Aberrant AKT activation drives well-differentiated liposarcoma
The MIT Faculty has made this article openly available.
Please share
how this access benefits you. Your story matters.
Citation
Gutierrez, A. et al. “Aberrant AKT Activation Drives
Well-differentiated Liposarcoma.” Proceedings of the National Academy
of Sciences 108.39 (2011): 16386–16391. Web. 13 Apr. 2012.
As Published
http://dx.doi.org/10.1073/pnas.1106127108
Publisher
Proceedings of the National Academy of Sciences (PNAS)
Version
Final published version
Citable link
http://hdl.handle.net/1721.1/70023
Terms of Use
Article is made available in accordance with the publisher's
policy and may be subject to US copyright law. Please refer to the
publisher's site for terms of use.
Aberrant AKT activation drives
well-differentiated liposarcoma
Alejandro Gutierrez
a,b,1, Eric L. Snyder
c,d,e,f, Adrian Marino-Enriquez
c, Yi-Xiang Zhang
f,g, Stefano Sioletic
f,g,
Elena Kozakewich
a, Ruta Grebliunaite
a, Wen-bin Ou
c, Ewa Sicinska
d,f, Chandrajit P. Raut
g,h,
George D. Demetri
f,g, Antonio R. Perez-Atayde
i, Andrew J. Wagner
f,g, Jonathan A. Fletcher
c,g,
Christopher D. M. Fletcher
c, and A. Thomas Look
a,b,1aDepartment of Pediatric Oncology,dDepartment of Medical Oncology and Center for Molecular Oncologic Pathology,fThe Ludwig Center at Dana-Farber/ Harvard Cancer Center, andgCenter for Sarcoma and Bone Oncology, The Dana-Farber Cancer Institute, MA 02215;bDivision of Hematology/Oncology, iDepartment of Pathology, Children’s Hospital, Boston, MA 02115;cDepartment of Pathology andhDepartment of Surgery, Brigham and Women’s Hospital, Boston, MA 02115; andeThe Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139
Edited* by Dennis A. Carson, University of California at San Diego, La Jolla, CA, and approved August 15, 2011 (received for review April 21, 2011) Well-differentiated liposarcoma (WDLPS), one of the most
com-mon human sarcomas, is poorly responsive to radiation and che-motherapy, and the lack of animal models suitable for experimen-tal analysis has seriously impeded functional investigation of its pathobiology and development of effective targeted therapies.
Here, we show that zebrafish expressing constitutively active Akt2
in mesenchymal progenitors develop WDLPS that closely
resem-bles the human disease. Tumor incidence rates were 8% in p53
wild-type zebrafish, 6% in p53 heterozygotes, and 29% in p53-homozygous mutant zebrafish (P = 0.013), indicating that aberrant
Akt activation collaborates with p53 mutation in WDLPS
patho-genesis. Analysis of primary clinical specimens of WDLPS, and of the closely related dedifferentiated liposarcoma (DDLPS) subtype, revealed immunohistochemical evidence of AKT activation in 27% of cases. Western blot analysis of a panel of cell lines derived from patients with WDLPS or DDLPS revealed robust AKT phosphoryla-tion in all cell lines examined, even when these cells were cultured in serum-free media. Moreover, BEZ235, a small molecule inhibitor of PI3K and mammalian target of rapamycin that effectively inhib-its AKT activation in these cells, impaired viability at nanomolar
concentrations. Ourfindings are unique in providing an animal
model to decipher the molecular pathogenesis of WDLPS, and im-plicate AKT as a previously unexplored therapeutic target in this chemoresistant sarcoma.
L
iposarcoma is the most common sarcoma of humans,
affect-ing
∼2,000 individuals per year in the United States (1).
These tumors are classified into five histopathologic subtypes,
with well-differentiated liposarcoma (WDLPS) accounting for
∼50% of cases, and dedifferentiated liposarcoma (DDLPS), a
closely related subtype that appears to arise from further
ma-lignant progression of WDLPS, accounting for an additional 9%
to 18% of cases (1–3). Liposarcomas are generally thought to
arise de novo rather than from preexisting benign lesions, and
most patients lack recognized causative factors. Although
com-plete surgical resection can be curative, WDLPS often develops
in deep anatomic locations, such as the retroperitoneum or
mediastinum, where its propensity to enwrap vital structures
typically makes complete surgical resection difficult or
impossi-ble, leading to high morbidity and mortality rates (1, 4).
Radia-tion and chemotherapy have limited efficacy in the treatment of
WDLPS (5, 6). Indeed, there are no systemic therapeutic
regi-mens known to improve survival when complete surgical
re-section is not feasible, underscoring the need for an improved
molecular understanding of WDLPS to stimulate the
develop-ment of effective targeted therapies.
