HAL Id: hal-03009927
https://hal.archives-ouvertes.fr/hal-03009927
Submitted on 24 Nov 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
E. Abou Nakad, J. Chaud, C. Morville, F. Bolze, A. Specht
To cite this version:
E. Abou Nakad, J. Chaud, C. Morville, F. Bolze, A. Specht. Monitoring of uncaging processes
by designing photolytical reactions. Photochemical & Photobiological Sciences , Royal Society of
Chemistry, 2020, 19 (9), pp.1122-1133. �10.1039/D0PP00169D�. �hal-03009927�
PERSPECTIVE
Received 00th January 20xx,
Accepted 00th January 20xx DOI: 10.1039/x0xx00000x
Monitoring of Uncaging Processes by design of the photolytical
reactions.
E. Abou Nakada, J. Chauda, C. Morville a, F. Bolzea* and A. Spechta*
The use of photolabile protecting groups (PPGs) has been growing in emphasis for decades, and they nowadays enable cutting-edge results in numerous fields ranging from organic synthesis to neurosciences. PPGs are chemical entities that can be conjugated to a biomolecule to hide its biological activity, forming a stable so called “caged compound”. This conjugate can be simply cleaved by light and therefore, the functionality of the biomolecule is restored with the formation of a PPG by-product. However, there is a sizeable need of PPGs able to quantify the “uncaging” process. In this review, we will discuss several strategies leading to an acute quantification of the uncaging events by fluorescence. In particular, we will focus on how molecular engineering of PPG could open new opportunities by an easy access to photoactivation protocols.
Introduction
Various strategies have been developed for triggering the release of a chemical or a biological effector by external stimuli,1
including light which is a particularly attractive source of stimulation. Indeed, light can be readily available and focused, allowing precise temporal and spatial definition of the stimulus. In addition, light exposure time and intensity can also be precisely controlled and, as a consequence, the strength of the stimulus can be finely tuned.2 Photoactivation techniques,
including photolysis (phototriggered release), sensitizer-assisted light-induced redox reactions, and photoisomerization, have proven to be attractive in various fields of chemistry3 and
biology.4 Even though the idea of regulating processes with light
is in no way new,5 recent years have witnessed technology
improvements allowing light to become one of the major orthogonal trigger used today in many fields of biology, such as neuroscience,6 cell biology,7 genetics or embryology.8 Chemical
photoswitches and phototriggers provide two strategies to rapidly cause the initiation of a wide range of dynamic biological processes.4 The first type is referring to reversible synthetic
photoisomerizable compounds using photoswitches in order to manipulate biological functions reversibly using light, and is wildly used in the opto-pharmacology field.4 The second type,
described as seminal opto-pharmacology, is related to irreversible “caged compounds” that release biological effectors after photolysis.
Chemical phototriggers can rapidly cause the initiation of a wide range of dynamic biological processes by a light induced and
localized concentration jump of a natural biological effector.4,6-8
This strategy uses light irradiation to induce a photolytical reaction, leading to the controlled release of chemically or biologically active compounds. Initially, PPGs were used mainly in organic synthesis.9 Kaplan, Forbush, and Hoffman have given
a new prospect to this particular class of protecting groups by developing the first so-called “caged compounds” for respectively the in vitro light-controlled release of adenosine tri-phosphate (ATP) and cyclic 3’,5’-adenosine mono-tri-phosphate (cAMP).10 This strategy has been successfully developed and
used to mask the activities of various biomolecules, including peptides, proteins, nucleic acids, and many other bioeffectors.4, 6-8 The use of UV irradiation (normally within power density
between 10−3 and 10−1 W.cm-2) to manipulate the functions of
biomolecules or mediate on-demand drug release in living systems via effective photoactivation with very high spatial and temporal control is a remarkable, well-developed and reviewed technique.4,6-8 During the last two decades, the challenge was to
overcome the difficulty that only high energy light can induce photochemical reactions. One strategy to lower the energy within the domain of one-photon excitation process is based on tailoring the caging groups with extended π-conjugation and introducing
heteroatoms and functional groups in the ring system, leading to blue light-sensitive photoremovable groups in the coumarinyl,11
o-nitrobenzyl and o-nitrophenethyl series.12 More recently, even
more red shifted wavelength can be used, for example with BODIPY’s (Scheme 1).13
Another developed strategy is based on two-photon (TP) induced photolysis, which enables long wavelength photoexcitation. This technique requires not only developing new photoremovable groups with larger molecular TP absorption cross-sections, but also expensive light sources to induce TP triggered photoreactions.14 TP absorption only occurs at the focal point of
a focalized laser beam, therefore enabling intrinsic 3D spatial resolution of the photolysis area (it can be as little as 1 fL and below).15
Dr. E. Abou Nakad, J. Chaud, C. Morville, Dr. F. Bolze, and Dr. A. Specht Laboratoire de Conception et Application de Molécules Bioactives, Equipe de Chimie et Neurobiologie Moléculaire,
Université de Strasbourg, CNRS, CAMB UMR 7199, F-67000 Strasbourg, France Fax: (+33) 368854306
Scheme 1: Chemical structures of nitrobenzyl, o-nitrophenethyl, coumarinyl and BODIPY PPGs, X=Photoreleased group.
Hence, for biomedical applications, two-photon absorption is a time-consuming spot-by-spot process to expose macroscopic materials like tissues or organs. These features have recently stimulated the need of in situ nanotransducers that can generate UV or visible light with a low-energy NIR excitation. Such nanotransducers can be used to trigger photochemical reactions spatially restricted to the nanometer range around the particle surface.16 The uses of uncaging techniques from a biology
perspective requires to fulfill several criteria. From a chemical, biochemical and photophysical point of view, caged compounds must: 1) render the parent molecule biologically inert, 2) be activated upon exposure to UV, visible or IR light that would not be harmful to live cells or tissues at the dose used, 3) release the parent molecule rapidly enough to trigger the biological process of interest, 4) be stable in a physiologically relevant aqueous solution without photoirradiation and 5) not have toxicity to live cells or tissues. All recent improvements of those criteria, including two-photon sensitivity optimization, have already been extensively discussed in excellent earlier reviews.6-8, 14 In
addition, from a technical point of view, the use of photoactivation techniques is very challenging on complex biological systems. In particular, it’s always extremely difficult to define a reproducible photoactivation protocol. Firstly, because the calibration of the triggering beam can be difficult in complex systems (like cells, tissues, or living organism). Secondly, because the local concentration of the inactive precursor is unknown, which makes it difficult to deliver a specific concentration of substrate. Accordingly, a straightforward quantification of uncaging reactions in living cells and the liberation of defined amounts of active biological effectors is sought-after. In this review, the basic strategies for designing caged compounds leading to acute quantification of the uncaging events by fluorescence are discussed.
