HAL Id: hal-00460169
https://hal.univ-brest.fr/hal-00460169
Submitted on 26 Feb 2010HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
neighbourhood of Algiers: use of the sea urchin,
Paracentrotus lividus, as a bioindicator
Dina Soualili, Philippe Dubois, Pol Gosselin, Philippe Pernet, Monique
Guillou
To cite this version:
Dina Soualili, Philippe Dubois, Pol Gosselin, Philippe Pernet, Monique Guillou. Assessment of sea-water pollution by heavy metals in the neighbourhood of Algiers: use of the sea urchin, Paracentrotus lividus, as a bioindicator. ICES Journal of Marine Science: Journal du Conseil, 2008, 65 (2), pp.132-139. �10.1093/icesjms/fsm183�. �hal-00460169�
The use of the sea urchin Paracentrotus lividus (L.) as bioindicator of the sea water quality of the Algerian coastal environment
Soualili D. Dubois Ph, Gosselin P., Pernet Ph., Guillou M.
Abstract
This study assessed marine contamination in heavy metals in the vicinity of the Algiers metropolis by combining chemical and toxicological data using the sea urchin Paracentrotus lividus. Metals were analyzed in the sediment and in the sea urchin gonads. The sediment toxicity was assessed by bioassays using sea urchin larval development. This study discriminated a site near the Algiers town as highly polluted in Pb. The contamination levels appeared to be toxic for the P. lividus larval development. The occurrence of other metals (Fe, Cd, Cu) was poor compared with that reported in the Mediterranean Sea, with exception of Zn that showed high values in female gonads.
Keywords : Algeria, bioassays, gonads, heavy metals, sediment, Paracentrotus lividus. Soualili Dina : Laboratoire de Biologie et d’Ecologie Marine, Faculté des Sciences de la Nature, Université des Sciences et Technologie Houari Boumedienne, Bab-Ezzouar, Algiers, Algeria.
Dubois Philippe, Pernet Philiipe : Laboratoire de Biologie Marine, Université Libre de Bruxelles, CP 160/15, Av. F.D. Roosevelt, B-1050 Bruxelles, Belgium
Gosselin Pol : Laboratoire de Biologie Marine, Université de Mons-Hainaut, Av. du Champs de Mars, Pentagone, 7000Mons, Belgium
Guillou Monique : Laboratoire des Sciences de l’Environnement Marin, UMR CNRS 6539, Institut Universitaire Européen de la Mer, Place N. Copernic, 29280 Plouzané, France.
Corresponding author : Guillou Monique, tel : 33 2 98 49 86 34 ; Fax 33 2 98 49 86 45 e-mail : mguillou@univ-brest.fr
Introduction 1
The Atlantic-Mediterranean sea urchin Paracentrotus lividus (Lamarck) 2
(Echinodermata : Echinoidea) is distributed from Ireland to southern Morocco, 3
including throughout the Mediterranean Sea. This opportunistic species has an 4
important ecological role in different ecosystems especially in Mediterranean Sea where 5
its grazing activity can locally control the dynamics of seaweeds (see review Lozano et 6
al., 1995) and seagrasses (see review Tomas et al., 2004). However human activities in 7
coastal zones can alter P. lividus morphology, biology and demography (Harmelin et 8
al., 1981; Delmas et Régis, 1984, Pancucci et al., 1992) and over the long-term can 9
modify its ecological role. The sedentary habit of P. lividus and its sensitivity to 10
pollutants has led to use this species as biological-biochemical indicator of local 11
pollution (Warnau et al. 1998, Coteur et al. 2001; Bayed et al. 2005). The sensitivity of 12
embryos and larvae of P. lividus prompted its use in embryotoxicity tests (Hagström 13
and Lönning, 1973; Pagano, 1986; Warnau et al. 1996). The embryonic and larval 14
development of sea urchins is one of the toxicity assays used in monitoring and risk 15
assessment programmes (see review Beiras et al., 2003). 16
On the Algerian coasts, P. lividus is a dominant species of the shallow water ecosystems 17
where it can reach 25 individuals.m-² (Semroud, 1993). It colonizes different biotop like 18
Posidonia oceanica and Cymodocea nodosa meadows, rocky substrate with photophile 19
algae and overgrazed rocky substrate (Semroud, 1993 ; Guettaf, 2000). It lives from the 20
low-water limit to about 10 m depth (Dieuzeide, 1933 ; Semroud, 1993). 21
Around the Bay of Algiers, the populations are exposed to anthropogenic disturbance. 22
More than 35% of the national littoral population live in the Algiers metropolitan area 23
(between Chenoua and Cap Djinet) or more than 4.3 million persons (950 habitants. 1
km-²) in a 115 km long coastal zone, This area is highly industrialized, concentrating 2
about 25 % of the Algerian factories with about 1000 factories in 2001 (Larid, 2003; 3
PAC, 2005) including metallurgical, chemical, pharmaceutical, building material and 4
hydrocarbon industries and in a lower proportion mechanical, electric and electronic 5
engineering industries and food and paper factories. A multitude of small polluting 6
activities, official or not, spreads also along the littoral. Two rivers flow in the bay of 7
Algiers, El Hamiz, and principally El Harrach (970 km-²; 6 m3 h-1) which drain the 8
domestic and industrial waste waters of the Algiers city. Among the wastewaters which 9
flow in the bay (225 millions m3 y-1) only 8% are treated (PAC, 2005). The marine area 10
under the influence of the Algiers metropolis catches the highest pollution flow of the 11
Algerian coasts (PAC, 2003) with 100 thousand t y-1 of organic chemical compounds, 12
175 thousand s t y-1 of suspended matter, 1 500 t y-1 of nitrogen and 4 thousand s t y-1 of 13
phosphorus (Larid, 2003). In 2004, 46 beaches of the Bay were prohibited from 14
swimming. The sediments of Algiers Bay are highly polluted in heavy metals, 15
