SHORTCOMMUNICATION
Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi
sensu lato in North Africa
ELYES ZHIOUA,1ALI BOUATTOUR,2CHANG MIN HU,3MOHAMED GHARBI,2 ANDRE AESCHLIMAN,3HOWARD S. GINSBERG,4ANDLISE GERN3
J. Med. Entomol. 36(2): 216Ð218 (1999)
ABSTRACT Free-living adult Ixodes ricinus L. were collected in Amdoun, situated in the Kroumiry
mountains in northwestern Tunisia (North Africa). Using direct ßuorescence antibody assay, the infection rate of Þeld-collected I. ricinus by Borrelia burgdorferi sensu lato was 30.5% (n 5 72). No difference in infection rate was observed between male and female ticks. Spirochetes that had been isolated from I. ricinus from Ain Drahim (Kroumiry Mountains) in 1988 were identiÞed as Borrelia
lusitaniae(formerly genospecies PotiB2). This is the Þrst identiÞcation of a genospecies of Borrelia
burgdorferisensu lato from the continent of Africa.
KEY WORDS Ixodes ricinus, Borrelia burgdorferi, Borrelia lusitaniae, Tunisia
INEUROPE, Ixodes ricinus L. is the principal vector of
Borrelia burgdorferi,the etiologic agent of Lyme bor-reliosis (Aeschlimann et al. 1986). Borrelia burgdorferi sensu lato is now known to include at least 5 geno-species associated with I. ricinus: Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii, Borrelia
valaisiana(formerly genospecies VS116), and Borrelia
lusitaniae(formerly genospecies PotiB2) (Baranton et al. 1992; Canica et al. 1993; Postic et al. 1994, 1997; Le Fle`che et al. 1997; Wang et al. 1997). The geographical distribution of I. ricinus covers southern Scandinavia, the British Isles, Central Europe, France, Spain, Por-tugal, Italy, the Balkans, East Europe, northern Iran, and North Africa (Perez and Rodhain 1977, Stanek et al. 1988). Infection of I. ricinus by B. burgdorferi sensu lato has been reported from many European coun-tries. In Africa, however, primarily serological and clinical results have been reported (Stanek et al. 1986, Ginsburg et al. 1991, Zahaf et al. 1994, Mhalu and Matre 1996), and few entomological studies have been performed (Zhioua et al. 1989). The objectives of this study were to determine the infection rate of I. ricinus by B. burgdorferi sensu lato at a sample site in North-ern Africa (Tunisia), and to identify the genospecies of Borrelia isolated from I. ricinus from Tunisia.
Materials and Methods
Study Site. In Tunisia, I. ricinus is localized in the
northwest, mainly in the mountains of Kroumiry
(At-las mountains), where it is abundant in oak forests (Zhioua et al. 1989, Bouattour and Darghouth 1996). These forests have rich undergrowth and harbor small mammals, deer, and wild boar (Gharaibeh 1997). The activity peak of adult I. ricinus in this area is in Feb-ruaryÐMarch (Senevet and Rossi, 1924, Sergent and Poncet 1937, YousÞ-Monod and Aeschlimann 1986), so we sampled ticks in March 1996. Unfed adult ticks were collected by ßagging or by direct collection from the grass in the region of Amdoun (Kroumiry).
Spirochete Detection. Adults were
surface-steril-ized and dissected to remove the gut. Midguts were chilled, placed on glass slides, and examined for the presence of spirochetes using direct ßuorescent anti-body assay (DFA) (Zhioua et al. 1994). Antigens were dried for at least 2 h at 348C and subsequently Þxed with acetone for 10 min. Preparations were stained directly with high-titered ßuorescein isothiocyanate conjugated anti-B. burgdorferi goat antibody (KPL, Gaithersburg, MD).
In 1988, spirochetes were isolated from Þeld-col-lected I. ricinus from the region of Ain Drahim (Kroumiry, Tunisia) (Zhioua et al. 1989). Unfortu-nately, identiÞcation of these spirochetes was not pos-sible in 1988 because the cultures were contaminated (Zhioua et al. 1989), and the isolates were stored at 2708C. Technical advances now allow characteriza-tion of these isolates. Borrelia isolates were identiÞed in 1996 by rrf (5S)-rrf (23S) spacer restriction frag-ment-length polymorphism (RFLP) analysis as de-scribed by Postic et al. (1994).
Results and Discussion
In total, 72 adult I. ricinus (41 females, 31 males) collected from Amdoun in 1996 were examined for the presence of B. burgdorferi sensu lato by DFA. The infection rate was 30.5% (22/72). No signiÞcant
dif-1Center for Vector-Borne Disease, Woodward Hall, University of
Rhode Island, Kingston, RI 02881.
2Institute Pasteur of Tunis, Department of Medical Entomology, 13
Place Pasteur, 1002 Tunis, Tunisia.
