Oldenbourg
Surface
Dynamics
of
the
Cyclohexadienyl
Radical
Adsorbed
onSilica Gel
Investigated
Using
Avoided
Level-Crossing
Muon
Spin
Resonance
By
MartinaSchwager,
Emil Roduner*Physikalisch-Chemisches
Institut derUniversitätZürich, Winterthurerstrasse 190,CH-8057 Zürich, Switzerland Ivan D. ReidPaul ScherrerInstitute,CH-5232VilligenPSI, Switzerland
Sydney
R. KreitzmanTRIUMF,4004 Wesbrook Mall,Vancouver, BritishColumbia, Canada V6T 2A3 Paul W.
Percival,
Jean-ClaudeBrodovitch,
Siu-Keung Leung
and
Sunny
Sun-MackChemistryDepartment, Simon FraserUniversity, Burnaby, BritishColumbia, CanadaV5A 1S6
Dedicatedto
Professor
Dr.Harry
Pfeifer
ontheoccasionof
his 65thbirthday
(Received
April
20, 1994)Surface
diffusion
ISilicaICyclohexadienyl
radical I Muonspin
resonanceThe
dynamic
behaviour of thecyclohexadienyl
radicalon asilica surface has beeninvesti-gated using
the avoidedlevel-crossing
muonspin
resonancetechnique.
Translationalmo-tion ofthe radical on a curved surface leads to
partial
averaging
of the muon-electronhyperfine
anisotropy
and thustomotionalnarrowing
of theexperimental signal.
A theo-retical model basedon astochasticLiouvilleformalism allowsinterpretation
of the datain terms ofradical diffusion. Arrheniusbehaviourwith an activation energy of(10.8 ±
* To whom
0.9)kJ/mol is observed. Specific deviations between theoreticalpredictions and experi-mental resultsindicate that thepotentialof thistechnique isnotyet
fully
exploitedand that the model canbe further refined.Das
dynamische
Verhalten des auf der Oberfläche von Silica adsorbiertenCyclohexa-dienylradikals
wurde untersucht mittelsAvoided-Level-Crossing Myon-Spin-Resonanz.
DieTranslation des Radikals auf dergekrümmtenOberfläche führtzueinerTeilausmitte-lungder
Anisotropie
derMyon-Elektron-Hyperfeinwechselwirkung.
Sie beeinflußt damit die Linienbreite deruntersuchten Resonanz. Diese wurdeinterpretiert
auf der Basis der stochastischenLiouville-Gleichung
für die Diffusion des Radikals, wobei Arrheniusver-haltenmit einerAktivierungsenergie
von(10.8±0.9)kJ/molgefunden
wurde. BestimmteAbweichungenzwischen theoretischenVoraussagenund
experimentellen
Resultaten zei-gen, daß das Potential derTechnik noch nichtvollständig ausgeschöpft
ist und daß das Modell nochverfeinertwerdenkann.1. Introduction
The motion of molecules both on and off the surface is of fundamental
interest in
heterogenous
catalysis
since it determines theturnoverfrequency
and the balance between unimolecular and bimolecular reactions. Nuclear
magnetic
resonance is well suitedtostudy
themobility
of adsorbeddiamag-netic
molecules,
andpioneering
work concerned with the surface diffusion ofbenzene on silicagel
was conducted inLeipzig
[1—4]
and in Hannover[5],
It was shown that intramolecular and intermoleculardipolar
proton-proton
interactions between adsórbatemolecules,
and furthermore the inter-action with surfacehydroxyl
groups and withparamagnetic impurities
in theadsorbent,
areimportant
for the relaxation of theprotons.
Relaxationtimes were found to lie in the range of 1
—
1000 ms
depending
on surfacecoverage and on the
degree
of deuteration of adsórbate and surface[3, 4].
Further studies of the surface
mobility
of benzene were undertaken for anA1203 [6, 7]
and agraphite
[8]
surface.Free radicals ate
potential
transient intermediates ofheterogeneously
catalysed
processes. Their surfacedynamics
isjust
as essential to theper-formance ofa
catalytic
process asis thedynamics
ofdiamagnetic
reactants andproducts.
