DETERMINATION OF F L U O R I N E BY PROTON- INDUCED GAMMA-RAY EMISSION (PIGE) SPECTROMETRY IN IGNEOUS AND METAMORPHIC CHARNOCKITIC
ROCKS F R O M R O G A L A N D (S. W. NORWAY)
I. ROELANDTS,* G, ROBAYE,** G, WEBER,* Jo M. DELBROUCK,** J. C. DUCHESNE*
*Geology, Petrology and Geochemistry **Experimental Nuclear Physics University o f LiOge, tt-4000 Sart Tilman (Belgium)
(Received December 12, 1986)
More than 200 specimens from different occurrences of the Rogaland igneous complex and surrounding granulite facies metamorphic rocks (S. W. Norway) have been analysed by a direct non-destructive proton induced gamma-ray emission (PIGE) technique. The fluorine contents vary from < 25 ppm to 3500 ppm. There is a good correlation between the concentration of fluorine and that of phosphorus for igneous rocks, suggesting a control of apatite on the F content, tn meta- morphic rocks, amphibole and biotite besides apatite are the principal cencentrators of fluorine indicating that fluorine in the system is controlled by granulite facies metamorphism conditions,
Introduction
F l u o r i n e is the t h i r t e e n e l e m e n t in o r d e r o f a b u n d a n c e in c r u s - t a l r o c k s o f the E a r t h , w i t h an a v e r a g e c o n t e n t o f 625 p p m /i/. It is an i m p o r t a n t c o n s t i t u e n t o f the f l u i d p h a s e a s s o c i a t e d w i t h g r a n i t i c r o c k s a n d v a r b o u s m i n e r a l i z a t i o n s . Its b e h a v i o u r
in the u p p e r p a r t of the crust, w h e r e t h e s e r o c k s g e n e r a l l y o c - cur, h a s b e e n e x t e n s i v e l y s t u d i e d /2~. In t h e d e e p e r p a r t o f the crust, it is l e s s w e l l d o c u m e n t e d In r e c e n t y e a r s , its i m - p o r t a n c e h a s h o w e v e r b e e n e m p h a s i z e d as a p o s s i b l ~ m a j o r c o n s t i - t u e n t b e s i d e s C02 o f the f l u i d p h a s e in e q u i l i b r i u m w i t h i g n e o u s a n d m e t a m o r p h i c r o c k s o f the g r a n u l i t e f a c i e s a n d s t r e s s e d the n e e d of f u r t h e r g e o c h e m i c a l d a t a in t h e s e m a t e r i a l s . T h i s p a p e r r e p o r t s the p r e l i m i n a r y d a t a of a g e o c h e m i c a l s t u d y of f l u o r i n e in t h e g r a n u l i t e f a c i e s r o c k s f r o m t h e R o g a - l a n d P r o v i n c e in S. N o r w a y . Analytical m e t h o d C o l o r i m e t r y a n d s p e c i f i c i o n - e l e c t r o d e , a n d to a l e s s e r e x t e n t r a d i o c h e m i c a l n e u t r o n a c t i v a t i o n a n a l y s i s are c o m m o n l y used. In all t h e s e t e c h n i q u e s the s a m p l e s m u s t be d i s s o l v e d a n d c h e m i c a l e l i m i n a t i o n of p o t e n t i a l l y i n t e r f e r i n g e l e m e n t s m u s t be c a r r i e d
:Research Associate of the NationaJ Fund for Scientific Research (Belgium)
12 * ~Tsevier, Amsterdam - Oxford New York
I. ROELANDTS et al.: DETERMINATION OF FLUORINE
out, that r e s t r i c t s the analysis of a large n u m b e r of samples in r e a s o n a b l e time as o f t e n r e q u i r e d in g e o c h e m i c a l studies.
B R E W E R S and F L A C K /3/ w e r e the first to d e m o n s t r a t e that p r o t o n i n d u c e d g a m m a ray e m i s s i o n (PIGE) s p e c t r o m e t r y was capa-
ble of m e a s u r i n g fluorine in rock samples. However, the place
of the PIGE t e c h n i q u e is not yet e s t a b l i s h e d in the g e o s c i e n c e s and g e o c h e m i c a l d a t a by this m e t h o d are still scarce /4/.
