Genetic variations in bile acid homeostasis are not overrepresented in alcoholic
cirrhosis compared to patients with heavy alcohol abuse and absent liver disease
Natalie Many
1, Felix Stickel
2, Johannes Schmitt
1,
Bruno Stieger
3,4, Michael Soyka
5,6, Pascal Frei
1,
Oliver Go¨tze
1, Beat Mu¨llhaupt
1,7and Andreas Geier
1,4,7,8,*
1Department of Gastroenterology and Hepatology, University Hospital Zurich (USZ), CH-8091 Zurich, Switzerland,2Department of Visceral Surgery and Medicine, Inselspital, University of Bern, CH-3010 Bern, Switzerland,
3
Department of Clinical Pharmacology, University Hospital Zurich (USZ), CH-8091 Zurich, Switzerland,4Zurich Center for Integrative Human Physiology
(ZIHP), University of Zurich, CH-8001 Zurich, Switzerland,5Private Hospital Meiringen, Willigen, CH-3860 Meiringen, Switzerland,6Psychiatric Hospital,
University of Munich, D-80336 Munich, Germany,7Swiss
Hepatopancreatobiliary (HPB)-Center, University Hospital Zurich (USZ), CH-8091 Zurich, Switzerland and8Division of Hepatology, Department of Medicine II, University Hospital Wu¨rzburg, D-97080 Wu¨rzburg, Germany.
*
To whom correspondence should be addressed. Department of Gastroenter-ology and HepatGastroenter-ology, University Hospital Zurich (USZ), Ra¨mistrasse 100, CH-8091 Zurich, Switzerland. Tel:þ41 44 255 2259; Fax: þ41 44 255 4503; Email: andreas.geier@usz.ch or geier_a2@medizin.uni-wuerzburg.de Received on December 23, 2011; revised on February 15, 2012; accepted on March 22, 2012
Increased serum bile salt levels have been associated to
a single-nucleotide polymorphism in the bile salt export
pump (BSEP; ABCB11) in several acquired cholestatic liver
diseases but there is little evidence in alcoholic liver disease
(ALD). Furthermore, a crosstalk between vitamin D and
bile acid synthesis has recently been discovered. Whether
this crosstalk has an influence on the course of ALD is
unclear to date. Our aim was to analyse the role of genetic
polymorphisms in BSEP and the vitamin D receptor gene
(NR1I1) on the emergence of cirrhosis in patients with ALD.
Therefore, 511 alcoholic patients (131 with cirrhosis and 380
without
cirrhosis)
underwent
ABCB11
genotyping
(rs2287622). Of these, 321 (131 with cirrhosis and 190
without cirrhosis) were also tested for NR1I1
polymor-phisms (bat-haplotype: BsmI rs1544410, ApaI rs7975232
and TaqI rs731236). Frequencies of ABCB11 and NR1I1
genotypes and haplotypes were compared between alcoholic
patients with and without cirrhosis and correlated to serum
bile salt, bilirubin and aspartate aminotransferase levels in
those with cirrhosis. Frequencies of ABCB11 and NR1I1
genotypes and haplotypes did not differ between the two
subgroups and no significant association between genotypes/
haplotypes and liver function tests could be determined for
neither polymorphism. We conclude that ABCB11 and
NR1I1 polymorphisms are obviously not associated with
development of cirrhosis in patients with ALD.
Introduction
Alcohol has been identified as the third leading global risk factor
for disease and disability (1) and represents a major cause of
liver cirrhosis (2). The fact that only up to 20% of alcoholics
develops cirrhosis (3) suggests that other factors such as age (4),
sex (4,5), genetics (6,7) and environmental influences (6) affect
the course of alcoholic liver disease (ALD) (8).
The mechanisms underlying alcohol-induced liver damage
are still largely unknown (9). However, the pathogenesis
involves multiple cell types and noxious factors such as
oxidative stress and proinflammatory cytokines, especially
tumour necrosis factor-alpha, which are directly involved in the
pathogenesis of alcoholic hepatitis (6,10,11) and at the same
time implicated in the downregulation of hepatobiliary
trans-port systems in both inflammatory and obstructive cholestasis
(12). Besides changes in transporter gene expression, ethanol
also functionally impairs bile salt secretion by hepatocellular
alterations in membrane fluidity and microtubule formation
(13–15). As expected from these experimental studies in
rodents, a single small heterogenous human study in 11 human
subjects with inflammatory cholestasis (7/11 with alcoholic
hepatitis) demonstrated the downregulation of major transport
systems in liver biopsy tissue as an acquired component of
cholestasis (16). Other studies underlining the importance of
hepatobiliary transport systems in alcoholic hepatitis have
shown that the severity of cholestatic alcoholic hepatitis is
associated with bile salt retention (17), which was suggested as
an even more relevant prognostic indicator of outcome than
jaundice (18). Functionally, bile salt accumulation contributes
to ethanol-induced hepatotoxicity since hydrophobic bile salts
aggravate steatosis, lipid peroxidation and cytolysis in rats fed
an ethanol-containing liquid diet (19). Endogenous bile salts
could therefore, in part, mediate ethanol-induced
hepatotoxic-ity. It has even been proposed that elevation of total serum bile
salt concentrations may actually correlate better with
histolog-ical progression of ALD than do standard biochemistries (13).
A possible interplay of bile acid homeostasis and vitamin D
has been suggested recently. At least in mice, vitamin D has
been involved in the activation of the intestinal hormone
fibroblast growth factor 19 (FGF19) and subsequent
receptor-mediated downregulation of hepatic cytochrome P450 7A1
(CYP7A1), the rate-limiting enzyme in bile acid synthesis (20).
Decreased 25 OH-vitamin D levels are a common phenomenon
in patients with alcoholism and have been described in several
studies even in the absence of advanced liver disease (21).
Furthermore, chronic ethanol consumption leads to the
in-duction of the renal 1,25 (OH)
2-vitamin D3-24-hydroxylase
(CYP24A1) which is responsible for the inactivation of the
active form of vitamin D3 (22). Considering the well-described
anti-inflammatory, antiproliferative and immunomodulatory
activities of vitamin D (23), hypovitaminosis D may play
a relevant role in the progression of ALD. Therefore, the
recently described crosstalk between vitamin D and bile acid
synthesis renders the vitamin D receptor (VDR) another
attractive target for genetic analysis in ALD.
Genetic case–control studies analysing single-nucleotide
polymorphisms (SNP) of genes related to oxidative stress
detected either no association with ALD or produced
conflicting results (6,7). Genetic variants in genes implicated in
vitamin D homeostasis and bile trafficking have not been
studied as yet, although the evidence detailed above renders
this hypothesis attractive. Thus, we set out to characterise the
role of frequent genetic polymorphisms in the bile salt export
pump (BSEP;
ABCB11), the major driving force for bile flow,
and the VDR (NR1I1) haplotype on the emergence of cirrhosis
in patients with chronic ALD.