Due to the open shell electronic character and the modified structure andenergetics
of the molecularorbitals,
the interaction of theradical with the surfacecan be
expected
todifferconsiderably
from that of theparent
diamagnetic
molecule from which it was derived. On the otherhand,
the radical and itsparent
should behavesimilarly
if the surface inter-action is dominatedby
van der Waals forces.Radicals are less
easily
amenable to studies of their surfacedynamics
than arediamagnetic
molecules,
since as soon asthey
are mobilethey
un-dergo
terminationreactions,
so that atcatalytically
relevanttemperatures
their concentration isusually
belowthe detection limitofmostconventionalexperimental techniques. Recently,
a newtechnique
which usespositive
muons substituted aspolarised spin
labels inorganic
radicals has beende-veloped
[9].
Themuons,obtained fromabeam line ofasuitableaccelerator,
arestopped
in thesample
andincorporated
in the radicalby
addition ofmuoniumatoms
(Mu
=µ+ß~
ischemically
alight isotope
ofhydrogen
with one-ninth the mass ofH)
across unsaturated bonds. The time evolution of muonspin polarisation
is theanalogue
ofa free inductiondecay
inpulsed
magnetic
resonance[10].
It carries information about themagnetic
interac-tion of the muon with the electron and with nuclear
spins.
Details of theexperimental technique
have beengiven
elsewhere[9,
11].
The extremesensitivity
allows the detection of radicals at concentrations down to asingle species
in the entiresample.
It thereforepermits
theinvestigation
of radicals adsorbed on surfaces under conditions wherethey
show trans-lationalmobility.
Thedynamics
is deduced from thepartial averaging
of the muon-electronhyperfine anisotropy.
This was demonstrated for the Muadductto
2,3-dimethyl-l,3-butadiene,
for which reorientational correlationtimes wereobtained in the motional
narrowing regime
[12].
Observation
using
the transverse fieldtechnique
is limitedby
there-quirements
that radicals are formed within 10"9 s,in order toavoid loss ofphase
coherence of the muonspin,
andby
the restriction that there is nomorethan one muon
present
in thesample
atany time. An alternativetech-nique,
avoidedlevel-crossing
muonspin
resonance(ALC-//SR)
inlongi-tudinal
magnetic
fields,
allows the detection ofradicals evenwhenthey
areformed over
periods
of the order ofamicrosecond,
provided
that thetran-sition rate is
high enough
toproduce
asiginificant
ALCsignal during
the muon lifetime[13].
It is atime-integral
technique
which can use the fullmuon flux of the accelerator since there is noconstraint on the number of muons within the
sample
at anygiven
time. Even under these conditions the concentrationof radicals isextremely
low,
and bimolecular termination reactions arenegligible.
It was thereforepossible
using
thistechnique
to observecyclohexadienyl
andethyl
radicals adsorbed on silica[14-16].
Overmostof the
region
ofastrong
externalmagnetic
field,
eigenstates
ofa
3-spin
system
(µ+,
e~, andnucleusp+
are allspin-V2 particles)
arepureZeeman states. Muons
injected
with theirspins
parallel
orantiparallel
tothis fieldarethus in an
eigenstate.
There is notime evolution and henceno muondepolarisation.
The behaviour is different in theregion
ofanavoidedcrossing,
where theeigenstates
are mixtures of two Zeeman states. This leads to an oscillation between the two states, and ifthey
differ in thez-component
of the muonspin,
to a decrease inpolarisation.
It is detected as a resonance in aplot
of the muonasymmetry
against
fieldstrength,
which is
given by
the normalised difference of thepositron
counts in two sets of detectorsplaced
in the forward and backward directions relative to the initial muonspin
[13].
There are different ALC transitions classified
according
to thechange
in the totalspin
quantum
number M of theparticipating
Zeeman states[14].
The resonance
obeying
the selection rule M =0,
essentially
amuon-proton
spin flip-flop,
occurs underisotropie
conditions to the first orderata resonancefield
A„-A„
A„
+A.
7m~ 7
(1)
where
Atn Ap
are theisotropie hyperfine coupling
constantsof themuon andthe
nucleus,
and yf„ yp theirgyromagnetic
ratios. This transition is drivenby
theisotropie
as well as theanisotropie
interaction. Inisotropie
systems
such as
liquids,
only
thistype
of resonance is observed.Increasing
ani-sotropy
of thesystem
broadens thesignal
until it reaches a static lineshape
which
is,
ingeneral,
asymmetric
forapowder,
having equal
probability
forall radical orientations.
A second
type
of transitionobeys
the selection rule\A M\
= 1. It
corre-sponds
toa muonspin flip
and occurs aroundBr
=
-2
1y„
ye(2)
It is driven
by
theanisotropie coupling
elements of the Hamiltonian[17],
and therefore vanishes when thesystem
reorients onatime scale faster thanthe inverse
hyperfine anisotropy.