The MDM2-p53 pathway plays a prominent role in WDLPS
pathogenesis, with the vast majority of human tumors harboring
either MDM2 amplifications or p53 mutations (6–10). Moreover,
individuals with germ-line p53 mutations appear to be at
in-creased risk of WDLPS development at a very young age (11).
Regions of chromosome 12q13-15 are often amplified in
well-differentiated and dewell-differentiated liposarcomas, typically
in-volving MDM2, CDK4, and HMGA2, along with several other
genes (6, 10, 12, 13); JUN can also be amplified in WDLPS cases
that have a dedifferentiated component (14). Further dissection
of WDLPS molecular pathogenesis has been greatly impeded by
the lack of animal models suitable for experimental analysis.
Oncogenic signal transduction through the PI3K-AKT
path-way, which is widely dysregulated in human cancer, is normally
down-regulated by the PTEN tumor suppressor (15). Individuals
with germ-line PTEN-inactivating mutations frequently develop
multiple lipomas (benign adipocytic neoplasms) (16), and AKT
activation has been described in human liposarcomas (17),
sug-gesting that the PI3K-AKT pathway is involved in adipocyte
transformation. Here we show that expression of constitutively
active Akt2 in zebrafish mesenchymal progenitors induces
WDLPS, thus being unique in providing an animal model for
future investigation of this disease. Moreover, we also show that
AKT pathway inhibition impairs viability in human cell lines
derived from patients with WDLPS and DDLPS, thus
implicat-ing AKT as a previously unexplored therapeutic target in these
chemoresistant sarcomas.
Results
Expression of Constitutively Active Akt2 Induces Well-Differentiated
Liposarcoma.
To test the hypothesis that Akt is a WDLPS
onco-gene that collaborates with p53 inactivation during adipocyte
transformation, we in-crossed zebrafish harboring heterozygous
p53
M214Kmutations, which encode a transactivation-defective
p53 protein (18), and all resultant embryos were microinjected at
the one-cell stage with a rag2:myr-mAkt2 expression construct
(Fig. 1A). This construct encodes a myristoylated, constitutively
active mouse Akt2 transgene (19) driven by a zebrafish rag2
Author contributions: A.G., E.L.S., A.M.-E., W.-b.O., J.A.F., C.M.D.F., and A.T.L. designed research; A.G., E.L.S., A.M.-E., Y.-X.Z., S.S., E.K., R.G., and W.-b.O. performed research; E.L.S., S.S., E.S., C.P.R., G.D.D., A.J.W., J.A.F., and C.M.D.F. contributed new reagents/ana-lytic tools; A.G., E.L.S., A.M.-E., Y.-X.Z., S.S., E.K., W.-b.O., C.P.R., G.D.D., A.R.P.-A., J.A.F., C.M.D.F., and A.T.L. analyzed data; and A.G. and A.T.L. wrote the paper.
Conflict of interest statement: C.P.R. is a consultant for Novartis and participates in clinical trials of Novartis. G.D.D. is a consultant for Novartis, Pfizer, Ariad, Johnson & Johnson, PharmaMar, Genentech, Infinity Pharmaceuticals, EMD-Serono, Glaxo Smith Kline, Am-gen, Daiichi-Sankyo, ArQule, Enzon, Millenium/Takeda; is a member of the scientific advisory board of Plexxikon, ZioPharm, Nereus, N-of-One, and Kolltan Pharmaceuticals; and participates in clinical trials of Novartis, Pfizer, Ariad, Johnson & Johnson, Pharma-Mar, and Infinity Pharmaceuticals. A.J.W. is a consultant for Novartis, Roche/Genentech, Sanofi, Pfizer, EMD-Serono, and participates in clinical trials supported by Novartis, Roche/Genentech, Pfizer, and Exelixis.
*This Direct Submission article had a prearranged editor.
1To whom correspondence may be addressed. E-mail: thomas_look@dfci.harvard.edu or
Alejandro_Gutierrez@dfci.harvard.edu.
This article contains supporting information online atwww.pnas.org/lookup/suppl/doi:10. 1073/pnas.1106127108/-/DCSupplemental.
promoter fragment that drives ectopic expression in
mesenchy-mal progenitors (20). Zebrafish injected with rag2:myr-mAkt2
developed externally visible solid tumors between 1 and 4 mo of
age; the tumor incidence rates were 29% in p53-homozygous
mutants, 6% in p53 heterozygotes, and 8% in their p53 wild-type
siblings (P = 0.01) (Fig. 1 B and C). Histologic analyses revealed
that 91% of these tumors consisted of locally invasive masses of
adipocytes showing considerable variation in cell size, together
with scattered atypical stromal cells with hyperchromatic nuclei
and lipoblasts characterized by multivacuolated cytoplasm and
large hyperchromatic pleomorphic nuclei,
findings that are
di-agnostic of WDLPS in humans (Fig. 1 D–F) (2), whereas the
remaining 9% of tumors were osteosarcomas, as described
be-low. Immunohistochemical analysis revealed strong reactivity for
phospho-AKT(Ser473) in the tumor cells of rag2:myr-mAkt2
transgenic zebrafish but not in the normal fat of control rag2:
GFP transgenic fish, indicating expression of the constitutively
active Akt2 transgene (Fig. 1 G–I). Moreover, none of the
con-trol p53-homozygous mutant zebrafish injected with rag2:GFP
(n = 60) developed tumors by 6 mo of age.