Materials and methods
Patients and study protocol
One hundred thirty-one Caucasian patients of central European descent with alcoholic liver cirrhosis from a previously reported cohort (24–26) were recruited and analysed (cirrhosis group). For those previous studies (24–26), consecutive alcoholic patients of whom sufficient clinical data were available and who reflected past and/or present heavy alcohol consumption as defined by at least 60 g/day for women and 80 g/day for men for.10 years were recruited between 2000 and 2009 in the participating centers. For confirming eligibility of patients, present alcohol consumption was quantified through interrogation during a face-to-face interview. All patients received a diagnosis of alcohol dependence (per Diagnostic and Statistical Manual of Mental Disorders-IV criteria) by the consensus of two clinical psychiatrists. All patients underwent careful clinical examination, standard laboratory testing and abdominal ultrasound. Alcoholic liver cirrhosis was detected through a liver biopsy or evidenced by unequivocal clinical and/or laboratory results including (i) standard blood tests (coagulation tests, serum albumin concentration and platelet count), (ii) cirrhosis-related complications, including encephalopathy or ascites, (iii) abdominal ultrasound and/or computed tomography and (iv) detection of esophageal varices via upper gastrointestinal endoscopy as described (24). Other causes of chronic liver disease were excluded in all patients. Testing for hepatitis B surface antigen, anti-hepatitis B core antibody and third-generation hepatitis C antibody enzyme-linked immunosorbent assay was negative. To rule out hereditary hemochromatosis, serum levels of ferritin and transferrin saturation were determined, and neither clinical nor serological signs of autoimmune liver disease were detectable (24).
Alcoholics with normal appearance of the liver on ultrasound and normal liver enzyme levels were recruited as controls for the mentioned previous studies. Of that cohort, 380 patients with sufficient available DNA were used as a control for our study.
The study was approved by all the relevant local ethics committees, and all the patients provided written informed consent prior to inclusion.
DNA isolation, storage and genotyping of ABCB11 and NR1I1 polymorphisms DNA was isolated from whole blood/peripheral blood mononuclear cells using the QIAamp DNA Minikit (Qiagen, Hilden, Germany) and stored at 80 C. Genotyping of the SNPABCB11 c.1331T . G (p.V444A, rs2287622) as well as ofNR1I1 polymorphisms forming the bat-haplotype (rs1544410, rs7975232 and rs731236) (27) was performed using standard TaqMan technology. Fluorogenic 5#-nuclease (TaqMan) assays on a ABIPRISM 7700 sequence detection system was used for SNP determination. Probe solution (0.25ll) and 2.5ll 2 Universal PCR Master-Mix (Applied Biosystems) were brought to 5 ll with 10 ng genomic DNA. All genotyping data were analysed with the SDS 2.3 software.
Haplotype analysis
For haplotype reconstruction, the freely available Haploview software (Broad Institute of MIT and Harvard, Massachusetts, USA; http://www.broadinstitu-te.org/mpg/haploview) was used.
Quantification of serum bile salt levels
Serum bile salts were quantified using a commercially available enzymatic assay (Bile Acids Procedure 450, Trinity Biotech, Bray, Ireland) with a linear range of serum bile salt concentrations between 0 and 200lmol/l (28). Blood was routinely drawn in a fasting state in the morning between 8 and 10 A.M. to avoid falsely high bile salt levels.
Serum liver function tests
Quantification of bilirubin and aspartate aminotransferase (AST) levels has been performed using standard autoanalyser techniques.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (The Predictive Analytics Company, Chicago, IL, USA). To evaluate the significance of
the differences in allelic and genotypic frequencies between alcoholic patients with and without cirrhosis, thev2test was used. The association of
serum markers (bile salts, bilirubin and AST) to genotypes and respective haplotypes was investigated using the Spearman Rho correlation. Effects of age and gender on bile salt level and emergence of cirrhosis were analysed using univariate and multiple logistic regression. Odds ratios with 95% confidence intervals were computed using logistic regression (Supplement 1, available atMutagenesis Online). The needed number of patients within the two different groups of alcoholic patients was estimated under the assumption of similar allelic frequencies as in a previous Caucasian cohort study in hepatitis C infected patients where the minor allele frequency for BSEP (minor allele: c.1331C) was 73.2% in cirrhotics and 60.3% in controls (29). The frequency ofNR1I1 bAt[CCA]-haplotype in cirrhotic hepatitis C infected patients was 63.5 and 48.6% in controls (30). Given these figures and our number of patients and controls, we expected a power of 0.73 for BSEP (a 5 0.05) and 0.94 for CCA haplotype (a 5 0.05) when designing this study.
Results
Patient characteristics
A total of 511 patients addicted to alcohol, with and without
cirrhosis, were included in this genetic cohort study for
ABCB11 genotyping. Additionally, NR1I1 genotyping was
performed for the 321 patients of the same cohorts with
sufficient remaining DNA. Cohort characteristics are shown in
Table I. Frequencies of the determined genotypes and
haplotypes in cirrhotic patients versus those without cirrhosis
were analysed. Furthermore, genotypes and haplotypes were
correlated to liver function tests in the cirrhosis group, where
individual data were available. By definition, all patients in the
control group had normal liver function tests.
Genotype and allelic distribution of the ABCB11 c.1331T . G
polymorphism according to fibrosis stage in alcoholic patients
We determined the genotype and allelic frequency of the
ABCB11 c.1331T . G SNP in the cohort of alcoholics with
cirrhosis (n 5 131) in comparison to the complete control
cohort of patients without cirrhosis (n 5 380) (Table II). The
ABCB11 genotype distribution was similar to other studies in
Caucasian European patients with intrahepatic cholestasis
of pregnancy (31) and chronic hepatitis C infection (29).
No difference between the two cohorts could be detected
for either genotype (data not shown) or allelic frequency
(Figure 1).
Influence of the ABCB11 c.1331T . G polymorphism on liver
function tests and serum bile salt levels in alcoholic cirrhosis
patients
The
ABCB11 c.1331T . G polymorphism was also
correlated with bilirubin and AST levels—markers for
cholestasis and liver cell damage—as well as with bile salt
levels in the subgroup of alcoholic cirrhosis patients
(bilirubin
n 5 105, AST n 5 105 and bile salts n 5 32).
Carriers of the C allele showed a slight tendency towards
higher bile salt levels compared to carriers of the T allele,
although without statistical significance (median bile salt
level for the C allele 58 versus 42
lmol/l for the T allele; P 5
0.413) (Figure 2). A similar trend was observed for the CC
genotype compared to
ABCB11 c.1331TC or TT (data not
shown). For both
ABCB11 c.1331 alleles and respective
genotypes, no relevant difference could be observed for either
serum bilirubin or AST levels (data not shown). In line with
this finding, no significant correlation of
ABCB11 genotype to
histological inflammatory activity was detectable in this
study.