The PIGE technique, c u r r e n t l y b e i n g d e v e l o p e d in Liege U- n i v e r s i t y /5/, p r o v e d to be v a l u a b l e for r o u t i n e fluorine deter- m i n a t i o n r a n g i n g from % to p p m levels in g e o l o g i c a l m a t e r i a l s of w i d e l y v a r y i n g c o m p o s i t i o n s i n c l u d i n g rocks, soils, sediments, m i n e r a l s a n d ores /6,7/. The n o n - d e s t r u c t i v e P I G E m e t h o d u s e d here is b a s e d on the n u c l e a r r e a c t i o n
19F(p,ey) 160.
The e x p e r i m e n t a l a r r a n g e m e n t for PIGE b a s i c a l l y c o n s i s t s of three c o m p o n e n t s : a V a n de G r a a f f a c c e l e r a t o r , a v a c u u m i r r a d i a - tion c h a m b e r and a d e t e c t i o n system w i t h a s s o c i a t e d e l e c t r o n i c s for c o u n t i n g the 7 rays. It is s c h e m a t i c a l l y r e p r e s e n t e d in Fig. i.
a
~
Proton beam ~ LEADCARBON 3"x 3" Na I (TI) Probe S c a l e 2 0 cm
b
Concrete 5 0 c mJ
t mS c a l e set up Local lead wa 5 0 m m thick Fig. 1. Schematic diagram of the experimental set-up
In t h i s w o r k a p e l l e t of g e o l o g i c a l m a t e r i a l m o u n t e d on an a l u m i n u m t a r g e t l a d d e r w i t h 16 p o s i t i o n s was b o m b a r d e d in v a c u - um w i t h a 500 n A b e a m of 1.5 M e V p r o t o n s from the 3 M e V V a n de G r a a f f a c c e l e r a t o r (HVEC) of the E x p e r i m e n t a l N u c l e a r I n s t i t u t e
( U n i v e r s i t y o f Liege). This b o m b a r d i n g e n e r g y w a s f o u n d e x p e r i -
m e n t a l l y to give the b e s t sensitivity. A b e a m s p o t a r e a at the
sample of a b o u t 20 m m 2 was chosen. The e m i t t e d y rays w e r e de-
t e c t e d w i t h a 3" x 3" N a I (TI) gamma p r o b e (AE/E = 7% FWKM) ca-, r e f u l l y s h i e l d e d a n d p l a c e d at a d i s t a n c e of 20 cm f r o m the tar- get w i t h a 0 . 0 4 9 sr s o l i d angle at i00 ~ LAB. The r e s u l t i n g p u l - ses w e r e c o l l e c t e d in a m u l t i c h a n n e l h e i g h t a n a l y s e r (INgO I n t e r -
technique) e m p l o y e d for d a t a a c q u i s i t i o n and analysis. E a c h
s a m p l e r e q u i r e d a b o u t 45 m i n u ~ s of r u n n i n g time.
S a m p l e p r e p a r a t i o n
T h e p o w d e r e d r o c k sample (carefully w e i g h e d w i t h a m i c r o b a l a n c e ) was i n t i m a t e l y m i x e d w i t h g r a p h i t e SP-IC p o w d e r (Union Carbide)
in a 9:1 w e i g h t ratio and r e g r i n d e d in an agate mortar. The m i x -
ture was t r a n s f e r r e d to a B e c k m a n e v a c u a b l e die and p r e s s e d at 4 . 1 0 8 p a in a h y d r a u l i c press. A p o i y p r o p y l e n e film was p l a c e d b e t w e e n the p e l l e t and the p r e s s i n g surface to p r e v e n t any con- t a m i n a t i o n and r e m o v e d afterwards. The r e s u l t i n g p e l l e t w h i c h was 13 m m in d i a m e t e r and 1-2 m m thick p r o v e d to be e a s y to h a n - dle~ F o r r o u t i n e analysis, 200 m g o f m i x t u r e w e r e p r e p a r e d
(180 m g s a m p l e and 20 m g graphite). C a c t e d as a b i n d e r and al-
so p r o v i d e d g o o d c o n d u c t i o n of the target.