In addition, one p53-homozygous mutant injected with rag2:
myr-mAkt2 developed a rigid mass at the base of the dorsal
fin
that demonstrated increased lobulation and vascularity
com-pared with the WDLPS tumors (Fig. 2 A and B). Histologic
analysis revealed that this mass consisted predominantly of
os-teoid interspersed with large malignant cells characterized by
pleomorphic nuclei, features that are diagnostic of osteosarcoma
in humans (Fig. 2 C and D) (2).
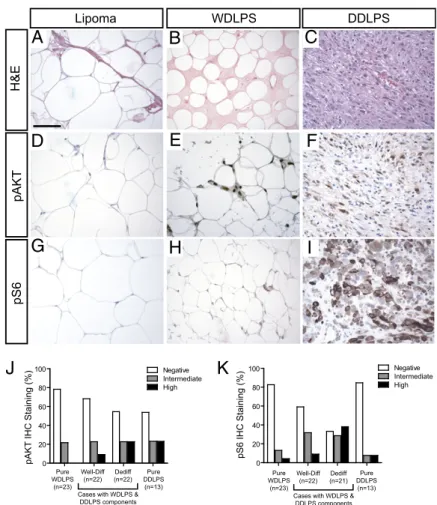
Aberrant AKT Pathway Activation in Primary Human Well-Differentiated
and Dedifferentiated Liposarcomas.
To test whether AKT
activa-tion is also involved in human liposarcoma pathogenesis, we
performed immunohistochemical analysis for phospho-AKT
(Ser473) on clinical specimens from 58 patients with
well-dif-ferentiated and dedifwell-dif-ferentiated liposarcomas. These studies
demonstrated AKT activation in a subset of the human WDLPS
or DDLPS cases (Fig. 3 A–F), including 22% of the pure
WDLPS (n = 23) and 46% of pure DDLPS (n = 13) tumors
analyzed (Fig. 3J). We also included human tumors containing
both well-differentiated and dedifferentiated liposarcoma
com-ponents in our analysis (n = 22), which revealed phospho-AKT
positivity in 32% of the well-differentiated components and 45%
in the dedifferentiated components of these cases (Fig. 3J).
Given that phospho-AKT is a labile epitope in clinical
speci-C
Control Well-Differentiated Liposarcoma
H&E phospho-AKT
D
E
F
I
H
G
0 2 4 6 8 0 10 20 30 p53 wt/wt (n=25) p53 mut/wt (n=52) p53 mut/mut (n=21) Age (months)Solid Tumor Incidence (%)
P = 0.013
A
XB
tp53
rag2 promoter-myr mAkt2
wt/mut tp53wt/mut
Inject:
Assess Tumor Onset Genotype
Fig. 1. Constitutive Akt activation drives WDLPS in the zebrafish. (A) Experimental design. (B) Solid tumor incidence in p53 wild-type, heterozygous, or p53M214K homozygous mutant siblings injected with a mAkt2 transgene at the one-cell stage. P value calculated via log-rank test. (C) Representative rag2:myr-mAkt2-injected zebrafish, which developed what appear to be two independent solid tumors. The animal shown was p53-homozygous mutant. (Scale bar, 5 mm.) Note that the image shown in (C) consists of merged adjacent photomicrographs. (D) Control H&E-stained zebrafish section. Arrow points to normal subcutaneous adipocytes. (E) Low-magnification view of an H&E section through the zebrafish shown in C, demonstrating a locally invasive mass consisting of well-differentiated adipocytes with significant variation in cell size. (F) High-magnification view of H&E section demonstrating a representative lipoblast scattered throughout these tumors, characterized by a multivacuolated cytoplasm and large hyperchromatic pleomorphic nuclei. (G) Phospho-AKT immuno-histochemistry on a control zebrafish section. Arrow points to normal subcutaneous adipocytes, which lacked detectable pAKT staining. (H and I) Phospho-AKT immunohistochemistry on tumor sections from the zebrafish shown in C, revealing strong immunoreactivity for phospho-AKT in tumor cells of rag2:myr-mAkt2-transgenic zebrafish. (Scale bars, 100 μm.)
Gutierrez et al. PNAS | September 27, 2011 | vol. 108 | no. 39 | 16387
MEDICAL
mens, we also performed immunohistochemistry for phospho-S6
(Ser235/236), a downstream target of the AKT pathway (21).
