high level of IAA production by Pseudomonas was noted in our study and by other researchers (Ahmad et al., 2008; Kumar et al., 2012). Other IAA producing bacteria belongs to Aeromonas (Halda-Alija, 2003) and Bacillus (Swain et
al., 2007) were also reported.
HCN is a volatile, secondary metabolite that suppresses the development of microorganisms. An important role of HCN produced by bacteria from rhizosphere in biological control of pathogens has been described (Siddiqui et al., 2006). In the current study isolates belonging to genus of Aeromonas, Burkholderia and Pseudomonas were able to produce HCN. To date various bacterial genera are able to produce HCN, including Pseudomonas, Aeromonas, Alcaligenes and
Rhizobium (Devi et al., 2007; Ahmad et al., 2008).
Siderophores produced by PGPR can promote plant growth either directly or indirectly, using radiolabeled ferric siderophores (Sujatha and Ammani, 2013). Our results showed that isolates belonging to genus Pseudomonas and Burkholderia were the best siderophore producers but isolates belonging to Bacillus sp. were unable to produce them. Also García-Gutiérrez et al. (2012) found that all
Pseudomonas strains isolated from soil were able to
produce siderophore, while only one strain among Bacillus was able to produce such compounds. Luvizotto et al. (2010) reported that B. cepacia exhibit a high levels of siderophore production.
The presence of nitrogen-fixing bacteria in soil along with its isolation and conversion into PGPR biofertilizer is an important strategy reducing the use of expensive chemical fertilizers especially in nutrient poor and degraded soils (Cakmakci et al., 2006). The majority of our rhizobacterial isolates have the ability to fix nitrogen. Currently there are evidences that plant stimulation effect by PGPR such as Azoarcus sp., Burkholderia sp.,
Gluconacetobacter is related to their ability to fix nitrogen
(Vessey, 2003). In the study of Cakmakci et al, (2006), the quality and yield of wheat, spinach and sugar beet was improved using nitrogen fixing bacterial isolates
Pseudomonas RC06 and Bacillus OSU-142 as
biofertilizers. Zhang et al. (1996) reported that A.
hydrophila have the ability to fix atmospheric nitrogen.
Ammonia production is an essential trait exhibited by PGPR, which can effect indirectly plants growth (Yadav et
al., 2010).All our isolates were able to produce ammonia. These results are similar with those of Ahmed et al. (2008) who revealed the production of ammonia commonly
detected in all the isolates of Pseudomonas, Bacillus and
Azotobacter. Similarly, all bacteria identified as Bacillus
and Pseudomonas isolated by Yadav et al. (2010) from chickpea rhizosphere in India have the ability to produce ammonia.
CONCLUSION
Phosphate solubilizing bacteria isolated from wheat rhizosphere from saline soil located in western region of Algeria showed a high potential to produce growth promoting traits. Isolates belonging to the Bacillus and
Pseudomonas showed a high phosphate solubilisation.
Pseudomonas sp. was the highest producer of IAA and siderophore and had the capacity to fix nitrogen. A.
hydrophila and B. cepacia showed high potential produce
HCN and other PGP traits also. Those bacterial isolates can be used as inoculants to enhance the phosphorus uptake by plants and reduce the utilization of phosphorus fertilizers and increase yield of crop production.
ACKNOWLEDGEMENT
This study was funded by the Laboratory of Microbiology and Plant Biology, Faculty of Natural and Life Sciences, University of Mostaganem, Algeria.
The authors wish to thank Dr. CHIBANI Abdelwaheb for his help in the finalization of the English version of this paper.
REFERENCES
Ahmad, F., Ahmad, I. and Khan, M. S. (2008). Screening
of free-living rhizobacteria for their multiple plant growth promoting activities. Microbiological Research
163, 173-181.
Ahmad, M. and Khan, M. S. (2012). Effect of fungicides
on plant growth promoting activities of phosphate solubilizing Pseudomonas putida isolated from mustard (Brassica campestris) rhizosphere.
Chemosphere 86, 945-950.
Alam, S. S., Khalil, N., Ayub, B. and Rashid, M. (2002).
In vitro solubilization of inorganic phosphate by
phosphate solubilizing microorganism (PSM) from maize rhizosphere. International Journal of Agricultural
Biology 4, 454-458.
Ashraf, M., Hasnain, S., Berge, O. and Mahmood, T.
(2004). Inoculating wheat seedlings with
exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress.
Biology and Fertility of Soils 40, 157-162.
Bakker, A. W. and Schippers, B. (1987). Microbial
cyanide production in the rhizosphere to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biology and Biochemistry 19,
451-457.