hydrocarbons and organic matter. The main metal detected in the coastal water are Hg, 16
Pb, Cu and Zn (PAC, 2003). The biological effects of the pollution on marine 17
ecosystem are important and the recent program on the management of the coastal 18
environment (PAC) pointed out a biodiversity decrease of 14% in the species of major 19
ecological interest (PAC, 2005). 20
The purpose of the present study was to assess the marine pollution in this area by 21
combining toxicological and chemical data using the sea urchin P. lividus. Embryo-22
larval sediment toxicity bioassays and metal contaminant analysis were used. The metal 23
were analyzed in the sediment. The toxicity of their bioavailable fraction was detected 24
by abnormalities in the P. lividus development as this species is a valuable indicator of 25
metal contamination and can accumulate metals as a function of the contamination level 1
of the environment (Warnau, 1998). The results of bioassays were compared to the 2
analysis of metal contamination in the sediments and in the gonads of adult sea urchin 3
inhabiting the sediments previously tested. Three sites were chosen in the vicinity of the 4
Bay of Algiers according to potential sources of pollution. The information obtained 5
will allow the ordination of the sampling stations in term of environmental quality. 6
7
Material and Methods 8
9
Sampling sites 10
Two stations, Algiers Beach and Tamentfoust, were located in the Bay of Algiers and 11
another Sidi Fredj, in the North of the Bay of Bou-Ismail (east of the Bay of Algiers) 12
(Fig. 1). The site of Algiers Beach should be the most polluted as it is the nearest of the 13
Algiers town. This site is characterized by a habitat mixing rocks with photophile algae 14
and degraded meadows. The site of Tamentfoust, situated in a ha lf closed creek, is 15
characterised by overgrazed rocks with a sparse Posidonia oceanica beds. The Bay of 16
Bou-Ismail where the site of Sidi Fredj is located has pollution levels much lower than 17
the levels of the Bay of Algiers (PAC, 2005). More distant from the very industrialized 18
area, it encloses a marine medical centre and shows a better health meadow. But 19
Guehioueche and Zelmat (in PAC, 2005) indicated a progressive meadow regression 20
which could be due to the mechanical and organic disturbance caused by the medical 21
centre. 22
23
Collection and preparation of samples 24
Forty sea urchins of 45-65 mm of diameter were sampled by scuba diving in March 1
2002 from each sites between 1 and 5 m depth. This period correspond to the maturity 2
period of the gonads (Semroud and Kada, 1987 ; Guettaf, 2000) before the main 3
spawning which can partly eliminate metal load in the gonad (Warnau et al., 1998). 4
Just after collection, the sea urchins were measured (ambital diameter) in the laboratory 5
and dissected. The sex was determined and the gonads were dried at 60°C for 48h and 6
stored separately in hermetically-sealed polyethylene containers. All the manipulations 7
were performed with stainless steel instruments. 8
Concomitantly to the sea urchin sampling, the upper 2 cm layer of the sediments 9
inhabit ed by sea urchins was collected by coring (5 cm diameter). The samples of 10
sediments were placed into sealed polyethylene bags, carried to the laboratory on ice 11
and immediately dried at 60°C for 48h until constant weight then stored in hermetically-12
sealed polyethylene containers at room temperature. 13
14
Metal analysis 15
The concentration of zinc (Zn), lead (Pb), cadmium (Cd), copper (Cu) and iron (Fe) 16
were measuring in the gonads of the urchins and in the sediment according to the 17
method described by Coteur et al. (2003). As the sediment was very heterogeneous with 18
big gravels, only the fraction with grain sizes < 1mm was retained and considered in 19
this study as the ‘total fraction’. Metal concentrations were also measured on the 20
fraction with grain sizes < 63 µm. This fraction allows to estimate the organic matter 21
content of the sediment. Pb, Cd and Cu concentrations were determined by graphite-22
furnace electrothermic atomic absorption spectrometry using a Varian GTA100 23
SpectrAA640Z spectrometer. Concentration of Zn and Fe were measured by flame 24
atomic absorption spectrometry using a GBC 906AA spectrometer. Accuracy of the 25
method was tested us ing certified reference material (Mytilus edulis tissues, BCR nr 1
278R from the Community Bureau of Reference, Commission of the European Union, 2
Brussels, Belgium). Detection limits for Zn, Pb, Cd, Cu and Fe were 0.002, 0.014, 3
0.001, 0.002 and 0.004 µg of metal per mL of digested sample respectively. 4
The gonads of 10 males and 10 females per site were analysed. For the sediment, 3 to 6 5
replicates of total fraction and 0 to 3 replicates of the fraction < 63 µm were used. 6
7
Embryo-larval bioassays 8
The toxicity of the sediments from the previous three sites was assessed by embryo-9
larval bioassays in May 2003. Natural sea water used for these tests was collected in 10
front of the marine station of Luc-sur-Mer (Normandy, France), the reference site of the 11
Laboratory of Marine Biology of the U.L.B. (Free University of Brussels, Belgium) 12
where the metal analysis where performed. 13
Sea-water was previously allowed to decant for 48 h, filtered by a 22 µm membrane and 14
maintained at 20 ± 1°C (FSW, filtered seawater). Adult Paracentrotus lividus genitors 15
were collected intertidally from a reference population inhabiting the rocky basins of 16
Morgat (Bay of Douarnenez, Brittany, France). Individuals were transferred in the 17