Genotype and haplotype distribution of common NR1I1 SNPs
according to fibrosis stage in alcoholic patients
In analogy to the
ABCB11 study, NR1I1 genotyping was
performed for alcoholics with cirrhosis (n 5 131) in order to
investigate the genotype and allelic frequency in our cohort and
compared to a control group of alcoholics without cirrhosis
(n 5 190 with sufficient DNA) (Table III). Again, the NR1I1
genotype distribution was comparable to previously published
data in Caucasian European patients with chronic hepatitis C
infection (30). No significant difference in the distribution of
genotypes (Table III) and haplotypes (Table IV) between
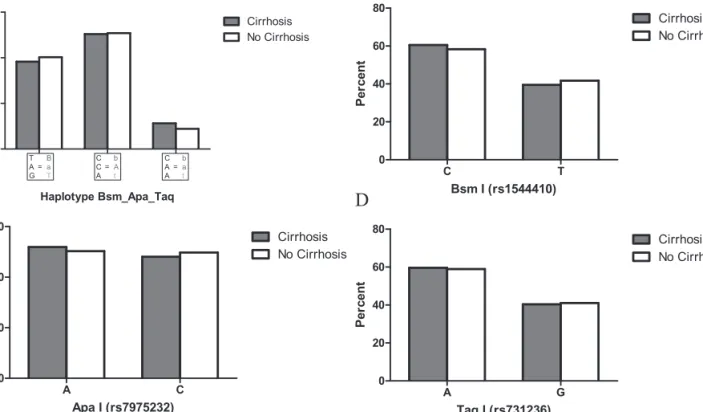
cirrhosis patients and controls was found (Figure 3).
Influence of NR1I1 genotype and haplotype on liver function
tests and serum bile salt levels in alcoholic cirrhosis patients
Subsequently,
NR1I1 genotypes and haplotypes were
corre-lated with bilirubin, AST and bile salt levels in the subgroup of
alcoholic cirrhosis patients (bilirubin
n 5 105, AST n 5 105
and bile salts
n 5 32). For the bAt[CCA]-haplotype, a slight
trend towards higher bile salt levels compared to all other
NR1I1 haplotypes could be observed (median bile salt level for
the bAt[CCA]-haplotype 58 versus 38
lmol/l for all other
NR1I1 haplotypes; P 5 0.216) (Figure 4A). However, no such
trend could be detected for the individual
NR1I1 SNPs (Figure
4B–D). Similarly, no correlation between
NR1I1 genotypes/
haplotype and serum bilirubin or AST levels was observed
(data not shown). In support, no significant correlation of the
bAt[CCA]-haplotype to histological inflammatory activity was
detectable.
Discussion
The present study investigated the genetic influence of the
ABCB11 c.1331T . G polymorphism and the common NR1I1
bAt[CCA]-haplotype on the presence of liver cirrhosis in
alcoholic patients. There are minor differences in the baseline
characteristics between the two cohorts. As expected from the
literature, median age in the cirrhosis group is
10 years higher
than in the control cohort since development of cirrhosis typically
Table I. Cohort characteristics
Cohort with cirrhosis forABCB11 and NR1I1 genotyping (n 5 131)
Gender (male/female) (n 5 122) 102/20
Median age (range) (n 5 119) 57 (26–77)
Alcohol intake (g/d) mean value (n 5 27) 159 102
AST (U/l) mean value (n 5 105) 104 312
Bilirubin (mg/dl) mean value (n 5 105) 30 41
Bile salts (lmol/l) mean value (n 5 32) 68 60
Cohort without cirrhosis forABCB11 genotyping (n 5 380)
Gender (male/female) (n 5 323) 249/74
Median age (range) (n 5 323) 44 (26–74)
Alcohol intake (g/d) mean value (n 5 42) 348 235
Cohort without cirrhosis forNR1I1 genotyping (n 5 190)
Gender (male/female) (n 5 152) 127/25
Median age (range) (n 5 152) 41 (27–74)
Alcohol intake (g/d) mean value (n 5 37) 362 234
Table II. Allelic and genotypic frequencies of ABCB11 SNP in alcoholic patients with and without cirrhosis
Genotype SNP Alcoholic patients with cirrhosis,n (%) Alcoholic patients without cirrhosis,n (%) BSEP (rs2287622) 131 (100) 380 (100) CC 52 (39.7) 139 (36.6) TT 21 (16.0) 66 (17.4) CT 58 (44.3) 175 (46.0) Frequency C Allele 162 (61.8) 453 (59.6) Frequency T Allele 100 (38.2) 307 (40.4)
Fig. 1. Comparison of allelic frequencies of ABCB11 c.1331T . G between alcoholic patients with and without cirrhosis.v2test revealed no significant
differences in the distribution of alleles between the groups of patients with and without cirrhosis (P 5 0.559).
Fig. 2. Correlation of ABCB11 c.1331T . G with serum bile salt levels. In alcoholic patients with cirrhosis, bile salt levels were correlated toABCB11 c.1331T and C alleles using Spearman Rho correlation. The C allele showed a trend towards higher bile salt levels compared to the T allele (median bile salt level for the C allele 58 versus 42lmol/l for the T allele; P 5 0.413).
conflicting results (6,7). Genetic variants in genes implicated in
vitamin D homeostasis and bile trafficking have not been
studied as yet, although the evidence detailed above renders
this hypothesis attractive. Thus, we set out to characterise the
role of frequent genetic polymorphisms in the bile salt export
pump (BSEP;
ABCB11), the major driving force for bile flow,
and the VDR (NR1I1) haplotype on the emergence of cirrhosis
in patients with chronic ALD.
Materials and methods
Patients and study protocol
One hundred thirty-one Caucasian patients of central European descent with alcoholic liver cirrhosis from a previously reported cohort (24–26) were recruited and analysed (cirrhosis group). For those previous studies (24–26), consecutive alcoholic patients of whom sufficient clinical data were available and who reflected past and/or present heavy alcohol consumption as defined by at least 60 g/day for women and 80 g/day for men for.10 years were recruited between 2000 and 2009 in the participating centers. For confirming eligibility of patients, present alcohol consumption was quantified through interrogation during a face-to-face interview. All patients received a diagnosis of alcohol dependence (per Diagnostic and Statistical Manual of Mental Disorders-IV criteria) by the consensus of two clinical psychiatrists. All patients underwent careful clinical examination, standard laboratory testing and abdominal ultrasound. Alcoholic liver cirrhosis was detected through a liver biopsy or evidenced by unequivocal clinical and/or laboratory results including (i) standard blood tests (coagulation tests, serum albumin concentration and platelet count), (ii) cirrhosis-related complications, including encephalopathy or ascites, (iii) abdominal ultrasound and/or computed tomography and (iv) detection of esophageal varices via upper gastrointestinal endoscopy as described (24). Other causes of chronic liver disease were excluded in all patients. Testing for hepatitis B surface antigen, anti-hepatitis B core antibody and third-generation hepatitis C antibody enzyme-linked immunosorbent assay was negative. To rule out hereditary hemochromatosis, serum levels of ferritin and transferrin saturation were determined, and neither clinical nor serological signs of autoimmune liver disease were detectable (24).
