GENETIC VARIATION IN SYMPATRIC AND ALLOPATRIC
POPULATIONS OF HYBRIDIZING FRESHWATER SNAIL
SPECIES (VIVIPARUS ATER AND V. CONTECTUS)
MASAYA KATOH
1and GEORG RIBI
2'Seikai National Fisheries Research Institute, Fukai-Ohta 148-446, Ishigaki 907-04, Japan. 2Zoologisches Museum der Universitat ZUnch, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland
(Received 21 June 1996; accepted 31 January 1997)
ABSTRACT
To estimate the geographical extent of introgression, we studied the genetic structure of sympatric and allopatric populations of hybridizing freshwater snail species Viviparus ater and V. contectus in central Europe. Six allozyme loci which were variable in Lake Garda, Italy in a previous study (five nearly diagnostic loci between the two species and one highly poly-morphic locus in V. contectus) were analyzed from ten sympatric locations and four allopatric populations each for the two species. Presumably introgressed genes (low allele frequencies) were found from at least one locus in seven out of the ten sympatric sites. These seven sites covered most of northern Italy. The data indicate that introgression has occurred from Viviparus contectus to V. ater and vice versa. There-fore, there is a possibility of widespread introgression or mosaic zones in nature. However, we cannot rule out that the observed patterns are due to the shared ancestry. V. ater possessed low genetic variation (the jackknifed mean of Wright's F^ ± S.E. over four loci was 0.041 ± 0.004). On the other hand, V. contectus showed high genetic differentiation (the jackknifed mean of Fgr ± S.E. over six loci was 0.546 ± 0.166). Although introgression may have caused evolution-ary changes in V. ater and V. contectus, it was not strong enough to level out the genetic differences between the two species, which may have originated from isolation among populations in V. contectus and a past bottleneck event in V. ater.
INTRODUCTION
Hybridization and introgression are at the
centre of evolutionary interest because they
can help to understand how reproductive
isola-tion evolves. Examples of hybridizaisola-tion and
introgression have been reported from many
plant (Rieseberg & Wendel, 1993) and animal
Address for correspondence: Dr. M Katoh, Ishigaki Tropical Station. Seikai National Fisheries Research Institute, Fukoi-Ohta \AS-M6, Ishi-gaki, Okinawa 907-04, Japan. +81-9806-8-2573 (fax) mkatoh®jnf-ilsjfTrc.go.jp (e-mail)species (Harrison, 1993) including molluscs
(Woodruff & Gould, 1987; McDonald, Seed &
Koehn, 1991; Falniowski, Kozik & Szarowska,
1993; Porter & Ribi, 1994; Katoh & Ribi, 1996;
Yokogawa, 1996). Natural hybridization takes
place in narrow area of contact zones or mosaic
zones (Rand & Harrison, 1989). Through
introgression, species may acquire genetic
polymorphism (Echelle & Connor, 1989).
Hybridization and introgression between
species may affect evolutionary events
includ-ing speciation and phylogenetic relationships.
First generation hybrids between the
fresh-water snails Viviparus ater and V. contectus
were collected in nature and their hybrid status
confirmed with allozyme markers (Katoh &
Ribi, 19%). The estimated frequency of the F,
hybrids was 0.74% in Lake Garda, Italy (Katoh
& Ribi, 19%). Allozyme data from Lake Garda
are consistent with the hypothesis of gene
introgression from V. ater into V. contectus
(Porter & Ribi, 1994) and vice versa (Katoh &
Ribi, 19%). Crossing experiments revealed a
Mendelian hybrid system with one pair of
linked loci (GPI and PNP; Katoh & Ribi,
19%). V. ater and V. contectus co-occur at high
densities in man-made structures such as
har-bours and canals. Their dispersal is limited
because of brooding. The purpose of this study
is to estimate the extent of introgression of
allozymes based on allele frequency data.