cultivation system of the marine laboratory of the U.M.H. (University of Mons-Hainaut, 18
Belgium). For the embryotoxicity test, reference sediment was sampled at Wimereux 19
(Nord-Pas-de-Calais, France). 20
The embryo- larval bioassays were performed at the laboratory of Mons-Hainaut. Those 21
bioassays were based on the method described by Coteur et al. (2003). Spawning was 22
induced by injection of 20 µl per gram of KCL 0.5N through the peristomial membrane. 23
Gametes from 3 females and 3 males were collected in FSW and their quality (i.e., 24
general eggs’ shape and sperm’s motility) was checked under Olympus TO41 inverted 25
light microscope. Eggs from each female were fertilised by the pooled sperm from the 3 1
males. Embryos at early gastrula stage (4-5 h after fertilization at 14°C ± 1°C) from the 2
3 females were mixed before to be used for the bioassays (this pooling leads to one 3
bioassay per site using the same mixed pool of zygotes). Experiments with embryos 4
were performed in six-well plates (Falcon, ref. 35-3046). O ne plate was dedicated to 5
each sampled sediments (Alger Plage, Tamenfoust, Sidi-Fredj and Wimereux). Each 6
well of those plates were filled with 0.1 g of dried sediment and 10 ml of FWS was 7
added before the bioassay. One plate filled with FWS was used as control. At the 8
beginning of the bioassay, batches of 250-300 embryos were transferred in each well. 9
After 72 h at 14 ± 1°C, larvae were fixed by adding 1 ml of formalin (commercial 10
solution, 35%) in each well and the plates were maintained in an oven at 70°C during 2 11
hours. Plates with fixed larvae were stored at 4°C. The frequency of developmental 12
stages was scored in each well on a random sample of 100 larvae. Developmental stages 13
were scored using an inverted light microscope according to the morphological criteria 14
adapted from Warnau and Pagano (1994). Our larvae were classified in 4 categories: 15
normal plutei (“Normal”, N), retarded plutei presenting a delayed development 16
(“Retarded”, R), abnormal plutei with skeletal malformations and/or gut abnormalities 17
(“Pathologic 1”, P1) and embryos whose development ended at the blastula stage or the 18
gastrula stage (“Pathologic 2”, P2). On our figures, the rates of “Viable” larvae were 19
obtained by summing the rates of “Normal” and “Retarded” larvae from each batch. 20
21
Statistical analyses 22
After arcsin transformation, the developmental rates of normal and viable larvae were 23
compared using a one-way ANOVA followed by a Bonferroni’s test (Zar 1996). 24
Dunnet’s tests were used to compare the different rates measured to their corresponding 1
controls (Zar 1996). Significant differences were determined at the 95% level. 2
Contamination levels in gonads were compared by 2-way analysis of variance 3
(ANOVA) and subsequent Tukey HSD multiple mean comparison test (effects: sex and 4
sampling site). 5
The relationship between contamination in gonad and sediment and percentage of viable 6
larvae was studied by factor analysis using the principal-component method with an 7
extraction matrix based on Pearson correlation coefficients and the ‘’varimax’’ method 8 of factor rotation. 9 10 Results 11 12
Contamination level in the sediment and in the sea urchin gonads 13
The fraction of sediment < 63 µm was more or less abundant according to the site, 0.03, 14
0.26, 1.56 % of the total fraction for Sidi Fredj, Tamenfoust and Algiers Beach 15
respectively. The sediment of Algiers Beach had an obviously higher mud content. 16
Tables 1 and 2 present the heavy-metal concentrations in the sediment. Only the levels 17
in the total sediment have been statistically compared because of the low level of mud 18
in the site of Tamentfoust and Sidi Fredj leading to a too low number of replicates in 19
these both sites. Fe, Pb and Zn concentrations in the total fraction of the sediment 20
differed according to the sampled sites. The site of Algiers Beach was the most 21
contaminated in Pb but the less contaminated in Fe and Zn. Fe concentrations were 22
significantly higher in the sediment from Sidi Fredj. 23
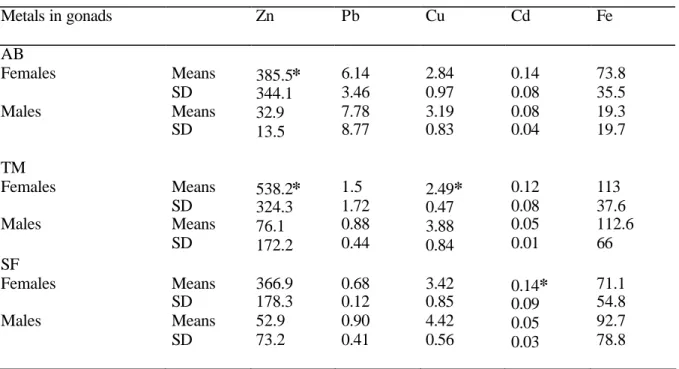
Tables 3 and 4 present the heavy- metal accumulation in the sea urchin gonads. Metal 24
concentrations in the gonads were compared according to sex and sampling site by 2-25
way ANOVA. Cd and Zn concentrations were significantly higher in female than in 1
male gonads while Cu was more concentrated in male gonads (p<10-4). Pb and Fe 2
concentrations did not significantly differ according to sex. Pb, Cu, and Fe 3
concentrations in the gonads differed according to the sampled sites (p=10-3). Pb and Cu 4
concentrations were significantly higher in sea urchins collected respectively in Algiers 5
Beach and Sidi Fredj (Table 3). Fe concentrations in sea urchins from Tamentfoust 6
significantly differed from those of Algiers Beach. No interaction was significant. 7
8
Embryo-larval development 9
A significant reduction of the percentages of both normal and viable larvae was only 10
observed when embryos were exposed to sediment samples from Alger Beach (Fig. 2 A, 11
B). The low rate of normal larvae induced in response to the sediment from Alger Beach 12
is related to an increase of the rates of abnormal plutei (P1) and retarded plutei (R) (see 13
Table 5). The high rate of retarded plutei induced in response to the sediment from 14
Alger Beach (Table 5) explains why the induced rate of viable plutei remains relatively 15
high (Fig 2B.). 16
17
Integrated data analysis 18
The metal concentrations in sediment, in male gonads, in female gonads and percentage 19
