ScienceDirect
EuropeanJournalofRadiologyOpen2(2015)32–38
Test-positive
rate
at
CT
colonography
is
increased
by
rectal
bleeding
and/or
unexplained
weight
loss,
unlike
other
common
gastrointestinal
symptoms
D.
Hock
a,∗,
R.
Materne
a,
R.
Ouhadi
a,
I.
Mancini
a,
S.A.
Aouachria
a,
A.
Nchimi
b aDepartmentofMedicalImaging,CentreHospitalierChrétien(CHC),RuedeHesbaye,75,B-4000Liège,BelgiumbDepartmentofThoracicandCardiovascularImaging,CHUdeLiège,DomaineUniversitaireduSartTilman,BâtimentB35,B-4000Liège,Belgium
Received19December2014;accepted23December2014 Availableonline8January2015
Abstract
Purpose: Weevaluatedtherateofsignificantcolonicandextra-colonicabnormalitiesatcomputedtomographycolonography(CTC),according
tosymptomsandage.
Materialsandmethods: Weretrospectivelyevaluated7361consecutiveaverage-risksubjects(3073males,averageage:60.3±13.9;range18–96
years)forcolorectalcancer(CRC)whounderwentCTC.Theyweredividedintothreegroupsaccordingtoclinicalsymptoms:1343asymptomatic individuals(groupA),899patientswithatleastone“alarm”symptomforCRC,includingrectalbleedingandunexplainedweightloss(group C),and5119subjectswithothergastrointestinalsymptoms(groupB).Diagnosticandtest-positiveratesofCTCwereestablishedusingoptical colonoscopy(OC)and/orsurgeryasreferencestandard.Inaddition,clinicallysignificantextra-colonicfindingswerenoted.
Results: 903outof7361(12%,95%confidenceinterval(CI)0.11–0.13)subjectshadatleastoneclinicallysignificantcolonicfindingatCTC.CTC
truepositivefractionandfalsepositivefractionwererespectively637/642(99.2%,95%CI0.98–0.99)and55/692(7.95%,95%CI0.05–0.09).The pooledtest-positiverateingroupC(138/689,20.0%,95%CI0.17–0.23)wassignificantlyhigherthaninbothgroupsA(79/1343,5.9%,95%CI 0.04–0.07)andB(420/5329,7.5%,95%CI0.07–0.08)(p<0.001).Agingandmalegenderwereassociatedtoahighertestpositiverate.Therate ofclinicallysignificantextra-colonicfindingswassignificantlyhigheringroupC(44/689,6.4%,95%CI0.04–0.08)versusgroupsA(26/1343, 1.9%,95%CI0.01–0.02)andB(64/5329,1.2%,95%CI0.01–0.02)(p<0.001).
Conclusion: Bothtest-positiveandsignificantextra-colonicfindingratesatCTCaresignificantlyincreasedinthepresenceof“alarm”
gastroin-testinalsymptomsespeciallyinolderpatients.
©2015TheAuthors.PublishedbyElsevierLtd.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
Keywords: Colorectalcancer;CTcolonography;Gastrointestinalsymptoms
1. Introduction
Colorectalcancer(CRC)isthesecondcauseofcancer-related death[1]andgenerallyresultsfromthetransformationof clini-callysilentadenomas[2]thataresoughtbyscreeningtests[3].
Persistenceorsuddenoccurrenceofvariousabdominal
symp-toms is often considered an indication to search or rule out
colonicabnormalities,including CRCor precancerouspolyps
[4].Literaturesuggeststhattheuseofopticalcolonoscopy(OC)
iswarrantedonlyfor subjects withrectal bleeding and
unex-plainedweightloss[5],whereastheothersymptoms’specificity
∗Correspondingauthor.Tel.:+3242248800;fax:+3242248810. E-mailaddress:danielle.hock@chc.be(D.Hock).
remain questionable[6–8].Meanwhile, the current diagnosis
guidelinesforindividualswithaverage-riskforCRConlyapply ifthereisnogastrointestinalsymptomorcomplain[2],raising potentiallyimportantconcerns.Indeed,aslongasallsymptoms areconsideredequivalentintermsofdiagnosticyield, individ-ualswithnonspecificgastrointestinalsymptomsareevaluated, whenneeded,byOC,causingpotentialcongestionofthe facil-itiesby lowresection-rateprocedures[9–11].Second,patient
compliancetocurrentCRCscreeningguidelinesislow.Almost
50%ofasymptomaticsubjects50yearsofageandolderescape
screeningprogramsoveraperiodof10years[12],whilesubjects
with nonspecific gastrointestinal symptoms agree toundergo
colonic explorations, for reassurance in a greater percentage
[7].
http://dx.doi.org/10.1016/j.ejro.2014.12.002
2352-0477/©2015TheAuthors.PublishedbyElsevierLtd.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
Computed tomography (CT) colonography (CTC) has
emerged over the past decade as an accurate and less
inva-sivealternativetoOCinseriesofsymptomaticpatients[13,14]. Similarlygoodresultswereobtainedinseriesofasymptomatic subjects[15].Toourknowledge,therearelittledataevaluating thetest-positiverateaccordingtogastrointestinalsymptomsat CTCintheliterature.Thishasimplicationsforrisk-stratification
andpotentiallyimpacts CRCscreening recommendation. We
thereforeevaluateinthisstudy,thedistributionofclinically sig-nificantcolonicfindingsandextra-colonicatCTC,accordingto
symptomsandagethroughareviewofa7-yearexperienceina
singlenon-academiccenter.