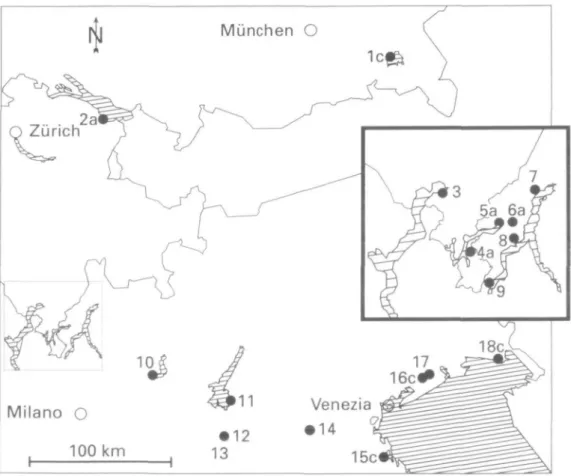
MATERIALS AND METHODS A total of 890 freshwater snails was collected from ten sympatric locations and four allopatric popula-tions each, of Viviparus ater (499 snails) and V. con-tectus (391 snails) in Italy, Switzerland, and Germany between October 1992 and August 1994 (Fig. 1 and Table 1). Each allopatric population was coded by a combination of a number which represents a specific site and a lower case letter which indicates an
exist-MASAYA KATOH & GEORG RIBI ing species. Sympatric sites were coded by a number
only. If both species were present at a sampling site or in a lake where a sampling site is located, we treated the site as sympatric in this study. Only V. ater was collected at the sympatric site Magadino, Lake Maggiore. Lake Chiem, Germany (Population lc) belongs to the Danube drainage system. Lake Constance (2a) is part of the Rhine system. The remaining sites are in the Po plain. Snails were trans-ported alive to the University of Zuurich-Irchel, Switzerland and frozen at -75°C until analyzed electrophoretically.
Standard horizontal starch-gel electrophoresis was performed. Procedures for tissue-extract preparation, and electrophoresis were similar to those of Murphy, Sites, Buth & Haufler (1990) with minor modifica-tions. Five allozyme loci were nearly diagnostic between the two species in Lake Garda, Italy in the previous study (Katoh & Ribi, 1996). One locus [PGDH] was highly polymorphic in V. conleclus. These six allozyme loci (fi-galactosidase, EC 3.2.1.22, fSGAL; glucose-6-phosphate isomerase, EC 5.3.1.9,
GPI; malate dehydrogenase-1, EC 1.1.1.37, MDH-1; phosphoglucomutase, EC 5.4.2.2, PGM; phosphoglu-conate dehydrogenase, EC 1.1.1.44, PGDH; purine-nucleoside phosphorylase, EC 2.4.2.1, PNP) were stained for the newly collected 890 snails. We used a single gel buffer system of 'JRP' following Avise, Smith & Ayala (1975) for the six allozyme loci studied. Alleles which were most abundant at each locus of V. ater in Lake Garda were designated 100, and the other alleles were named according to their relative mobilities. /•'-statistics (Weir & Cockerham, 1984) were calculated for each locus to determine genetic structure among collections within species. The method by Weir & Cockerham (1984) gives an unbiased estimate of Wright's (1978) F^.
RESULTS
Genes presumed to be introgressed were found
at one or two loci in seven out of ten sympatric
Figure 1. Collection sites for sympatric and allopatric populations of Viviparus ater and Viviparus contectiis in
507
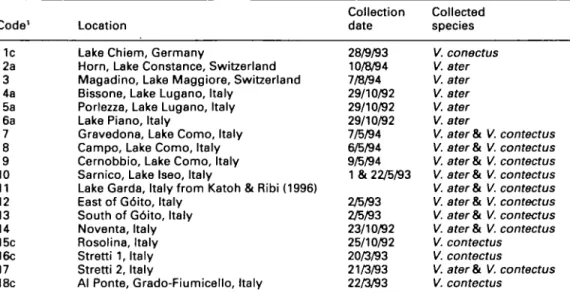
Table 1. Sampling sites and dates for sympatric and allopatric populations of Viviparus ater and
Viviparus contectus in central Europe.