Development of caged pro-fluorophores
Amongst the described applications of the uncaging concept, a spatio-temporal controlled release of fluorescence by photochemical activation does represent a powerful tool for accurate quantification of uncaging reaction (Figure 1). Therefore, the use of PPGs for the release of a fluorophore can lead to an efficient monitoring of the uncaging process in complex biological systems. This technology is also extremely useful for cellular biologists, especially when the fluorescent signal allows the tracking of a cellular component in living systems or when the photoactivation enables the use of super resolution microscopy.17 Two strategies have been used to
develop photoactivatable fluorophores. The first is based on the direct uncaging of a fluorophore, which uses the property of some fluorophores able to present a fluorescence quenching after fluorophore alkylations. The second strategy is based on fluorescence extinction achieved by chemically bound quenching systems to the fluorophores via photocleavable linkers. If the quenching system of such continuously quenched fluorophore can be effectively and specifically switched off (which then restores the fluorescence) as response to light, then the fluorophore-quencher pair is an interesting photoactivatable fluorescence reporter. The quenching mechanisms used in such fluorescence reporting systems are based either on a photoinduced electron transfer or on a resonance energy transfer. Those strategies have already been covered by us18 and others19
and would exceed the scope of this review, therefore we will not further develop this aspect of fluorescence uncaging reports here. In the following chapters, we will describe several strategies enabling the release of a functional biological (or chemical) effector together with a florescent report of the uncaging event in a single photochemical probe designed to allow such dual properties.
Figure 1: Principle of caged pro-fluorophores: A) A PPG non-fluorescent chromophore can be used to liberate a fluorophore B) Fluorophore emission is quenched using a photocleavable quencher, hp = light excitation leading to photolysis, hf = light excitation leading to fluorescence.
Figure 2: Principle of fluorescent uncaging monitoring using Fluorescent PPGs and biological effectors as quencher, hp = light excitation leading to photolysis, hf = light excitation leading to fluorescence.
Fluorescent PPG and biological effectors as quenchers In most cases, caged compounds and the uncaging secondary product exhibit similar fluorescence wavelength and brightness leading to light induced responses without quantification of the biological effector delivery. The first example of uncaging that occurs with an increase in fluorescence associated with the release of the desired substrate was reported by Hagen’s group on coumarinyl caged cyclic nucleotides.20 In this case, the
absence of fluorescence emission of the caged compound was accomplished through the quenching of the coumarin fluorescence by the nucleotide substrate moiety. This system is characterized by the difference in fluorescence between the starting caged compound and the uncaging by-product (as described in figure 2). Normally, coumarin-based caged compounds are fluorescent before photocleavage but when attached to cyclic adenosine or guanine nucleotides they show a very weak fluorescence due to an accelerated internal conversion from the excited state caused by mixing the -* and the n-*
states.20 This energy transfer between the coumarinyl moiety and
the nucleobase moiety leads to a drop in the fluorescence or so-called fluorescence quenching.
This quenching is advantageous in this case because it helps in quantifying the release of cAMP or cGMP from the fluorescence observed after photocleavage due to the liberation of the coumarin by-product. Of note, the group of Hagen has been able to used coumarinyl caged cyclic nucleotides for in vitro cell imaging and real time monitoring of the uncaging event.20d More
recently this concept was used by Pradeep Singh and co-workers.
Figure 3: Principle of uncaging report using tethered fluorescent PPGs, hp = Light excitation leading to photolysis, hf = light excitation leading to fluorescence.
In a very interesting piece of work, the fluorescence of coumarinyl PPGs was quenched by non-fluorescent o-nitrobenzyl PPG in order to develop “dually locked” photoresponsive drug delivery systems.21 Therefore,
chlorambucil (an anticancer drug) was caged using a fluorescent 7-hydroxycoumarinyl. This PPG was “locked” on his phenolic function using an o-nitrobenzyl PPG. The “locking” of 7-hydroxycoumarin by a o-nitrobenzyl PPG switches off the fluorescence of 7-hydroxycoumarin, but after brief illumination (360 nm or 720 nm using one and TP excitation respectively) the fluorescence is restored, and this can be used to “unlock” the coumarinyl uncaging reaction. Therefore, once this second phototrigger is unlocked, extended irradiation can be used to release the drug. This probe was used on cells, and one- and two-photon excitation rapidly activated an increased fluorescence in living cells. This property was employed for the real-time monitoring of a prodrug by in vitro cellular imaging. Extended irradiation of this tool resulted in release of the anticancer drug chlorambucil, leading to a light controlled increased cytotoxicity towards the MDA-MB-231 cell line.
Tethered Fluorescent PPG for the monitoring of uncaging events
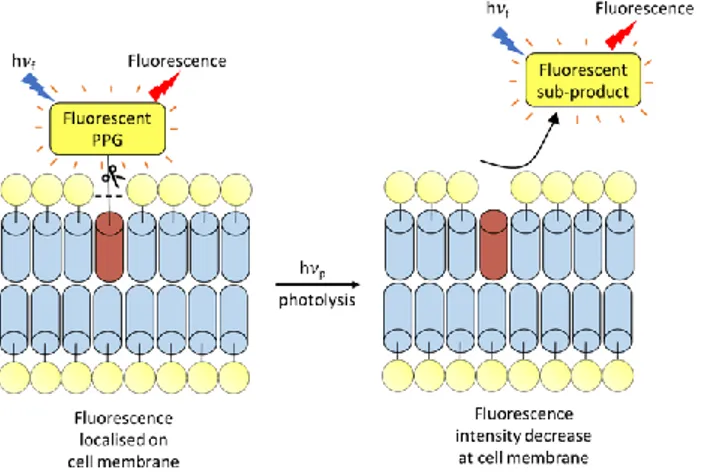
Another interesting use of fluorescent PPGs (like coumarinyl PPGs) was demonstrated by Schultz and co-workers in the end of 2015, with a focus on the use of the fluorescence characteristic of coumarinyl PPGs for plasma membrane-specific photoactivation.22 The principle of this method is based on the
use of hydrophilic coumarinyl based PPG tethered to fatty acids. Such caged fatty acids led to a localized fluorescent signal before uncaging at the cellular membrane, followed by a lipid flip-flop. Upon photoactivation by UV light, the fatty acid remains in the plasma membrane enabling the trigger of physiological events, whereas the coumarin-OH by-product is released inside the cellular space. Consequently, a fluorescence is observed at the vesicles level due to the entrance of the highly hydrophilic coumarin-OH to these vesicles by endocytosis.