2. Materialsandmethods
2.1. Patients
Ourinstitutionalreviewboardapprovedthestudyand autho-rized this retrospective patient data analysis without further
consent. We searched our hospital records for all subjects
who completed a CTC procedure between June 2003 and
August2010.Thissearchyielded9122subjects(3822males,
5300females,averageage:60.11±13.75years,range:18–96
years). Indications for CTC included screening and direct
referral (n=8573), secondary referral after incomplete OC
(n=285),andDoubleContrastBariumEnema(DCBE)referral
change(n=264).Thisreferralchangewasjustifiedbythe
non-superiorityof DCBEoverCTC forcolonic lesionsinseveral
studies[16,17].
Writteninformedconsentwasgivenbyallsubjectspriorto procedures.1761subjectswithafamilialorpersonalhistoryof
polypsor colorectal cancer,genetic conditions, inflammatory
boweldisease,whowereatincreased-orhigh-riskfor colorec-talcancer[2]wereexcluded.Theremaining7361subjects,with
average-risk [18]for CRC (general population)(3073 males,
4288 females, average age: 60.3±13.9 years, range 18–96
years)wereevaluated. Theirclinicalstatus withregardtothe
presence ofthe following gastrointestinalsymptoms, prior to
CTCwasretrievedfromthereferralformsand/orgatheredby
patient’sanamnesisandallotheravailablepatientdata, includ-ing: (i) abdominal pain, (ii) constipation, (iii) diarrhea, (iv) irregularbowelmovement,(v)bloating,(vi)melena,(vii)rectal bleeding,and(viii)unexplainedweightloss.Weretrospectively assignedthesubjectstothreemaingroups,accordingtothe pur-portedclinicalimportanceofthesesymptomsregardingthelevel ofspecificityforCRC[5]:groupAincludedtheasymptomatic
subjects; groupB, the patients withone or morenonspecific
symptom(s) (i–vii) in the absence of an established “alarm”
symptom(viiandviii),whowereassignedtogroupC. 2.2. CTCtechnique
Allpatientsunderwentthesamestandardizedprocedurethat consistedintothree stepsincluding patientpreparation, scan-ninganddatainterpretation.Thepreparationinvolvedtwosteps
including cathartic colonic cleansing and residual fluid
tag-ging.Forpatientsingoodgeneralcondition,coloniccleansing
was achieved by a one-day clear liquid diet, one bottle of
sodium phosphate preparation (Fleet-Phospho-soda®, Wolfs,
Zwijndrecht,Belgium)and4tabletsofbisacodyl(Dulcolax®, BoehringerIngelheim,Ingelheim,Germany).Forfrailpatients,
cleansing consistedinto2 days of low-residuediet combined
to8gofmagnesium–sulphateontheexaminationday’s
morn-ing,inadditionto2tabletsofbisacodyland100mlofcontrast
agent(Gastrografin®,ScheringAG,Berlin,Germany)twicea
day.Inpatientswithrenalinsufficiency,cardiacfailureorsevere hypertension,preparationconsistedin3daysoflow-residuediet with2lofMoviprep®(Norgine,Heverlee,Belgium) (propylene-glycol+ascorbicacid)and4tabletsofbisacodylthedaybefore thestudy.Residualfluidtaggingwasobtainedbyingestionof 100mlGastrografin®theeveningbeforetheprocedureandtotal
colonicresidualfluidvolumewasreducedbyusinga
supposi-toryofbisacodylapproximately2hbeforeexamination,except
for patientswhounderwentCTCafter incompleteOC. These
patientsdrank100mlofGastrografinandinsertedasuppository ofbisacodyl1hbeforetheprocedure.Beforedataacquisition,an
ivinjectionof20mg/1mlofBuscopan®(butylhyoscinbromid
– Boehringer Ingelheim, Bruxelles,Belgium) was performed
andarectal cannula was inserted for colonicdistensionwith
an automaticcarbondioxideinsufflatorVMX-1010A(Vimap
technologiesTM,Girona,Spain).
A32-row(GELightspeedVCTTM,GEHealthcare,
Milwau-kee, WI)until09/2010,thena64-row(GEDiscoveryCT750
HDTM, GE Healthcare, Milwaukee, WI) multislice scanners
were used for image acquisitions. Parameters consisted into
1.2mm-thick slices with a 0.625mm reconstruction interval,
usinga50mAslow-doseprotocolswithvariablekV,adjusted
tobody-densityfordosereduction,supplementedsince2010by anadaptivestatisticaliterativereconstructionalgorithm(ASIR)
(GEHealthcare,Milwaukee,WI).Twoacquisitionswere
per-formed: the first in supine position andthe second, either in proneposition,or rightdecubitusfor unfitandobesepatients.