The resonance is thus absent underiso-tropie
conditions but veryprominent
in solids[18],
and it isexpected
tobea sensitive indicator of small
anisotropies.
A
strong
transitionobeying
the selection rule A M = 0wasdetected for
muonated
cyclohexadienyl
andmethoxycyclohexadienyl
radicals adsorbedon
quartz
powders
[15].
It was observed that the resonance broadens withdecreasing
temperature,
and this was ascribed to slower reorientation of the radical. A reorientational correlation time was estimated on the basis of ajump
model.Since
then,
the theoretical model has beengreatly
improved.
This workprovides
the firstexample
where theanalysis
ofexperimental
data is basedon a stochastic Liouville formalism which was
adapted by
Kreitzman[19]
to describe the
dynamics
at avoided levelcrossings.
It treats the effects of translational diffusion on aspherical
surface,
electronspin exchange
andchemical
reaction,
andittakesintoaccount interactionbetweenresonances.The
system
underinvestigation
is the muonatedcyclohexadienyl
radical,
C6H6Mu,
adsorbed on the surface of silicagel.
It differs from thediamag-netic benzene molecule
only by
the presence of the Mu atom and shouldthereforehave
closely
thesamevander Waals interaction. Since theadsorb-ent is also a silica
gel,
as in theearly
work on benzeneby
theLeipzig
group, this should allow a
comparison
between the surfacemobility
of adiamagnetic
molecule anda closeto related radical. In thelatter,
the650
stronger
than that of theproton.
This has the consequence that the nuclear relaxation rates increaseby
alarge
factor. Allproton-proton
dipolar
interactions are now
negligible,
instead,
thedipolar
part of the electronnuclear
hyperfine coupling
isthe relevant interaction that causesrelaxation.Depending
onthedistance oftheproton from the radicalcentre, thehyper-fine
anisotropy
may amount toas much as 30 MHz[20].
Theexperimental
time scale of
dynamic
processes whichpartly
average outthedipolar
inter-actions are thustypically
a few tens ofnanoseconds,
which is the sameorder of
magnitude
as correlation times amenable to thestudy
ofdiamag-netic molecules
by
means of NMR[3].
For benzene adsorbed on silica
gel
it wasproposed by
Michel[3]
thatthe adsorbed molecules
undergo
fast rotation about the sixfold axis down totemperatures
ofat least 130 K. This was necessary toexplain
thetem-perature
dependence
of the intramolecular part of theproton ,
relaxation data. It wassupported
furtherby
2H NMR results ofbenzene-<i6
adsorbed ongraphite
[8]
andonalumina[6],
which demonstrated thedynamic
behav-iour and the axial
symmetry
downto lowtemperature.
Thecyclohexadienyl
radical is also flat andrigid,
and it isplausible
that in amonolayer
ofbenzeneon silica its behaviour is the same as that ofbenzene. Its energy is
lowest when ituses the maximum
possible
contact area. Itis thereforeas-sumed to lie flaton the surface andto rotate
rapidly
about the axisperpen-dicular to the molecular
plane,
leading
to ananisotropie
system
of axialsymmetry.
Additional motionby
translation around thegrains
orinside thepores leads to an
averaging
of the axialanisotropy
and renders thesystem
isotropie.
2.
Experimental
A stainless steel sample cell with a window of 25µ thickness was füled with 3 g of
silica gel (Aldrich Chemical Company, Inc.) which hada
particle
size of 5 —25µ ,a
BET surface areaof 500
m2/g,
apore volume of 0.75cm3/g,
anaverage pore diameterof 6.0nm, and
ferromagnetic impurities
of 10 ppm.Itwas heatedat383 for24 hours undervacuum until a pressure of less than 10"5 mbarwas reached. This treatmentre-moves physisorbedwaterand leaves behindafully hydroxylatedsurface.
Benzene was
degassed
with three freeze-pump-thaw cycles,then adsorbed onto the silicagel togivea nominal monolayer, assumingamolecularcrosssection of 0.40 nm2[21].The isosteric heat of
adsorption
of benzeneon ahydroxylated
silica surfaceamounts toca. 43 kJ/mol [22].The heat ofvaporisation
ofliquid
benzeneatroomtemperatureisonly
34 kJ/mol. This makessurethatC6H6condensesandspreads
onthesurface and thatit does notform
droplets
orescape into the gasphase
toasignificant
extent at roomtemperature.
