HAL Id: hal-03031069
https://hal.archives-ouvertes.fr/hal-03031069
Submitted on 7 Dec 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Dynamic expression of MMP28 during cranial
morphogenesis
Nadege Gouignard, Eric Théveneau, Jean-Pierre Saint-Jeannet
To cite this version:
Nadege Gouignard, Eric Théveneau, Jean-Pierre Saint-Jeannet. Dynamic expression of MMP28 during cranial morphogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences, Royal Society, The, 2020, 375, �10.1098/rstb.2019.0559�. �hal-03031069�
Dynamic expression of MMP28 during cranial morphogenesis
12
Nadege Gouignard1,2, Eric Theveneau*1, Jean-Pierre Saint-Jeannet*2
3 4
1 Centre de Biologie du Développement (CBD), Centre de Biologie Intégrative (CBI), Université
5
de Toulouse, CNRS, UPS, France 6
2 Department of Basic Science and Craniofacial Biology, New York University College of
7
Dentistry, New York NY 10010, USA 8
* Corresponding authors: jsj4@nyu.edu and eric.theveneau@univ-tlse3.fr
9 10 11
Abstract
12
Matrix metalloproteinases (MMP) are a large family of proteases comprising 24 members in 13
vertebrates. They are well known for their extracellular matrix remodelling activity. MMP28 is 14
the latest member of the family to be discovered. It is a secreted MMP involved in wound 15
healing, immune system maturation, cell survival and migration. MMP28 is also expressed 16
during embryogenesis in human and mouse. Here we describe the detailed expression profile of 17
MMP28 in Xenopus laevis embryos. We show that MMP28 is expressed maternally and 18
accumulates at neurula and tailbud stages specifically in the cranial placode territories adjacent 19
to migrating neural crest cells. As a secreted MMP, MMP28 may be required in neural crest-20
placode interactions. 21
22
Keywords: MMP, embryogenesis, cranial placodes, neural crest, Xenopus 23
Background
25
Matrix metalloproteinases form a large family of proteases regrouping 23 members in 26
human and 24 in Xenopus laevis (1). MMPs are translated as zymogens, or pro-enzymes, where 27
the N-terminal part of the protein, called domain, blocks the catalytic domain. This pro-28
domain can either change conformation (a process known as allosteric activation) or be removed 29
by proteolytic cleavage to ensure the activity of the protein (2). MMPs are Zn2+-dependent
30
proteases mostly known for their extracellular matrix remodelling activity but they are also 31
capable of cleaving a wide range of substrates including growth factors and their cognate 32
receptors, adhesion molecules and chemokines (3). For decades, MMPs have been studied in the 33
context of cardiovascular diseases, rheumatoid arthritis and neurological disorders. MMPs are 34
also of prime interest in cancer since MMPs are present in virtually all human cancers and most 35
members of the MMP family have been found to be dysregulated in human cancers (4). 36
MMPs are essential for key cellular processes from extracellular matrix regulation to cell 37
migration and invasion to cell proliferation and apoptosis. Therefore, they are important in adults 38
for wound healing, angiogenesis, tissues homeostasis and immunity. In addition, MMPs are 39
expressed during embryogenesis and are involved throughout development (5). Indeed, about 40
half of all MMP family members are expressed during Xenopus laevis embryogenesis (see Table 41
1). 42
MMP28 was first identified in human and is the latest member of the family to be 43
discovered (6, 7). MMP28 is a soluble MMP with endopeptidase activity and the ability to 44
degrade casein, N-CAM and Nogo-A proteins (8). MMP28 is involved in nerve repair and 45