Immediatereviewof theimageswasperformed bya
radiolo-gistinallcases.In897patients(10%),athirdacquisitionwas
orderedbecauseof asegmentalcollapse preventingconfident
analysis.
Reading was performed offline on a workstation
(Advan-tageWindows,GEHealthcare,Milwaukee,WI)withasoftware
(Colon VCAR) allowing filet-view, supplemented by
“com-puter aided diagnosis” (CAD) assistancefrom January 2009,
andelectroniccleansingfromJune2010.Reconstruction
algo-rithms,imagedisplaypreferencesandreadingprinciplesused
forinterpretationaredescribedelsewhere[19].WeusedC-RAD reportingclassificationforallfindings[20].Eachfinding was
assigned tobothacolonicsegmentandadistancetotheanal
margin.
2.3. Dataanalysis
Clinicallysignificantcolonicfindingsweredefinedaseither
≥6mmpolyps,massesorothersrequiringwork-uportreatment
[20].Clinicalfiles,andreportsweresearchedforrepeatCTC, OCandsurgicalproceduresaftertheinitialCTC,when
standardfindings,usingavailablelocationbysegment,and/or distancefromtheanalmargin.Wedidnotattempttomatchusing
thesizecriteriabecause ofknownsizediscrepanciesbetween
OC and CTC polyp measurement [21]. Using an “intention
totreat”algorithm,per-patient CTCdiagnostic valuesforthe diagnosisofclinicallysignificantcolonicfindingswere calcu-lated.Patientswithatleastonematchedfindingwereconsidered
true-positivewhilepatientswithCTCfindingsunmatchingthe
referencestandardwereconsideredasfalse-positive.Thosewith nofindingatCTCandatleastonepositivefindingonthe refer-encestandardswereconsideredfalse-negative.
For patients with several colonic findings, two clinicians
having access to all available data were requested to
deter-mine inconsensus, the most significant withregard toCRC.
Forexample,an individualwithbotha>6mmcolonic polyp
andanon-neoplasticcolonicmassaccounted,foronlythefirst. In addition, theywere requested to establish a potential
cor-respondencebetweentheclinicalsymptomsandCTCcolonic
andsignificantextra-colonicfindings(i.e.:E4gradeoftheCTC ReportingandDataSystem,unrelatedtoacolonicdisease). 2.4. Statisticalanalysis
Percentages are given with their 95% Confidence
Inter-vals(CI).Continuousvariablesarecomparedusinganalysisof
variance,andtwo-tailedt-testsareusedfor directcomparison
betweentwogroups.Pearsonchi-squaretestsareusedto
com-pareproportionsandpercentages.Weusedaregressionanalysis toevaluatetheimpactofthegroupofsymptoms,ageandgender onthetest-positiverate.Ap-valueoflessthan0.05denotesa statisticalsignificance.
3. Results
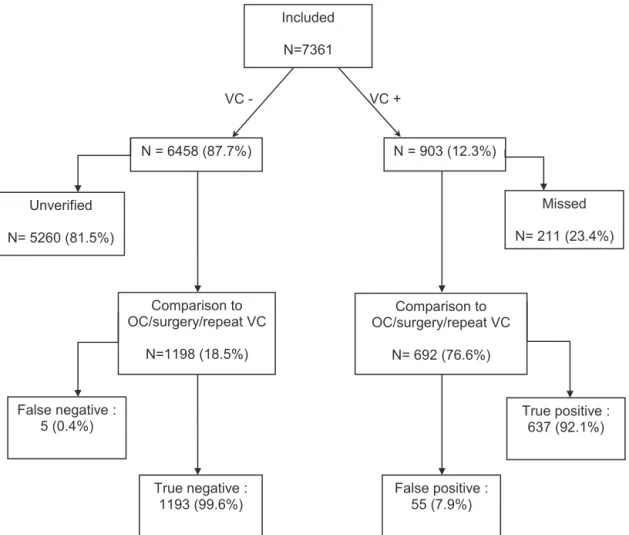
Fig.1summarizesthepatientflowchartinthisstudy.903out
of the 7361(12%,95%CI 11–13%)subjects hadatleastone
clinicallysignificantcolonicfindingatCTC.Histopathological diagnosesand/oretiologyfortheconfirmedfindingsaregivenin
Table1,showingthattheabnormalitiesweremostcommonlyof
mucosalorigin.ComparisonofCTCtoOCand/orsurgerywas
possible for692/903(77%;95%CI0.074–0.79) ofthese
sub-jects. CTCfindingswereconfirmedin637,while55 patients
werefalse-positive.Inaddition,repeatCTC,OCand/orsurgery
was performed within arange of 6–60 months in1198
sub-jectswithnegativeCTCfindingsforthefollowingreasons:656
patients underwentOCincludingprogrammedscreeningtests
in 168, exacerbation of a“non-alarm” gastrointestinal
symp-tom oroccurrenceof anewsymptomin443,andoccurrence
of an “alarm” symptomin48.527 patientsunderwent a
sec-ondCTCincludingprogrammedscreeningtestsin287,newor