3Institute of Zoology, Emile Argand 11, University of Neuchaˆtel,
Neuchaˆtel, Switzerland.
4USGS Patuxent Wildlife Research Center, Woodward Hall-PLS,
ference was observed between the infection rates of females (36.6%, n 5 41) and males (22.6%, n 5 31) (x2 5 1.63, df 5 1, P 5 0.2).
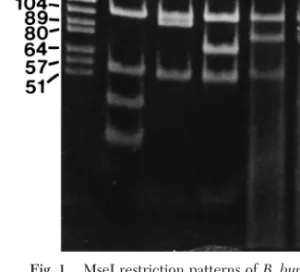
IdentiÞcation of 2 strains of spirochetes isolated from Tunisia in 1988 showed 1 genospecies, B.
lus-itaniae(Fig. 1). Borrelia burgdorferi sensu stricto, B.
garinii, B. afzelii,and B. valaisiana were not observed. Here, we report the presence of B. burgdorferi sensu lato in I. ricinus on the African continent. On a sub-tropical island in the Atlantic, northwest of continen-tal Africa, the infection rate of I. ricinus by B.
burg-dorferiwas 1.3% (Matuschka et al. 1994). The 30.5% infection rate we observed in Tunisia is within the range reported from European countriesÑ13.8% in Spain (Mareuez and Constan 1990), 9.6% in Belgium (Martin et al. 1990), 5Ð50% in Switzerland (Aeschli-mann et al. 1986, Gern et al. 1992), 20% in the Neth-erlands (Rijpkema et al. 1994), 18% in Germany (Ma-tuschka et al. 1992), 13% in Slovenia (Strle et al. 1995), 45% in Croatia (Rijpkema et al. 1996), and 12.4% in France (Zhioua et al. 1996). Additional studies are needed to determine the infection rates of immatures and to identify the reservoir hosts of B. burgdorferi sensu lato in Tunisia.
Borrelia burgdorferisensu stricto is the only patho-genic genospecies currently known to be present in the United States (Postic et al. 1994). In Eurasia, B.
afzeliiand B. garinii are widely distributed (Fukunaga and Hamase 1995, Postic et al. 1997). In Europe, 3 genospecies associated with Lyme borreliosis have been recognized to dateÑB. burgdorferi sensu stricto,
B. garinii,and B. afzelii. These genospecies have been isolated from patients, rodents, birds, and I. ricinus from various areas in Europe (Humair et al. 1995, Olsen et al. 1995, Picken et al. 1996, Zhioua et al. 1996, Postic et al. 1997). Two additional genospecies have been observed in I. ricinus and identiÞed as B.
val-aisianaand B. lusitaniae (formerly the genospecies
VS116 and PotiB2, respectively) (Postic et al. 1994, 1997; Pe´ter et al. 1995; Rijpkema et al. 1995; Le Fle`che et al. 1997; Wang et al. 1997). To date, only a few isolates have been identiÞed as B. lusitaniae; 3 from Portugal, 2 from Moldavia, 1 from Ukraine, 1 from Czech Republic (Postic et al. 1994, 1997), and now 1 from North Africa. The roles of B. lusitaniae and B.
valaisianain the etiology of Lyme borreliosis remain to be clariÞed.
In western, central, and northern Europe, I. ricinus is commonly infected with various Borrelia genospe-cies, but rarely with B. lusitaniae, which appears to be limited to the southern part of the northern Hemi-sphere. Additional isolates from I. ricinus are neces-sary to describe the heterogeneity of spirochetes and the distribution of the various genospecies in southern Europe and North Africa. In sub-Saharan Africa, the existence of B. burgdorferi sensu lato remains unclear, but seems to be improbable because of the absence of ticks of the I. ricinus species complex (Mason et al. 1994, Marjolet et al. 1995).
Acknowledgments
The authors thank K. Dellagi (Director, Pasteur Institute of Tunis) for his constant support, including logistics and laboratory facilities. We also thank R. A. LeBrun, P. Logan, D. Dennis, and A. Spielman for constructive comments on early drafts of the manuscript. Raquel Martinez and Paul Johnson provided technical assistance. This work beneÞted from support by the Swiss National Science Foundation (Grant Nos. 3.975.87 and 32-29964.90), the U.S. National Biological Service, and the Department of Plant Sciences. This is Contribution No. 3446 of the College of Resource Development, University of Rhode Island.
References Cited
Aeschlimann, A., E. Chamot, F. Gigon, J.-P. Jeanneret, D. Kesseler, and C. Walter. 1986. Borrelia burgdorferi in
Switzerland. Zentralbl. Bakteriol. Microbiol. Hyg. Ser. A 263: 450Ð458.
Baranton, G., D. Postic, I. Saint-Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P.A.D. Grimont. 1992.