Barber, S. A. (1995). Soil nutrient bioavailability: A
mechanistic approach, 2nd Edn. John Wiley. New York, USA. pp. 245-248.
Brick, J. M., Bostock, R. M. and Silverstone, S. E.
(1991). Rapid in situ assay for indole acetic acid
production by bacteria immobilized on nitrocellulose membrane. Applied and Environmental Microbiology
57, 535-538.
Caballero-Mellado, J., Onofre-Lemus, J., Estrada-de Los Santos, P. and Martínez-Aguilar, L. (2007). The
tomato rhizosphere, an environment rich in nitrogen-fixing Burkholderia species with capabilities of interest for agriculture and bioremediation. Applied and
Environmental Microbiology 73, 5308-5319.
Cakmakci, R., Donmez, F., Aydın, A. and Sahin, F.
(2006). Growth promotion of plants by plant growth
promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biology and
Biochemistry 38, 1482-1487.
Cappuccino, J. G. and Sherman, N. (1992). Biochemical
activities of microorganisms. In: Microbiology, A Laboratory Manual. The Benjamin/Cummings Publishing Co. California, USA. pp.188-247.
Cattelan, A. J., Hartel, P. G. and Fuhrmann, J. J.
(1999). Screening of plant-growth promoting
rhizobacteria to promote early soybean growth. Soil
Science Society of America Journal 63, 1670-1680. Chen, Y. P., Rekha, P. D., Arun, A. B, Shen, F. T., Lai,
W. A. and Young, C. C. (2006). Phosphate
solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied Soil
Ecology 34, 33-41.
Devi, K. K., Seth, N., Kothamasi, S. and Kothamasi, D. (2007). Hydrogen cyanide producing rhizobacteria kill
subterranean termite Odontotermesobesus (Rambur) by cyanide poisoning under in vitro conditions. Current
Microbiology 54, 74-78.
Dobereiner, J. and Day, J. M. (1976). Associative
symbiosis in tropical grasses: Characterization of microorganisms and dinitrogen fixing sites. In: Newton, W.E. and Nyman, C.J. (eds.). Proceedings of the 1st International Symposium on Nitrogen Fixation, Washington State University Press, Pullman, USA.
pp. 518-538.
García-Gutiérrez, L., Romero, D., Zeriouh, H., Cazorla, F. M., Torés, J. A., Vicente, A. and Pérez-García, A. (2012). Isolation and selection of plant growth
promoting rhizobacteria as inducers of systemic resistance in melon. Plant and Soil 358, 201-212.
Grattan, S. R. and Grieve, C. M. (1999). Salinity mineral
nutrient relations in horticultural crops. Scientia Horticulturae78, 127-157.
Gyaneshwar, P. and Naresh, K. G. (2002). Role of soil
microorganisms in improving P nutrition of plants.
Plant Soil 245, 83-93.
Halda-Alija, L. (2003). Identification of indole-3- acetic
acid producing freshwater wetland rhizosphere bacteria associated with Juncus effusus L. Canadian
Journal of Microbiology 49, 781-787.
Herman, R. P., Provencio, K., Torrez, T. and Seager, G. M. (1994). Seasonal and spatial population
dynamics of the nitrogen efficient guild in a desert bajada grassland. Applied and Environmental
Idikut, L. Z., Dumlupinar, S. N., Kara, C., Yururdurmaz, A. and Çolkese, M. (2012). The effect of different
temperatures and salt concentrations on some popcorn landraces and hybrid corn genotype germinations. Pakistan Journal of Botany 44, 579-587.
Jackson, M. L. (1973). Soil chemical analysis. Prentice
Hall of Englewood cliffs. New Jersey, USA. pp.
378-398.
Khan, M. S., Zaidi, A. and Ahmad, E. (2014).
Mechanism of phosphate solubilisation and physiological functions of phosphate-solubilizing microorganisms. In: Phosphate Solubilizing Microorganisms. Khan, M. S., Zaidi, A. and Mussarrat, J. (eds.). Springer International Publishing, Switzerland. pp. 34-45.
Khan, M. S., Zaidi, A., Ahmed, M., Oves, M. and Wani, P. M. (2010). Plant growth promotion by phosphate
solubilizing fungi-current respective. Archives of
Agronomy and Soil Science 56, 73-98.
Khan, M. S., Zaidi, A., Ahmed, M., Oves, M. and Wani, P. M. (2010). Plant growth promotion by phosphate
solubilizing fungi-current respective. Archives of
Agronomy and Soil Science 56, 73-98.
Kumar, A., Kumar, A., Devi, S., Patil, S., Payal, C. and
Negi, S. (2012). Isolation, screening and
characterization of bacteria from Rhizospheric soils for different plant growth promotion (PGP) activities: An in
vitro study. Recent Research in Science and Technology 4, 01-05.