Alcoholics with normal appearance of the liver on ultrasound and normal liver enzyme levels were recruited as controls for the mentioned previous studies. Of that cohort, 380 patients with sufficient available DNA were used as a control for our study.
The study was approved by all the relevant local ethics committees, and all the patients provided written informed consent prior to inclusion.
DNA isolation, storage and genotyping of ABCB11 and NR1I1 polymorphisms DNA was isolated from whole blood/peripheral blood mononuclear cells using the QIAamp DNA Minikit (Qiagen, Hilden, Germany) and stored at 80 C. Genotyping of the SNPABCB11 c.1331T . G (p.V444A, rs2287622) as well as ofNR1I1 polymorphisms forming the bat-haplotype (rs1544410, rs7975232 and rs731236) (27) was performed using standard TaqMan technology. Fluorogenic 5#-nuclease (TaqMan) assays on a ABIPRISM 7700 sequence detection system was used for SNP determination. Probe solution (0.25ll) and 2.5ll 2 Universal PCR Master-Mix (Applied Biosystems) were brought to 5 ll with 10 ng genomic DNA. All genotyping data were analysed with the SDS 2.3 software.
Haplotype analysis
For haplotype reconstruction, the freely available Haploview software (Broad Institute of MIT and Harvard, Massachusetts, USA; http://www.broadinstitu-te.org/mpg/haploview) was used.
Quantification of serum bile salt levels
Serum bile salts were quantified using a commercially available enzymatic assay (Bile Acids Procedure 450, Trinity Biotech, Bray, Ireland) with a linear range of serum bile salt concentrations between 0 and 200lmol/l (28). Blood was routinely drawn in a fasting state in the morning between 8 and 10 A.M. to avoid falsely high bile salt levels.
Serum liver function tests
Quantification of bilirubin and aspartate aminotransferase (AST) levels has been performed using standard autoanalyser techniques.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (The Predictive Analytics Company, Chicago, IL, USA). To evaluate the significance of
the differences in allelic and genotypic frequencies between alcoholic patients with and without cirrhosis, thev2test was used. The association of
serum markers (bile salts, bilirubin and AST) to genotypes and respective haplotypes was investigated using the Spearman Rho correlation. Effects of age and gender on bile salt level and emergence of cirrhosis were analysed using univariate and multiple logistic regression. Odds ratios with 95% confidence intervals were computed using logistic regression (Supplement 1, available atMutagenesis Online). The needed number of patients within the two different groups of alcoholic patients was estimated under the assumption of similar allelic frequencies as in a previous Caucasian cohort study in hepatitis C infected patients where the minor allele frequency for BSEP (minor allele: c.1331C) was 73.2% in cirrhotics and 60.3% in controls (29). The frequency ofNR1I1 bAt[CCA]-haplotype in cirrhotic hepatitis C infected patients was 63.5 and 48.6% in controls (30). Given these figures and our number of patients and controls, we expected a power of 0.73 for BSEP (a 5 0.05) and 0.94 for CCA haplotype (a 5 0.05) when designing this study.
Results
Patient characteristics
A total of 511 patients addicted to alcohol, with and without
cirrhosis, were included in this genetic cohort study for
ABCB11 genotyping. Additionally, NR1I1 genotyping was
performed for the 321 patients of the same cohorts with
sufficient remaining DNA. Cohort characteristics are shown in
Table I. Frequencies of the determined genotypes and
haplotypes in cirrhotic patients versus those without cirrhosis
were analysed. Furthermore, genotypes and haplotypes were
correlated to liver function tests in the cirrhosis group, where
individual data were available. By definition, all patients in the
control group had normal liver function tests.
Genotype and allelic distribution of the ABCB11 c.1331T . G
polymorphism according to fibrosis stage in alcoholic patients
We determined the genotype and allelic frequency of the
ABCB11 c.1331T . G SNP in the cohort of alcoholics with
cirrhosis (n 5 131) in comparison to the complete control
cohort of patients without cirrhosis (n 5 380) (Table II). The
ABCB11 genotype distribution was similar to other studies in
Caucasian European patients with intrahepatic cholestasis
of pregnancy (31) and chronic hepatitis C infection (29).
No difference between the two cohorts could be detected
for either genotype (data not shown) or allelic frequency
(Figure 1).
Influence of the ABCB11 c.1331T . G polymorphism on liver
function tests and serum bile salt levels in alcoholic cirrhosis
patients
The
ABCB11 c.1331T . G polymorphism was also
correlated with bilirubin and AST levels—markers for
cholestasis and liver cell damage—as well as with bile salt
levels in the subgroup of alcoholic cirrhosis patients
(bilirubin
n 5 105, AST n 5 105 and bile salts n 5 32).
Carriers of the C allele showed a slight tendency towards
higher bile salt levels compared to carriers of the T allele,
although without statistical significance (median bile salt
level for the C allele 58 versus 42
lmol/l for the T allele; P 5
0.413) (Figure 2). A similar trend was observed for the CC
genotype compared to
ABCB11 c.1331TC or TT (data not
shown). For both
ABCB11 c.1331 alleles and respective
genotypes, no relevant difference could be observed for either
serum bilirubin or AST levels (data not shown). In line with
this finding, no significant correlation of
ABCB11 genotype to
histological inflammatory activity was detectable in this
study.
Genotype and haplotype distribution of common NR1I1 SNPs
according to fibrosis stage in alcoholic patients
In analogy to the
ABCB11 study, NR1I1 genotyping was
performed for alcoholics with cirrhosis (n 5 131) in order to
investigate the genotype and allelic frequency in our cohort and
compared to a control group of alcoholics without cirrhosis
(n 5 190 with sufficient DNA) (Table III). Again, the NR1I1
genotype distribution was comparable to previously published
data in Caucasian European patients with chronic hepatitis C
infection (30). No significant difference in the distribution of
genotypes (Table III) and haplotypes (Table IV) between
cirrhosis patients and controls was found (Figure 3).
Influence of NR1I1 genotype and haplotype on liver function
tests and serum bile salt levels in alcoholic cirrhosis patients
Subsequently,
NR1I1 genotypes and haplotypes were
corre-lated with bilirubin, AST and bile salt levels in the subgroup of
alcoholic cirrhosis patients (bilirubin
n 5 105, AST n 5 105
and bile salts
n 5 32). For the bAt[CCA]-haplotype, a slight
trend towards higher bile salt levels compared to all other
NR1I1 haplotypes could be observed (median bile salt level for
the bAt[CCA]-haplotype 58 versus 38
lmol/l for all other
NR1I1 haplotypes; P 5 0.216) (Figure 4A). However, no such
trend could be detected for the individual
NR1I1 SNPs (Figure
4B–D). Similarly, no correlation between
NR1I1 genotypes/
haplotype and serum bilirubin or AST levels was observed
(data not shown). In support, no significant correlation of the
bAt[CCA]-haplotype to histological inflammatory activity was
detectable.