Included N=7361 N= 6458 (87.7%) N= 903 (12.3%) VC - VC + Comparison to OC/surgery/repeat VC N=1198 (18.5%) Comparison to OC/surgery/repeat VC N= 692 (76.6%) True negative: 1193 (99.6%) False positive: 55 (7.9%) True positive: 637 (92.1%) Missed N= 211 (23.4%) False negative : 5 (0.4%) Unverified N= 5260 (81.5%)
Table1
HistopathologyandcausesofconfirmedCTCfindingsin642patients. Causesoffindings Confirmed
findings(N=642)
% 95%CI
Tumorsandpolyps
Adenocarcinoma 97* 15.10 0.12–0.18 Carcinoidtumor 1 0.15 NA Gastrointestinalstromal tumor 1 0.15 NA Peritonealmetastasis 1 0.15 NA Bladdercancerinfiltration 1 0.15 NA
Villous 11 1.71 0.01–0.03 Tubulovillous 69 10.75 0.08–0.13 Tubular 70** 10.90 0.08–0.14 Hyperplasic 35** 5.45 0.04–0.07 Hamartomatous 1 0.15 NA Fibrolipoma 1 0.15 NA Lipoma 4 0.62 0.00–0.01 Post-hemorrhoidscar 1 0.15 NA Others Diverticulitis 24 3.73 0.02–0.05 Ischemia 7 1.09 0.00–0.02 Endometriosis 7 1.09 0.00–0.02 Non-specificcolitis 1 0.15 NA Post-radiationcolitis 1 0.15 NA Leiomyoma 1 0.15 NA
Unknown(notrecovered foranalysis)
308 48.0 0.44–0.52
* Including3virtualcolonoscopyfalse-negative. ** Including1virtualcolonoscopyfalse-negative.
exacerbated “non alarm symptoms in 216 and“alarm
symp-toms” in 24. Twelve patients (all in group B) had surgical
resectionafteracutediverticulitis.
Fivepatientswerefalse-negative(2with>6mmpolypsand 3withcancers).Allcontrolledcases,yielda637/642(99.2%,
95%CI0.98–0.99)true-positivefractionanda55/692(7.95%,
95%CI0.05–0.09)false-positivefraction.
Theaveragenumberofsymptomsperpatientwas1.54±0.35 (3800,1524,662,29and3patientshadrespectively1,2,3,4and
5symptoms).Abdominalpainandconstipationwerethemost
frequent,whileunexplainedweightlosswastheleastfrequent
symptom.GroupsA,BandCincludedrespectively1343,5329
and689patients;theirdemographiccharacteristicsaregivenin
Table2.TheaverageageingroupC(64.37±14.61years)was significantlyhigherthaninbothgroupsA(60.96±11.22years) andB(59.43±14.31years)(p<0.001).Themale/femaleratio wasloweringroupB(0.63)versusgroupA(1.03)(p<0.001). TherateofE4findingsintheC-RADreportingsystemwas
sig-nificantlyhigheringroupC(44/689,6.4%,95%CI0.04–0.08)
versus groups A (26/1343, 1.9%, 95%CI 0.01–0.03) and B
(64/5329,1.2%,95%CI0.01–0.02)(p<0.001).Theregression
analysis showed that the group of symptom (B<C), gender
(F<M)andageallsignificantlyimpactedthetest-positiverateat CTC.Whenpoolingpatientsbyagegroups(Table3),the
test-positiverate ingroupC (138/689,20.0%, 95%CI 0.17–0.23)
was significantly higher than in groups A (79/1343, 5.8%,
95%CI0.04–0.07)andB(420/5329,7.8%,95%CI0.07–0.08)
(p<0.001),then, acrossall ages, exceptfor patients younger
than 50 years of age whose test-positive rate in groups C,
A andBwere respectively(6/96, 6.25%, 95%CI 0.01–0.11),
(5/173,2.9%,95%CI0.00–0.05)and(47/1241,3.8%,95%CI
0.02–0.04) (p=0.412). Diagnostic values of CTC within the
threegroupsaregiveninTable4.Boththetrue-positive frac-tionthatwasconsistentlyabove97%inallgroups(p=0.991) andthe false-positivefractiondid nodifferamongallgroups (p=0.240).
4. Discussion
In a recent meta-analysis evaluating the value of various
symptomsforCRCinprimarycare,Jellemaetal.foundlarge
heterogeneitiesinthesensitivityandspecificityofmost
symp-toms [6]. Rectal bleeding and unexplained weight loss have
consistently higher specificity than the others, according to
another recent meta-analysis [5]. Similarly to studies using
OC, the test-positive rate at CTC inour study washigher in
patientswiththese“alarm”symptomsthaninasymptomatic sub-jectsandthosewithminorgastrointestinalsymptoms(p<0.05).