C a l i b r a t i o n was e f f e c t e d via p r i m a r y p o w d e r standards. T h e y w e r e f a b r i c a t e d simply by a d d i n q k n o w n q u a n t i t i e s of s o d i u m
f l u o r i d e (Merck, A R g r a d e purity) to pure q u a r t z (Merck, A R gra- de purity) and a g i t a t i n g the w h o l e in a T u r b u l a s y s t e m Schatz for 15 h o u r s to e n s u r e a u n i f o r m fluorine d i s t r i b u t i o n . T h r e e r e p l i c a t e p e l l e t s from e a c h c a l i b r a t i o n s t a n d a r d W e r e p r e p a r e d f o l l o w i n g the p r o c e d u r e used for the rocks. The ~ r a y i n t e n s i - ty was a l i n e a r f u n c t i o n of the f l u o r i n e c o n t e n t o v e r a w i d e range of c o n c e n t r a t i o n s . "Blank" p e l ~ e t s were m a d e from p u r e q u a r t z and graphite.
Ganur~a ray spect~rum
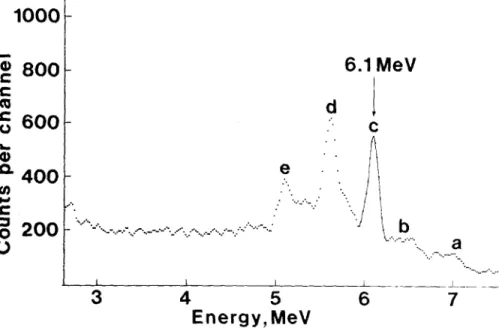
A t y p i c a l e x a m p l e of a g a m m a s p e c t r u m is shown in Fig.2. It is m a i n l y c o m p o s e d b y thel 7.1 and 6.~ M e V g a m m a - r a y s c o r r e s p o n d i n g to the d e e x c i t a t i o n of 160 n u c l e i p r o d u c e d in the r e a c t i o n 19F(p,a7)160. Due to the p a i r p r o d u c t i o n p h e n o m e n o n - i m p o r t a n t at t h e s e e n e r g i e s - five g a m m a - p e a k s can be seen c o r r e s p o n d i n g
to full e n e r g y loss, single escape and d o u b l e escape. T h e 6.1
M e V p e a k was p r e f e r r e d for fluorine a n a l y s i s b e c a u s e the b a s e line m a y be m o r e a c c u r a t e l y drawn in this g a m m a s p e c b r u m region. For a u n i f o r m a r e a sample, the c o n c e n t r a t i o n of f l u o r i n e can be d e t e r m i n e d d i r e c t l y from the counts of the g a m m a ray p e a k a f t e r b a c k g r o u n d c o r r e c t i o n s and b l a n k C o n t r i b u t i o n s u b t r a c t i o n .
I. ROELANDTS et al.: DETERMINATION OF FLUORINE
1 0 0 0
m8 0 0
c - C =6 0 0
i . . _ 0=" 4 0 0
t - O2 0 0
0
e ," ",,. -',. ,6 . 1 M e V
C I I 3_3
4
5
6
E n e r g y , M e V
a .J7
Fig. 2. Typical ~ m m a - r a y spectrum; a - 7 . I - M e V full-energy peak; b - 7 . I - M e V single-escape peak; c - 7 . I - McdV double-escape + 6 , 1 - M e V full-energy peak; d - 6 , 1 - M e V single-escape peak; e - 6 . 1 - M e V double-esc~ape peak L i m i t of d e t e c t i o n - P r e c i s i o n - A c c u r a c y A d e t a i l e d e v a l u a t i o n of t h e s e t h r e e p a r a m e t e r s w h i c h are i m p o r - t a n t a t t r i b u t e s w h e n j u d g i n g the m e r i t s of a m e t h o d has b e e n p u - b l f s h e d e l s e w h e r e /6,7/. T h e l i m i t of d e t e c t i o n , d e f i n e d h e r e as t h e c o n c e n t r a t i o n c o r r e s p o n d i n g to 3 / B p u l s e s in the p e a k (B is t h e n u m b e r of p u l - ses in the b a c k g r o u n d ) , w a s c a l c u l a t e d to be e q u a l to 25 p p m of f l u o r i n e . T h i s l o w l i m i t of d e t e c t i o n is m a i n l y due to a c a r e - ful s t u d y of t h e g a m m a s h i e l d i n g of the NaI(T1) p r o b e . T h i s v a - lue is q u i t e a c c e p t a b l e for m o s t g e o c h e m i c a l a p p l i c a t i o n s .