This pioneer work using tethered fluorescent PPG for the monitoring of uncaging events (Figure 3) has been recently extended respectively to the organelle‐targeting of such photochemical tools and to other PPGs families. Firstly, the group of Nadler was able to develop coumarin-based PPGs that can be modified with organelle-targeting moieties by click chemistry.23
Figure 4: Principe of uncaging report using self-immolative spacers, hp = Light excitation leading to photolysis, hf = light excitation leading to fluorescence.
Those new caged lipids thus enable precise photorelease of lipid messengers in distinct organelles, a crucial behavior for cellular signaling studies. Secondly, Yushchenko and co-workers have also recently described o-nitrobenzyl PPGs (usually non-fluorescent PPGs) modified by hydrophilic fluorophores. Those fluorescent o-nitrobenzyl PPGs (called ONB-Rh) have been applied to the development of caged oleates leading to a semi-orthogonal application of ONB-Rh PPGs and coumarinyl PPGs for a sequential photorelease of lipids.24
In summary, this method acts as a tool for membrane imaging of caged lipids. But yet it has shown limitations since it provides only a qualitative information about the concentration jump of the biological effector.
Self-Immolative spacer for Uncaging with
Fluorescence Reporting.
In 2012, Jullien and co-workers were able to address the quantification of the uncaging event using a simultaneous release of a reporter and an effector from a common precursor in an equimolar ratio.25 This very elegant approach is based on the uses
of branched self-immolative spacers able to release two molecules after a single triggering photocleavage.26
For this purpose, an o-nitrobenzyl PPG was coupled to the self-immolative 4,6-dibromo-2-hydroxy-5-methyl-1,3-phenylene-dimethanol motif.
Scheme 2: Chemical structure of caged self-immolative spacers (in red) with fluorescent uncaging report. CPT = camptothecin, DDAO = 3-dichloro-9,9-dimethyl-9H-acridin-2(7)-one, and DCM= dicyanomethylene-4H- pyran. The harrow isthe photocleavable bound, and the dotted lines are related to the cleaved bond under control of the self-immolative spacers, hp = Light excitation leading to photolysis.
It enables the simultaneous release of two substrates upon photoliberation of a phenolate function (Figure 4). In particular, using confocal microscopy, quantitative analysis was performed for the concomitant release of two fluorophores on HEK 293 cells.
Using the same strategy, a prodrug featuring real-time prodrug localization and monitoring of the release event by dual fluorescence has been reported by Wu and co-workers in 2017.27
The authors have therefore developed a cleavable structure which comprises of a coumarinyl PPG, an anticancer drug (camptothecin), a self-immolative linker and a red fluorescent dye (dicyanomethylene-4H-pyran). In this smart photochemical tool, the fluorescence of coumarinyl and camptothecin was completely quenched by the dicyanomethylene-4H-pyran moiety via fluorescence resonance energy transfer. The internalization of the prodrug by the cells and its subsequent intracellular location was tracked using the red fluorescence signal of dicyanomethylene-4H-pyran while the release of active camptothecin was performed using one- or two-photon excitation. The uncaging process can be monitored using the appearance of the blue fluorescent signal of free camptothecin. The photoactivatable prodrug showed photocontrollable cytotoxicity toward HeLa cells and A549 cells, with low IC50 values of 4.01 and 2.53 μM respectively upon light irradiation and with IC50 values > 40 μM without light excitation.
More recently, the group of Choi was also able to validate this principle for the release of anticancer drugs (taxol or doxorubicine) with a fluorescent reporter (fluorescein or coumarin).28 In particular, the authors have developed a minimal
spacer since a dual arm PPG was used, leading to a sequential dual release of two substrates. A thioacetal ortho-nitrobenzaldehyde (TNB) was designed to be cleaved in response to UV light exposure leading to C–S bond cleavage, followed by intramolecular self-immolation leading to the release of the free anticancer drugs (Scheme 2). This sophisticated PPGs has been successfully used to follow the light induced cytotoxicity by in situ fluorescence monitoring on KB cells for the first time. This new property for PPGs was a great achievement toward future applications of such photochemical tools. For example in the field of theranostic medicine for drug delivery imaging.29
This versatile strategy allows to select any type of PPG and fluorophore (based on their photochemical and photophysical properties), thus leading to the development of photochemical tools for a wide range of applications. However, for any type of application, a time consuming synthesis is required.
Pro-fluorescent PPGs
Interestingly, PPGs can be designed to release a fluorophore as a side product. Therefore, different PPGs have been designed to allow direct monitoring of the uncaging event by the release of an easily detectable fluorescent side-product (Figure 5, Table 1).