Although theaverageagewasalsosignificantlyhigher inthe
“alarm”symptomsgroup(p<0.05),agewasnotakey determi-nant,sincesimilardifferenceswereobservedinallagegroups
above 50 years. In terms of CRC diagnostic
recommenda-tions,atest-positiveratearound20%makesOCtheprocedure
of choice in patients with “alarm” symptoms, owing to the
high resection-rate. An exceptionto thisrule, though requir-ingstrongerevidence,maybepatientsagedlessthan50years.
Theirfindingsprevalencewas6/96(6.25%;95%CI0.01–0.11),
i.e.:higherthaninage-matchedpatients,butcomparabletothe overallprevalenceintheothergroups.
Duetothelowspecificityoftheremaininggastrointestinal
symptoms (alone or in combination), diagnostic
recommen-dations currently fails to avoid OC facilities congestion by
proceduresyieldinglowratesofresection[9–11,22,23].
Excep-tion made of melena and hemorrhoids, the “non-alarm”
symptoms evaluated in this study are commonly described
in patients with irritable bowel syndrome (IBS) which is a
functionalboweldisorderaffecting7–15%ofthegeneral
pop-ulationin the USA,women being twice more oftenafflicted
thanmen[24,25].Itsdiagnosisnicelyillustratesthedichotomy
between the daily management of gastrointestinal symptoms
and evidence-baseddiagnostic recommendations. Indeed, the
diagnosisof IBSisbasedexclusivelyonclinicalcriteria[26],
but, up to 75% of clinicians believe that it should be
ascer-tainedbyexclusionof organicdisease [27].Forthispurpose,
approximately half of the patients with known or suspected
IBShaveundergoneatleastonediagnosticOCprocedure[28].
Lastly, it hasbeen estimated that 25% of the OCprocedures
performed inthe USAarefor patientswith“non-alarm”
gas-trointestinalsymptoms,althoughtheactualriskofCRCinthese patientsisnothigherthaninasymptomaticindividualsin sev-eralstudiesreportingvery closerangesoftest-positive rateat
OCamongIBS(range1.9–9.3%)andasymptomaticindividuals
(range4.5–12.1%)[29,30].Severalreasonscouldbeadvocated forthisdichotomy,includingphysiciansandpatient’sanxiety,
Table2
Patientgroupsdemographics.
Age±SD(years) Agerange(years) Sexratio C-RADreportingclassification
C0 C1 C2 C3 C4 E4 GroupA (N=1343) 60.96±11.22 21–91 683M/660F(1.03) 11 1179 (87%) 58 (4%) 55 (4%) 40 (3%) 26 (2%) GroupB (N=5329) 59.43±14.31 18–96 1975M/3144F(0.63) 21 4587 (86%) 269 (5%) 287 (5%) 165 (3%) 64 (1%) GroupC (N=689) 64.37±14.61 20–95 318M/371F(0.86) 0 594 (86%) 34 (5%) 38 (5%) 23 (3%) 44 (6%) p-value <0.0001* NA <0.0001** NA 0.906 0.573 0.174 0.916 <0.001*
* Significantlyhigher(p<0.05)ingroupCversusgroupBandgroupCversusgroupA. **TheproportionofmalesissignificantlyhigheringroupAversusgroupB(p<0.0001).
NA=notapplicable.
C-RADreportinganddatasystem.
• C0=inadequatestudy.
• C1=normalcolon/benignlesion.
• C2=polyps6–9mmindiameteror<3innumber.
• C3=polyp>10mmindiameteror>3polypswitheach6–9mm. E4=potentiallyimportantfinding:communicatetoreferringphysician.
symptomsseverityandthelackofevidencethatotherdiagnostic proceduresmaybeasaccurateorcost-effectivethanOC.
Toourknowledge,ourstudy isthefirstevaluatingcolonic
findings according to gastrointestinal symptoms using CTC.
Thiswaspossibleas,since2002,CTChasprogressivelybecome
aroutine procedurein ourinstitution, acknowledgedboth by
ourgastroenterologists,generalpractitionersandpatients.The
ranges of test-positive rate in asymptomatic individuals and
those with“non-alarm”symptoms werelow andcomparable
to thosereported using OC. In bothgroups, the test-positive
rate increased roughly linearly with aging (p<0.05). Given theirrelativelylowtest-positiverate,patientswith“non-alarm”
Table3
CTCtest-positiveratebyageinallgroups.