The ALC
experiments
wereperformed using
a surface muonbeam at TRIUMF in Vancouver. Theexperimental
setup andtechniques
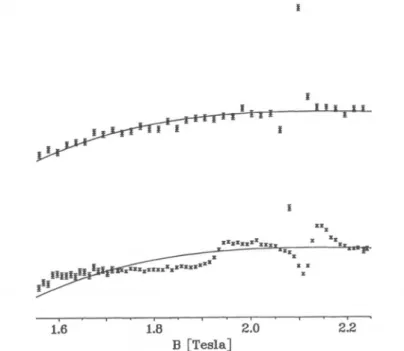
have beendescribed elsewhere [23].The
applied magnetic
fieldwasmodulatednearly rectangularly by
nominally
±9.75mT,I
ß
1.8 2.0 2.2[Tesla]
Fig.
1.RawALC-¿fSR
spectrumof differentialasymmetry (arbitrary units)against
longi-tudinalmagnetic
field,takenat295 (upper), with thefitted background,andat 193(lower)with thesame background.
figurestoenhance thevisibility of thesignals by removing the
sloping
baseline. For the analysis, the theoretical fit function was convoluted with the response function of themagneticfieldto accountfortheexactexperimentalconditions.
3. Results and discussion
For the
cyclohexadienyl
radical weexpect
to find four ALC resonancescorresponding
tothe selection rule AM =0,
which involve theméthylène
proton
(at
around 2.1T)
and theortho,
meta, and paraprotons
(between
2.7 and 3.0T),
and the\A M\
= 1 resonance around 1.9 T.Figure
1displays
the rawspectrum
obtained in the range of 1.55-2.25 for twotemperatures.
Asharp
resonance isclearly
observed at 2.1 T. Two furtherlines were seen at 2.904 and at 2.964
(ortho
and paraprotons).
They
clearly identify
theradical,
butthey
were too weak forquantitative
work andarethereforenot used. At 295 thereseemstobeasmoothbackground
(solid line)
with no indication ofa\A M\
= 1 line. At 193K,
however,
a
clear but broad feature is observed around 1.9 T. In order to follow the
spectral changes
withtemperature
the identicalexperimental background
as1. 1.8 2.0 2.2 1.6 1.8 2.0 2.2
[Teala] [Tesla]
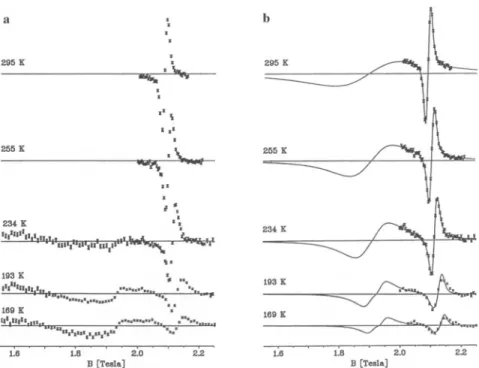
Fig.
2.a)ALC-//SRspectraobtained with anominal monolayerof benzene adsorbedonsilica gel at varioustemperatures. The differential asymmetry, with the
background
ofFig.
1 subtracted,isdisplayed.
Theprominent
resonanceatound 2.1 Tesla is the M=0 muon-proton spin
flip-flop
transition of the Mu, and the H bound in the méthylène position of thecyclohexadienyl
radical. The weak feature around 1.94 Tesla is the\ M\
= 1 muonspin flip
resonance, b) Fit of the M = 0 resonance with a modelconsideringsmallanglerotationaldiffusion,and simulation of the lower field
region
with the derived fitparameters.Figure
2a showsaselection oftypical
spectra
takenatdifferenttempera-tures in therange of 132—295 K. Around 2.1 Tesla the M = 0
transition,
due to theméthylène
proton
coupling,
is observedclearly
down to 169 K. Withdecreasing
temperature
abroadening together
with loss ofintensity
occurs, and at 132 this
signal
is nolonger
observable. At 234 a weak\A M\
= 1 transition appears,becoming
stronger
at lowertemperature,
witha maximum relative
intensity
around 193 K. Itsposition,
which isdirectly
proportional
tothe muonspin coupling, Eqn
(2),
shifts tohigher
fields withdecreasing
temperature.
The
region
of the M = 0 transition wasanalysed
in terms of the theoretical modelofsmallangle
rotational diffusion of thecyclohexadienyl
radical. Thefitting
function is available in numerical formonly.