Code' 1c 2a 3 4a 5a 6a 7 8 9 10 11 12 13 14 15c 16c 17 18c Location
Lake Chiem, Germany
Horn, Lake Constance, Switzerland Magadino, Lake Maggiore, Switzerland Bissone, Lake Lugano, Italy
Porlezza, Lake Lugano, Italy Lake Piano, Italy
Gravedona, Lake Como, Italy Campo, Lake Como, Italy Cernobbio, Lake Como, Italy Sarnico, Lake Iseo, Italy
Lake Garda, Italy from Katoh & Ribi (1996) East of G6ito, Italy
South of G6ito, Italy Noventa, Italy Rosolina, Italy Stretti 1, Italy Stretti 2, Italy
Al Ponte, Grado-Fiumicello, Italy
Collection date 28/9/93 10/8/94 7/8/94 29/10/92 29/10/92 29/10/92 7/5/94 6/5/94 9/5/94 1 & 22/5/93 2/5/93 2/5/93 23/10/92 25/10/92 20/3/93 21/3/93 22/3/93 Collected species V. conectus V. ater V. ater V. ater V. ater V. ater
V. ater & V. contectus V. ater Si V. contectus V. ater&t V. contectus V. ater & V. contectus V. ater & V. contectus V. ater Si V. contectus V. ater & V. contectus V. ater & V. contectus V. contectus
V. contectus
V. ater &. V, contectus V. contectus
'a and c indicate the existing species Viviparus ater and Viviparus contectus, respectively, at the allopatric sites.
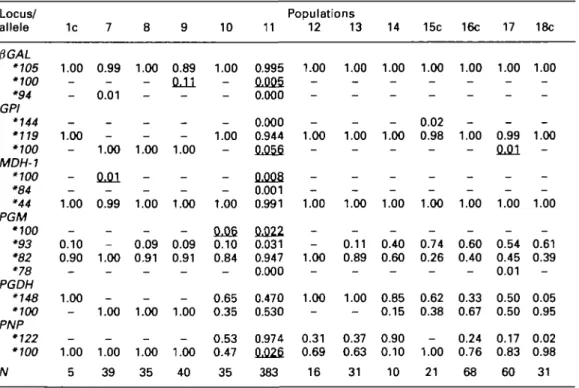
Table 2. Allele frequencies of allopatric and sympatric populations of Viviparus aferfrom Switzerland and Italy. See Table 1 for names of the population sites.
Locus/ allele 2a 3 4a 5a 6a Populations 7 8 9 10 11 12 13 14 17 PGAL '105 '100 •94 GPI •119 •100 MDH-1 •143 '100 *44 PGM '100 *93 *82 PGDH '148 '100 PNP '100 N - - - £LQ5 -0.99 1.00 1.00 1.00 1.00 1.00 0.95 1.00 1.00 -0.998 1 0.01 _ - - _ _ _ - - 0.000 - - - 0.Q07 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 0.993 1 - - - 0.01 -1.00 -1.00 -1.00 -1.00 -1.00 -1.00 0.99 -1.00 -1.00 0.997 1 - - - 0.003 1.00 1.00 0.93 0.92 1.00 1.00 0.99 1.00 1.00 0.998 1 - - - 0.002
0.07 0.08 - - M l - - 0.000
0.01 - - - 0.0Q2 1.00 1.00 0.99 1.00 1.00 1.00 1.00 1.00 1.00 0.998 1 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.000 1 80 34 58 60 15 45 40 40 42 293 .00 .00 .00 .00 .00 .00 5 1.00 1.00 1.00 0.96 0.04 1.00 1.00 28 0,02 0.86 0.05 1.00 1.00 1.00 1.00 1.00 11 1.00 1.00 1.00 0.99 0.01 1.00 1.00 41 Frequencies of proposed introgressed alleles are underlined.The most common allele in V. ater is designated as '100; "-" means 0.00. Data for Population 11 (Lake Garda, Italy) are from Katoh & Ribi (1996).
Table 3. Allele frequencies of allopatric and sympatric populations of Viviparus contectus from Germany and Italy. See Table 1 for names of the population sites.