Figure 5: Principle of uncaging report using pro-fluorescent PPGs
- Hydroxycinnamates pro-fluorescent PPGs In 2007, inspired by the work of Porter’s group in the late 1980s,30 Jullien and co-workers investigated the use of
hydroxycinnamates for alcohol uncaging.31 This work focuses on
the release of a coumarin uncaging by-product leading to a fluorescence signal used to quantify the release and report the uncaging event for the first time using pro-fluorescent PPGs. The light induced (330 – 370 nm for one-photon irradiation) transformation of the (E)-isomer to the (Z)-isomer, leads to the liberation of an alcohol along with a coumarin by-product (strong fluorescent by-product with emission wavelength between 391 – 482 nm, Scheme 3). This strong fluorescent signal of the coumarin uncaging side-product is therefore helpful to quantify the release of alcohols from these caged-systems. A water-soluble version of this pro-fluorescent PPG was reported in 2012 by Song and co-workers.32Two examples of the use of
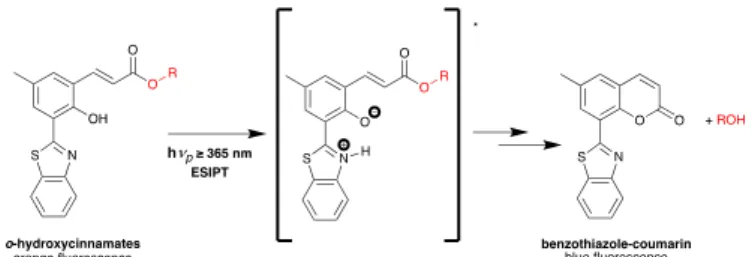
o-hydroxycinnamates were recently reported by Pradeep Singh and co-workers.33, 34 The first one is based on excited-state
intramolecular proton transfer (ESIPT) inducing fluorescent o-hydroxycinnamates formation.33 In this study, an ESIPT is
followed by a Z-E isomerization followed by a rearrangement yielding to a fluorescent benzothiazole-coumarin by-product, allowing the uncaging event monitoring in real time. The ESIPT phenomena leads to a huge stokes shift only for the PPG leading to a distinct fluorescence color change upon photorelease. In other words, the uncaging (of alcohol or phenol here) can be monitored by the drastic change in fluorescence emission from orange (for the hydroxycinnamates PPG due to the ESIPT phenomena) to blue (benzothiazole-coumarin) after photocleavage (Scheme 4).
Scheme 3: Mechanism of the uncaging process leading to the release of the caged alcohol (ethanol in this case) along with a Fluorescent Coumarinyl by-product.
Scheme 4: Photoinduced uncaging of alcohols from the ESIPT-induced fluorescence of o-hydroxycinnamates.
The second hydroxycinnamate based recently described work, allows sequential uncaging of two molecules with a concomitant liberation of a fluorescent coumarinyl by product.34 Therefore,
the photoisomerization of the PPG and the formation of the coumarin, associated with the release of the first molecule (containing an alcohol function), correspond to an in situ generation of a second phototrigger capable to release (in a second step) an second molecule (containing an acid function). This system was applied to the release of two different cosmeceuticals, e.g. ferulic acid ethyl ester (FEAA) and salicylic acid (SA) (Scheme 5). The formation of the coumarin chromophore during the first photochemical reaction leads to the establishment of a fluorescent reporter allowing the monitoring of the uncaging by fluorescence at 400 nm.
To our knowledge, o-hydroxy-cinnamate PPGs have not been used yet on cells for the release of a biological effector with a concomitant quantification of the uncaging reaction.
- Thio-chromone pro-fluorescent PPGs
Another work on pro-fluorescent PPGs has been described by Kakiuchi and co-workers for quantitative alcohols,35a amines, 35a
phosphoric acids,35b carboxylic acids35c or thymidine
nucleobases35d uncaging. They introduced the thiochromone
S,S-dioxyde (TCM) as a pro-fluorescent PPG (Scheme 6). Of note, for the release of aliphatic alcohol or amine functions, respectively carbonate or carbamate linkages between the PPG and those functions are required. For all photolytical reactions, a highly fluorescent by-product derived from the thiochromone is formed as a uncaging by-product (scheme 6).
Scheme 5: In situ generation of the second phototrigger for sequentially controlled uncaging of two different functional groups.
Scheme 6: Photodeprotection using thio-chromones S,S-dioxydes.
More recently this PPG, was used to quantitatively release propargylic alcohols.36 This thiochromone derivative was
examined with various substrates to determine its application scope. In particular, using several propargyl derivatives (R1=R2=
methyl or phenyl / R3= phenyl, p-bromophenyl, p-toluene,
n-Butyl in Scheme 6), the deprotection reaction was carried out under irradiation (at 310 nm) to give the corresponding propargyl alcohol with >99% yields.
In parallel, UV-Vis and fluorescence spectra were recorded to monitor the formation of the by-product. An intense fluorescence emission at 445 nm appears after uncaging while the starting materials are non-fluorescent.
Another research work on thiochromone-type pro-fluorescent PPGs has been introduced by the same group, in 2017, for the release of biologically active alcohol functions.37 Interestingly in
this recent study, the authors have used thiochromone-type PPG for the release of resveratrol by light excitation with a concomitant release of the fluorescent thio-chromone by-product (Scheme 6). Of note, resveratrol is known to be an antioxidant or an inhibitor of luciferase thus blocking the luciferin−luciferase reaction, inhibiting the formation of oxyluciferin and diminishing its generation. Therefore, a luciferin−luciferase reaction, able to generate chemiluminescent (CL) product oxyluciferin, was used to monitor the biological effector’s release by luminescence too.
Thio-chromone pro-fluorescent PPGs have not been used so far on cells for the release of a biological effector with a concomitant quantification of the uncaging reaction. This type of pro-florescent PPGs still needs to be improved. In particular, hydrophylic versions of this PPG are highly sought after in order to be able to perform uncaging experiments in water solution and on cell culture.
- BODIPY pro-fluorescent PPGs
One recent work by Winter and co-workers describes a family of pro-fluorescent BODIPY PPGs for Visible/Near IR light uncaging.38 The photocleavage of these BODIPY PPG was
realized first using different alcohols as solvents and under light excitation in order to cleave the carbamate or carbonate linkage and release the attached molecule (Scheme 7). This group was able to design BODIPY PPGs following a structure-activity relationship. They described the synthesis of a set of BODIPY PPGs by chemically modifying several positions aiming to tune the photophysical and photochemical properties (Scheme 7).
Scheme 7: Photolytical reaction of BODIPY-type PPG.