of viable larvae were used in the factor analysis. The variables were distributed along 20
axes representing factors according to the correlation coefficient of the variable with the 21
factors (Fig. 3). The first two factors accounted for 99.9 % of the total variance of the 22
data. On the x-axis, the concentrations in Fe, Cu and Zn in the sediment were opposed 23
to the concentrations in Cd and Pb in the sediment. The relationships between the metal 24
level in the sediment and the metal level in the gonads was dependent on the metal. For 25
Pb the link was strong between sediment and the both gonads, for Cd and Cu it was 1
high between sediment and the male gonads. On the x-axis the percentage of viable 2
larvae was closed to the concentrations in Fe, Cu and Zn in the sediment (e.i located on 3
the other end of the x-axis compared to the Pb and Cd contaminations in sediment and 4
gonads). This suggests a close relationship between Pb and Cd levels in sediment and in 5
gonads and a strongly negative interaction with these latter parameters and the 6
percentage of viable larvae. 7
The y-axis comprised, at the negative end, the levels of Zn in the male and female 8
gonads, and the level of Fe in the female gonads, and at the positive end, the levels of 9
Cd and Cu in the female gonads. The percentage of viable larvae was located on the 10
negative part of this axis suggesting that this parameter was negatively intercorrelated 11
with Cd and Cu levels in the female gonads. 12
13
Discussion 14
The main goal of the present study was to assess the marine pollution in the vicinity of 15
the Bay of Algiers by combining chemical and toxicological data using the sea urchin P. 16
lividus as bioindicator. 17
No site was distinguishable from others by a generalized higher concentration of metals 18
in the total sediments or in the sea urchin gonads. The sediments of Algiers Beach was 19
separated from the others by the highest concentration in Pb but the lowest 20
concentrations in Zn and Fe, the site of Sidi Fredj by the highest concentration in Fe. 21
Algiers Beach is the most muddy site but its present a significant increase in metal 22
contamination only for a metal, Pb, while usually the load of metal in the sediments 23
increase along with the percentage of the fine fraction. This fact can be due to the low 24
sediment contamination in the other metals (see below). In the gonads, Pb also isolated 25
the population of Algiers Beach from the others. In this site, Fe was low but not 1
different from Sidi Fredj. This latter site presented the highest concentrations in Cu. Cd 2
did not discriminate any site. 3
In the sediment, Cd and Cu values can be considered in the background concentrations 4
of the Mediterranean range. Cd values were ranged between to 0.12 to 0.76 µg g-1 dry 5
wt in the total fraction of the sediment while the background concentrations of the 6
Mediterranean were estimated between 0.05 and 1 µg g-1 dry wt (EEA, 1999). We note 7
however a higher concentration in the fraction with grain sizes <63 µm of the site of 8
Algiers Beach (1.22 µg g-1 dry wt). The Cu values ranged from 4.1 to 6.4 µg g-1 dry wt 9
in the total fraction of the Algerian sediment and to 20.9 µg g-1 in the fraction with grain 10
sizes <63 µm (Algiers Beach) and the background concentrations of the Mediterranean 11
ranged between 5 to 30 µg g-1 (EEA, 1999). The Zn (between 0.02 and 0.08 µg g-1 dry 12
wt) and Fe concentrations (between 7.1 and 41.5 µg g-1) were very low compared to 13
values usually observed in the Mediterranean Sea ranged from 35 to 150 µg g-1 for Zn 14
(Saad et al. 1981, Hoogstraten and Nolting, 1991; Storelli et al. 2001) and > 103 µg g-1 15
for Fe (Saad et al. 1981, Storelli et al. 2001, Menchi et al., 2002). Only Pb values in the 16
total fraction of the sediment of Algiers Beach (39.6 µg g-1) and in the fraction with 17
grain sizes <63 µm of Sidi Fredj (40.6 µg g-1) exceeded the background concentrations 18
of the Mediterranean range detected between 5.2 to 23.2 µg g-1(EEA, 1999) or the 19
background (4-17 µg g-1) given by the reference tables of NOAA (Buchman,1999). 20
In the gonads, comparisons with P. lividus data available from the literature (Table 6) 21
and especially the study of Warnau et al (1998), confirms the conclusions on the level 22
of metal contamination deduced from the sediment analysis except for Zn. The gonad 23
concentrations in Cd, Cu and Fe from the three sampling locations averaged background 24
concentrations in P. lividus gonads. Only the gonad contamination in Zn from the 3 25
sites and in Pb from Algiers Beach were obviously higher that the Zn and Pb 1
background concentrations for this species. The Zn concentrations, not significantly 2
different among the sites, were high, comparable to the concentrations estimated in 3
female gonads of Sphaerechinus granularis from the bay of Bay of Brest (between 190 4
and 700 µg g-1) (Guillou et al. 2000). This bay is known to be contaminated by this 5
metal which covers the whole roof of the town (Troadec,1995). But in the present study 6
the Zn gonad levels appeared disproportionate with the Zn levels in sediment indicating 7
that sediment possibly is not the most contaminated abiotic compartment. But as Zn was 8
dominant in the gonads of the females, the results about this essential element for 9
animal metabolism must be cautiously interpreted (Hambidge et al., 1986). On the 10
contrary, the high Pb levels in gonads reflected the high Pb levels in the sediment. 11
Among the compared sites, only Rabat (Morocco) presented higher concentrations due 12