Luvizotto, D. M., Marcon, J., Andreote, F. D, Neves, A. A. C., Araύjo, W. L. and Pizzirani-Kleiner, A. A. (2010). Genetic diversity and plant-growth related
features of Burkholderia spp. from sugarcane roots.
World Journal of Microbiology and Biotechnology 26,
1829-1836.
Mardad, I., Serrano, A. and Soukri, A. (2013).
Solubilisation of inorganic phosphate and production of organic acids by bacteria isolated from a Moroccan mineral phosphate deposit. African Journal of
Microbiology Research 7, 626-635.
Mittler, R. (2002). Oxidative stress, antioxidants and
stress tolerance. Trends in Plant Sciences 7, 405-410.
Nautiyal, C. S. (1999). An efficient microbiological growth
medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters 170,
265-270.
Oteino, N., Lally, R. D., Kiwanuka, S. K, Lloyd, A., Rayan, D., Germaine, K. J. and Dowling, D. N. (2015). Plant growth promotion induced by phosphate
solubilizing endophytic Pseudomonas isolates.
Frontiers in Microbiology 6, 745-755.
Oves, M., Khan, M. S. and Zaidi., A. (2013). Chromium
reducing and plant growth promoting novel strain
Pseudomonas aeruginosa OSG41 enhance chickpea
growth in chromium amended soils. European Journal
of Soil Biology 56, 72-83.
Pandey, P. and Maheshwari, D. K. (2007). Two species
microbial consortium for growth promotion of Cajanus Cajan. Current Science 8, 92-25.
Peix, A., Mateos, P. F., Rodriguez-Barrueco, C., Martinez-Molina, E. and Velazquez, E. (2001).
Growth promotion of common bean (Phaseolus
vulgaris L.) by a strain of Burkholderia cepacia under
growth chamber conditions. Soil Biology and Biochemistry 33, 1927-1935.
Pikovskaya, R. I. (1948). Mobilization of phosphorus in
soil inconnection with vital activity of some microbial species. Microbiologia 17, 362-370.
Rajkumar, M. and Freitas, H. (2008). Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinuscommunis in soil contaminated with heavy metals. Chemosphere 71, 834-842.
Schwyn, B. and Neilands, J. B. (1987). Universal assay
for the detection and determination of siderophores.
Analytical Biochemistry 160, 47-56.
Siddiqui, I. A, Shaukat, S. S, Hussain Sheikh, I. and Khan, A. (2006). Role of cyanide production by
Pseudomonas fluorescens CHAO in the suppression
of root-knot nematode, Meloidogyne javanica in tomato. World Journal of Microbiology and Biotechnology 22, 641-650.
Srinivasan, R., Yendigeri, M. S., Kashyap, S. and Alagawadi, A. R. (2012). Effect of salt on survival and
P solubilization potential on phosphate solubilizing microorganisms from salt affected soils. Saudi Journal
of Biological Sciences 19, 427-434.
Sujatha, N. and Ammani, K. (2013). Siderophore
production by the isolates of fluorescent Pseudomonads. International Journal of Current Research and Academic Review 5, 1-7.
Swain, M. R., Naskar, S. K. and Ray, R. C. (2007).
Indole-3-acetic acid production and effect on sprouting of Yam (Dioscorea rotundata L.) minisetts by Bacillus
subtilis isolated from culturable cowdung microflora. Polish Journal of Microbiology 56, 103-110.
Vessey, J. K. (2003). Plant growth promoting
rhizobacteria as biofertilizers. Plant and Soil 255,
571-586.
Xiao, C., Chi, R., Huan, H. E. and Zhang, W. (2009).
Characterization of tricalcium phosphate solubilization by Stenotrophomonas maltophilia YC isolated from phosphate mines. Journal of Central South University
of Technology 16, 0581-0587.
Yadav, J., Verma, J. P. and Tiwari, K. N. (2010). Effect
of plant growth promoting rhizobacteria on seed germination and plant growth chickpea (Cicerarietinum L.) under in vitro conditions. Biological Forum An
International Journal 2(2), 15-18.
Zhang, F., Dashti, N., Hynes, R. K. and Smith, D. L. (1996). Plant growth promoting rhizobacteria and
soybean [Glycine max (L.) Merr.] nodulation and nitrogen fixation at suboptimal root zone temperatures.
Annals of Botany 77, 453-459.
Zhou, K., Binkley, D. and Doxtader, K.G. (1992). A new
method estimating gross phosphorus mineralization and immobilisation rates in soils. Plant and Soil 147,