Similar results (Fig. 3 G–I) were obtained, with 17% of pure
WDLPS (n = 23), 41% of well-differentiated WDLPS/DDLPS
components (n = 22), and 47% of DDLPS (pure DDLPS, n =
13; dedifferentiated WDLPS/DDLPS components, n = 21),
demonstrating evidence of S6 activation (Fig. 3K). We found no
immunohistochemical evidence of AKT or S6 phosphorylation in
lipomas or in normal adipose tissue (Fig. 3 D and G, and
Fig. S1
).
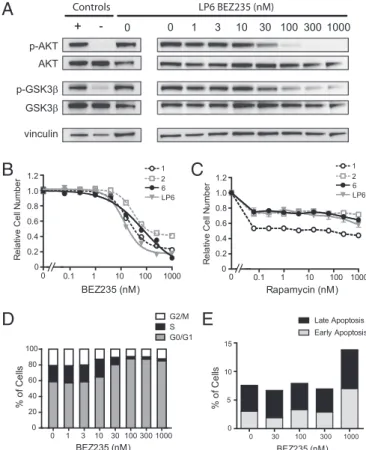
To determine whether AKT activation is aberrant in human
WDLPS and DDLPS, we took advantage of a panel of cell lines
derived from patients with these sarcomas to evaluate
phos-phorylation of AKT and of its downstream target GSK3β after
4 h of growth in serum-free conditions. Strikingly, Western blot
analysis revealed persistent phosphorylation of AKT and of its
downstream target GSK3β in all eight cell lines examined, even
under such serum-starved conditions. In contrast, serum
starva-tion resulted in silencing of AKT and GSK3β phosphorylastarva-tion in
control SU-CCS-1 clear cell sarcoma cells (Fig. 4).
PI3K-AKT-Mammalian Target of Rapamycin Pathway Inhibition
Im-pairs the Viability of Human Liposarcoma Cells.
To determine
whether human WDLPS and DDLPS cells are dependent on
aberrant AKT pathway activation, we treated four cell lines (two
WDLPS and two DDLPS) with BEZ235, a dual-specificity
in-hibitor of PI3K and both mammalian target of rapamycin
(mTOR) complexes (22) that effectively silences AKT pathway
activation in these cells (Fig. 5A). BEZ235 treatment for 72 h
decreased the viability of all cell lines tested, with IC50 values
ranging from 13 to 75 nM (Fig. 5B). Treatment with rapamycin,
an mTORC1 inhibitor, had less of an effect on viability (Fig. 5C),
suggesting that the PI3K-AKT pathway plays both
mTORC1-dependent and -inmTORC1-dependent roles in the pathobiology of
WDLPS and DDLPS. Analysis of cell-cycle profiles revealed that
BEZ235 treatment induced G1 arrest at nanomolar
concen-trations (Fig. 5D), whereas apoptosis was induced only at a 1-μM
concentration (Fig. 5E).
A
Lipoma
H&E
pAKT
WDLPS
DDLPS
B
C
D
E
F
J K
pS6
G
H I
Pure WDLPS (n=23) Pure DDLPS (n=13) 0 20 40 60 80 100 Negative Intermediate HighpAKT IHC Staining (%)
Well-Diff (n=22)
Dediff (n=22) Cases with WDLPS &
DDLPS components 0 20 40 60 80 100 pS6 IHC Staining (%) Pure WDLPS (n=23) Pure DDLPS (n=13) Negative Intermediate High Well-Diff (n=22) Dediff (n=21) Cases with WDLPS &
DDLPS components
Fig. 3. AKT pathway activation in primary human well-dif-ferentiated and dedifwell-dif-ferentiated liposarcomas. (A–C) H&E staining of human lipoma, WDLPS, and DDLPS specimens. (D–F) Immunohistochemistry for Ser473-phosphorylated AKT in a representative lipoma and AKT-positive WDLPS and DDLPS clinical specimens. (Scale bar, 100μm.) (G–I) Immunohisto-chemistry for phospho-S6 ribosomal protein (Ser235/236) in a representative lipoma, as well as AKT-positive WDLPS and DDLPS clinical specimens. (J and K) Quantitation of phospho-AKT and phospho-S6 immunohistochemistry in pure WDLPS, pure DDLPS, and in human tumors with mixed well-differen-tiated and dedifferenwell-differen-tiated liposarcoma components.
A
B
C
D
Fig. 2. Osteosarcoma development in a rag2:myr-mAkt2-injected, p53-ho-mozygous mutant zebrafish. (A and B) A p53-homozygous mutant zebrafish injected with the rag2:myr-mAkt2 expression construct developed a solid lobulated mass at the base of the dorsalfin. Note that the image shown in A consists of merged adjacent photomicrographs. (Scale bars, 1 mm.) (C and D) H&E-stained sections at low and high magnification, respectively, demon-strate a mass consisting predominantly of osteoid matrix interspersed with large malignant cells with pleomorphic nuclei, features that in humans are diagnostic of osteoblastic osteosarcoma. (Scale bars, 100μm.)