to the untreated pottery activity of this town (Bayed et al., 2005). 13
However when metal discriminates the site in term of contamination, the response given 14
by the sediment can differ from that given by the gonad. The geographical Pb gradient 15
observed in the sediment matches closely the gradient reported by the gonad 16
accumulation, pointing out the site of Algiers Beach as the most contaminated in this 17
metal. But Fe and Zn bioaccumulation did not discriminate the sites in the same way as 18
Fe and Zn sediment concentrations. Three explanations can explain the differential 19
metal pattern: i) the bioavailability of the metals is different ii) essential elements as Fe 20
and Zn have different accumulation pattern that the other metals iii) Fe, Zn and Cu 21
concentrations were too low in the sediment to be suitably expressed in the biological 22
compartment. Although the two first hypothesis are plausible, the third one is clear (see 23
above). 24
The high contamination level in Pb in the site of Algiers Beach expressed by the 1
sediment and gonad analysis was confirmed by a recent study in the water and stream 2
sediment of Oued El Harrach which flows in the Algiers Bay. Levels of 21 to 41 µg g-1 3
dry wt, close to the levels detected in the total fraction of the sediment of Algiers Beach 4
(39.6 µg g-1) were detected in the sediments in the mouth of the Oued. This pollution 5
was probably caused by the discharge of an Algiers untreated industrial wastewater 6
(Yoshida et al., 2005). 7
The results of the bioassays pointed out Algiers Beach as the only site showing toxic ity 8
on sea-urchins embryos with 24.3 % of abnormal larvae (vs 7.9 ± 2.8 % for the 3 other 9
sites) and 47,8 % of retarded larvae (vs 22.56 ± 4.4 % for the 3 other sites). No 10
difference was detected between the two other Algerian sites and the reference site and 11
the control. According to the Kobayashi criteria (1991), the level of normal plutei of 12
Algiers Beach (20.5%) expressed a strong inhibition of the sea urchin development in 13
relation with a high disturbance. The results of these assays appeared negatively related 14
with the Pb concentrations in the male and female gonads which were positively 15
correlated with the Pb concentration in the total fraction of the sediment. The integrated 16
data analysis confirms these conclusions. The high positive relationship between the 17
rate of viable larvae and the Cu and Fe concentrations in the sediment and male gonad 18
and the Zn concentration in the sediment would not express a positive effect of these 19
metals on the larval development but more precisely a lack of negative effect du to the 20
low levels of these metals. Nevertheless the position of the viable larvae suggests a 21
negatively correlation with Cd and Cu levels in the female gonads. Although these 22
contamination levels were low, a possible higher influence of the female go nad on the 23
larval viability would be considered when these gonads were contaminated by metals so 24
toxic as Cd and Cu. 25
The Pb pollution was not spread out as the sediment and the sea urchins of the site of 1
Tamenfoust, a little farther from the Oued El Harrach than the site of Algiers Beach, did 2
not present high Pb accumulation or developmental anomalies. In a semi- closed creek, 3
this former site could be protected from the sea water and sediment flows coming from 4
the Oued. The site of Sidi Fredj considered as less contaminated because more distant 5
from the very industrialized area influenced by Algiers metropolis cannot be 6
distinguished from the site of Tamenfoust according to the criteria used in this study. 7
8
In conclusion, this study confirms the link between developmental abnormalities, metal 9
accumulations in the gonads and metal contamination in the sediment when the metal 10
concentration in sediment is sufficiently high. It accredits the indicator value of P. 11
lividus. In the present study the analysis discriminates a site and a metal, Pb, but the 12
method could be improved by increasing the number of toxicants analysed and by 13
extending the metal analysis to other sea urchin storage organs as the gut which can 14
better reflect a heavy metal accumulation (Warnau et al, 1998; Guillou et al, 2000). 15
16
Acknowledgments. 17
This paper is a part of the thesis of D. Soualili; this study was carried out in the 18
« Laboratoire de Biologie Marine de l’Université Libre de Bruxelles, ULB », in the 19
« Laboratoire de Biologie Marine de l’Université de Mons-Hainaut, UMH » (Belgium), 20
and ended in the « laboratoire des Sciences de l’Environnement Marin de l’Université 21
de Bretagne Occidentale, UBO » (France). The study in Belgium was funded by a grant 22
of the “Institut des Sciences Agronomiques et Vétérinaires de l’Université de Blida 23
(Algeria)”, the stay in France by a grant of the Algerian Department of Higher 24
Education and Research. D. Soualili gratefully acknowledges Professeur M. Jangoux 25
(ULB) for his hospitality, for access to facilities and helpful discussions. The authors 1
also thank Dr F. Boukroufa for assistance in the field, M. Briand (UBO) for her help in 2
the preparation of figures and M.P. Friocourt (UBO) for fruitful discussions about the 3
writing of the English manuscript. 4
References
Bayed, A., Quiniou, F., Benrha, A., Guillou, M. 2005. The Paracentrotus lividus populations from the Northern Moroccan Atlantic coast: growth, reproduction and health condition. Journal of Marine Biological Association of the United Kingdom 85, 999-1007.