Age(years) GroupA GroupB GroupC Total p-value*
<50 5/173 47/1241 6/96 58/1510 0.412
(2.89%) (3.78%) (6.25%) (3.84%)
95%CI 95%CI 95%CI 95%CI
0.00–0.05 0.02–0.04 0.01–0.11 0.02–0.0.5
50–59 23/441 91/1362 22/159 136/1962(6.93%) 0.003*
(5.21%) (6.68%) (13.83%) 95%CI
95%CI 95%CI 95%CI 0.05–0.08
0.03–0.07 0.05–0.08 0.08–0.19
60–69 30/420 107/1251 42/151 179/1822(9.82%) <0.0001*
(7.14%) (8.55%) (27.81%) 95%CI
95%CI 95%CI 95%CI 0.08–0.11
0.04–0.09 0.07–0.10 0.21–0.35
70–79 14/244 134/1077 42/180 190/1501(12.65%) <0.0001*
(5.73%) (12.44%) (23.33%) 95%CI
95%CI 95%CI 95%CI 0.10–0.14
0.02–0.08 0.10–0.14 0.17–0.29
>80 7/65 41/398 26/103 74/566(13.07%) 0.003**
(10.77%) (10.30%) (25.24%) 95%CI
95%CI 95%CI 95%CI 0.10–0.16
0.03–0.18 0.07–0.13 0.17–0.33
Total 79/1343 420/5329 138/689 637/7361 <0.0001*
(5.88%) (7.88%) (20.02%) (8.65%)
95%CI 95%CI 95%CI 95%CI
0.04–0.07 0.07–0.08 0.17–0.23 0.08–0.09
p-value 0.176 <0.0001 =0.002 <0.0001 NA
* SignificantdifferencebetweengroupCversusgroupsAandB. **SignificantdifferencebetweengroupCversusgroupsB.
Table4
DiagnosticvaluesofCTCversusOCand/orsurgeryingroupsA,BandC. Abbreviationsasinthetext.
True-positivefraction False-positivefraction GroupA 79/79(100%) (95%CI=NA) 10/89(11%) (95%CI0.04–0.17) GroupB 420/422(99.5%) (95%CI0.98–1.00) 38/458(8%) (95%CI0.05–0.11) GroupC 138/141(97.9%) (95%CI0.95–1.00) 7/145(5%) (95%CI0.01–0.08) p-value 0.991 0.240
symptoms may indeed benefit from accurate noninvasive
diagnosticprocedures.Inourseriesof5329patientswith
“non-alarm” symptoms, 4088 were older than 50 years of age: it
maythusbeemphasizedthatthesesymptoms werean
incen-tivetocomplywithscreeningguidelines andshouldtherefore
becarefullysought.Althoughpatientsunder50yearsofageand
suspectedofIBSshouldnotundergoanycolonicexploration,
manyof themunfortunatelydoinmostinstitutions,including
ours.Inourseries,thismalpracticeappliesto1241patients(23%
referredbygastroenterologists)forwhomCTChasalow
test-positiverateingoodcorrelationwithotherseries,butrepresent themostcomprehensiveandthelessharmfuloptionascompared toOC,thestandardofreference.
Inthissetting,ourstudyconfirmedthatCTChasahigh true-positivefractionandalowfalse-positivefractionforpolypsand CRCinallgroups[15,31]clearlyadvocatingforits recommen-dationinpatientswith“non-alarm”gastrointestinalsymptoms.
Consideringtherecommendationsguidelines forCRC,the
main disadvantage of CTC, namely the inability to perform
polypectomy,mayraisecost-effectivenessissues.Inourstudy,at least420additionalOCwereperformedinthegroupofpatients
with“non-alarm”symptoms,whileapproximately5000
poten-tialdiagnosticOCprocedureswerereplacedbylessexpensive
CTCprocedures,puttingobviouslythecost-effectiveness
bal-anceinfavorofaprimarydiagnosticprocedurebyCTCinour
institution.In addition,offeringasame-dayOCafter positive
CTCscenario currently prevent the needfor asecond bowel
preparationinmostinstitutions includingours[32].The abil-ityto detect clinicallysignificant extra-colonic abnormalities
(E4)mayalsoimpactCTCcost-effectiveness.Atotalnumber
of 134 individuals hadE4findings, whichrepresentan asset
forCTC.Interestingly,therateofE4findingsdistributionper group was quite similar to the test-positive rate distribution, prevailingthus in olderpatients. Thisindicates that E4 find-ingsratearenoteitherincreasedbynonspecificgastrointestinal symptoms.Lastly,theconcernofexposuretoionizingradiation
during CTC,has dropped significantly with the latest
gener-ationof scanners [33]. In our institution, the combined dose
forsupine andproneacquisitions of anaverage-sizedsubject
isapproximately3mSv (i.e.: levelscomparabletotheannual
environmentalscatterdoses).
Somelimitationsmaybeappliedtothisstudyandits conclu-sions,thefirstofwhichisitsretrospectivenaturethatresulted intoseveralpatientslosttofollow-upandclinicaldatamissing.