Discussion
The present study investigated the genetic influence of the
ABCB11 c.1331T . G polymorphism and the common NR1I1
bAt[CCA]-haplotype on the presence of liver cirrhosis in
alcoholic patients. There are minor differences in the baseline
characteristics between the two cohorts. As expected from the
literature, median age in the cirrhosis group is
10 years higher
than in the control cohort since development of cirrhosis typically
Table I. Cohort characteristics
Cohort with cirrhosis forABCB11 and NR1I1 genotyping (n 5 131)
Gender (male/female) (n 5 122) 102/20
Median age (range) (n 5 119) 57 (26–77)
Alcohol intake (g/d) mean value (n 5 27) 159 102
AST (U/l) mean value (n 5 105) 104 312
Bilirubin (mg/dl) mean value (n 5 105) 30 41
Bile salts (lmol/l) mean value (n 5 32) 68 60
Cohort without cirrhosis forABCB11 genotyping (n 5 380)
Gender (male/female) (n 5 323) 249/74
Median age (range) (n 5 323) 44 (26–74)
Alcohol intake (g/d) mean value (n 5 42) 348 235
Cohort without cirrhosis forNR1I1 genotyping (n 5 190)
Gender (male/female) (n 5 152) 127/25
Median age (range) (n 5 152) 41 (27–74)
Alcohol intake (g/d) mean value (n 5 37) 362 234
Table II. Allelic and genotypic frequencies of ABCB11 SNP in alcoholic patients with and without cirrhosis
Genotype SNP Alcoholic patients with cirrhosis,n (%) Alcoholic patients without cirrhosis,n (%) BSEP (rs2287622) 131 (100) 380 (100) CC 52 (39.7) 139 (36.6) TT 21 (16.0) 66 (17.4) CT 58 (44.3) 175 (46.0) Frequency C Allele 162 (61.8) 453 (59.6) Frequency T Allele 100 (38.2) 307 (40.4)
Fig. 1. Comparison of allelic frequencies of ABCB11 c.1331T . G between alcoholic patients with and without cirrhosis.v2test revealed no significant
differences in the distribution of alleles between the groups of patients with and without cirrhosis (P 5 0.559).
Fig. 2. Correlation of ABCB11 c.1331T . G with serum bile salt levels. In alcoholic patients with cirrhosis, bile salt levels were correlated toABCB11 c.1331T and C alleles using Spearman Rho correlation. The C allele showed a trend towards higher bile salt levels compared to the T allele (median bile salt level for the C allele 58 versus 42lmol/l for the T allele; P 5 0.413).
develops with this interval during chronic liver damage. Given
the absence of any clinicochemical or imaging pathologies in the
non-cirrhotic controls, the likelihood of chronic liver damage and
subsequent development of cirrhosis is very low. Nevertheless,
the unknown long-term outcome of non-cirrhotic controls is
a given limitation of this study. Differences in alcohol intake are
not relevant for our findings since mean intake in the
non-cirrhosis group even exceeds the non-cirrhosis group.
Despite a slight trend towards bile salt retention in alcoholic
cirrhosis patients carrying the
ABCB11 c.1331T . G
poly-morphism and the common
NR1I1 bAt[CCA]-haplotype, no
increased frequency could be observed in the present study
compared to our control cohort without liver damage. Similarly,
earlier genetic studies analysing SNP in oxidative stress-related
genes did not show a clear association with ALD (6,7). The only
robust genetic rsik factor for progressive ALD so far is an SNP
in the gene coding for patatin-like phospholipase
domain-containing protein 3 (PNPLA3; rs738409), which has been
established quite recently (7,26,32).
The major driving force for bile flow represents the BSEP
located in the canalicular membrane of hepatocytes (33). Several
studies have analysed the role of SNPs and mutations in
hepatobiliary transport systems. Low BSEP protein expression
levels have been associated with the non-synonymous c.1331C
allele (p.444A) of
ABCB11 (34) and elevated serum bile salt
levels have been found in carriers of the ‘cholestatic’ c.1331CC
genotype in independent studies (29,31). The particular
pathogenetic role of bile salt retention in ALD clearly correlates
with histological inflammatory scores and histological
pro-gression (13,17).
Although largely negative, this study contains pathogenetic
information for disease progression in ALD, at least in the
differentiation from other forms of chronic liver disease. As
such, polymorphisms in
ABCB11 encoding for BSEP have
been identified as risk factors for intrahepatic cholestasis of
pregnancy (31). Further evidence exists for a role of BSEP in
hereditary forms of cholestasis (33) as well as drug-induced
cholestasis (35). However, this polymorphism does not
exclusively affect cholestatic liver disease since the c.1331C
allele (p.444A) of
ABCB11 has recently also been associated
with the progression to cirrhosis in chronic viral hepatitis C
(HCV) patients (29). The same trend towards increased serum
bile salt levels observed for patients with intrahepatic
cholestasis of pregnancy (31) and chronic viral hepatitis C
(29) carrying the c.1331C allele (p.444A) of
ABCB11 in
independent studies can be detected in the present cohort. The
fact that no correlation of
ABCB11 c.1331C with advanced
fibrosis has been observed in another large cohort of patients
with non-alcoholic fatty liver disease (29) allows the
conclusion that bile salt retention may not play a central role
in the disease progression to cirrhosis in neither alcoholic nor
non-alcoholic fatty liver disease which are histologically
indiscernible. Nevertheless, at least in ALD, the pivotal role
of bile salt retention in the pathogenesis of acute liver injury
and its prognosis remains unchallenged.
Hypovitaminosis D is a common feature of alcoholism (21).
The well-described anti-inflammatory, antiproliferative and
immunomodulatory activities of vitamin D and a recently
described crosstalk between vitamin D and bile acid synthesis
(20) render the VDR (NR1I1) a second attractive target gene in
ALD. In fact, genetic variations in the VDR (NR1I1) gene have
been characterised as important modulators of multiple
diseases, including hepatic disorders such as primary biliary
cirrhosis and autoimmune hepatitis (36,37). Recently, our
group has identified a significant association between the
NR1I1 bAt[CCA]-haplotype and fibrosis progression rate as
well as development of cirrhosis in HCV patients (30). VDR
(NR1I1) haplotypes are also significantly associated with the
occurrence of hepatocellular carcinoma in patients with liver
cirrhosis (38). Of note, this relationship is even more specific
for patients with an alcoholic aetiology. From these data, it
appeared attractive to hypothesise a pathogenetic role of VDR
(NR1I1) haplotypes in ALD. However, the present study does
not support this assumption since no increased frequency could
be observed in alcoholic cirrhosis patients compared to
a control cohort without liver damage.
Certainly, it would have been attractive to study the
influence of
NR1I1 SNPs on vitamin D levels but unfortunately
blood samples were no longer available. However, another
recent study of our group in HCV-infected patients showed no
correlation between VDR (NR1I1) polymorphism and 25-OH
vitamin D levels (30).