Beiras, R., Fernandez, N., Bellas, J., Besada, V., Gonzalez-Quijano, A., Nunes, T. 2003. Integrative assessment of marine pollution in Galician estuaries using sediment chemistry, mussel bioaccumulation, and embryo- larval toxicity bioassays. Chemosphere 52(7), 1209-1224.
Buchman, M.F. 1999. NOAA Screening Quick Reference Tables, NOAA HAZMAT Report 9-1, Seattle WA, Coastal Protection and Restoration Division, National Oceanic and Atmospheric Administration, 12pp.
Coteur, G., Danis, B., Fowler, S.W., Teysiié, J.L., Dubois, Ph., Warnau, M. 2001. Effects of PCB’s on reactive oxygen species (ROS) production by the immune cells of Paracentrotus lividus (echinodermata). Marine Pollution Bulletin 42, 667-672.
Coteur, G., Gosselin, P., Wantier, P., Chambost-Manciet, Y., Danis, B., Pernet, Ph., Warnau, M., Dubois, Ph. 2003. Echinoderms as bioindicators, bioassays and impact assessment tools of sediment-associated metals and PCBs in the North Sea. Archives of Environmental Contamination and Toxicology 45, 190-202.
Dieuzeide, R. 1933. Les Echinoides réguliers de la baie de Castiglione. Bulletin des Travaux Scientifiques de la Station Aquacole et de Pêche de Castiglione, 1-9.
European Environment Agency (EEA),. 1999. Environmental State and Threats. In : State and pressures of the marine and coastal Mediterranean environment. UNEP, 6-104.
Guettaf, M., Gustavo, A., San Martin, G.H., Francour, P. 2000. Interpopulation variability of the reproductive cycle of Paracentrotus lividus (Echinodermata: Echinoidea) in the south-western Mediterranean. Journal of Marine Biological Association of the United Kingdom 80, 899-907.
Guillou, M., Quiniou, F., Huart, B., Pagano, G. 2000. Comparison of embryonic development and metal contamination in several populations of the sea urchin Sphaerechinus granularis (Larmarck) exposed to anthropogenic pollution. Archives of Environmental Contamination and Toxicology 39, 337-344.
Hagström, B.E., Lönning, S. 1973. The sea urchin egg as testing object in toxicology. Acta Pharmacologica and Toxicologica 13 : 7-49.
Hambrige, K.M., Casey, C.E., Krebs, N.F. 1986. Zinc. In: Mertz, W. (Ed), Trace elements in human and animal nutrition. Vol 2, 5th edn., Academic Press, Orlando, FL.
Hoogstraten van, R.J., Nolting, R.F. 1991. Trace and major elements in sediments and inporewaters from the North Western basin of the Mediterranean Sea. Netherlands Institute for Sea Research (NIOZ), Rapport 1991 10, 1-72.
Kobayashi, N. 1991. Marine pollution bioassay by using sea urchin eggs in the Tanabe Bay, Wakayama Perfecture, Japan, 1970-1987. Marine Pollution Bulletin 23, 709-713. Larid, M. 2003. Analyse de la durabilité dans le cadre du PAC “Zone côtière algéroise” (Algérie). Rapport de la première étape 36 pp.
Lozano, J., Galera, J., Lopez, S., Turon, X., Palacin, C., Morera, G. 1995. Biological cycles and recruitment of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats. Marine Ecology Progress Series 122, 179-191.
Menchi, V., Balocchi, C., Pozo, K., Perra, G., Graziozi, M., Focardi, S. 2002. Distribution of PCBs, PAHs and heavy metals in bottom sediments of the Eastern Mediterranean Sea. In : Challenges in Environmental risk Assessment and Modelling, Proceedings of the SETAC, Europe 12 th Annual Meeting. Vienna, Austria p.147. Pagano, G., Cipollaro, M., Corsale, G., Esposito, E., Ragucci, E., Giordano G.G., Trieff, N.M. 1986. The sea urchin: Bioassay for the assessment of damage from environmental contaminants. In : Cairns, J. JR. (ed), Community toxicity testing. American Society for testing and materials, Philadelphia, PA, USA, 66-92.
PAC (Rapport d’Aménagement Côtier) 2003. Rapport sur l’état et l’avenir de l’environnement. Ministère de l’Aménagement du Territoire et de l’Environnement. République Algérienne Démocratique et Populaire. 463pp.