Discussion
We have demonstrated that expression of activated Akt2 in
mes-enchymal progenitors drives WDLPS in transgenic zebrafish, and
that nearly one-third of clinical specimens from primary cases of
human WDLPS and DDLPS showed immunohistochemical
evi-dence of AKT pathway activation. Moreover, treatment with the
PI3K-AKT pathway inhibitor BEZ235 inhibited viability in all cell
lines derived from patients with WDLPS and DDLPS that we
tested. Taken together, our
findings implicate a central role for
oncogenic AKT signaling in the molecular pathogenesis of human
WDLPS and DDLPS, and suggest the need for clinical trials of
AKT pathway inhibitors in patients with unresectable disease, for
whom there are currently no known effective therapies.
Our analyses of primary human tumors revealed an increased
frequency and intensity of staining for AKT and
phospho-S6 ribosomal protein in dedifferentiated liposarcomas
com-pared with their well-differentiated counterparts. These
findings
suggest the intriguing possibility that AKT activation may define
a subset of WDLPS, which is particularly prone to
dediffer-entiation, a process that may be caused in part by the acquisition
of additional oncogenic abnormalities further potentiating
sig-naling through the AKT pathway. However, we cannot rule out
the possibility that this apparent difference may simply be related
to the greater difficulty of detecting phosphorylated epitopes in
WDLPS sections, in which most of the tumor mass consists of
large fat vacuoles within malignant yet well-differentiated
adipo-cytes, whereas the densely cellular DDLPS tumors have a much
greater number of cellular elements in which AKT
phosphoryla-tion can be assessed per secphosphoryla-tion. Further studies will be required
to establish the mechanisms underlying this observation.
Recent work has revealed that 18% of myxoid/round-cell
lip-osarcomas harbor activating mutations in PIK3CA, encoding the
catalytic subunit of class IA PI3K (13). Myxoid/round-cell
lip-osarcomas are characterized by t(12;16)(q13-14;p11)
transloca-tions, resulting in expression of TLS-CHOP fusion proteins,
whereas these tumors lack the 12q amplifications characteristic
of WDLPS/DDLPS, leading most investigators to believe that
these liposarcoma subtypes are biologically distinct (1, 2). S6K1,
a direct target of mTORC1 downstream of PI3K-AKT, has
re-cently been shown to be required for the earliest stages of
adi-pogenesis (23), providing one plausible mechanism to explain
selection for AKT pathway activation in diverse liposarcoma
subtypes. Nevertheless, the fact that expression of activated AKT
in p53-mutant zebrafish drives development of WDLPS but not
myxoid/round-cell liposarcomas indicates that AKT activation
and p53 mutation can be early events in WDLPS pathogenesis,
whereas expression of the TLS-CHOP fusion may be required in
addition to PI3K-AKT pathway activation in the genesis of
myxoid/round-cell liposarcomas.
Current knowledge of the molecular pathogenesis of WDLPS
has been driven by genetic analyses of human tumors, which have
revealed that almost all cases harbor MDM2 amplification or TP53
mutations (7–10). Evidence also suggests that individuals with
germ-line TP53 mutations are at increased risk of WDLPS
de-velopment at a very young age (11). By demonstrating that
acti-vated Akt2 and p53 mutations collaborate in the zebrafish, we have
now experimentally demonstrated the long-suspected role of p53
as a tumor suppressor in WDLPS. These tumors are also
charac-terized by recurrent amplifications of distinct regions of
chromo-some 12q13-15 (6, 10, 12), but until now it has not been possible to
identify which of the involved genes are WDLPS oncogenes
driving the selection for these amplifications, and which are merely
nonpathogenic
“passengers.” Furthermore, although
dediffer-entiated liposarcoma is thought to arise because of further
ma-lignant transformation of WDLPS, it has previously been
im-possible to directly test the ability of candidate genetic lesions to
drive this transformation in a physiological context. The zebrafish
model we describe now provides a platform for experimental
vinculin GSK3β p-GSK3β AKT p-AKT WDLPS DDLPS 1 1 2 3 4 5 6 7 8 Serum+
ControlFig. 4. Aberrant AKT activation in human cell lines derived from patients with WDLPS and DDLPS. Western blot analysis for phosphorylation of AKT and its downstream target GSK3β was performed in a panel of cell lines derived from patients with WDLPS (samples 1–3) or DDLPS (samples 4–8), grown in serum-free conditions for 4 h before analysis. Control is the SU-SSC-1 clear-cell sarcoma cell line, shown in the presence and absence of serum. Sample 1 was run on both Western blots as a control for interblot variability.