T h e ~ t t a i n e d p ~ e c i s i o n w a s f o u n d to be w i t h i n a c c e p t a b l e l i m i t s : l O % at iO0 p p m level, 6 % at i000 p p m l e v e l a n d 3 % at 1 % c o n c e n t r a t i o n l e v e l of fluorine. In the p r e s e n t study, d i f - f e r e n t p e l l e t s of the g r a n o d i o r i t e G S P - I (U.S.G.S.) w e r e s y s t e m a - t i c a l l y i n c l u d e d in e v e r y i r r a d i a t i o n . T h e m e a n v a l u e of o u r G S P - I a n a l y s e s w a s 3 4 7 7 ~ 1 9 5 p p m (n = 48). E i g h t y . i n t e r n a t i o n a l geochemica'l r e f e r e n c e s a m p l e s (GRS) f r o m d i f f e r e n t o r g a n i s m s , i n c l u d i n g i g n e o u s , m e t a m o r p h i c a n d s e d i - m e n t a r y s i l i c a % @ 8 , c a r b o n a t e s , p h o s p h a t e s , m i n e r a l s , s o i l s a n d o r e s h a v e b e e n a n a l y s e d to c h e c k the a c c u r a c y of o u r d e t e r m i n a - t i o n s /6/. M o s t of o u r r e s u l t s w e r e w i t h i n i0 % (or better) of t h e q u o t e d v a l u e s . T h i s a c c u r a c y is q u i t e s a t i s f a c t o r y e s p e c i a l - ly c o n s i d e r i n g the r a n g e of v a r i a t i o n of the a v a i l a b l e l i t e r a t u - re v a l u e s .
Data
206 igneous and 31 m e t a m o r p h i c rocks from R o g a l a n d w e r e analysed. This p r o v i n c e has been e x t e n s i v e l y s t u d i e d by P . M Z C K O T /8/ who u n r a v e l l e d the o v e r a l l g e o l o g i c a l h i s t o r y and b y m a n y authors who have dealt w i t h g e o c h e m i c a l and isotopic aspects (see the r e v i e w by D U C H E S N E et al. /9/).
Th_ e i~neous rocks
The igneous rocks b e l o n g to the anorthosite suite and comprise anorthosite, leuconOrite, norite, monzonorite, m a n g e r i t e , quartz m a n g e r i t e and c h a r n o c k i t e (nomenclature after S T R E C K E Z S E N /iO/), all m e m b e r s b e l o n g i n g to the c h a r n o c k i t i c family.
C o n t r a r i l y to famous provinces e.g. in the t y p i c a l region near M a d r a s in I n d i a /ii/ where charnockites were defined, Roga- land igneous c h a r n o c k i t e s h a v e not been m e t a m o r p h o s e d and thus have p r e s e r v e d t h e i r p r i m a r y m a g m a t i c c h a r a c t e r s a n d g e o c h e m i c a l features.
The igneous rocks are coming from the various g e o l o g i c a l
units of the province. These one e s s e n t i a l l y c o m p r i s e
(i) three large m a s s i f - t y p e a n o r t h o s i t i c bodies e s s e n t i a l l y m a d e up of a n o r t h o s i t e (more than 90 % of andesine plagioclase) w i t h s u b o r d i n a t e amounts of leuconorite and n o r i t e
(2) a l a y e r e d l o p o l i t h (the B j e r k r e i m Sokndal massif) m a d e up of a series of c u m u l a t e s g r a d i n g from a n 0 r t h o s i t e to m a n g e r i t e and on top of the series quartz m a n g e r i t e and charnockite.
(3) s e v e r a l large dykes and intrusions o f o v e r a l l m o n z o n o r i t i c characters, some of them v a r y i n g in c o m p o s i t i o n from n o r i t e to quartz m a n g e r i t e /12/.