Locus/ allele 1c 10 Populations 11 12 13 14 15c 16c 17 18c PGAL •105 •100 •94 GPI •144 •119 •100 MDH-1 •100 •84 •44 PGM '100 '93 •82 •78 PGDH •148 •100 PNP •122 '100 N 1.00 -_ 1.00 -1.00 -0.10 0.90 _ 1.00 _ -1.00 5 0.99 -0.01 _ -1.00 0.01 -0.99 -_ 1.00 _ _ 1.00 -1.00 39 1.00 -1.00 -— 1.00 -0.09 0.91 _ _ 1.00 -1.00 35 0.89 QA1 — _ -1.00 -1.00 -0.09 0.91 _ _ 1.00 -1.00 40 1.00 -— — 1.00 -— 1.00 0.06 0.10 0.84 _ 0.65 0.35 0.53 0.47 35 0.995 0.005 0.000 0.000 0.944 0.056 OJJOS 0.001 0.991 PJJ22 0.031 0.947 0.000 0.470 0.530 0.974 0JJ26 383 1.00 -1.00 -_ 1.00 -— 1.00 _ 1.00 _ 0.31 0.69 16 1.00 -1.00 -_ 1.00 -0.11 0.89 _ 1.00 _ 0.37 0.63 31 1.00 -1.00 -1.00 -0.40 0.60 _ 0.85 0.15 0.90 0.10 10 1.00 -0.02 0.98 -1.00 -0.74 0.26 _ 0.62 0.38 -1.00 21 1.00 -1.00 -1.00 -0.60 0.40 _ 0.33 0.67 0.24 0.76 68 1.00 -0.99 0.01 -1.00 -0.54 0.45 0.01 0.50 0.50 0.17 0.83 60 1.00 -1.00 -1.00 -0.61 0.39 _ 0.05 0.95 0.02 0.98 31 Frequencies of proposed introgressed alleles are underlined.
The most common allele in V. ater is designated as '700; "-" means 0.00. Data for Population 11 (Lake Garda, Italy) are from Katoh & Ribi (1996).
sites (Viviparus ater in Table 2 [Populations 8,
13, 14 and 17; V. contectus in Table 3
[Popula-tions 7,9,10 and 17]). These seven sites covered
most of northern Italy. Inferred directions of
introgresson were from V. contectus to V. ater
(Table 2) and vice versa (Table 3). However,
two allopatric populations (Table 2;
Popula-tions 4a and 5a) of V. ater in Lake Lugano
pos-sessed PGM*82 and PGDH*148, which were
rather common alleles in V. contectus (Table 3),
at low frequencies.
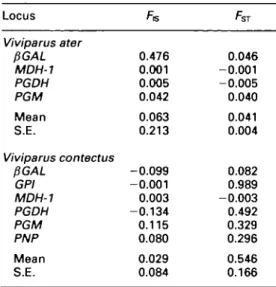
Viviparus ater had low mean heterozygosity
(overall mean H,. ± S.D. = 1.1 ± 1.4%) and
little genetic differentiation (Table 2). The
jackknifed mean of Wright's F^ ± S.E. over
four loci was low (0.041 ± 0.004), which also
indicates genetic homogeneity (Table 4). On
the other hand, V. contectus showed high mean
expected heterozygosity (overall mean H
c±
S.D. = 11.5 ± 7.6%) and high genetic
differ-entiation especially at four loci (GPI, PGM,
PGDH, and PNP, Table 3). The jackknifed
mean of F^ ± S.E. over six loci was very high
(0.546 ± 0.166), which indicates high genetic
differentiation among populations (Table 4).
Sympatric populations of V. contectus in Lake
Como, Italy (populations 7, 8 and 9) possessed
only GPI*100 and PNP*100 at one pair of the
linked loci (Table 3). GPI at the other
sym-patric and allosym-patric populations were nearly
substituted by *119 (Table 3). Frequencies of
PNP*100, which was a common allele in V. ater
and only 2.6% in V. contectus in Lake Garda,
were more than 50% in most populations of V.
contectus in this study.