The param-eters are theisotropie hyperfine couplings,
µ
andAp,
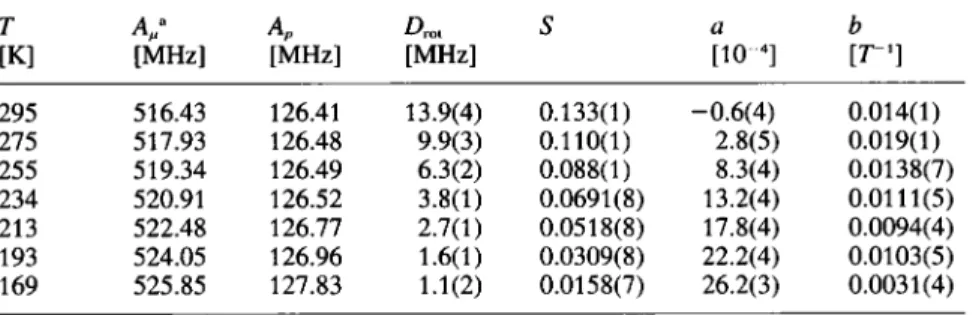
their axialTable 1. Results of least squares analysis forthe isotropie hyperfine couplingconstants
of the muon(A„),theméthylèneproton (Ap),the rotational diffusion coefficient Dra,the
experimental
scale factorS, andthebackground given by
a + b (B-Br).[ ] A " [MHz] [MHz] [MHz] a [KT4] b [ "1] 295 275 255 234 213 193 169 516.43 517.93 519.34 520.91 522.48 524.05 525.85 126.41 126.48 126.49 126.52 126.77 126.96 127.83 13.9(4) 9.9(3) 6.3(2) 3.8(1) 2.7(1) 1.6(1) 1.1(2) 0.133(1) 0.110(1) 0.088(1) 0.0691(8) 0.0518(8) 0.0309(8) 0.0158(7) -0.6(4) 2.8(5) 8.3(4) 13.2(4) 17.8(4) 22.2(4) 26.2(3) 0.014(1) 0.019(1) 0.0138(7) 0.0111(5) 0.0094(4) 0.0103(5) 0.0031(4) *
Parameterfixedinthe fit.
was called vrin
[19])
forisotropie
rotational diffusionby
translation of the radical on the outer or inner surface of asphere
of radiusR,
thesignal
amplitude
S,
which isproportional
tothe radicalyield,
andasloping
back-ground.
Ofthese,
only
Ap,
DrM,
S,
and thebackground
parameters
weretreated as variables.
They
are listed in Table 1. Theanisotropie
hyperfine
parameters
weretaken asconstant. An electron relaxationrateof 0.25 MHzas determined for the
liquid by
Heming
etal.[24]
was usedatalltempera-tures, but it has no
significant
effect on the results. This rate is notstrictly
temperature
independent
and has a maximum for € =1,
which is thecasewhen is of the order ofa few
picoseconds
atthemagnetic
field used here. It is notexpected
that our systems are on the fast side of thismaxi-mum, sothatlower
temperatures
have a lowerrelaxation rate, and theroomtemperature
value usedhererepresents
the maximumtobeexpected
inourtemperature
range. Basedontheexperience
ofexperiments
with bulkliquid
benzene,
chemical reaction wasneglected.
Over the entire
temperature
range, thepositions
of the|zl M\
= 0reso-nances are shifted to
higher
fieldsby
ca. 9.2 mTcompared
with those inbulk
liquid
benzene at the sametemperature
[25],
which is inagreement
with
previous
observations[15]. B,
depends
on the differenceAf—Ap
Eqn. (1)
and,
since the two nucleiparticipating
in the resonance arechemi-cally equivalent,
it is assumed that bothcoupling
constants contributepro-portionally
to this shift and that an identical resonanceposition
re-presents
identical values ofµ
andAp.
On thisbasis,
µ( )
was estimatedfrom its value at 295
(taken
from theliquid
at thetemperature
with identicalBr)
and itstemperature
dependence
on theSi02 surface,
dA/dT=
—0.075(6)
MHz/K,
and used as a non-variableparameter.
µ
is thusslightly
higher
on a silica surface thanin the bulkliquid
at the sametem-perature,
as has been notedpreviously
[15].