0 1 3 10 30 100 300 1000 0 20 40 60 80 100 G0/G1 S G2/M BEZ235 (nM) % o f C e lls 0.1 1 10 100 1000 0 BEZ235 (nM) 0 0.2 0.4 0.6 0.8 1.0 1.2 Re la tiv e C e ll Nu m b e r
A
B
D
C
E
0 30 100 300 1000 0 5 10 15 % o f C e lls BEZ235 (nM) Early Apoptosis Late Apoptosis vinculin GSK3β p-GSK3β AKT p-AKT 0 1 3 10 30 100 300 1000 0.1 1 10 100 1000 0 Rapamycin (nM) 0 0.2 0.4 0.6 0.8 1.0 1.2 R e la tiv e C e ll N u m b e r LP6 BEZ235 (nM) 0 Controls + -1 2 LP6 6 1 2 LP6 6Fig. 5. PI3K-AKT-mTOR pathway inhibitors impair viability in human WDLPS and DDLPS cells. (A) Western blot analysis of the LP6 cell line, derived from a patient with DDLPS, demonstrating that BEZ235 effectively inhibits phos-phorylation of AKT and its downstream target GSK3β at nanomolar concen-trations. Positive and negative controls are the SU-SSC-1 cell line grown in the presence and absence of serum, respectively. (B) Viability of four cell lines derived from patients with WDLPS (cells 1 and 2) or DDLPS (cells 6 and LP6) was assessed after 72 h of BEZ235 treatment using the CellTiter-Glo luminescent cell viability assay. Values represent mean± SEM (n = 3 replicates). IC50 values were 19, 32, 14, and 75 nM, respectively, for cells 1, 2, 6, and LP6. (C) Viability of WDLPS/DDLPS cell lines after 72 h of rapamycin treatment. (D) Effect of BEZ235 treatment on cell cycle distribution of LP6 cells examined byflow cytometry analysis at 24 h. Data shown are representative of two independent experi-ments. (E) Effect of BEZ235 treatment on apoptosis of LP6 cells, assessed by Annexin V and 7-AAD double-staining. Data shown are representative of two independent experiments.
Gutierrez et al. PNAS | September 27, 2011 | vol. 108 | no. 39 | 16389
MEDICAL
studies to dissect molecular pathogenesis and discover novel
therapeutic targets in this chemoresistant tumor.
Materials and Methods
Zebrafish Husbandry, Mutant Lines, and Imaging. Zebrafish husbandry was performed as previously described (24), in accord with protocols approved by the Dana-Farber Cancer Institute Animal Care and Use Committee. The p53M214K-mutant zebrafish line was previously described (18). Zebrafish images were obtained using a Nikon SMZ1500 microscope, Nikon DS2MBWc camera, and NIS-Elements F Package Ver. 3.00 (Nikon Instruments Inc.). Expression Constructs. The rag2:myr-mAkt2 construct was generated by placing a myristoylated murine Akt2 transgene (19), which is constitutively activated as a result of constitutive membrane localization, downstream of a zebrafish rag2 promoter fragment (25) in a modified pBluescript vector, wherein the rag2:myr-mAkt2 construct isflanked by recognition sequences for I-SceI meganuclease.
Generation of Transgenic Zebrafish. Circular rag2:myr-mAkt2 plasmid DNA (30 pg) was microinjected along with I-SceI meganuclease (New England Biolabs) into one-cell stage zebrafish embryos from the AB wild-type strain, as previously described (26).
Zebrafish Paraffin Embedding and Sectioning. Zebrafish were killed in tricaine anesthetic,fixed in 4% paraformaldehyde at 4 °C for 2 d, decalcified with 0.25 M EDTA (pH 8.0) for 2 d, dehydrated in alcohol, cleared in xylene, and embedded in paraffin. Tissue sections from paraffin-embedded tissue blocks were placed on charged slides, deparaffinized in xylene, rehydrated through graded alcohol solutions, and stained with H&E or analyzed by immunohistochemistry.
Patient Samples. Human well-differentiated liposarcoma, dedifferentiated liposarcoma, lipoma, and normal fat specimens were removed at surgery and collected from patients treated at Brigham and Women’s Hospital, who gave informed consent for use of anonymized surgical specimens for research purposes after all clinically relevant evaluations were performed, with ap-proval of the Partners Health Care Institutional Review Board. The diagnosis of well-differentiated liposarcoma/atypical lipomatous tumor, dediffer-entiated liposarcoma, or lipoma was made by institutional pathologists and reviewed by E.L.S. and C.D.M.F. to ensure diagnostic accuracy based on cri-teria of the World Health Organization (2).