Histopathologicaldatawerealsomissinginseveralpatientfrom
mis-retrievedpolypectomiesthatare,however,notuncommon
[33].Reportedsymptomswerenotbasedonachecklistofall
gastrointestinalsymptoms.Thesamedesignforcedustopool
symptomsintwogroups,resultingintopotentialmaskingofthe effectofoneorseveralunderrepresentedindividualsymptom(s) ontheCTCtest-positiverate.Moreover,ageofsymptomsonset, theirintensityanddurationlacked,preventingabetterdetailed relationshipbetweensymptomsandtest-positiverate.Reported
diagnosticvaluesofCTC,thoughcomparablewithpreviously
reportedstudies,aresubjecttoverificationbias,sinceOCwas
mostlyperformed when CTC waspositive. In addition, most
oftheunverifiedCTCpositivecasesweresmallpolyps,which
probably decreased artificially the false-positive rate. Lastly, patientswithatleastonegastrointestinalsymptomlargely
out-numberedasymptomaticsubjectsinourstudy,with6929outof
the7361average-risksubjectsforCRChavingatleastone
symp-tom. Thisselection biaswas causedbyaprogressive referral
replacementofDCBEbyCTCduringthestudyperiod.
5. Conclusion
In conclusion, asymptomaticsubjects, patients with
“non-alarm” gastrointestinal symptoms and even young patients
with “alarm” symptoms for CRC have nearly similarly low
test-positive rates at CTC. Overall, the rate of colonic and
extra-colonicfindingsincreasedwithage,malegenderandthe
presence of“alarm” symptomsfor CRC.The highdiagnostic
valueofCTCinallpatientgroupsmakesittheexaminationof choiceinlow-yieldpatients;whoincludeasymptomaticsubjects
andthosewith“non-alarm”gastrointestinalsymptoms.
Conflictofinterest
Theauthorsdeclarethatthereisnoconflictofinterest.
References
[1]FerlayJ,Steliarova-FoucherE,Lortet-TieulentJ,RossoS,CoeberghJW, ComberH,etal.CancerincidenceandmortalitypatternsinEurope: esti-matesfor40countriesin2012.EurJCancer2013;49(6):1374–403.
[2]LevinB,LiebermanDA,McFarlandB,SmithRA,BrooksD,AndrewsKS, etal.Screeningandsurveillancefortheearlydetectionofcolorectalcancer andadenomatouspolyps,2008:ajointguidelinefromtheAmericanCancer Society,theUSMulti-SocietyTaskForceonColorectalCancer,andthe AmericanCollegeofRadiology.CACancerJClin2008;58(3):130–60.
[3]McFarlandEG,LevinB,LiebermanDA,PickhardtPJ,JohnsonCD,Glick SN,etal.Revisedcolorectalscreeningguidelines:jointeffortofthe Amer-icanCancerSociety,U.S.MultisocietyTaskForceonColorectalCancer, andAmericanCollegeofRadiology.Radiology2008;248(3):717–20.
[4]ArditiC, Peytremann-BridevauxI, BurnandB, EckardtVF, BytzerP, AgreusL,etal.AppropriatenessofcolonoscopyinEurope(EPAGEII). Screeningforcolorectalcancer.Endoscopy2009;41(3):200–8.
[5]AdelsteinBA,MacaskillP,ChanSF,KatelarisPH,IrwigL.Mostbowel cancersymptomsdonotindicatecolorectalcancerandpolyps:asystematic review.BMCGastroenterol2011;11:65.
[6]JellemaP,vanderWindtDA,BruinvelsDJ,MallenCD,vanWeyenberg SJ,MulderCJ,etal.Valueofsymptomsandadditionaldiagnostictests forcolorectalcancerinprimarycare:systematicreviewandmeta-analysis. BMJ2010;340:c1269.
[7]LiebermanDA,WilliamsJL,HolubJL,MorrisCD,LoganJR,EisenGM, etal.Colonoscopyutilizationandoutcomes2000to2011.Gastrointest Endosc2014;80(1):133–43.
[8]AstinM,GriffinT,NealRD,RoseP,HamiltonW.Thediagnosticvalueof symptomsforcolorectalcancerinprimarycare:asystematicreview.BrJ GenPract2011;61(586):e231–43.
[9]RexDK,LiebermanDA.Feasibilityofcolonoscopyscreening:discussion ofissuesandrecommendationsregardingimplementation.Gastrointest Endosc2001;54(5):662–7.
[10]Hoffman RM, Espey D, Rhyne RL. A public-health perspective on screeningcolonoscopy.ExpertRevAnticancerTher2011;11(4):561–9.
[11]LevinTR.Colonoscopycapacity:canwebuildit?Willtheycome? Gas-troenterology2004;127(6):1841–4.
[12]ShapiroJA,SeeffLC,ThompsonTD,NadelMR,KlabundeCN,Vernon SW.Colorectalcancertestusefromthe2005NationalHealthInterview Survey.CancerEpidemiolBiomarkersPrev2008;17(7):1623–30.
[13]Halligan S,Wooldrage K,Dadswell E, Kralj-Hans I, vonWagner C, EdwardsR,etal.Computedtomographic colonographyversus barium enema for diagnosis of colorectal cancer or large polyps in symp-tomatic patients (SIGGAR): a multicentre randomised trial. Lancet 2013;381(9873):1185–93.