It is consistent with the aboveAp
oftheméthylène
proton
(Table
1)
are alsolarger
for the adsorbed casecompared
with theliquid
[25],
and thatthey
exhibit a similartemperature
dependence.
The error inµ
is estimated to be^0.5%,
which can affectDTOt
by
asmuch as 5%.£y±
for the muonium-substitutedcyclohexadienyl
radical was estimated on the basis of the tensor measured for thecorresponding
Mu adduct todurene
[26].
It was scaledby
the ratio of theisotropie coupling
constants of the tworadicals,
and then thedynamic
average was taken for fast rota-tion about the axisperpendicular
to the molecularplane, leading
toZ>[
= 3.18 MHz. Thecorresponding
proton
anisotropy,
Ty±
= 1.00MHz,
was
estimated
by multiplication
of the muonhyperfine
value with the ratio ofmagnetic
moments,/ µ
= 0.3141. Theseparameters
influenceDrot
ap-proximately according
to(Z>[-Z>1)2,
so that anuncertainty
of 0.1 MHz in£>l-affects
the diffusion coefficientby
6%. The result of the fit over the AM = 0resonance and its
extrapolation
tolower fields is shown in
Fig.
2 b. The model works well for the A M = 0 transition athigher
temperatures,
but below 240 it deviatessignificantly.
Whereas theexperimental
A M = 0 line is of Lorentzianshape
atalltem-peratures,
theshape
of the fit function becomesincreasingly asymmetric.
The theoretical
prediction
deviates from theexperimental
spectrum
also in theregion
of the\A M\
= 1resonance,and in
Figure
2b thediscrepancy
becomes most severe for the 255spectrum.
While apronounced
featureis
predicted, only
a weaksignal
is seen in theexperimental
data. At lowertemperatures,
the resonance is indeedpresent
in theexperiment
butalways
broader thanpredicted.
It isgenerally
notpossible
toobtain aconsistent fitoverthe entire field range. In the fit overthe restricted range the mismatch
is accommodated in the
temperature
dependence
of thebackground
(coef-ficients a and b in Table
1).
Since the resonanceis the mostprominent
onein solid benzene
[18, 15],
its nearabsence for surface adsorbed benzene isjudged
to be verysignificant
andobviously
ofdynamic origin.
The exactphysical
process which causes the line tobe so weak is unclearatpresent.
Quantitative
analysis
therefore had torely
on the M = 0 line alone. The orientationaldynamics
of thehyperfine
tensor are reflected in therotational diffusion coefficients.Forthe surface adsorbed radical the
averag-ing
over all orientationsdepends
on thegrain
and pore size as well as onits
shape, whereby
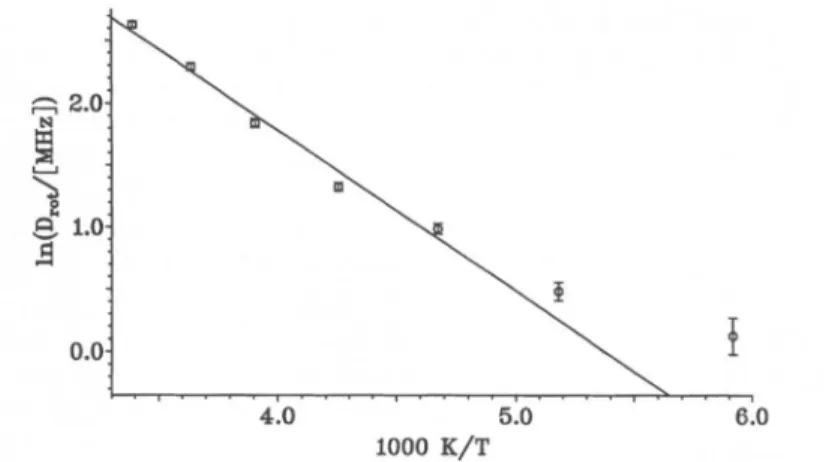
smaller diameters lead to faster rotational diffusion. An Arrheniusplot
of the rotational diffusion coefficients is shown inFigure
3. Theslightly
curvedplot
isapproximately represented by
theequation
Drot
=(1.1
±°oi)X
109s-1 ·exp(-1300
±100)
KVT.(3)
The activation energy of(10.8
±0.9)
kl/mol is similar to values for the difiusion of the benzene molecule onhydroxylated
porousA1203
(11.5
kJ/mol
[7])
andonsilicagel
HR(10.5
kl/mol[4]).
Although
both thesubstrateacti-1000
K/T
Fig.