wound healing, immune system maturation, cell migration and invasion (9, 10). MMP28 was 46
detected by PCR in human foetal tissues including skeletal muscle, lung, kidney and brain, and 47
by in situ hybridization in mouse heart, central nervous system and peripheral nervous system 48
from stage E.10 onward (7, 8, 11). In early Xenopus embryogenesis, MMP28 was first identified 49
in a comparative microarray screen performed on dissected neural border explants to identify 50
neural crest (NC)-specific transcripts (12). NC cells arise from the neural plate border alongside 51
the cranial placodes. They give rise to a wide variety of derivatives including smooth muscles, 52
melanocytes, craniofacial bones and cartilages, and together with placode cells, all the peripheral 53
nervous system of the head (13). So far MMP2, 11, 13, 14 and 16 were described as being 54
expressed by the NC or by tissues surrounding the NC (see Table1). However, a detailed 55
expression profile of MMP28 during Xenopus development is still lacking. 56
In this study, we used qPCR analyses, single and double in situ hybridization to assess 57
the expression of MMP28 during Xenopus laevis embryogenesis. We found that MMP28 has a 58
well-defined expression domain in the pre-placodal region at early neurula stage. During NC cell 59
migration, MMP28 is then detected in some placodes and NC subpopulations. Given that NC and 60
placodes interact to form the cephalic peripheral nervous system (13) and that MMP28 is 61
secreted, we propose that MMP28 may play a role in mediating these interactions to direct 62
cranial morphogenesis. 63
64
Materials and Methods
65
Xenopus manipulation and in vitro fertilization 66
All experiments were performed in accordance with the guidelines of the Guide for the 67
Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by 68
the Institutional Animal Care and Use Committee of New York University (animal protocol 69
#IA16-00052). Female Xenopus laevis were injected with 500 to 1000 Units of chorionic 70
gonadotrophin and kept overnight at 18°C. For fertilization, a suspension of minced testis was 71
added to the oocytes collected in a petri dish in 0.1X Normal Amphibian Medium (NAM): NaCl 72
(110mM), KCl (2mM), Ca(CO3)2 (1mM), MgSO4 (1mM), EDTA (0.1mM), NaHCO3 (1mM),
73
Sodium Phosphate (2mM). Embryos were staged according to Nieuwkoop and Faber, 1967. 74
75
qPCR
76
Embryos were collected by quick-freeze into liquid nitrogen. Total RNAs from batches 77
of 3 to 5 embryos were extracted with the RNeasy Micro Kit (Qiagen, Valencia CA). Relative 78
quantitative PCR was performed on a QuantStudio 3 Real-Time PCR System (Applied 79
Biosystems, Foster City, CA) using Power SYBR™ Green RNA-to-CT 1-Step Kit according to
80
manufacturer instructions and the following primers: Twist1qPCR_fdw: 5’-81
CGACTTTCTCTGCCAGGTCT-3’, Twist1qPCR_rev: 5’-TCCACACGGAGAAGGCATAG-3’, 82
MMP28-E7_fdw: 5’-TGCAGTGGTATCGGGTTTAG-3’, MMP28-E8_rev: 5’-83
AAAGTGCAGTGTCAGGACGA-3’, Sox10_fdw: 5’-CTGTGAACACAGCATGCAAA-3’ Sox10_rev: 84
5’-TGGCCAACTGACCATGTAAA-3’, Six1_fdw: 5’-CTGGAGAGCCACCAGTTCTC-3’, Six1_rev: 85
5’-AGTGGTCTCCCCCTCAGTTT-3’ ODC_fdw: 5’-ACATGGCATTCTCCCTGAAG-3’, ODC_rev: 86
5’-TGGTCCCAAGGCTAAAGTTG-3’. All primer pairs were validated using the standard curve 87
method. Data from embryos collected from three different females were normalized to ODC 88
using the ΔCt method(14) and expressed as mean with standard deviation error bars. 89
90
In situ hybridization and histology 91
Embryos were fixed for 1h at room temperature in MEMFA (0.1 M MOPS pH 7.42 mM 92
EGTA pH 7.0, 1 mM MgSO4, 3.7 % (wt:vol) Formaldehyde) and stored in 100% methanol.