Based on the assumption of a significant difference in
previous genetic studies investigating
ABCB11 and VDR
genotypes/haplotypes in HCV patients, the present study was
a priori adequately powered. Given the minimal difference
in minor allele frequencies for both genes even a 10-fold
number of patients would be marginal to detect significant
differences with then questionable relevance. In conclusion,
Table III. Allelic and genotypic frequencies of NR1I1 SNPs in alcoholic patients with and without cirrhosis
Genotype SNP Alcoholic patients with cirrhosis,n (%) Alcoholic patients without cirrhosis,n (%) Bsm I (rs1544410) 124 (100) 187 (100) CC 47 (37.9) 65 (34.8) TT 21 (16.9) 34 (18.2) CT 56 (45.2) 88 (47.0) Frequency C Allele 150 (60.5) 218 (58.3) Frequency T Allele 98 (39.5) 156 (41.7) Apa I (rs7975232) 131 (100) 189 (100) AA 38 (29.0) 52 (27.5) CC 33 (25.2) 51 (27.0) CA 60 (45.8) 86 (45.5) Frequency A Allele 136 (51.9) 190 (50.3) Frequency C Allele 126 (48.1) 188 (49.7) Taq I (rs731236) 130 (100) 189 (100) AA 47 (36.2) 65 (34.4) GG 22 (16.9) 31 (16.4) AG 61 (46.9) 93 (49.2) Frequency A Allele 155 (59.6) 223 (59.0) Frequency G Allele 105 (40.4) 155 (41.0)
Table IV. NR1I1-haplotype frequencies in alcoholic patients with and without cirrhosis
NR1I1-haplotype (Bsm_Apa_Taq)
Haplotype distribution in the cohort with cirrhosis,n (%)
Haplotype distribution in the cohort without cirrhosis,n (%) Haplotype (Bsm_Apa_Taq) 248 (100) 372 (100) TAG (BaT) 94 (37.9) 149 (40.0) CCA (bAt) 121 (48.8) 183 (49.2) CAA (bat) 27 (10.9) 33 (8.9) CAG (baT) 2 (0.8) 1 (0.3) TAA (Bat) 2 (0.8) 4 (1.1) TCG (BAT) 2 (0.8) 2 (0.5)
VDR polymorphisms and the corresponding haplotype may
not play a central role in cirrhosis development in patients
with chronic alcohol consumption but this gene locus could
contribute to the development of hepatocellular carcinoma as
the most serious complication of end-stage ALD.
Taking into account the complexity of the pathogenesis of
ALD and the fact that many genetic association studies have
failed to show an association to disease progression (6,7) one
could hypothesise that one SNP alone may not be enough to
significantly influence the course of the disease.
Fig. 3. Comparison of NR1I1 allelic haplotype and individual allele frequencies between alcoholics with and without cirrhosis. (A)v2
test revealed no significant differences in the distribution of the three most frequent haplotypes between the two cohorts (P 5 0.630). (B–D) Neither of the individual NR1I1 allele frequencies significantly differed between cirrhosis patients and controls (P 5 0.618 for BsmI, P 5 0.689 for ApaI and P 5 0.935 for TaqI).
Fig. 4. Correlation of NR1I1 allelic haplotype and individual alleles with serum bile salt levels. (A) In alcoholic patients with cirrhosis, bile salt levels were correlated toNR1I1 haplotypes using Spearman Rho correlation. The bAt[CCA]-haplotype showed a slight trend towards higher bile salt levels compared to otherNR1I1 haplotypes (median bile salt level for the bAt[CCA]-haplotype 58 versus 38lmol/l for all other NR1I1 haplotypes; P 5 0.216). (B–D) No correlation between the alleles of individualNR1I1 SNPs and serum bile salt levels could be detected (P 5 0.223 for BsmI, P 5 0.303 for ApaI and P 5 0.265 for TaqI).
develops with this interval during chronic liver damage. Given
the absence of any clinicochemical or imaging pathologies in the
non-cirrhotic controls, the likelihood of chronic liver damage and
subsequent development of cirrhosis is very low. Nevertheless,
the unknown long-term outcome of non-cirrhotic controls is
a given limitation of this study. Differences in alcohol intake are
not relevant for our findings since mean intake in the
non-cirrhosis group even exceeds the non-cirrhosis group.
Despite a slight trend towards bile salt retention in alcoholic
cirrhosis patients carrying the
ABCB11 c.1331T . G
poly-morphism and the common
NR1I1 bAt[CCA]-haplotype, no
increased frequency could be observed in the present study
compared to our control cohort without liver damage. Similarly,
earlier genetic studies analysing SNP in oxidative stress-related
genes did not show a clear association with ALD (6,7). The only
robust genetic rsik factor for progressive ALD so far is an SNP
in the gene coding for patatin-like phospholipase
domain-containing protein 3 (PNPLA3; rs738409), which has been
established quite recently (7,26,32).
The major driving force for bile flow represents the BSEP
located in the canalicular membrane of hepatocytes (33). Several
studies have analysed the role of SNPs and mutations in
hepatobiliary transport systems. Low BSEP protein expression
levels have been associated with the non-synonymous c.1331C
allele (p.444A) of
ABCB11 (34) and elevated serum bile salt
levels have been found in carriers of the ‘cholestatic’ c.1331CC
genotype in independent studies (29,31). The particular
pathogenetic role of bile salt retention in ALD clearly correlates
with histological inflammatory scores and histological
pro-gression (13,17).
Although largely negative, this study contains pathogenetic
information for disease progression in ALD, at least in the
differentiation from other forms of chronic liver disease. As
such, polymorphisms in
ABCB11 encoding for BSEP have
been identified as risk factors for intrahepatic cholestasis of
pregnancy (31). Further evidence exists for a role of BSEP in
hereditary forms of cholestasis (33) as well as drug-induced
cholestasis (35). However, this polymorphism does not
exclusively affect cholestatic liver disease since the c.1331C
allele (p.444A) of
ABCB11 has recently also been associated
with the progression to cirrhosis in chronic viral hepatitis C
(HCV) patients (29). The same trend towards increased serum
bile salt levels observed for patients with intrahepatic
cholestasis of pregnancy (31) and chronic viral hepatitis C
(29) carrying the c.1331C allele (p.444A) of
ABCB11 in
independent studies can be detected in the present cohort. The
fact that no correlation of
ABCB11 c.1331C with advanced
fibrosis has been observed in another large cohort of patients
with non-alcoholic fatty liver disease (29) allows the
conclusion that bile salt retention may not play a central role
in the disease progression to cirrhosis in neither alcoholic nor
non-alcoholic fatty liver disease which are histologically
indiscernible. Nevertheless, at least in ALD, the pivotal role
of bile salt retention in the pathogenesis of acute liver injury
and its prognosis remains unchallenged.
Hypovitaminosis D is a common feature of alcoholism (21).