DISCUSSION
Presumably introgressed alleles were found
from most sympatric populations of Viviparus
ater and V. contectus in northern Italy. Some
allopatric populations had genes which may
have had their origin in introgression. But
be-cause shared alleles between species can be
due to mutation or to common ancestry, the
Table 4. Hierarchical F-statistics for Viviparus ater and Viviparus contectus with jackknifed means and standard errors (S.E.).
Locus Viviparus ater PGAL MDH-1 PGDH PGM Mean S.E. Viviparus contectus PGAL GPI MDH-1 PGDH PGM PNP Mean S.E. FG 0.476 0.001 0.005 0.042 0.063 0.213 -0.099 -0.001 0.003 -0.134 0.115 0.080 0.029 0.084 0.046 -0.001 -0.005 0.040 0.041 0.004 0.082 0.989 -0.003 0.492 0.329 0.296 0.546 0.166
origin of each allele cannot be inferred from
allele frequency data (Harrison, 1990).
There-fore, we cannot rule out that observed patterns
are due to shared ancestry. In fact, although
PNP*100 in V. contectus from Lake Garda was
interpreted as introgressed allele in Katoh &
Ribi (19%), the PNP*100 in Lake Garda may
be a simple polymorphic allele because PNP
was highly variable at other populations in this
study. However, V. ater and V. contectus can
hybridize in nature and frequency of F] hybrids
was 0.74% in Lake Garda (Katoh & Ribi,
19%). F! hybrids are viable and mainly males
(Trllb, 1990; Katoh & Ribi, 19%). Females
crossed with male F, hybrids produced
back-crossed offspring (Triib, 1990). Therefore,
there is a possibility of widespread
intro-gression or mosaic zones in nature. Moreover,
the alleles PGM*82 and PGDH*148 of V. ater
in Lake Lugano (allopatric sites) may have
introgressed in the past when the two species
coexisted.
Even if hybridization and introgression
occur at many places, we do not expect that
frequencies of introgressed alleles increase in
time because Viviparus ater and V. contectus
have reduced fitness of F, hybrids. In
Vivi-parus, crosses between V. ater females and V.
contectus males produced 50% of offspring of
normal intraspecific V. ater fecundity (Trilb,
1990). The reverse cross produced only 2% of
intraspecific V. contectus fecundity. The
fecun-dity of the back-crossed snails was about half of
normal conspecific matings. In spite of these
disadvantages, these species could maintain a
low level of gene leakage through interspecific
and back-cross matings.
The six allozyme loci indicated high genetic
differentiation in Viviparus contectus and low
genetic differentiation in Viviparus ater. The
presence of many isolated small populations
seems to cause high differentiation in V.
con-tectus. V. contectus prefers small streams and
swamps where populations tend to be small
and V. ater lives primarily in larger bodies of
water such as lakes and rivers where
popula-tions are large (Franz, 1932; personal
observa-tion). Other brooding Viviparus species in the
United States show large genetic differentiation
among and within drainage systems (Katoh
& Foltz, 1994). In general, freshwater
gastro-pod populations tend to be genetically
differ-entiated (Brown & Richardson, 1988; Jarne &
Delay, 1991). In addition, the low genetic
varia-tion found in V. ater based on 13 allozyme loci
suggests a severe bottleneck event in the past
(Porter & Ribi, 1994). Although low
introgres-sion may have happened several times at many
places where V. ater and V. contectus coexisted,
the genetic differences which were observed
between V. ater and V. contectus in this study
can be explained by the presence of many
isolated small populations of V. contectus and a
past bottleneck event in V. ater.
ACKNOWLEDGEMENTS
We thank R.T. Dillon and B.R. Anholt for helpful comments on the manuscript. D.W. Foltz (LSU) pro-vided us his computer program for genetic analysis. We also thank D. Furrer for running gels and P. Brauchli for drawing a map. This work was supported by Swiss NSF grants 25310.88 and 31-36363.92 to G.R.
REFERENCES
AVISE, J.C., SMITH, JJ. & AYALA, FJ. 1975. Adaptive
differentiation with little genetic change between two native California minnows. Evolution, 29: 411-426.