Embryos were then rehydrated in methanol solutions of decreasing concentrations, and processed 94
for single or double in situ hybridization as previously described(15). The following probes were 95
used: Snai2 (16), MMP28 (this study), Sox10 (17), Six1 (18), Foxi4.1 (19), Xl-96
Sox2 (20) and Xl-Dmrta1 (21). Each staining was performed at least three times on batches of 97
embryos collected from different females. For histology, stained embryos were embedded in 98
Paraplast+, sectioned (12 µm) on an Olympus rotary microtome, counter stained with Eosin and 99 mounted in Permount. 100 101 Results 102
Xenopus has two homologous copies (22) of MMP28 gene on chromosome 2L 103
(MMP28.L) and 2S (MMP28.S), which are composed of 8 exons each and encode a 1977 bp and 104
1916 bp mRNAs, respectively. Both mRNA sequences share 84.71% identity, and 92.9% 105
identity for the open reading frame. MMP28.L and MMP28.S code for proteins of 496 and 497 106
amino acids, respectively, with a predicted molecular weight of 57 kDa. Both protein sequences 107
share 91.15% identity and 97.58% similarity. As the sequences share high identity, we designed 108
primers and probes for in situ hybridization that detect both MMP28.L and MMP28.S. 109
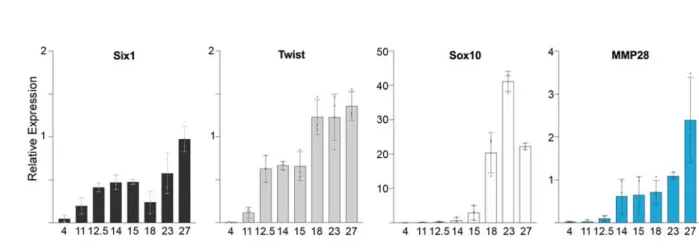
We assessed the temporal expression of MMP28 by relative qPCR at different stages of 110
Xenopus development from stage 4 (8-cell stage) to tail bud stage (Figure 1, blue bars), and 111
compared its expression profile to that of Twist (Figure 1, light grey bars), and Sox10 (Figure 1, 112
white bars), two markers of cephalic NC (17, 23) and Six1, a marker for cranial placodes (24) 113
(Figure 1, black bars). MMP28 is maternally expressed (stage 4) although at relatively low 114
levels, similar to Six1, while Twist and Sox10 do not appear to have a maternal contribution. 115
After the mid-blastula transition the expression of embryonic genes is initiated. MMP28 116
expression levels remain relatively low at stage 11 and stage 12.5 similar to Sox10, while both 117
Twist and Six1 expression increase significantly during that period until stage 15 when they both 118
transiently reach a plateau of expression. MMP28 shows a marked increased expression at stage 119
14, which is maintained until stage 18. Later in development, MMP28 expression progressively 120
increases up to stage 27, the last stage examined in this study. Sox10 expression increases 121
strongly between stages 15 and 23 before declining at stage 27. Twist reaches its higher 122
expression level at stage 18 without any further increase later in development. Six1 expression 123
decreases significantly at stage 18 and at later stage progressively increases in a similar manner 124
as MMP28. These data indicate that MMP28 expression is strongly upregulated at the neurula 125
stage (stage 14) and the escalation of Twist and Six1 expressions precedes that of MMP28 and 126
Sox10. 127
128
We next performed in situ hybridization to characterize the spatial expression of MMP28 129
during Xenopus development. MMP28 is not detected at gastrula stage (Figure 2B, C). MMP28 130
transcripts first accumulate at detectable levels at the end of gastrulation (NF stage 12.5), in the 131
form of a thin horseshoe shape domain at the anterior part of the embryo, which represents the 132
neural plate border (Figure 2 E), the region that gives rise to both cranial placodes and NC cells. 133
A control sense probe confirmed the specificity of this expression (Figure 2D). At stage 14/15, 134
MMP28 expression is more defined into two bilateral domains on either side of the neural plate 135
(Figure 2F-H). This staining strongly suggests a placodal expression since the neural fold is 136
devoid of staining. At this stage, MMP28 is also detected in a more discrete domain along the 137
border of the neural plate (Figure 2H and I’, arrowhead). To substantiate these observations, 138
stage 15 embryos were sectioned to visualize MMP28 expression in more detail (Figure 2H, H’, 139
H’’ and H’’’). MMP28 expression is localized in the deep ectoderm layers consistent with 140
cranial placodes expression (Figure 2H’’’ and I’, arrow). By contrast, the thin line of expression 141