The well-described anti-inflammatory, antiproliferative and
immunomodulatory activities of vitamin D and a recently
described crosstalk between vitamin D and bile acid synthesis
(20) render the VDR (NR1I1) a second attractive target gene in
ALD. In fact, genetic variations in the VDR (NR1I1) gene have
been characterised as important modulators of multiple
diseases, including hepatic disorders such as primary biliary
cirrhosis and autoimmune hepatitis (36,37). Recently, our
group has identified a significant association between the
NR1I1 bAt[CCA]-haplotype and fibrosis progression rate as
well as development of cirrhosis in HCV patients (30). VDR
(NR1I1) haplotypes are also significantly associated with the
occurrence of hepatocellular carcinoma in patients with liver
cirrhosis (38). Of note, this relationship is even more specific
for patients with an alcoholic aetiology. From these data, it
appeared attractive to hypothesise a pathogenetic role of VDR
(NR1I1) haplotypes in ALD. However, the present study does
not support this assumption since no increased frequency could
be observed in alcoholic cirrhosis patients compared to
a control cohort without liver damage.
Certainly, it would have been attractive to study the
influence of
NR1I1 SNPs on vitamin D levels but unfortunately
blood samples were no longer available. However, another
recent study of our group in HCV-infected patients showed no
correlation between VDR (NR1I1) polymorphism and 25-OH
vitamin D levels (30).
Based on the assumption of a significant difference in
previous genetic studies investigating
ABCB11 and VDR
genotypes/haplotypes in HCV patients, the present study was
a priori adequately powered. Given the minimal difference
in minor allele frequencies for both genes even a 10-fold
number of patients would be marginal to detect significant
differences with then questionable relevance. In conclusion,
Table III. Allelic and genotypic frequencies of NR1I1 SNPs in alcoholic patients with and without cirrhosis
Genotype SNP Alcoholic patients with cirrhosis,n (%) Alcoholic patients without cirrhosis,n (%) Bsm I (rs1544410) 124 (100) 187 (100) CC 47 (37.9) 65 (34.8) TT 21 (16.9) 34 (18.2) CT 56 (45.2) 88 (47.0) Frequency C Allele 150 (60.5) 218 (58.3) Frequency T Allele 98 (39.5) 156 (41.7) Apa I (rs7975232) 131 (100) 189 (100) AA 38 (29.0) 52 (27.5) CC 33 (25.2) 51 (27.0) CA 60 (45.8) 86 (45.5) Frequency A Allele 136 (51.9) 190 (50.3) Frequency C Allele 126 (48.1) 188 (49.7) Taq I (rs731236) 130 (100) 189 (100) AA 47 (36.2) 65 (34.4) GG 22 (16.9) 31 (16.4) AG 61 (46.9) 93 (49.2) Frequency A Allele 155 (59.6) 223 (59.0) Frequency G Allele 105 (40.4) 155 (41.0)
Table IV. NR1I1-haplotype frequencies in alcoholic patients with and without cirrhosis
NR1I1-haplotype (Bsm_Apa_Taq)
Haplotype distribution in the cohort with cirrhosis,n (%)
Haplotype distribution in the cohort without cirrhosis,n (%) Haplotype (Bsm_Apa_Taq) 248 (100) 372 (100) TAG (BaT) 94 (37.9) 149 (40.0) CCA (bAt) 121 (48.8) 183 (49.2) CAA (bat) 27 (10.9) 33 (8.9) CAG (baT) 2 (0.8) 1 (0.3) TAA (Bat) 2 (0.8) 4 (1.1) TCG (BAT) 2 (0.8) 2 (0.5)
VDR polymorphisms and the corresponding haplotype may
not play a central role in cirrhosis development in patients
with chronic alcohol consumption but this gene locus could
contribute to the development of hepatocellular carcinoma as
the most serious complication of end-stage ALD.
Taking into account the complexity of the pathogenesis of
ALD and the fact that many genetic association studies have
failed to show an association to disease progression (6,7) one
could hypothesise that one SNP alone may not be enough to
significantly influence the course of the disease.
Fig. 3. Comparison of NR1I1 allelic haplotype and individual allele frequencies between alcoholics with and without cirrhosis. (A)v2
test revealed no significant differences in the distribution of the three most frequent haplotypes between the two cohorts (P 5 0.630). (B–D) Neither of the individual NR1I1 allele frequencies significantly differed between cirrhosis patients and controls (P 5 0.618 for BsmI, P 5 0.689 for ApaI and P 5 0.935 for TaqI).
Fig. 4. Correlation of NR1I1 allelic haplotype and individual alleles with serum bile salt levels. (A) In alcoholic patients with cirrhosis, bile salt levels were correlated toNR1I1 haplotypes using Spearman Rho correlation. The bAt[CCA]-haplotype showed a slight trend towards higher bile salt levels compared to otherNR1I1 haplotypes (median bile salt level for the bAt[CCA]-haplotype 58 versus 38lmol/l for all other NR1I1 haplotypes; P 5 0.216). (B–D) No correlation between the alleles of individualNR1I1 SNPs and serum bile salt levels could be detected (P 5 0.223 for BsmI, P 5 0.303 for ApaI and P 5 0.265 for TaqI).
Although the present study could not detect a significant
genetic influence of the
ABCB11 p.V444A polymorphism and
the common
NR1I1 bAt[CCA]-haplotype on the presence of
liver cirrhosis in patients with chronic alcohol abuse, our
findings do not preclude a relevant role of bile salts and vitamin
D in acute ALD. Whereas treatment with hydrophilic bile acids
such as ursodeoxycholic acid has beneficial effects to limit bile
acid toxicity but does not represent a therapeutic standard,
substitution of vitamin D may be discussed in patients with any
stage of ALD to correct for hypovitaminosis.
Supplementary data
Supplement 1 is available at
Mutagenesis Online.
Funding
Hartmann Mu¨ller Foundation, Zurich, Switzerland (to P.F. and
A.G.).
Acknowledgements
The authors thank Jyrki Eloranta, Katharina Baur and Claudia Gottier for advice and excellent technical support, respectively.
Conflict of interest statement: None declared.
References
1. WHO (2011)Global Status Report on Alcohol and Health. World Health Organization, Geneva, Switzerland, p. 85.
2. Perz, J. F., Armstrong, G. L., Farrington, L. A., Hutin, Y. J. and Bell, B. P. (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide.J. Hepatol., 45, 529–538.
3. Altamirano, J. and Bataller, R. (2011) Alcoholic liver disease: pathogenesis and new targets for therapy.Nat. Rev. Gastroenterol. Hepatol., 8, 491–501. 4. Raynard, B., Balian, A., Fallik, D., Capron, F., Bedossa, P., Chaput, J. C. and Naveau, S. (2002) Risk factors of fibrosis in alcohol-induced liver disease.Hepatology, 35, 635–638.