Immunohistochemistry. Immunohistochemistry on human samples was per-formed on a tissue microarray containing three 0.4-mm cores from each individual tumor, and on selected whole tumor sections. Zebrafish immu-nohistochemistry was performed on slides of whole zebrafish sections. Slides were deparaffinized and pretreated with 10 mM citrate (pH 6.0) in a steam pressure cooker (Decloaking Chamber, BioCare Medical) according to the manufacturer’s instructions, followed by washing in distilled water. Slides were pretreated with Peroxidase Block (Dako) for 5 min, followed by serum-free protein block (Dako) for 20 min. Primary rabbit antibody to Ser473 AKT (#4058; Cell Signaling Technology) or to Ser235/236 phospho-S6 ribosomal protein (#4858; Cell Signaling Technology) was applied at a 1:50 dilution in Antibody Diluent (Dako) and incubated at 4 °C overnight (phospho-AKT) or at room temperature for 1 h (phospho-S6). Anti-rabbit horseradish peroxidase-labeled polymer (Dako) was applied for 30 min. Immunoperoxidase staining was performed with diaminobenzidine (DAB)+ chromogen kit (Dako), according to the manufacturer’s instructions.
Immunohistochemistry was independently scored by two pathologists (E.L.S. and S.S.), who then reviewed the few discordant cases together to arrive at a consensus score. Tumors were scored as positive if>10% of sarcoma cells exhibited evidence of specific staining for phospho-AKT or phospho-S6. Positive tumors were further subclassified based on intensity of staining into either high (strong staining) or intermediate (weak-to-moderate staining) categories. Normal adipose tissue was used as a negative control.
Inhibitors. BEZ235 and rapamycin were purchased from AXON Medchem and Calbiochem, respectively.
Western Blotting. Whole-cell lysates were prepared using lysis buffer (1% Nonidet P-40, 50 mM Tris-HCl pH 8.0, 100 mM sodiumfluoride, 30 mM sodium pyrophosphate, 2 mM sodium molybdate, 5 mM EDTA, and 2 mM sodium orthovanadate) containing protease inhibitors (10μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride). The lysates were then rocked overnight at 4 °C. Lysates were cleared by centrifugation at 16,100× g for 30 min at 4 °C, and protein concentrations were determined with a Bio-Rad protein assay (Bio-Rad Laboratories). Equal amounts of pro-tein were separated by SDS/PAGE, blotted to nitrocellulose membranes (Schleicher and Schuell) and then stained with the following antibodies: AKT (Cell Signaling Technology; #9272; 1:500), phospho-AKT(Ser473) (Cell Signaling Technology; #9271; 1:500), phospho-GSK3β(Ser9) (Cell Signaling Technology; #9336; 1:1,000), GSK3 (Santa Cruz; #sc-7291; 1:500), and vinculin (Sigma-Aldrich; #V4505; 1:500). The hybridization signals were detected by chem-iluminescence (ECL, Amersham Biosciences) and captured using a LAS1000-plus chemiluminescence imaging system (Fujifilm).
Cell Viability Assays. Liposarcoma cells were plated in 96-well plates at 2,000 cells per well in 100μL of medium containing 15% FBS. After 24 h, cells were exposed to increasing concentrations of compounds. Each concentration was tested in triplicate. Cell viability was determined after 72 h using the Cell-Titer-Glo Luminescent Cell Viability Assay Kit (Promega) with a modification to the manufacturer’s protocol wherein the CellTiter-Glo reagent was di-luted 1:3 with PBS. The relative luminescence units (RLU) were measured using the FLUOstar Optima plate reader (BMG Labtech GmbH) and relative cell number was calculated by normalization to the RLU of the control treated cells. The inhibitory concentrations 50 (IC50) were calculated using Sigmoidal dose-response (variable slope) curvefitting with Prism version 5.0 (GraphPad Software).
Cell Cycle and Apoptosis Analyses. Human liposarcoma cells were exposed to inhibitors or 0.1% DMSO for 24 h and harvested. For cell cycle analysis, cells were washed with ice-cold PBS,fixed in 70% ethanol at 4 °C overnight, and stained in PBS containing 10μg/mL RNase A and 20 μg/mL propidium io-dide (Sigma) in the dark. DNA content analysis was performed by flow cytometry (FACScan; Becton Dickinson) with CellQuest and ModFIT LT soft-ware (Becton Dickinson).
For apoptosis analysis, cells were exposed to BEZ235 or 0.1% DMSO for 38 h and harvested. Annexin V and 7-aminoactinomycin D (7-AAD) staining was performed using the PE Annexin V Apoptosis Detection Kit I (#558763; BD Pharmingen) according to the manufacturer’s instructions. Stained cells were quantitated as viable (Annexin V−/7-AAD−), early apoptotic (Annexin V+/ 7-AAD−), or late apoptotic (Annexin V+/ 7-AAD+) by flow cytometry (FACScan; BD Biosciences) with CellQuest software (BD Biosciences). Statistical Analyses. Differences in sarcoma incidence between zebrafish of different p53 genotypes were assessed by the log-rank test. Differences in categorical data were assessed via Fisher’s exact test.