along the neural plate is localized in the most superficial ectoderm layer (Figure 2I’ and H’’’, 142
arrowhead), which presumably correspond to the medial NC (25, 26). At stage 17, the expression 143
is broader and outlines the prospective eyes (Figure 2J, arrowhead). At stage 23, during NC cells 144
migration, MMP28 expression seems to be restricted to discrete domains around the eyes and in 145
territories posterior to that region, likely to correspond to the epibranchial placodes (Figure 2L, 146
L’). At stage 25, strong MMP28 expression is detected in the first stream of NC as well as what 147
appears to be the third and fourth NC streams (Figure 2N, arrows). The specificity of MMP28 148
expression was confirmed using a control sense probe (Figure 2M). 149
150
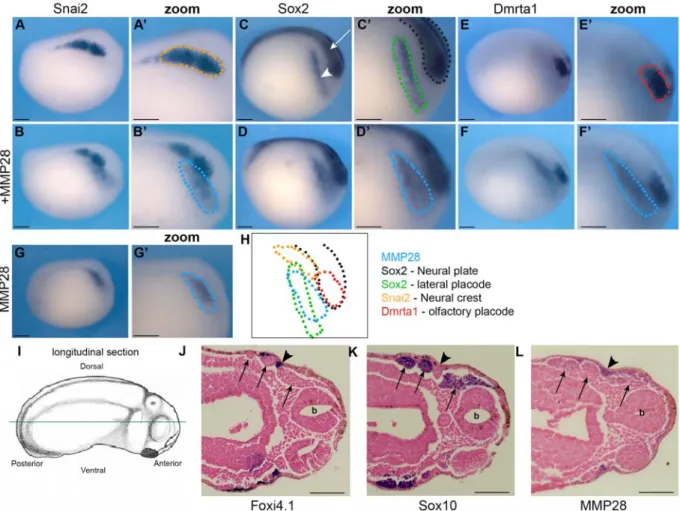
In order to identify more precisely which tissues express MMP28, we performed in situ 151
hybridization at stage 15 and 25 for MMP28 and genes expressed in similar regions of the 152
ectoderm, including Sox2 (neural plate and cranial placodes), Snai2/Slug (NC), Dmrt1a 153
(olfactory placodes), Foxi4.1 (epibranchial placodes) and Sox10 (migrating NC). The probes 154
were used alone or in combination as indicated (Figure 3). Spatially, there is no overlap between 155
MMP28 and Snai2, as Snai2 expression domain (Figure 3A, A’) is medial to that of MMP28 156
(Figure 3G, G’ and B, B’). Sox2 is expressed in two regions, the neural plate (Figure 3C, arrow) 157
and lateral placodes (Figure 3C and C’, arrowhead). MMP28-positive territory overlaps 158
exclusively with Sox2 placodal expression domain (Figure 3 D, D’). Since cranial placodes are a 159
broad area giving rise to different derivatives, we also compared MMP28 to Dmrta1, a gene 160
restricted to the most anterior placodal region, the prospective olfactory placodes (Figure 3E, E’). 161
We found that MMP28 and Dmrta1 are expressed in adjacent but non-overlapping domains 162
(Figure 3F, F’). At stage 25, Foxi4.1 is strongly expressed in the epibranchial placodes and 163
sensory layer of the ectoderm (Figure 3J, arrowheads) but not in the NC streams (Figure 3J, 164
arrows). In contrast, Sox10 is expressed strongly in all NC streams (Figure 3, arrows) and 165
excluded from the branchial ectoderm. This histological analysis indicates that MMP28 is not 166
expressed in the posterior NC streams (Figure 3L, arrows) but shows clear expression in the 167
epibranchial placodes and sensory layer of the ectoderm (Figure 3L, arrowheads). 168
Our results indicate that, at neurula stage, MMP28 is not expressed in the NC but is 169
confined to the cranial placodes territory overlapping with Sox2 (Figure 3 H) and that its 170
expression is maintained in the epibranchial placodes and sensory layer of the ectoderm at tail 171
bud stage (Figure 3L). 172
173
Discussion
174
Here we report the developmental expression of MMP28, a member of the matrix 175
metalloproteinases family, in Xenopus. Our results showed that MMP28 is expressed maternally 176
even though faintly and is upregulated at the end of gastrulation (St. 12.5) at which point it 177
becomes detectable by in situ hybridization. We observed that MMP28 is a secreted MMP 178
uniquely expressed at the neurula stage in the pre-placodal region adjacent to the prospective NC 179
territory. 180
We showed that MMP28 expression is limited to the neural plate border at early neurula 181
stage, then to the lateral placodes. This placodal expression is sustained in the epibranchial 182
placodes at tail bud stage. In addition, MMP28 expression is detected along the migratory 183
pathway of the first NC stream. As indicated earlier, MMP28 was first isolated in a screen for 184
genes activated in the NC tissue at neurula stage (12). However, as discussed by the authors of 185
this study, because the microarray-based approach was performed on manually dissected 186
embryonic tissues at different stages, potential contamination by surrounding tissues could not be 187
excluded. In fact, the authors reported that MMP28 transcripts were detected at the edge of the
188
NC domain, not the NC per se (12). At neurula stage, our double in situ hybridization show that 189