Delin-eation of Borrelia burgdorferi sensu stricto, Borrelia
ga-riniisp. nov., and Group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42: 378Ð383.
Bouattour, A., and M. A. Darghouth. 1996. First report of
Babesia divergensin Tunisia. Vet. Parasitol. 63: 161Ð165.
Canica, M. M., F. Nato, L. du Merle, J. C. Mazie, G. Baranton, and D. Postic. 1993. Monoclonal antibodies for the
iden-tiÞcation of Borrelia afzelii sp. nov., associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25: 441Ð448.
Fukunaga, M., and A. Hamase. 1995. Outer surface protein
C gene sequence analysis of Borrelia burgdorferi sensu lato isolates from Japan. J. Clin. Microbiol. 33: 2415Ð2420.
Gern, L., W. Burgdorfer, A. Aeschlimann, and H. E. Krampitz. 1992. The ecology of Lyme borreliosis in
Eu-rope, pp. 59Ð69. In K. Weber and W. Burgdorfer [eds.], Aspects of Lyme borreliosis. Spring, Berlin.
Gharaibeh, B. M. 1997. Systematics, distribution, and
zoo-geography of mammals of Tunisia. Ph.D. dissertation, Texas Tech University, Lubbock, TX.
Fig. 1. MseI restriction patterns of B. burgdorferi sensu
lato. Lane 1, DNA size standards; lane 2, B. burgdorferi sensu stricto; lane 3, B. garinii; lane 4, B. afzelii; lane 5, B. lusitaniae; lanes 6 and 7, Tunisian isolates.
Ginsburg, Ch., C. Saint-Val, J. F. Eizenbaum, and J. D. Degos. 1991. Maladie de Lyme africaine. Pres. Med. 20: 2161Ð
2162.
Humair, P. F., O. Pe´ter, R. Wallich, and L. Gern. 1995.
Strain variation of Lyme disease spirochetes isolated from
Ixodes ricinusticks and rodents collected in two endemic areas in Switzerland. J. Med. Entomol. 32: 433Ð438.
Le Fle`che, A., D. Postic, K. Girardet, O. Pe´ter, and G. Baran-ton. 1997. Characterization of Borrelia lusitaniae sp. nov.
by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47: 921Ð925.
Mareuez, F. J., and M. C. Constan. 1990. Infection dÕIxodes
ricinus(L. 1758) et Haemaphysalis punctata Canestrini et Franzo, 1877 (Acarina, Ixodidae) par Borrelia burgdorferi dans le nord de la pe´ninsule ibe´rique (Pays Basque Es-pagnol et Navarre). Bull. Soc. Fr. Parasitol. 8: 323Ð330.
Marjolet, M., B. Gueglio, and M. Traoure. 1995. Does Lyme
disease (or an analogous disease) exist in Mali, West Africa? Trans. R. Soc. Trop. Med. Hyg. 89: 387.
Martin, Ph., G. Bigaignon, A. Thirion, and A. Fain. 1990.
Fre´quence de Borrelia burgdorferi (maladie de Lyme) et re´partition de son vecteur Ixodes ricinus (Acari: Ixodidae) dans le district de Mosan de Belgique. Bull. Soc. Fr. Parasitol. 8: 331Ð338.
Mason, P. R., P. J. Kelly, I. Nilson, and T. Wadstrom. 1994.
Apparent absence of Lyme borreliosis in Zimbabwe. Trans. R. Soc. Trop. Med. Hyg. 88: 412.
Matuschka, F. R., P. Fisher, M. Heiler, S. Blu¨mcke, and A. Spielman. 1992. Stage-associated risk of transmission of
the Lyme disease spirochete by European Ixodes ticks. Parasitol. Res. 78: 695Ð698.
Matuschka, F. K., H. Eiffer, A. Ohlenbusch, D. Richter, E. Schein, and A. Spielmann. 1994. Transmission of the
Lyme disease on a subtropical island. Trop. Med. Para-sitol. 45: 39Ð44.
Mhalu, F. S., and R. Matre. 1996. Serological evidence of
Lyme borreliosis in Africa: results from studies in Dar es Salem, Tanzania. East. Afr. Med. J. 73: 583Ð585.
Olsen, B., T.G.T. Jaenson, and S. Bergstro¨m. 1995.
Preva-lence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 61: 3082Ð3087.
Perez, C., and F. Rodhain. 1977. Biologie dÕIxodes ricinus L.,
1758. I. Ecologie, cycle e´volutif. Bull. Soc. Pathol. Exot. 70: 187Ð201.
Pe´ter, O., A. G. Bretz, and D. Bee. 1995. Occurrence of
different genospecies of Borrelia burgdorferi sensu lato in ixodid ticks of Valais, Switzerland. Eur. J. Epidemiol. 11: 463Ð467.