3.Arrheniusplotof therotational diffusion coefficient of thecyclohexadienyl
rad-ical,arisingfrom translational diffusionon a sphericalsurface.vation
energies
isstriking. Obviously,
the radical and itsparent
molecule have the same activation energy for diffusion on ahydroxylated
surface,
and,
quite unexpectedly,
it does not seem to be ofprimary importance
whether this is on a surface ofA1203
or of silica. Ifthis coincidence is notjust by
chance,
then it wouldimply
that thebinding
of the radical to the surface is dominatedby
van der Waals forces and that the open shellelec-tronic structure
plays
a minor role. It would also mean that the interactionis
mainly
with the surfacehydroxyl
groupswhich mask the different natureof the
underlying
substrates. A finalinterpretation
cancertainly
not be drawn at thispoint, especially
since the extent of surfacehydroxylation
depends
critically
onthe conditions of adsorbentpretreatment
[1].
Theearly
NMR work used up to 300
higher
temperatures
inheating
out the silicagel
beforeadsorption
of the benzene[2—5].
Thispoint
will be further ad-dressed inforthcoming
work[21].
Small
angle
rotational diffusion on the surface of asphere
with radiusR is
equivalent
to two-dimensional translational diffusion onthe samesur-face which for small
angles
isapproximately planar.
The two diffusionequations
can be interconvertedusing
the substitution =r/R,
where is the rotationangle
and r themagnitude
of thedisplacement
vector on thesurface. This leads to
Dtrans
=R2Drot [28].
Itgives
aprefactor
ofD£ai»
= 1X10 8 m2/sfor translational surface diffusion. Due to the
irregular
shape
of the pores[29],
and since the molecule could inpart
also roll instead ofjust
slide on thesurface,
this is considered to beonly
arough
estimate. Itis
expected
to be affectedby
features ofthe transition state ofdiffusion,
e.g.
by
radical-surfacevibrationalmodes,
andby
the averagejump
distanceand with
neighbouring
molecules[30].
At 298 we obtainDtrans
= 1.3X10~10 m2/s. The
corresponding
value in three dimensions amounts toAram
= 2.0 X10"10m2/s,
which isonly
a factor of 11 below the value fordiffusion of benzene in the bulk
liquid
at the sametemperature
[31].
TheArrhenius
prefactor
will be further discussed inupcoming
work[27].
The number of sites within reach for the
diffusing
adsorbedspecies
depends
on itsroot-mean-square
displacement
on the surface. This is ingeneral
alsotemperature
dependent
and can cause aprefactor
whichin-creases with
temperature.
The curvaturein the Arrheniusplot
(Fig.
3)
may have tobe ascribed to this effect. A furtherpossible origin
is theneglect
ofthe
inhomogeneity
of the surface: Different surface sites lead to adistri-bution of
isotropie
muon andproton
couplings.
This causes aninhomo-geneous line
broadening
which is alsoaveraged
out asthespecies
becomes more mobile. The effectwouldexplain
why
under near-static conditions atlow
temperatures
the theoretical linecannot account for the fullexperimen-tal width.
It is evident from Table 1 that the
signal amplitude
also shows a cleartrend with
temperature.
Under the conditions of ourexperiments
5(7)
isproportional
to the radicalyield.
Interestingly,
it shows agood
Arrheniusbehaviour,
and its activation energy of 6.8 kl/mol is within error the same as that of the Mu addition reaction to benzene in the gasphase
[32].
It isunlikely
that this isa purecoincidence,
rather it couldbe taken asevidencethat the activated reaction of radical formation
competes
for Mu withan-other,
unspecified
reaction offirst orpseudo-first
orderkinetics,
so that theradical is
simply
not formed at lowtemperatures.
Inagreement
with thisinterpretation
is the fact that radicals formed on silicaby
Mu addition todienes,
which is much less activated[32],
are observed down to far lowertemperatures.
Further measurements with benzene on non-porous
spherical
silicagrains
of narrow size distribution arebeing
written up[27].
They
includemeasurements of the coverage
dependence,
andexploit
alsoplatinum
andpalladium
loadedmaterial,
i. e. realcatalysts.
The systems are moreaccu-rate
representations
of thepresent
theoretical model and are thusexpected
toallow a more detailed
interpretation.
Acknowledgments
Financial
support
by
the Swiss National Science Foundation and the Natural Sciences andEngineering
Research Council of Canada isgratefully
acknowledged.