The fluorine content of 206 rocks is s u m m a r i z e d in T a b l e i. The m o s t s t r i k i n g feature is the r e l a t i o n s h i p b e t w e e n the F and
TabM 1
Fluorine and P20s contents of igneous rocks of the charnockitir family F p p m M a s s i f - t y p e a n o r t h o s i % e a n o r t h o s i t e , l e u c o n o r i t e , n o r i t e B 2 e r k r e i m - S o k n d a l l o p o l i t h L a y e r e d a n o r t h o s i t e , l e u c o n o r i t e and a p a t i t e - f r e e n o r i t e L a y e r e d a p a t i t e - b e a r i n g n o r i t e L a y e r e d m a n g e r i t e Q u a r t z m a n g e r i t e ( + o l i v i n e ) A m p h i b o l e c h a r n o c k i t e M o n z o n o r i t i c dykes and i n t r u s i o n s A n t i p e r t h i t i c n o r i t e ( ~ quartz) M o n z o n o r i t e M o n z o n i t e /Mangeritel Quartz m a n g e r i t e / c h a r n o c k i t e < 25 P205 % < 0.01 45-110 O . 0 4 - 0 . 1 4 1 5 7 0 - 2 8 0 0 1 . 9 0 - 3 . 3 0 3 5 0 - 8 0 0 0.4 - 0 . 9 100-750 0. i - i.O 270-620 0 . 0 7 - 0.3 1370-2380 1.7 - 3.00 1 3 2 0 - 2 4 7 0 1.5 - 3.1 800-1380 1.0 - 1.7 370-630 0.4 - i.O 4 5 7
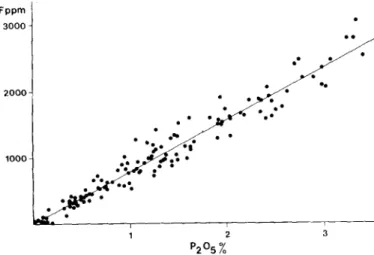
P205 c o n t e n t s of the rock, w h i c h is a p p e a r e d to be l i n e a r in Fig.3. This c l e a r l y indicates that F is e s s e n t i a l l y c o n t a i n e d in the m i n e r a l a p a t i t e C a 5 ( P O 4 ) 3 ( F , C I , O H ) . This b e h a v i o u r is w e l l i l l u s t r a t e d by the e v o l u t i o n in the B j e r k r e i m S o k n d a l m a s - sif, w h e r e a p a t i t e s u d d e n l y rises in the n o r i t e s from v e r y l o w c o n t e n t (0.O4-0.15%)P205 to the % level as soon as it b e c o m e s a liquidus m i n e r a l in process of f r a c t i o n a l c r y s t a l l i z a t i o n w h i c h is i n v o k e d to e x p l a i n the m a s s i f /13,14/. F u r t k e r on in tke se- ries of rocks, the a p a t i t e and F contents d e c r e a s e p r o g r e s s i v e l y . A similar t r e n d is also o b s e r v e d in the dykes and i n t r u s i o n s where the a p a t i t e c o n t e n t decreases w i t h the e v o l u t i o n t o w a r d s m o n z o n i t e / m a n g e r i t e and quartz mangerite. A g a i n the c r y s t a l l i -
zation of a p a t i t e can account for such a b e h a v i o u r in s u c c e s s i v e
liquids /12/ The a v e r a g e F/P205 ratio w h i c h can be d e d u c e d
from the fig[3 is 785.10 -4 w h i c h c o r r e s p o n d s to a F c o n t e n t of the apatite b f 3.4 %, a value w h i c h c l o s e l y m a t c h e s t h o s e m e a s u - red by B A U M E R et al. / 1 5 / on apatite s e p a r a t e d from B j e r k r e i m - S o k n d a l norites. Fppm 3 0 0 0 2 0 0 0 . 1 0 0 0
..j
9 Q i i i 1 2 3P2 ~
Fig. 3. F content vs. P~ Os in igneous rocks of the charnockitic family
The m e t a m o r p h i c rocks
A c o l l e c t i o n of rocks from the g r a n u l i t e facies t e r r a n e s in w h i c h
the igneous rocks w e r e e m p l a c e d have b e e n analysed. T h e y e s s e n -
tially c o m p r i s e m e t a b a s i t e s , m e t a p e l i t e s , g r a n i t e - g n e i s s e s , m e t a - c h a r n o c k i t e s and a u g e n - g n e i s s e s . The results are r e p o r t e d in
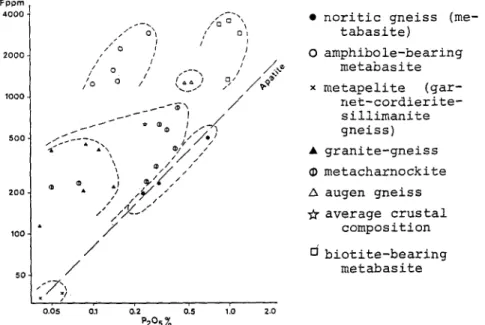
Table 2. Fig.4 c l e a r l y shows that c o n t r a r i l y to the igneous
rocks, the a p a t i t e c o n t e n t is not the sole f a c t o r w h i c h controls the F c o n t e n t of a rock.