Picken, R. N., Y. Cheng, F. Strle, J. Cimperman, V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, D. Han, J. A. Nelson, M. M. Picken, and G. M. Trenholme. 1996. Molecular
characterization of Borrelia burgdorferi sensu lato from Slovenia revealing signiÞcant differences between tick and human isolates. Eur. J. Clin. Microbiol. Infect. Dis. 15: 313Ð323.
Postic, D., M. V. Assous, P.A.D. Grimont, and G. Baranton. 1994. Diversity of Borrelia burgdorferi sensu lato
evi-denced by restriction fragment length polymorphism of
rrf(5S)-rrf (23S) intergenic spacer amplicons. Int. J. Sys. Bacteriol. 44: 743Ð752.
Postic, D., E. Korenberg, N. Gorelova, Y. V. Kovalevski, E. Bellenger, and G. Baranton. 1997. Borrelia burgdorferi
sensu lato in Russia and neighbouring countries: high incidence of mixed isolates. Res. Microbiol. 148: 691Ð702.
Rijpkema, S.G.T., J. Nieuwenhuiis, F.F.J. Franssen, and F. J. Jongejan. 1994. Infection rates of Borrelia burgdorferi in
different instars of Ixodes ricinus from the Duch North Sea Island of Ameland. Exp. Appl. Acarol. 18: 531Ð542.
Rijpkema, S.G.T., M.J.C.H. Molkenboer, L. M. Schouls, F. J. Jongean, and J.F.P. Schellekens. 1995. Simultaneous
de-tection and genotypic of three genomic groups of Borrelia
burgdorferisensu lato in Dutch Ixodes ricinus ticks by characterization of the ampliÞed intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33: 3091Ð3095.
Rijpkema, S.G.T., D. Golubic, M. Molkenboer, N. Ver-beek-De Kruif, and J. Schellekens. 1996. IdentiÞcation
of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 20: 23Ð30.
Senevet, G., and P. Rossi. 1924. Contribution a` lÕe´tude des
Ixodide´s (XII Note). Etude saisonnie`re des ixodide´s dans la re´gion de Bouira (Alge´rie). Arch. Inst. Pasteur Alger. 2: 223Ð232.
Sergent, E., and A. Poncet. 1937. Tableau de la re´partition
saisonnie`re des tiques les plus re´pandues en Alge´rie. Arch. Inst. Pasteur Alger. 37: 220Ð224.
Stanek, G., A. Hirschl, H. Stemberger, G. Wewalka, and G. Wiedermann. 1986. Does Lyme borreliosis occur in
tropical and subtropical areas? Zentralbl. Bakteriol. Mi-crobiol. Hyg. Ser. A 263: 491Ð495.
Stanek, G., M. Pletschette, H. Flamm, A. M. Hirschl, E. Aberer, W. Kristoferitsch, and E. Schmutzhard. 1988.
European Lyme borreliosis. Ann. N.Y. Acad. Sci. 539: 274Ð282.
Strle, F., Y. Cheng, M. Picken, J. K. Bouseman, and R. N. Picken. 1995. Infection rates of Ixodes ricinus ticks with
Borrelia afzelii, Borrelia garinii,and Borrelia burgdorferi sensu stricto in Slovenia. Eur. J. Clin. Microbiol. Infect. Dis. 14: 994Ð1001.
Wang, G., A. P. van Dam, A. Le Fle`che, D. Postic, G. Baran-ton, R. de Boer, L. Spanjaard, and J. Dankert. 1997.
Ge-netic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19). Int. J. Syst. Microbiol. 47: 926Ð932.
Yousfi-Monod, R., and A. Aeschlimann. 1986. Recherche sur
les tiques (Acarina, Ixodidae) parasites des bovide´s dans lÕouest alge´rien. I- Inventaire syste´matique et dynamique saisonnie`re. Ann. Parasitol. Hum. Comp. 61: 341Ð358.
Zahaf, A., S. Bouassida, S. Boudaya, and H. Turki. 1994. La
maladie de Lyme a` Sfax. Ann. Dermatol. Venereol. 121: 177Ð179.
Zhioua, E., L. Gern, and A. Aeschlimann. 1989. Isolement
dÕun spiroche`te a` partir dÕIxodes ricinus de Tunisie. Bull. Soc. Fr. Parasitol. 7: 107Ð110.
Zhioua, E., A. Aeschlimann, and L. Gern. 1994. Infection of
Þeld-collected Ixodes ricinus (Acari: Ixodidae) larvae with Borrelia burgdorferi in Switzerland. J. Med. Entomol. 31: 763Ð766.
Zhioua, E., D. Postic, F. Rodhain, and C. Perez-Eid. 1996.
Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia
burgdorferiin Ile de France. J. Med. Entomol. 34: 694Ð 697.
Received for publication 27 March 1998; accepted 13 October 1998.