PAC (Rapport d’Aménagement Côtier) 2005. “Zone côtière algéroise”. Protection des sites sensibles naturels marins du secteur Cap Djinet au Mont Chenoua. Impact des activités anthropiques. Ministère de l’Aménagement du Territoire et de l’Environnement. République Algérienne Démocratique et Populaire. 88 pp.
Pancucci, M.A., Panayotidis, P., Zenetos, A. 1993. Morphological changes in sea urchin populations as a response to environmental stress. In Aldrich, J.C. (ed), Quantified phenotypic responses in morphology and physiology. JAPAGA, Ashford. pp. 247-257. Saad, M.A., El-Rayis, O.A. , El-Nady, F.E. 1981. Occurrence of organic matter and heavy metals in sediments from the Mediterranean. In : Stuckey, D., Hamza, A. (eds), Management of Industrial Wastewater in Developing Nations. Pergamon Press, Oxford. pp.127–139.
Semroud, R. 1993. Contribution à la connaissance de l’écosystème à Posidonia oceanica (L) Delile dans la région d’Alger (Algérie): étude de quelques compartiments. PhD thesis, Institut des Sciences de la Nature, Université des Sciences et de la Technologie Houari Boumediene, Algeria.
Semroud, R., Kada, H. 1987. Contribution à l’étude de l’oursin Paracentrotus lividus (Larmarck) dans la région d’Alger (Algé rie): indice de réplétion et indice gonadique. In:
Boudouresque, C.F. (ed), Colloque international sur Paracentrotus lividus et les oursins comestibles. GIS Posidonie Publications, Marseille, France, pp 117-124.
Storelli, M.M., Storelli, A., Marcotrigiano, G.O. 2001. Heavy metals in the aquatic environment of the Southern Adriatic Sea, Italy Macroalgae, sediments and benthic species. Environment International 26, 505-509.
Tomas, F., Romero, J., Turon, X. 2004. Settlement and recruitment of the sea urchin Paracentrotus lividus in two contrasting habitats in the Mediterranean. Marine Ecology Progress Series 282, 173-184.
Troadec, P. 1995. La qualité du milieu marin: la rade de Brest. Communauté urbaine de Brest, rapport interne, 165 pp.
Warnau, M.., Pagano, G. 1994. Developmental toxicity of PbC12 in the echinoid Paracentrotus lividus (Echinodermata). Bulletin of Environmental Contamination and Toxicology 53, 434-441.
Warnau, M., Iaccarino, M., De Biase, A., Temara, A., Jangoux, M., Dubois, Ph. 1998. Spermiotoxicity and embryotoxicity of heavy metals in the echinoid Paracentrotus lividus. Environmental Toxicology and Chemistry 15, 1931-1936.
Warnau, M., Biondo, R., Temara, A., Bouquegneau, J.M., Jangoux, M., Dubois, Ph. 1998. Distribution of heavy metal in the echinoid Paracentrotus lividus (Lmk) from the Mediterranean Posidonia oceanica ecosystem: seasonal and geographical variations. Journal of Sea Research 39, 267-280.
Yoshida, M., Moali, M., Houas, O., Lakhdari, M., Nechaoui, L., Guerrida, D., Chatal, A., Oussalem, S., Makour, F., Khelifi, F., Laleg, A. 2005. Environmental Pollution in Oued El Harrach area, Alger A Preliminary Report on Mercury and Heavy Metals Contaminations. Compte-Rendu du Séminaire “Pollution et Protection de l’Environnement en Algérie”. ONEDD, JICA, Alger, pp 19-37.
Zar, J.H. 1996. Biostatistical analysis. Prentice-Hall Inc., Upple Saddle River, New Jersey.
Legends of figures
Figure 1 : Location of the three sampling sites: Algiers Beach, Tamenfoust and Sidi Fredj.
Figure 2. Percentages (mean ± S.D.) of normal (A) and viable (B) larvae of Paracentrotus lividus after exposition to dried sediments and control throughout
embryogenesis (72 h). 6 replicates by exposure, 100 larvae scored by replicate. For each species, there were no significant differences between the series designed by the same letter (Bonferroni, p = 0.05). Normal larvae 61,67 62,17 60,67 20,50 59,17 0 10 20 30 40 50 60 70 80 90 100
Control Wimereux Tamentfoust Sidi Fredj Algiers Beach
% Viable larvae 89,33 82,33 80,50 68,33 82,83 0 10 20 30 40 50 60 70 80 90 100
Control Wimereux Tamentfoust Sidi Fredj Algiers Beach
% ab b c a a a b a A B Viable larvae 89,33 82,33 80,50 68,33 82,83 0 10 20 30 40 50 60 70 80 90 100
Control Wimereux Tamentfoust Sidi Fredj Algiers Beach
% ab b
c B
Fig. 3. Factor analysis (principal-component method) showing the relationships between the metal concentrations in the sediment (metal and suffix SED), in the male gonads (metal and suffix MG), in the female gonads (metal and suffix FG) and the percentage of viable larvae (VIABLARV). The first (x-axis) and the second (y-axis) factors accounts 70% and 29.9%. of the total variance respectively.
Legends of tables
Table 1. Metal concentration (mean ± SD ; µg g-1 dw) in the total fraction (n =3 to 6) and in the <63µm grain-size fraction (n= 0 to 3) of the sediment collected in the three Algerian sites (AB: Algiers Beach; TM: Tamenfoust; SF: Sidi Fredj) .