In the m e t a b a s i t e s , the--presence of the h y d r o x y l d b e a r i n g m i n e r a l s a m p h i b o l e and b i o t i t e rises the F/P205 values from 785
iO-4~n a m p h i b o l e and b i o t i t e - f r e e m e t a b a s i t e s to 2800 10 -4 and 11400 iO-4 in b i o t i t e - b e a r i n g and a m p h i b o l e ' b e a r i n g m e t a b a s i t e s
Table 2
Fluorine, P20s and K20 contents of metamorphic rocks
n (i) F (ppm) P205 (%) F/P205 K20 (%) 10 -4 M e t a b a s i t e s N o r i t i c 3 A m p h i b o l i t e / a m p h i b o l e - 5 b e a r i n g n o r i t i c gneiss B i o t i t e - b e a r i n g n o r i - 4 t i c - g n e i s s M e t a p e l i t e s G a r n e t - c o r d i e r i t e - s i l l i m a n i t e gneiss 3 G r a n i t e - ~ n e i s s e s 6 M e t a - c h a r n o c k i t e s 8 A u g e n - g n e i s s e s 2 190-5OO 0.2-0.7 785 0.38 1230-29OO O.1-0.3 1 1 4 0 0 0.78 1330-3570 O.8-1.2 2800 1.77 ~30 ~O.O4 ~750 3.7 100-450 0.O4-0.14 600-8200 5.31 220-830 0.05-0.42 1050-43OO 4.92 1250 0.50 2500 3.06 (i) n u m b e r of s p e c i m e n s F p p m , 4 0 0 0 2 0 0 0 1 0 0 0 - 5 0 0 - 2 0 0 - 100 - 5 0 //~ o\% / I ' o [ i / / i I 0 ii1
i/0 0
---J*oo
/ / / / / //f
y ~ /
/
/
/
/ - 7 ~ . % 9 ', 7 / 0,05 / o / o i I J / /"~L"
/ ~
/
o'.I 0:2 o.s (.o 2.0
P20s
9 noritic gneiss (me- tabasite) O a m p h i b o l e - b e a r i n g m e t a b a s i t e x m e t a p e l i t e (gar- n e t - c o r d i e r i t e - sillimanite gneiss) 9 g r a n i t e - g n e i s s m e t a c h a r n o c k i t e A augen gneiss average crustal composition b i o t i t e - b e a r i n g m e t a b a s i t e
Fig. 41 F content versus PzOs in metamorphic rocks around the RogaIand igneous bodies. (The dashed line gives the
F/P2 05 ratio of the common apatite in igneous rocks)
I. ROELANDTS et al.: DETERMINATION OF FLUORINE
respectively. It is g e n e r a l l y admitted that the m e t a b a s i t e s re-
sult from the m e t a m o r p h i s m of basaltic igneous rocks. The F con-
tent of common b a s a l t i c rocks/16/ in the range of K20 values re- p o r t e d here (K20 = 0.38 - 1.77) varies a p p r o x i m a t e l y from 300 p p m
to 800 ppm. The h i g h values reported here (up to 3500 ppm) indi-
cate that the c h e m i c a l s y s t e m did not remain closed w i t h respect to F d u r i n g the m e t a m o r p h i s m . Moreover, the s y s t e m a t i c correla- tion w i t h the h y d r o x y l - m i n e r a l s suggests that the F and H20 w e r e a s s o c i a t e d in the fluid p h a s e which was carried into the s y s t e m d u r i n g the g r a n u l i t e facies metamorphism.