TRIUMF is fundedthrough
the National Research Council of Canada.References
1. H.Pfeifer, inNMR,Basic
Principles
andProgress, Vol.7,P. Diehl,E. Ruck and R. Kosfeld, eds.,Springer,
Berlin, 1972.2. D.Freude, .Phys. Chem. (Leipzig)242(1969)57. 3. D. Michel,Z. Naturforsch. 23a(1968)339.
4. H. Winkler, M. Nagel, D. Michel and H. Pfeifer, .
Phys.
Chem.(Leipzig)
248 (1971) 17.5. B.
Boddenberg,
R. Haul andG.Opperman,Naturwissenschaften 56 (1969)635. 6. . Boddenbergand . Beerwerth,J.Phys. Chem. 93(1989) 1435.7. B. Boddenbergand B. Beerwerth,J.Phys. Chem. 93(1989) 1440. 8. B.
Boddenberg
and R. Grosse,Z. Naturforsch. 43a (1988)497.9. E. Roduner,ThePositive Muonas aProbe in FreeRadicalChemistry. Potential and
Limitations ofthe//5/?
Techniques,
Lecture Notes in Chemistry, Vol. 49,Springer,
Heidelberg,
1988.10. E. Roduner,Chem. Soc. Rev.22(1993)337.
11. E. Roduner,M.SchwagerandM.Shelley,in RadicalsonSurfaces,eds. A. Lund and
C. J.Rhodes, seriesonMolecularOrganization andEngineering, Klewer Academic
Publishers, inpress.
12. M. Hemingand E. Roduner,Surf. Sci. 189/190(1987)535.
13. M. Heming, E. Roduner, . D.Patterson, W. Odermatt, J. Schneider, H. Baumeler and H.Keller, Chem. Phys.Lett. 128(1986) 100.
14. I. D. Reid,T. Azuma and E. Roduner, Nature 345(1990)328.
15. I. D. Reid,T.Azuma and E.Roduner, HyperfineInteract. 65(1990) 879.
16. M.
Schwager,
E. Roduner, L D. Reid,P. W. Percival, J.-C. Brodovitch, S. Wlodek and R. F. Marzke,Hyperfine
Interact. 87(1994) 859.17. E. Roduner,I. D. Reid,M. Riccoand R. DeRenzi, Ber. Bunsenges. Phys.Chem. 93
(1989) 1194.
18. E. Roduner,
Hyperfine
Interact. 65(1990) 857.19. S. KreitzmanandE. Roduner,Chem.
Phys.
189(1994)in press.20. A. Carrington and A. D. McLachlan, Introduction to Magnetic Resonance, Harper,
NewYork,1967.
21. S. I. Greggand K. S. W. Sing, Adsorption, SurfaceArea andPorosity, Academic, London, 1967.
22. A. V. Kiselev and V. I. Lygin, Infrared Spectra of Surface Compounds,
Wiley,
New York, 1975.23. P. W. Percival,J. C. Brodovitch, S. K. Leung, D. Yu, R. F. Kiefl, G. M. Luke, K. Venkateswaran and S. F. J. Cox,Chem. Phys. 127(1988) 137.
24. M. Heming, E. Roduner, I. D. Reid, P. W. F. Louwrier, J. W. Schneider, H. Keller,
W.Odermatt, . D. Patterson,H.Simmler, . Pümpinand I. M.Savie,Chem. Phys.
129(1989)335.
25. D. Yu,P. W. Percival,J. C. Brodovitch,S. K.Leung,R. F. Kiefl,K. Venkateswaran andS. F. J. Cox, Chem. Phys. 142(1990) 229.
26. E.Roduner, Chem.Phys. Lett.81 (1981)191.
27. M.Schwager, H.Dilger,E.Roduner,I. D.Reid,P. W. Percival and A. Baiker,Chem.
Phys. 189(1994) in press.
28. P. A. Flinn, Diffusion in Solids and
Liquids,
in: Application ofMòssbauerSpec-troscopy, R. L.Cohen, ed., Vol.2,Academic Press, NewYork, 1980. 29. J. M. Drake and J. Klafter,
Phys.
Today(1990)46.30. K. D.Dobbs and D. J. Doren,J. Chem. Phys. 97(1992)3722. 31. A. F.
Collings
andR. Mills,Trans.Faraday
Soc. 66(1970)2761.32. E. Roduner, P. W. F. Louwrier, G. A. Brinkman, D. M. Garner, I. D. Reid,D.