MMP28-positive signal does not overlap with the NC marker Snai2/Slug, but co-localizes with 190
the lateral placodal domain of Sox2. While we have detected expression in the medial NC, 191
overall MMP28 expression is primarily confined to the cranial placodes at early neurula stages. 192
The fact that MMP28 is strongly expressed in placodes is very interesting and unique. 193
MMP1, 2, 3, 7, 9, 11, 13, 14, 15, 18, 20 and 24 are expressed during Xenopus embryogenesis 194
(Table 1). Almost half of them are expressed by the NC or surrounding tissues and their 195
derivatives, but none of them has been described in placode cells at the stages studied here. 196
MMP28 expression profile over time is comparable to that of Six1. However, the MMP28-197
positive territory only represents a subdomain of Six1 (24). Notably, MMP28 was not detected in 198
the most anterior placodal domain giving rise to olfactory placodes. By contrast, MMP28 199
expression is maintained in the epibranchial placodes that originate from the lateral placodes 200
(Sox2-positive territory) and later is detected in between migratory NC streams. 201
NC cells and cranial placodes cooperate to form the cephalic peripheral nervous system. 202
NC cells give rise to glial cells while neurons have a dual origin coming from either NC, 203
placodes or both depending on the cranial ganglion of interest (27). Interestingly, NC and 204
placode interaction involved in peripheral nervous system patterning starts during NC migration. 205
In Xenopus, placodal cells are the source of Stromal cell-derived factor 1 (Sdf1/Cxcl12) a key 206
regulator of NC cell migration (28). Sdf1 promotes NC cell motility, which in the context of the 207
constraints of the developing head leads to migration of NC cells towards the placodes (29). 208
However, physical interactions between the two cell types leads to a repulsion. This short range 209
repulsion mid-range attraction system, coined “chase-and-run”, leads to a sustained directional 210
migration of both NC and placode cells towards the ventral regions of the face (30). Placodal 211
cells are less motile than NC cells and are progressively pushed aside while NC cells migrate 212
ventrally. This leads to a pattern of accumulation of placode cells in between NC streams (30, 213
31). Therefore interfering with NC migration leads to defects in placode patterning and this 214
relationship is conserved in vertebrates (30, 32-34). The expression of MMP28, a secreted MMP, 215
in the placodes prior to the onset for NC migration raises the possibility that this enzyme might 216
be required in proper NC-placode interactions. 217
218
Conclusion
219
Overall our data indicate that the onset of MMP28 expression in placodes occurs after the 220
NC and pre-placodal domains have been specified at the neural plate border. MMP28 expression 221
is excluded from the anterior-most placodes and persists in epibranchial placodes strongly 222
suggesting a role for this molecule in NC-placode interactions at early stages of the cranial 223
peripheral nervous system formation. 224
225
Acknowledgment: The authors would like to thank Ms. Allison Williams for excellent technical 226
assistance. 227
228
Funding: This work was supported by a grant from the National Institutes of Health to J-P.S-J. 229
(R01-DE25806), a pilot grant from the NYU CSCB which was established by NIH to N.G. 230
(1P30DE020754) and funding from the Fondation pour la Recherche Medicale 231
(FRMAJE201224), the Midi-Pyrenees regional council (grant 13053025) and the CNRS to E.T. 232
233
References
234
1. Woessner JF. MMPs and TIMPs‐An Historical Perspective. Molecular biotechnology. 235 2002;22(1):033‐50. 236 2. Ra H‐J, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biology. 237 2007;26(8):587‐96. 238 3. Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: What do they not do? 239 New substrates and biological roles identified by murine models and proteomics. Biochimica et 240 Biophysica Acta (BBA) ‐ Molecular Cell Research. 2010;1803(1):39‐54. 241
4. Cathcart J, Pulkoski‐Gross A, Cao J. Targeting matrix metalloproteinases in cancer: 242