The m e t a p e l i t e s are strongly d e p l e t e d in F w i t h respect to
t h e i r n o n m e t a m o r p h i c equivalents. Averages in the range of 500-
800 p p m F are c u r r e n t l y r e p o r t e d for shales /17/. This s t r o n g l y suggests that F has been removed from the system t o g e t h e r w i t h w a t e r d u r i n g m e t a m o r p h i s m .
The g r a n i t e - g n e i s s e s and the m e t a c h a r n o c k i t e s show large and
o v e r l a p p i n g domains of F-P205 variations. F appears to be c o n -
t a i n e d not o n l y in apatite but also in b i o t i t e and/or amphibole,
w h i c h u s u a l l y o c c u r as m i n o r constituents of the rocks. F is
d e p l e t e d in g r a n i t e , g n e i s s e s w i t h regards to the average crustal
of 625 p p m and the average value adopted for g r a n i t e s (about
800 ppm) /1,2/. A g a i n this feature can result from granulite
facies m e t a m o r p h i s m . Some m e t a m o r p h o s e d charnockites and a u g e n - gneisses can h o w e v e r be more F and P205 e n r i c h e d than granite- g n e i s s e s .
We are grateful to the "Institut Interuniversitaire des Sciences Nucl6aires" for the financial support of this
research.
References
1. B. MASON and C. B. MOORE, Principles of Geochemistry, John Wiley, New York, 1982, p. 46. 2. J. C. BAILEY, Chem. Geol, 19 (1977) 1.
3. J. M. BREWERS, F. C. FLACK, Analyst, 94 (1969) 7.
4. R. O. ALLEN, P. J. CLARK Geochim. Cosmochim, Acta, 41 (1977) 581.
5. G. ROBAYE, J. M. DELBROUCK-HABARU, I. ROELANDTS, G. WEBER, L, GIRARD-REYDET, J. MORELLI, J. P. QUISEFIT, Nucl. lnstr. Methods, B6 (1985) 558.
6. I. ROELANDTS, G. ROBAYE, G. WEBER, J. M. DELBROUCK, Geostand. Newsl., 9 (1985) 191.
7. I. ROELANDTS, G. ROBAYE, G. WEBER, J. M. DELBROUCK.HABARU, Chem. Geol., 54 (1986) to be puslished.
8. P. MICHOT, Norges Geol. Unders., 212g (1960) 1.
9. J. C. DUCHESNE, R. MAQUIL, D. DEMAIFFE, in: The Deep Proterozoic Crust in the North Atlantic Provinces, A. C. TOBBI and J. L. R. TOURET (Eds,), D. Reidel Publ. Cy, 1985, p. 449.
10. A. STRECKEISEN, in: Geologic des domaine~s cdstallins, J. BELLIERE and J. C. DUCHESNE (Eds.), Soci6t6 G6ologique de Belgique, Li6ge, 1974, p. 349.
11. R. D. GAVRILIN, R. N. LEELANANDA, Ye. N. SAVINOVA, IL B. REDDI, Geochem. Intern. 21 (6) (1984) 35.
12. J. C. DUCHESNE, I. ROELANDTS, D. DEMAIFFE, D. WEISS, Contrib. Mineral. Petrol., 90 (1985) 214. 13. I. ROELANDTS and J. C. DUCHESNE, in: Origha and distribution of the elements, L. H. AHRENS (Ed.),
Pergamon, 1979, p. 199.
14. J. C. DUCHESNE, Contrib, Mineral. Petrol., 66 (1978) 175.
i5. A. BAUMER, D. LAPRAZ, J. C. DUCHESNE, W. E. KLEE, IMA Communication, 13th General Meeting Varna (Bulgaria), Sep. 19-25, 1982, p. 171.
16. K. AOKI, K. ISHIWAKA, S. KANISAWA, Contrib. Mineral. Petrol., 76 (1981) 53.
17. R. ALLMANN, S. KORITNIG, in: Handbook of Geochemistry II-1, K. H. WEDEPOHL (Ed,), Springer Verlag Berlin, 1978, Ch. 9.