Metals in sediments Zn Pb Cu Cd Fe Total fraction AB TM SF Means SD Means SD Means SD 0.023 0.005 0.045 0.010 0.050 0.006 39.63 7.93 14.59 1.55 12.37 4.07 4.08 0.62 5.73 1.65 6.43 1.32 0.76 1.05 0.15 0.06 0.12 0.01 7.11 2.18 17.85 3.71 31.32 6.49 <63 µm fraction AB SF Means SD Means SD 0.081 0.008 0.08 --- 23.76 0.75 40.63 --- 20.89 0.75 10.48 --- 1.22 0.04 0.25 --- 19.70 2.08 41.50 ---
Table 2. Comparison of the metal concentrations in the total fraction of the sediments measured in the three Algerian sites (AB: Algiers Beach; TM: Tamenfoust; SF:Sidi Fredj) .
Metal in sediments Total fraction
p ANOVA Level of contamination b
+
-
Zn Pb Cu Cd Fe 0.03 > 10 -2 NS a NS > 10 -2 SF TM AB AB TM SF TM SF AB AB TM SF SF TM AB a NS, no significant difference bstations which do not differ in metal concentration are underlined (p >0.05, Tukey HSD test)
Table 3. Metal concentrations (mean ± SD ; µg g-1 dw; n=10) in the gonads of Paracentrotus
lividus collected in the three Algerian sites (AB: Algiers Beach; TM: Tamenfoust; SF: Sidi
Fredj) . Metals in gonads Zn Pb Cu Cd Fe AB Females Males Means SD Means SD 385.5* 344.1 32.9 13.5 6.14 3.46 7.78 8.77 2.84 0.97 3.19 0.83 0.14 0.08 0.08 0.04 73.8 35.5 19.3 19.7 TM Females Males Means SD Means SD 538.2* 324.3 76.1 172.2 1.5 1.72 0.88 0.44 2.49* 0.47 3.88 0.84 0.12 0.08 0.05 0.01 113 37.6 112.6 66 SF Females Males Means SD Means SD 366.9 178.3 52.9 73.2 0.68 0.12 0.90 0.41 3.42 0.85 4.42 0.56 0.14* 0.09 0.05 0.03 71.1 54.8 92.7 78.8 * indicates a significant difference between the sexes
Table 4 Comparison of metal concentrations in the gonads of Paracentrotus lividus measured in the three Algerian sites (2-factor ANOVA: sex and site) (AB: Algiers Beach; TM: Tamenfoust; SF:Sidi Fredj) .
p ANOVA
Metal in gonads Sex Site Interaction n Level of contamination b
+ - Zn Pb Cu Cd Fe <10-4 0.69 a <10-4 <10-4 0.40 a 0.26 a <10-4 <10-2 0.45 a <10-3 0.53 0.66 0.11 0.80 0.06 60 60 60 60 60 TM SF AB AB TM SF SF TM AB SF AB TM TM SF AB a no significant difference b
stations which do not differ in metal concentration are underlined (p >0.05, Tukey HSD test)
Table 5. Frequencies (means ± standard errors) of developmental stages in Paracentrotus
lividus larvae exposed to sediments samples throughout embryogenesis.
N R P1 P2 Du1 V Du2 Control 59.2 ± 9.7 23.7 ± 5.9 8.7 ± 4.7 8.5 ± 3.2 82.8 ± 6.3 Wimereux 61.7 ± 5.8 27.7 ± 6.2 5.0 ± 2.3 5.7 ± 2.2 = 0.6 89.3 ± 2.4 = 0.05 Sidi Fredj 60.7 ± 8.1 19.8 ± 2.6 10.7 ± 3.1 8.8 ± 4.2 = 0.6 80.5 ± 6.8 = 0.4 Tamentfou st 62.2 ± 6.6 20.2 ± 3.9 8.2 ± 3.3 9.5 ± 2.6 = 0.6 82.3 ± 4.8 = 0.5 Algiers Beach 20.5 ± 17.4 47.8 ± 13.5 24.3 ± 7.9 7.3 ± 3.3 < 0.0005 68.3 ± 6.4 < 0.0005
N: normal plutei; R: retarded plutei; P1: abnormal plutei; P2; Blastula; V: viable plutei; Du1: result of the Dunnett test comparing the rate of normal plutei to the control; Du2: result of the Dunnett test comparing the rate of viable plutei to the control.
Table 6. Comparison of the mean concentrations (µg g-1 dw) of metals in the gonads of
Paracentrotus lividus.. Authors (sampling date) Sites Zn Pb Cu Cd Fe Algiers Beach Females Males 385.5 32.9 6.14 7.78 2.84 3.19 0.14 0.08 73.8 19.3 Tamentfoust Females Males 538.2 76.1 1.5 0.88 2.49 3.88 0.12 0.05 113 112.6 Present study March 2002 Sidi Fredj Females Males 366.9 52.9 0.68 0.90 3.42 4.42 0.14 0.05 71.1 92.7 Bayed et al 2005 March 2000 Atlantic Morocco Rabat Females Males Bouznika Females Males Mohammedia Females Males 35.32 12.41 11.3 7.18 5.22 6.14 3.34 0.52 5.56 1.52 2.51 1.18 25.15 2.58 2.21 1.51 2.24 2.32 Warnau et al 1998 Calvi Ischia Marseille 124 140 109 2.25 3.02 3.68 0.15 0.41 0.19 3.47 3.41 3.51 51 90 39 Storelli et al 2001 April 1998 Adriatic Sea 157.1 0.86 0.24 5.19 18.37