Bringing new life to old ideas. Genes & Diseases. 2015;2(1):26‐34. 243
5. Cui N, Hu M, Khalil RA. Biochemical and Biological Attributes of Matrix 244
Metalloproteinases. Elsevier; 2017. p. 1‐73. 245
6. Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase 246
(MMP‐28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 247 2001;276(13):10134‐44. 248 7. Marchenko GN, Strongin AY. MMP‐28, a new human matrix metalloproteinase with an 249 unusual cysteine‐switch sequence is widely expressed in tumors. Gene. 2001;265(1‐2):87‐93. 250 8. Werner SR, Mescher AL, Neff AW, King MW, Chaturvedi S, Duffin KL, et al. Neural MMP‐ 251
28 expression precedes myelination during development and peripheral nerve repair. 252
Developmental dynamics : an official publication of the American Association of Anatomists. 253
2007;236(10):2852‐64. 254
9. Saarialho‐Kere U, Kerkela E, Jahkola T, Suomela S, Keski‐Oja J, Lohi J. Epilysin (MMP‐28) 255
expression is associated with cell proliferation during epithelial repair. J Invest Dermatol. 256
2002;119(1):14‐21. 257
10. Reno F, Sabbatini M, Stella M, Magliacani G, Cannas M. Effect of in vitro mechanical 258
compression on Epilysin (matrix metalloproteinase‐28) expression in hypertrophic scars. 259
2005;13(3):255‐61. 260
11. Illman SA, Keski‐Oja J, Lohi J. Promoter characterization of the human and mouse 261
epilysin (MMP‐28) genes. 2001;275(1):185‐94. 262
12. Plouhinec JL, Roche DD, Pegoraro C, Figueiredo AL, Maczkowiak F, Brunet LJ, et al. Pax3 263
and Zic1 trigger the early neural crest gene regulatory network by the direct activation of 264
multiple key neural crest specifiers. Developmental biology. 2014;386(2):461‐72. 265
13. Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP 266
and Wnt signalling during gastrulation and neurulation define two steps in neural crest 267
induction. Development. 2009;136(5):771‐9. 268
14. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real‐Time 269
Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402‐8. 270
15. Saint‐Jeannet J‐P. Whole‐Mount In Situ Hybridization of Xenopus Embryos. Cold Spring 271 Harbor protocols. 2017;2017(12):pdb.prot097287. 272 16. Mayor R, Morgan R, Sargent MG. Induction of the prospective neural crest of Xenopus. 273 Development. 1995;121(3):767‐77. 274
17. Aoki Y, Saint‐Germain N, Gyda M, Magner‐Fink E, Lee Y‐H, Credidio C, et al. Sox10 275
regulates the development of neural crest‐derived melanocytes in Xenopus. Developmental 276 biology. 2003;259(1):19‐33. 277 18. Ghanbari H, Seo H‐C, Fjose A, Brändli AW. Molecular cloning and embryonic expression 278 of Xenopus Six homeobox genes. 2001;101(1‐2):271‐7. 279
19. Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. 280
2004;271(2):439‐66. 281
20. Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic‐related‐1 and Sox‐2, 282
two factors induced by chordin, have distinct activities in the initiation of neural induction. 283 Development. 1998;125(4):579. 284 21. Huang X, Hong CS, O'Donnell M, Saint‐Jeannet JP. The doublesex‐related gene, XDmrt4, 285 is required for neurogenesis in the olfactory system. 2005;102(32):11349‐54. 286
22. Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, et al. Genome 287
evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538(7625):336‐43. 288
23. Hopwood ND, Pluck A, Gurdon JB. A Xenopus mRNA related to Drosophila twist is 289
expressed in response to induction in the mesoderm and the neural crest. 1989;59(5):893‐903. 290
24. Pandur PD, Moody SA. Xenopus Six1 gene is expressed in neurogenic cranial placodes 291
and maintained in the differentiating lateral lines. 2000;96(2):253‐7. 292
25. Spokony RF, Aoki Y, Saint‐Germain N, Magner‐Fink E, Saint‐Jeannet J‐P. The 293
transcription factor Sox9 is required for cranial neural crest development in 294
<em>Xenopus</em>. Development. 2002;129(2):421. 295
26. Sadaghiani B, Thiébaud CH. Neural crest development in the Xenopus laevis embryo, 296
studied by interspecific transplantation and scanning electron microscopy. Developmental 297 biology. 1987;124(1):91‐110. 298 27. Theveneau E, Mayor R. Collective cell migration of the cephalic neural crest: The art of 299 integrating information. 2011;49(4):164‐76. 300
28. Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective 301
chemotaxis requires contact‐dependent cell polarity. Developmental cell. 2010;19(1):39‐53. 302
29. Bajanca F, Gouignard N, Colle C, Parsons M, Mayor R, Theveneau E. In vivo topology 303
converts competition for cell‐matrix adhesion into directional migration. Nature 304
communications. 2019;10(1):1518. 305
30. Theveneau E, Steventon B, Scarpa E, Garcia S, Trepat X, Streit A, et al. Chase‐and‐run 306
between adjacent cell populations promotes directional collective migration. Nature Cell 307
Biology. 2013;15(7):763‐72. 308
31. Szabó A, Theveneau E, Turan M, Mayor R. Neural crest streaming as an emergent 309
property of tissue interactions during morphogenesis. PLoS computational biology. 310
2019;15(4):e1007002. 311
32. Culbertson MD, Lewis ZR, Nechiporuk AV. Chondrogenic and Gliogenic Subpopulations 312
of Neural Crest Play Distinct Roles during the Assembly of Epibranchial Ganglia. PLoS ONE. 313
2011;6(9):e24443. 314
33. Freter S, Fleenor SJ, Freter R, Liu KJ, Begbie J. Cranial neural crest cells form corridors 315 prefiguring sensory neuroblast migration. 2013;140(17):3595‐600. 316 34. Golding JP, Trainor P, Krumlauf R, Gassmann M. Defects in pathfinding by cranial neural 317 crest cells in mice lacking the neuregulin receptor ErbB4. 2000;2(2):103‐9. 318 319 320 321 Figure Legends 322 323 324
Figure 1: Expression of MMP28 assessed by qPCR.
325
Relative expression of Six1 (black), Twist (light grey), Sox10 (white), MMP28 (blue) during 326
Xenopus embryogenesis from stage 4 to stage 27. Results were generated using Ct method with 327
the house-keeping gene ODC as reference and expressed as mean of at least three independent 328
biological samples, error bars correspond to standard deviation. For representation purposes, 329
relative expression values results were multiplied by 1000. 330
331
Figure 2: Developmental expression of MMP28 by in situ hybridization.
332
ISH for MMP28 in lateral view (B, C, I, I’, L, M, N), anterior view (D-E, J, L’), dorsal view (F-333
H) and transversally sectioned embryos (H’ and H’’). A and K: schematic representation of 334
placodes (dark grey), neural crest (light grey) and neural plate (grey). (B-C) At St 11 MMP28 is 335
not detected. (D-E) MMP28 is first detected at the anterior neural plate border at St 12.5. (F-J) 336
Neurula stage embryos show expression in the lateral placodes (arrow) and medial NC (arrow-337
head). (H’-H’’’) Transversal sections as indicated in H, placodes (arrow), medial NC 338
(arrowhead). (L-L’) Expression in the epibranchial placodes (arrow heads). (M-N) At tail bud 339
stage MMP28 expression is strongly detected in the first NC stream (arrows) and epibranchial 340
placodes (arrow head). The embryonic stage is indicated in the lower right corner of each panel. 341
When MMP28 sense probe is used (Sense) it is indicated in the upper right corner of the panel. 342
Scale bar: 0.25mm. Number of embryos per experiment >20. 343
344
345
Figure 3: Comparative analysis of MMP28, Snai2, Sox2, Dmrta1, Foxi4.1 and Sox10
346
expression by single and double in situ hybridization.
347
A-H: Xenopus embryos at stage 15 (lateral view, anterior to right, dorsal to top) after single in 348
situ hybridization for Snai2 (A-A’), Sox2 (C-C’), Dmrta1 (E-E') and MMP28 (G-G’), or double 349
in situ hybridization for MMP28/Snai2 (B-B’), MMP28/Sox2 (D-D’) and MMP28/Dmrta1 (F-350
F’). Summary of the expression domains of these genes (H). Doted lines represent expression 351
territories of MMP28 (blue), Snai2 (orange), Sox2 (black, neural plate; green, placodes) and 352
Dmrta1 (red). MMP28 (blue) overlaps with the placodal domain of Sox2 (green). I-L: Xenopus 353
embryos at stage 25 (anterior to the right, posterior to the left). Diagram illustrating the plane of 354
section (green line; I). Single in situ hybridization for Foxi4.1 (J), Sox10 (K) and MMP28 (L). 355
Arrows show neural crest streams, arrowheads show epibranchial placodes, b: brain. Scale bar 356
0.25 mm. Number of embryos per experiment >20. 357
358 359
Table1: Developmental expression and putative substrates of matrix metalloproteinases
360
identified in Xenopus laevis
361 362