BROWN, K.M. & RICHARDSON, T.D. 1988. Genetic
polymorphism in gastropods: a comparison of methods and habitat scales. American Malaco-logical Bulletin, 6: 9-17.
ECHELLE, A.A. & CONNOR, PJ. 1989. Rapid,
geo-graphically extensive genetic introgression after secondary contact between two pupfish species (Cyprinodon, Cyprinodontidae). Evolution, 43: 717-727.
FALNIOWSKJ, A., KOZIK, A. & SZAROWSKA, M. 1993.
Two common'European viviparid species hybrid-ize. American Malacological Bulletin, 10: 161-164. FRANZ, V. 1932. Viviparus: Morphometrie, Phyloge-nie und Geographie der europSischen, fossilen und rezenten Paludinen. Denkschriften der Medi-zinisch-Natunvissenschaftlichen Cesellschaft zu Jena (Gustav Fischer), 18:1-160.
HARRISON, R.G. 1990. Hybrid zones: windows on evolutionary process. Oxford Surveys in Evolu-tionary Biology, 7: 69-128.
HARRISON, R.G. (ED.). 1993. Hybrid zones and the
evolutionary process. Oxford University Press, Oxford.
JARNE, P. & DELAY, B. 1991. Population genetics of
freshwater snails. Trends in Ecology and Evolu-tion, 6: 383-386.
KATOH, M. & FOLTZ, D.W. 1994. Genetic subdivision
and morphological variation in a freshwater snail species complex formerly referred to as Viviparus georgianus (Lea). Biological Journal of the Lin-nean Society, 53: 73-90.
KATOH, M. & RIBI, G. 1996. Genetic evidence for
natural hybridization and apparent introgression between freshwater snail species (Viviparus ater and V. contectus). Journal of Evolutionary Biology, 9: 67-82.
MCDONALD, J.H., SEED, R. & KOEHN, R.K. 1991.
Allozymes and morphometrie characters of three species of Mytilus in the Northern and Southern Hemispheres. Marine Biology, 111: 323-333.
MURPHY, R.W., SITES, J.W., JR., BUTH, D.G. &
HAUFLER, C.H. 1990. Proteins I: isozyme electro-phoresis. In: Molecular Systematics (D.M. Hillis
& C. Mortiz eds), 45-126. Sinauer, Sunderland, Massachusetts.
PORTER, A. & RIBI, G. 1994. Population genetics of
Viviparus (Mollusca: Prosobranchia): Homogeneity of V. ater and apparent introgression into V. con-tectus. Heredity, 73:170-176.
RAND, D.M. & HARRISON, R.G. 1989. Ecological
genetics of a mosaic hybrid zone: mitochondria], nuclear, and reproductive differentiation of crickets by soil type. Evolution, 43:432-449.
RIESEBERG, L.H. & WENDEL, J.F. 1993. Introgression
and its consequences in plants. In: Hybrid zones and the evolutionary process (R.G. Harrison, ed.), 70-109. Oxford University Press, Oxford.
TRUB, H. 1990. ZUchtung von Hybriden zwischen Viviparus ater und V. contectus (Mollusca, Proso-branchia) im ZUrichsee und Okologische Unter-suchungen in einer gemischten Population im Gardasee. Ph.D. Dissertation, Universitat Zurich.
WEIR, B.S. & COCKERHAM, C.C. 1984. Estimating
F-statistics for the analysis of population structure. Evolution, 38:1358-1370.
WOODRUFF, D.S. & GOULD, SJ. 1987. Fifty years of
interspecific hybridization: genetics and morpho-metrics of a controlled experiment on the land snail Cerion in the Florida Keys. Evolution, 41: 1022-1045.
WRIGHT, S. 1978. Evolution and the genetics of populations, vol. 4: variability within and among natural populations. University of Chicago Press, Chicago.
YOKOGAWA, K. 1996. Genetic divergence in two forms of pen shell Atrina pectinata. Venus, 55: 25-39. (In Japanese with English abstract.)