5. Becker, U., Deis, A., Sørensen, T. I., Grønbaek, M., Borch-Johnsen, K., Mu¨ller, C. F., Schnohr, P. and Jensen, G. (1996) Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology, 23, 1025–1029.
6. Wilfred de Alwis, N. M. and Day, C. P. (2007) Genetics of alcoholic liver disease and nonalcoholic fatty liver disease.Semin. Liver Dis., 27, 44–54. 7. Stickel, F. and Hampe, J. (2012) Genetic determinants of alcoholic liver
disease.Gut, 61, 150–159.
8. Lucey, M. R., Mathurin, P. and Morgan, T. R. (2009) Alcoholic hepatitis. N. Engl. J. Med., 360, 2758–2769.
9. Miller, A. M., Horiguchi, N., Jeong, W. I., Radaeva, S. and Gao, B. (2011) Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines.Alcohol Clin. Exp. Res, 35, 787–793.
10. Yin, M., Wheeler, M. D., Kono, H., Bradford, B. U., Gallucci, R. M., Luster, M. I. and Thurman, R. G. (1999) Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice.Gastroenterology, 117, 942–952.
11. McClain, C. J., Song, Z., Barve, S. S., Hill, D. B. and Deaciuc, I. (2004) Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease.Am. J. Physiol. Gastrointest. Liver Physiol., 287, G497–G502.
12. Geier, A., Dietrich, C. G., Voigt, S., Kim, S. K., Gerloff, T., Kullak-Ublick, G. A., Lorenzen, J., Matern, S. and Gartung, C. (2003) Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis.Hepatology, 38, 345–354.
13. Tung, B. Y. and Carithers, R. L. (1999) Cholestasis and alcoholic liver disease.Clin. Liver Dis., 3, 585–601.
14. Mills, P. R., Meier, P. J., Smith, D. J., Ballatori, N., Boyer, J. L. and Gordon, E. R. (1987) The effect of changes in the fluid state of rat liver plasma membrane on the transport of taurocholate.Hepatology, 7, 61–66. 15. Gregory, D. H., Vlahcevic, Z. R., Prugh, M. F. and Swell, L. (1978) Mechanism of secretion of biliary lipids: role of a microtubular system in hepatocellular transport of biliary lipids in the rat.Gastroenterology, 74, 93–100.
16. Zollner, G., Fickert, P., Zenz, R.et al. (2001) Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases.Hepatology, 33, 633–646.
17. Trinchet, J. C., Gerhardt, M. F., Balkau, B., Munz, C. and Poupon, R. E. (1994) Serum bile acids and cholestasis in alcoholic hepatitis. Relationship with usual liver tests and histological features.J. Hepatol., 21, 235–240. 18. Nissenbaum, M., Chedid, A., Mendenhall, C. and Gartside, P. (1990)
Prognostic significance of cholestatic alcoholic hepatitis. VA Cooperative Study Group #119.Dig. Dis. Sci., 35, 891–896.
19. Montet, A. M., Oliva, L., Beauge´, F. and Montet, J. C. (2002) Bile salts modulate chronic ethanol-induced hepatotoxicity. Alcohol Alcohol., 37, 25–29.
20. Schmidt, D. R., Holmstrom, S. R., Fon Tacer, K., Bookout, A. L., Kliewer, S. A. and Mangelsdorf, D. J. (2010) Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem., 285, 14486–14494.
21. Malik, P., Gasser, R. W., Kemmler, G., Moncayo, R., Finkenstedt, G., Kurz, M. and Fleischhacker, W. W. (2009) Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study.Alcohol Clin. Exp. Res., 33, 375–381. 22. Shankar, K., Liu, X., Singhal, R., Chen, J. R., Nagarajan, S., Badger, T. M.
and Ronis, M. J. (2008) Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3-24-hydroxylase (CYP24A1). Endocrinology, 149, 1748–1756.
23. Nagpal, S., Na, S. and Rathnachalam, R. (2005) Noncalcemic actions of vitamin D receptor ligands.Endocr. Rev., 26, 662–687.
24. Stickel, F., Osterreicher, C. H., Halangk, J. et al. (2008) No role of matrixmetalloproteinase-3 genetic promoter polymorphism 1171 as a risk factor for cirrhosis in alcoholic liver disease.Alcohol Clin. Exp. Res., 32, 959–965.
25. Treutlein, J., Cichon, S., Ridinger, M.et al. (2009) Genome-wide association study of alcohol dependence.Arch. Gen. Psychiatry, 66, 773–784. 26. Stickel, F., Buch, S., Lau, K.et al. (2011) Genetic variation in the PNPLA3
gene is associated with alcoholic liver injury in Caucasians.Hepatology, 53, 86–95.
27. Uitterlinden, A. G., Fang, Y., Van Meurs, J. B., Pols, H. A. and Van Leeuwen, J. P. (2004) Genetics and biology of vitamin D receptor polymorphisms.Gene, 338, 143–156.
28. Roda, A., Kricka, L. J., DeLuca, M. and Hofmann, A. (1982) Bio-luminescence measurement of primary bile acids using immobilized 7 alpha-hydroxysteroid dehydrogenase: application to serum bile acids. J. Lipid Res., 23, 1354–1361.
29. Iwata, R.et al. (2011) A common polymorphism in the ABCB11 gene is associated with advanced fibrosis in hepatitis C but not in non-alcoholic fatty liver disease.Clin. Sci. (Lond.), 120, 287–296.
30. Baur, K., Baur, K., Stieger, B.et al. (2012) Combined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patients.Liver Int, 32, 635–643. 31. Meier, Y., Zodan, T., Lang, C., Zimmermann, R., Kullak-Ublick, G. A.,
Meier, P. J., Stieger, B. and Pauli-Magnus, C. (2008) Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T.C polymorphism in the bile salt export pump.World J. Gastroenterol., 14, 38–45.
32. Tian, C., Stokowski, R. P., Kershenobich, D., Ballinger, D. G. and Hinds, D. A. (2010) Variant in PNPLA3 is associated with alcoholic liver disease.Nat. Genet., 42, 21–23.
33. Stieger, B., Meier, Y. and Meier, P. J. (2007) The bile salt export pump. Pflugers Arch., 453, 611–620.
34. Meier, Y., Pauli-Magnus, C., Zanger, U. M.et al. (2006) Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expres-sion in human liver.Hepatology, 44, 62–74.
35. Lang, C., Meier, Y., Stieger, B., Beuers, U., Lang, T., Kerb, R., Kullak-Ublick, G. A., Meier, P. J. and Pauli-Magnus, C. (2007) Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet. Genomics, 17, 47–60.
36. Valdivielso, J. M. and Fernandez, E. (2006) Vitamin D receptor polymorphisms and diseases.Clin. Chim. Acta, 371, 1–12.
37. Vogel, A., Strassburg, C. P. and Manns, M. P. (2002) Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis.Hepatology, 35, 126–131.
38. Falleti, E., Bitetto, D., Fabris, C.et al. (2010) Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis.World J. Gastroenterol., 16, 3016–3024.