In particular, by adding strong electron-donating groups to two styryl linkers, a red-shifted absorption maximum up to 50 nm (Scheme 8) of the BODIPY PPG, compared to less conjugated systems, was observed. More interestingly, a fluorescent increase (at 600 nm) was generated after photoactivation of this class of BODIPY PPGs.38 Therefore, the red shifted PPG
derivative was chemically modified with oligo-ethylene glycol chains for an increased water solubility in order to study the PPG optical properties in live cells. Of note, a 4-nitrobenzoic acid was used as leaving group.38 The authors were able to demonstrate
that a 4-nitrobenzoic acid caged compound does not exhibit detectable fluorescence before uncaging, whereas upon irradiation at 635 nm, the nitrobenzoic acid is released and the PPG by-product retains its fluorescence signal in cells at 605 nm. These PPGs exhibit very interesting absorbance wavelengths that fall in the transparency window for biological experiments. However, the uncaging efficiency of this profluorescent PPGs need to be improved since relatively long irradiation time are required for the uncaging reaction to proceed (6-8 minutes irradiation using a microscope light source at 635 ± 15nm).
Scheme 9: ESIPT-assisted photorelease on a pro-fluorescent pHP PPG
- p-hydroxyPhenacyl pro-fluorescent PPGs
p-Hydroxyphenacyl (pHP) group is a well-known PPG due to its
fast and highly efficient release of active molecules. These PPGs have shown less interest for biological applications because of their excitation wavelength below 400 nm and because the released by-product doesn’t have any useful fluorescent properties. The group of Pradeep Singh was able to overcome these limitations by adding a 2-(2’-hydroxyphenyl) benzothiazole (HBT) core to the pHP.39 This HBT core induces
a rapid ESIPT phenomenon which can assist the deprotonation of the pHP group for a faster release, with excitation higher than 400 nm as well as a distinct fluorescence shift upon photorelease. Using this pro-fluorescent PPG, the authors were able to develop a drug release system leading to the liberation of Chlorambucil (Cbl) using light with real time monitoring of the uncaging process. Interestingly, the pHP-HBT-Cbl system shows a green emission before uncaging, shifted to a blue one after photoliberation. The origin of this blue emission is the release of
pHP-HBT-COOH by-product along with the drug according to
the reaction presented in scheme 9. This system was used more recently by the same group for the development of a dual responsive drug release system (H2O2 and light responsive) and
for the release of hydrogen sulfide.40
Using this extremely interesting pro-florescent PPG, the group of Pradeep Singh was able to nicely follow the uncaging reaction by fluorescence microscopy on cells. Of note, the reaction can be followed whether by the decrease of a green-emission band (517 nm for the PPG) whereas by the increase of the blue emission band (450nm for the uncaging by-product). However, this system is still very sensitive to triplet quenchers and its application on cells still requires relatively long irradiation time (around 15 min using a ≥ 410nm UV-visible lamp).
- o-nitrobenzyl pro-fluorescent PPGs
The first example of pro-fluorescent o-nitrobenzyl PPG was introduced in 2018 by our group.41 One of the most commonly
used PPGs for the triggering of diverse biological responses is based on the o-nitrobenzyl photochemistry. Since the photolytical release mechanism of o-nitrobenzyl PPG leads to the formation of a nitrosoketone derivative, the introduction of an aryl substituent at the benzylic position yielded to a photolysis by-product able to rearrange in a conjugated -hydroxystilbene fluorophore (by a keto-enol tautomerism). Using this new class of o-nitrobenzyl PPGs, the photocleavage leads to an important increase of a specific fluorescence signal originating from the
Scheme 10: Photolytical reaction of o-nitrobenzyl based pro-fluorescent PPGs.
liberated o-nitroso by-products (Scheme 10).41 Four different
o-nitrobenzyl PPGs were designed and their photophysical properties were evaluated with the liberation of carboxylic acid upon irradiation at 405 nm. Interestingly, the keto-enol equilibrium is favored towards a more red-shifted fluorescent by-product when the system is more conjugated (R= 4-dimethylamino, 4-nitro and 4-methoxy phenyl) compared to a control compound (R = Br). This conjugation of the system after cleavage induces a tunable fluorescent signal generated from the
o-nitroso enol by-product (Scheme 10).
Pro-fluorescent PPG Excitation wavelength for the photolysis Caged compound Em. By-product structure (photorelease d chemical functions) By-product Em. (Uncaging monitoring on cells) o-Hydroxy-cinnamate 330-400 nm (750 nm) (TP) Only for ESIPT derivatives Coumarin (Al) 391-482 nm (no) Thio-chromones 280-375 nm / Methylene- thio-chromone (Al., Am., C.A. P.A., T.) 445 nm (no) Pro-fluorescent BODIPYs 450-700 nm 526-745 nm BODIPY (C.A.) 605-610 nm (yes) Pro-fluorescent p-hydroxy -phenacyl 300-450 nm 517 nm p-hydroxy -phenyl- acetic acid (C.A.) 450 nm (yes) Pro-fluorescent o-nitrobenzyl 350-480 nm / -hydroxy-stilbenes (C.A.) 480-526 nm (yes)
Table 1: Pro-Fluorescent PPGs: Photophysical and photochemical properties, Em. = Fluorescence emission wavelength ; Al. = Alcohol; Am. : Amine ; C.A. : Carboxylic acid; P.A. : Phosphoric acid; T. : Thymine nucleobase
One interesting example to mention is the 4-methoxyphenyl derivatives (Scheme 10, R = OMe), for which a 200-fold fluorescence increase was observed at 526 nm between the starting compound and the photolytical by-product.
Therefore, a water-soluble version of this compound has been successfully developed for real time monitoring of the uncaging event in cell culture.41 However, the uncaging efficiency of the
described o-NB profluorescent PPGs are still very low, leading to long irradiation time for the uncaging reaction to proceed (15 minutes using a microscope light source at 360 ± 20nm). In the future, this strategy should therefore be applied to the development of more efficient visible light sensitive photoremovable groups in the o-NB series, to be able to follow the uncaging events by fluorescent microscopy with a concomitant release of a biological effector.