ACKNOWLEDGMENTS. We thank G. Molind and L. Zhang for zebrafish husbandry, J. Testa for the myristoylated mouse Akt2 transgene used in these studies, and D. E. Fisher for the SU-CCS-1 cell line. This work was conducted with support from Harvard Catalystj The Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1 RR 025758 andfinancial contributions from Harvard University and its affiliated academic health care centers), as well as the Ludwig Center at Dana-Farber/ Harvard Cancer Center and Harvard Medical School; A.G. is supported by National Institutes of Health Grant 1K08CA133103 and is a scholar of the American Society of Hematology-Amos Medical Faculty Development pro-gram; and A.M.-E. is supported by Fundacion Alfonso Martin Escudero.
1. Dalal KM, Antonescu CR, Singer S (2008) Diagnosis and management of lipomatous tumors. J Surg Oncol 97:298–313.
2. Fletcher CDM, Unni KK, Mertens F eds (2002) Pathology and Genetics of Tumours of Soft Tissue and Bone (IARC Press, Lyon).
3. Kransdorf MJ (1995) Malignant soft-tissue tumors in a large referral population: Distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol 164:129–134. 4. Dei Tos AP, Pedeutour F (2002) Atypical lipomatous tumor/well differentiated lip-osarcoma; Dedifferentiated liposarcoma. Pathology and Genetics of Tumors of Soft
Tissue and Bone: World Health Organization Classification of Tumors., ed Fletcher CDM (IARC press, Lyon), pp 35–39.
5. Jones RL, Fisher C, Al-Muderis O, Judson IR (2005) Differential sensitivity of lip-osarcoma subtypes to chemotherapy. Eur J Cancer 41:2853–2860.
6. Conyers R, Young S, Thomas DM (2011) Liposarcoma: Molecular genetics and thera-peutics. Sarcoma 2011:483154.
7. Leach FS, et al. (1993) p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res 53(10, Suppl):2231–2234.
8. Pilotti S, et al. (1997) Distinct mdm2/p53 expression patterns in liposarcoma sub-groups: Implications for different pathogenetic mechanisms. J Pathol 181:14–24. 9. Schneider-Stock R, et al. (1998) MDM2 amplification and loss of heterozygosity at Rb
and p53 genes: no simultaneous alterations in the oncogenesis of liposarcomas. J Cancer Res Clin Oncol 124:532–540.
10. Italiano A, et al. (2008) HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent am-plicon. Int J Cancer 122:2233–2241.
11. Debelenko LV, et al. (2010) p53+/mdm2- atypical lipomatous tumor/well-differenti-ated liposarcoma in young children: an early expression of Li-Fraumeni syndrome. Pediatr Dev Pathol 13:218–224.
12. Pedeutour F, et al. (1999) Structure of the supernumerary ring and giant rod chro-mosomes in adipose tissue tumors. Genes Chrochro-mosomes Cancer 24:30–41. 13. Barretina J, et al. (2010) Subtype-specific genomic alterations define new targets for
soft-tissue sarcoma therapy. Nat Genet 42:715–721.
14. Snyder EL, et al. (2009) c-Jun amplification and overexpression are oncogenic in lip-osarcoma but not always sufficient to inhibit the adipocytic differentiation pro-gramme. J Pathol 218:292–300.
15. Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8:627–644.
16. Marsh DJ, et al. (1999) PTEN mutation spectrum and genotype-phenotype correla-tions in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet 8:1461–1472.
17. Hernando E, et al. (2007) The AKT-mTOR pathway plays a critical role in the de-velopment of leiomyosarcomas. Nat Med 13:748–753.
18. Berghmans S, et al. (2005) tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA 102:407–412.
19. Tan Y, et al. (2008) A novel recurrent chromosomal inversion implicates the homeo-box gene Dlx5 in T-cell lymphomas from Lck-Akt2 transgenic mice. Cancer Res 68: 1296–1302.
20. Langenau DM, et al. (2007) Effects of RAS on the genesis of embryonal rhabdo-myosarcoma. Genes Dev 21:1382–1395.
21. Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18: 1926–1945.
22. Maira SM, et al. (2008) Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 7:1851–1863. 23. Carnevalli LS, et al. (2010) S6K1 plays a critical role in early adipocyte differentiation.
Dev Cell 18:763–774.
24. Westerfield M (1994) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) (University of Oregon Press, Eugene, OR); 2.1 Ed.
25. Jessen JR, Jessen TN, Vogel SS, Lin S (2001) Concurrent expression of recombination activating genes 1 and 2 in zebrafish olfactory sensory neurons. Genesis 29:156–162. 26. Gutierrez A, et al. (2011) Pten mediates Myc oncogene dependence in a conditional zebrafish model of T cell acute lymphoblastic leukemia. J Exp Med 208:1595–1603.
Gutierrez et al. PNAS | September 27, 2011 | vol. 108 | no. 39 | 16391
MEDICAL