[14]Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus colonoscopyforinvestigationofpatientswith symptomssuggestiveof colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet 2013;381(9873):1194–202.
[15]PickhardtPJ,ChoiJR,HwangI,ButlerJA,PuckettML,HildebrandtHA, etal.Computedtomographicvirtualcolonoscopytoscreenfor colorec-talneoplasiainasymptomaticadults.NEnglJMed2003;349(23):2191– 200.
[16]JohnsonCD,MacCartyRL,WelchTJ,WilsonLA,HarmsenWS,Ilstrup DM,etal.ComparisonoftherelativesensitivityofCTcolonographyand double-contrastbariumenemaforscreendetectionofcolorectalpolyps. ClinGastroenterolHepatol2004;2(4):314–21.
[17]SosnaJ,Bar-ZivJ,LibsonE,EligulashviliM,BlacharA.Criticalanalysis oftheperformanceofdouble-contrastbariumenemafordetecting colorec-talpolyps≥6mmintheeraofCTcolonography.AJRAmJRoentgenol 2008;190(2):374–85.
[18]Summariesforpatients.Screeningforcolorectalcancer:recommendations fromtheUnitedStatesPreventiveServicesTaskForce.AnnInternMed 2002;137(2):I38.
[19]HockD,OuhadiR,MaterneR,AouchriaAS,ManciniI,BroussaudT, etal.VirtualdissectionCTcolonography:evaluationoflearningcurves andreadingtimeswithandwithoutcomputer-aideddetection.Radiology 2008;248(3):860–8.
[20]ZalisME,BarishMA,ChoiJR,DachmanAH,FenlonHM,FerrucciJT, etal.CTcolonographyreportinganddatasystem:aconsensusproposal. Radiology2005;236(1):3–9.
[21]deVriesAH,BipatS,DekkerE,LiedenbaumMH,FlorieJ,FockensP, etal.PolypmeasurementbasedonCTcolonographyandcolonoscopy: variabilityandsystematicdifferences.EurRadiol2010;20(6):1404–13.
[22]BrownML,KlabundeCN,MysliwiecP.Currentcapacityforendoscopic colorectalcancerscreeningintheUnitedStates:datafromtheNational CancerInstituteSurveyofColorectalCancerScreeningPractices.AmJ Med2003;115(2):129–33.
[23]Seeff LC, Manninen DL, DongFB, Chattopadhyay SK, NadelMR, TangkaFK,etal.Isthereendoscopiccapacitytoprovidecolorectalcancer screeningtotheunscreenedpopulationintheUnitedStates? Gastroenter-ology2004;127(6):1661–9.
[24]MeleineM,MatriconJ.Gender-relateddifferencesinirritablebowel syn-drome:potentialmechanismsof sexhormones.WorldJGastroenterol 2014;20(22):6725–43.
[25]KhanS,ChangL.DiagnosisandmanagementofIBS.NatRev Gastroen-terolHepatol2010;7(10):565–81.
[26]LongstrethGF,ThompsonWG,CheyWD,HoughtonLA,MearinF,Spiller RC.Functionalboweldisorders.Gastroenterology2006;130(5):1480–91.
[27]Spiegel BM, Farid M, Esrailian E, Talley J, Chang L. Is irritable bowelsyndrome a diagnosis of exclusion?: a surveyof primarycare providers, gastroenterologists, and IBS experts. Am J Gastroenterol 2010;105(4):848–58.
[28]Talley NJ, Boyce P, Owen BK. Psychological distress and seasonal symptom changes in irritable bowel syndrome. Am J Gastroenterol 1995;90(12):2115–9.
[29]LiebermanDA,HolubJ,EisenG,KraemerD,MorrisCD.Prevalenceof polypsgreaterthan9mminaconsortiumofdiverseclinicalpractice sett-ingsintheUnitedStates.ClinGastroenterolHepatol2005;3(8):798–805.
[30]CashBD,Schoenfeld P, CheyWD. Theutilityof diagnostic tests in irritablebowelsyndromepatients:asystematicreview.AmJGastroenterol 2002;97(11):2812–9.
[31]JohnsonCD,ChenMH,ToledanoAY,HeikenJP,DachmanA,KuoMD, etal.AccuracyofCTcolonographyfordetectionoflargeadenomasand cancers.NEnglJMed2008;359(12):1207–17.
[32]PickhardtPJ,TaylorAJ,KimDH,ReichelderferM,GopalDV,PfauPR. ScreeningforcolorectalneoplasiawithCTcolonography:initial expe-riencefromthe1styearofcoveragebythird-partypayers.Radiology 2006;241(2):417–25.
[33]BerringtondeGonzalezA,KimKP,KnudsenAB,Lansdorp-VogelaarI, RutterCM,Smith-BindmanR,etal.Radiation-relatedcancerrisksfrom CTcolonographyscreening:arisk-benefitanalysis.AJRAmJRoentgenol 2011;196(4):816–23.