Summary, conclusions, and future outlook
Photoactivatable tools have been developed during the last decades leading to significative outputs for the study of dynamic processes in biology. In particular, sophisticated photoremovable groups have been successfully used for in vivo
neurophysiological studies.42 Therefore, newer PPGs have been
described with improved photophysical properties, especially shifting the absorption maxima further into the visible/NIR region and enhancing the uncaging efficiency. The transfer of the ‘‘caged” compounds technique to near-infrared region is the cornerstone for in vivo applications. A fine control of the photoactivation process is highly sought-after in order to precisely assess the amount of effectors locally photoreleased. However, classical PPGs and the uncaging secondary products exhibit generally similar fluorescence properties, preventing the possibility of quantification of the biological effector delivery. It would be very advantageous to monitor the uncaging event, for example, by the emergence of a fluorescent signal (e.g. optical reporting). In this review the different strategies allowing an acute quantification of the uncaging events by fluorescence have been described.
Firstly, photoactivatable fluorophores enabling the quantification of the uncaging event without a concomitant release of an effector have been described. Then, the seminal strategy developed for the simultaneous release of cyclic nucleotides and fluorescent uncaging by-products based on fluorescence quenching on coumarinyl caged cyclic nucleotides was exposed. Then, a more general strategy was described based on the uses of tethered fluorescent PPGs, leading to a qualitative quantification of the uncaging events, by decrease of localized fluorescence signals.
Finally, two strategies allowing a precise quantification of the uncaging events by fluorescence, together with a concomitant release of effectors, have been reported: the first one is based on the uses of PPGs and self-immolative spacers for the release of two substrates; the second strategy is substrate independent and is based on the release of a fluorescent uncaging by-product using pro-fluorescent PPGs.
The first strategy allows to select any type of PPG and fluorophore (based on their photochemical and photophysical properties), therefore this versatile approach can lead to the development of photochemical tools for a wide range of applications.
The second strategy is more complex, since any pro-fluorescent PPG has its own photochemical and photophysical properties (see table 1). Up to date this strategy has already been able to release a large number of functional groups with the concomitant release of fluorescent side-products with fluorescence emission between 390 to 610 nm as summarize in table 1. Only 3 type of pro-fluorescent PPGs have already been successfully used for in
vitro cell imaging and real time monitoring of the uncaging
event. Therefore, those recent photochemical tools still need to be optimized. In particular, pro-fluorescent PPGs with better uncaging efficiencies together with a highly specific fluorescent signal generated by the by-product need to be developed.
In the future, if those type of photochemical tools can be implemented to caged biological effectors, this should lead first to an easy access to photoactivation protocols and therefore to more sophisticated cellular signaling studies,43 in particular if
those photochemical tools are combined with super resolution microscopy.17 Furthermore, those new generations of PPGs
should find significant applications in the field of nanomedicine29 and in the development of light sensitive
biomaterials.4d
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Université de Strasbourg (IdEx Grant to A.S.) the CNRS and by Grants from the Agence Nationale de la Recherche (Contract No. ANR-13-JSJV-0009-01 and ANR-18-CE09-0ANR-13-JSJV-0009-016-ANR-13-JSJV-0009-01 to A.S.).
Notes and references
1. a) E. K. Lim, T. Kim, S. Paik, S. Haam, Y. M. Huh, K. Lee, Nanomaterials for Theranostics: Recent Advances and Future Challenges, Chem Rev, 2015, 115, 327-394, b) S. Mura, J.
Nicolas, P. Couvreur, Nanomaterials for Theranostics: Recent Advances and Future Challenges, Nature materials, 2013, 12, 991-1003, c) M. Wei, Y. Gao, X. Li, M. J. Serpe, Stimuli-responsive polymers and their applications, Polymer Chemistry, 2017, 8, 127-143.
2. S. H. Yun, S. J. J. Kwok, Light in diagnosis, therapy and surgery, Nature Biomedical Engineering, 2017, 1, 0008
.
3. V. San Miguel, C. G. Bochet, A. del Campo,Wavelength-Selective Caged Surfaces: How Many Functional Levels Are Possible?, J Am Chem Soc, 2011, 133, 5380-5388, b) P. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov, J. Wirz, Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy, Chemical Reviews, 2013, 113, 119-191, c) R. J. T. Mikkelsen, K. E. Grier, K. T. Mortensen, T. E. Nielsen, K.
Qvortrup, Photolabile Linkers for Solid-Phase Synthesis, ACS
Combinatorial Science, 2018, 20, 377-399.
4. a) C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer, A. Heckel, Light-Controlled Tools, Angew Chem Int Ed Engl, 2012, 51, 8446-8476, b) A. Gautier, C. Gauron, M. Volovitch, D. Bensimon, L. Jullien, S. Vriz, How to control proteins with light in living systems, Nat Chem Biol, 2014, 10, 533-541, c) M. M. Lerch, M. J. Hansen, G. M. van Dam, W. Szymanski, B. L. Feringa, Emerging Targets in Photopharmacology, Angew
Chem Int Ed Engl, 2016, 55, 10978-10999, d) N. Ankenbruck, T.
Courtney, Y. Naro, A. Deiters, Optochemical Control of Biological Processes in Cells and Animals, Angew Chem Int Ed
Engl, 2018, 57, 2768-2798. d) Y. Zheng, A. Farrukh, A. del
Campo, Optoregulated Biointerfaces to Trigger Cellular Responses, Langmuir, 2018, 34, 14459-14471, e) C. L. Fleming, M. Grøtli, J. Andréasson, On-Command Regulation of Kinase Activity using Photonic Stimuli, ChemPhotoChem, 2019, 3, 318-326.
5. F. H. C. Crick, Thinking about the Brain, Scientific American, 1979, 241, 219-233.
6. a) S. Berlin, E. Y. Isacoff, Synapses in the spotlight with synthetic optogenetics, EMBO reports, 2017, 18, 677-692, b) C. K. Kim, A. Adhikari, K. Deisseroth, Integration of optogenetics with complementary methodologies in systems neuroscience,
Nature Reviews Neuroscience, 2017, 18, 222-235, c) K. Hüll, J.
Morstein, D. Trauner, In Vivo Photopharmacology, Chemical
Reviews, 2018, 118, 10710-10747, d) P. Paoletti, G. C. R.
Ellis-Davies, A. Mourot, Optical control of neuronal ion channels and receptors, Nature Reviews Neuroscience, 2019, 20, 514-532. 7. a) A. Deiters, Principles and Applications of the Photochemical Control of Cellular Processes, ChemBioChem, 2010, 11, 47-53, b) D. Höglinger, A. Nadler, C. Schultz, Caged lipids as tools for investigating cellular signaling, Biochimica et Biophysica Acta
(BBA)- Molecular and Cell Biology of Lipids, 2014, 1841,
1085-1096, c) G. Guglielmi, H. J. Falk, S. De Renzis, Optogenetic Control of Protein Function: From Intracellular Processes to Tissue Morphogenesis, Trends in Cell Biology, 2016, 26, 864-874.
8. a) B. Goegan, F. Terzi, F. Bolze, S. Cambridge, A. Specht, Synthesis and Characterization of Photoactivatable Doxycycline Analogues Bearing Two-Photon-Sensitive Photoremovable Groups Suitable for Light-Induced Gene Expression, ChemBioChem, 2018, 19, 1341-1348, b) W. Brown, A. Deiters, Chapter Thirteen - Light-activation of Cre recombinase in zebrafish embryos through genetic code expansion, in Methods in Enzymology, ed. A. Deiters, Academic Press, 2019, vol. 624, pp. 265-281, c) F. Hamouri, W. Zhang, I. Aujard, T. Le Saux, B. Ducos, S. Vriz, L. Jullien, D. Bensimon, Chapter One - Optical control of protein activity and gene expression by photoactivation of caged cyclofen, in Methods
in Enzymology, ed. A. Deiters, Academic Press, 2019, vol. 624,
pp. 1-23, d) P. Müller, P. Seyfried, A. Frühauf, A. Heckel, Chapter Five - Phosphodiester photo-tethers for the (multi-)cyclic conformational caging of oligonucleotides, in Methods
in Enzymology, ed. A. Deiters, Academic Press, 2019, vol. 624,
pp. 89-111, e) S. Pattanayak, L. A. Vázquez-Maldonado, A. Deiters, J. K. Chen, Chapter Four - Combinatorial control of gene function with wavelength-selective caged morpholinos, in Methods in Enzymology, ed. A. Deiters, Academic Press, 2019, vol. 624, pp. 69-88.
9. V. N. Rajasekharan Pillai, Photoremovable Protecting Groups in Organic Synthesis, Synthesis, 1980, 1980, 1-26.
10. a) J. Engels, E. J. Schlaeger, Synthesis, structure, and reactivity of adenosine cyclic 3',5'-phosphate-benzyltriesters, Journal of
Medicinal Chemistry, 1977, 20, 907-911, b) J. H. Kaplan, B.
Forbush, J. F. Hoffman, Rapid photolytic release of adenosine
5'-triphosphate from a protected analog: utilization by the sodium:potassium pump of human red blood cell ghosts,
Biochemistry, 1978, 17, 1929-1935.
11. a) V. Hagen, B. Dekowski, N. Kotzur, R. Lechler, B. Wiesner, B.
Briand, M. Beyermann,
{7-[Bis(carboxymethyl)amino]coumarin-4-yl}methoxycarbonyl derivatives for photorelease of carboxylic acids, alcohols/phenols, thioalcohols/thiophenols, and amines,
Chemistry A European Journal, 2008, 14, 1621-1627, b) L.
Fournier, I. Aujard, T. Le Saux, S. Maurin, S. Beaupierre, J. B. Baudin, L. Jullien, Coumarinylmethyl caging groups with redshifted absorption, Chemistry A European Journal , 2013, 19, 17494-17507, c) M. T. Richers, J. M. Amatrudo, J. P. Olson, G. C. R. Ellis-Davies, Cloaked Caged Compounds: Chemical Probes for Two-Photon Optoneurobiology, Angew Chem Int Ed
Engl, 2017, 56, 193-197, d) Q. Lin, L. Yang, Z. Wang, Y. Hua,
D. Zhang, B. Bao, C. Bao, X. Gong, L. Zhu, Coumarin Photocaging Groups Modified with an Electron-Rich Styryl Moiety at the 3-Position: Long-Wavelength Excitation, Rapid Photolysis, and Photobleaching, Angew Chem Int Ed Engl , 2018, 57, 3722-3726.
12. a) A. Specht, F. Bolze, L. Donato, C. Herbivo, S. Charon, D. Warther, S. Gug, J. F. Nicoud, M. Goeldner, The donor-acceptor biphenyl platform: a versatile chromophore for the engineering of highly efficient two-photon sensitive photoremovable protecting groups, Photochem Photobiol Sci, 2012, 11, 578-586, b) S. Jakkampudi, M. Abe, N. Komori, R. Takagi, K. Furukawa, C. Katan, W. Sawada, N. Takahashi, H. Kasai, Design and Synthesis of a 4-Nitrobromobenzene Derivative Bearing an Ethylene Glycol Tetraacetic Acid Unit for a New Generation of Caged Calcium Compounds with Two-Photon Absorption Properties in the Near-IR Region and Their Application in Vivo, ACS Omega, 2016, 1, 193-201, c) M. T. Richers, S. Passlick, H. Agarwal, G. C. R. Ellis-Davies, Dendrimer Conjugation Enables Multiphoton Chemical Neurophysiology Studies with an Extended π-Electron Caging Chromophore, Angew Chem Int Ed Engl, 2019, 58, 12086-12090.
13. a) P. P. Goswami, A. Syed, C. L. Beck, T. R. Albright, K. M. Mahoney, R. Unash, E. A. Smith, A. H. Winter, BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light, J Am Chem Soc, 2015, 137, 3783-3786, b) A. Y. Vorobev, A. E. Moskalensky, Long-wavelength photoremovable protecting groups: On the way to in vivo application, Computational and Structural Biotechnology Journal, 2020, 18, 27-34.
14. a) T. Furuta, S. S.-H. Wang, J. L. Dantzker, T. M. Dore, W. J. Bybee, E. M. Callaway, W. Denk, R. Y. Tsien, Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis, Proceedings of the National Academy of Sciences, 1999, 96, 1193-1200, b) G. Bort, T. Gallavardin, D. Ogden, P. I. Dalko, From One-Photon to Two-Photon Probes: “Caged” Compounds, Actuators, and Photoswitches, Angew Chem Int
Ed Engl, 2013, 52, 4526-4537, c) M. Abe, Y. Chitose, S.
Jakkampudi, P. T. T. Thuy, Q. Lin, B. T. Van, A. Yamada, R. Oyama, M. Sasaki, C. Katan, Design and Synthesis of Two-Photon Responsive Chromophores for Near-Infrared Light-Induced Uncaging Reactions, Synthesis A European Journal, 2017, 49, 3337-3346, d) G. C. R. Ellis-Davies, Two-Photon Uncaging of Glutamate, Frontiers in Synaptic Neuroscience, 2019, 10.
15. a) L. García-Fernández, C. Herbivo, V. S. M. Arranz, D. Warther, L. Donato, A. Specht, A. del Campo, Dual Photosensitive Polymers with Wavelength-Selective Photoresponse,
Weyel, S. Junek, F. Schafer, C. Herbivo, M. Goeldner, A. Specht, J. Wachtveitl, A. Heckel, Three-Dimensional Control of DNA Hybridization by Orthogonal Two-Color Two-Photon Uncaging, Angew Chem Int Ed Engl, 2016, 55, 8948-8952.
C.-16. a) J. Carling, F. Nourmohammadian, J.-C. Boyer, N. R.Branda, Remote-Control Photorelease of Caged Compounds Using Near-Infrared Light and Upconverting Nanoparticles, Angew Chem Int Ed Engl, 2010, 49(22), 3782-3785, b) J. Cao, S. Huang, Y. Chen, S. Li, X. Li, D. Deng, Z. Qian, L. Tang, Y. Gu, Near-infrared light-triggered micelles for fast controlled drug release in deep tissue, Biomaterials, 2013, 34, 6272-6283.
17. a) P. Sengupta, S. B. van Engelenburg, J. Lippincott-Schwartz, Superresolution imaging of biological systems using photoactivated localization microscopy, Chemical Reviews, 2014, 114, 3189-3202, b) E. Betzig, Single Molecules, Cells, and Super-Resolution Optics, Angew Chem Int Ed Engl, 2015,
54, 8034-8053, c) S. W. Hell, Nanoscopy with Focused Light,
Angew Chem Int Ed Engl, 2015, 54, 8054-8066, d) W. E. Moerner, Single-Molecule Spectroscopy, Imaging, and Photocontrol: Foundations for Super-Resolution Microscopy,
Angew Chem Int Ed Engl, 2015, 54, 8067-8093.
18. D. Puliti, D. Warther, C. Orange, A. Specht, M. Goeldner, Small photoactivatable molecules for controlled fluorescence activation in living cells, Bioorganic & Medicinal Chemistry,
2011, 19, 1023-1029.
19. a) H. Li, J. C. Vaughan, Switchable Fluorophores for Single-Molecule Localization Microscopy, Chemical Reviews, 2018,
118, 9412-9454, b) F. M. Raymo, Y. Zhang, Live-Cell Imaging
at the Nanoscale with Bioconjugatable and Photoactivatable Fluorophores, Bioconjugate Chemistry, 2020, 31, 4, 1052-1062. 20. a) J. Bendig, S. Helm, V. Hagen, (Coumarin-4-yl)Methyl Ester of cGMP and 8-Br-cGMP: Photochemical Fluorescence Enhancement Journal of Fluorescence, 1997, 7, 357-361, b) V. Hagen, J. Bendig, S. Frings, B. Wiesner, B. Schade, S. Helm, D. Lorenz, U. Benjamin Kaupp, Synthesis, photochemistry and application of (7-methoxycoumarin-4-yl)methyl-caged bromoadenosine cyclic 3′,5′-monophosphate and 8-bromoguanosine cyclic 3′,5′-monophosphate photolyzed in the nanosecond time region, Journal of Photochemistry and
Photobiology B: Biology, 1999, 53, 91-102, c) B. Schade, V.
Hagen, R. Schmidt, R. Herbrich, E. Krause, T. Eckardt and J. Bendig, Deactivation Behavior and Excited-State Properties of (Coumarin-4-yl)methyl Derivatives. 1. Photocleavage of (7-Methoxycoumarin-4-yl)methyl-Caged Acids with Fluorescence Enhancement, The Journal of Organic Chemistry, 1999, 64, 9109-9117, d) V. Hagen, S. Frings, J. Bendig, D. Lorenz, B. Wiesner, U.B. Kaupp, Fluorescence Spectroscopic Quantification of the Release of Cyclic Nucleotides from Photocleavable [Bis(carboxymethoxy)coumarin‐4‐yl]methyl Esters inside Cells, Angew Chem Int Ed Engl, 2002, 41, 3625-3628.
21. S. Karthik, B. N. Prashanth Kumar, M. Gangopadhyay, M. Mandal, N. D. P. Singh, A targeted, image-guided and dually locked photoresponsive drug delivery system, Journal of
Materials Chemistry B, 2015, 3, 728-732.
22. A. Nadler, D. A. Yushchenko, R. Müller, F. Stein, S. Feng, C. Mulle, M. Carta, C. Schultz, Exclusive photorelease of signalling lipids at the plasma membrane Nature Communications, 2015, 6, 10056.
23. N. Wagner, M. Stephan, D. Höglinger, A. Nadler, A Click Cage: Organelle-Specific Uncaging of Lipid Messengers, Angew Chem Int Ed Engl, 2018, 57, 13339-13343.
24. P. Gaur, O. A. Kucherak, Y. G. Ermakova, V. V. Shvadchak, D. A. Yushchenko, Nitrobenzyl-based fluorescent photocages for
spatial and temporal control of signalling lipids in cells,
Chemical Communications, 2019, 55, 12288-12291.
25. R. Labruère, A. Alouane, T. Le Saux, I. Aujard, P. Pelupessy, A. Gautier, S. Dubruille, F. Schmidt, L. Jullien, “Self-Immolative” Spacer for Uncaging with Fluorescence Reporting, Angew Chem Int Ed Engl, 2012, 51, 9344-9347
.
26. a) R. J. Amir, N. Pessah, M. Shamis, D. Shabat, Self-Immolative Dendrimers, Angew Chem Int Ed Engl, 2003, 42, 4494-4499, b) F. M. H. de Groot, C. Albrecht, R. Koekkoek, P. H. Beusker, H. W. Scheeren, “Cascade-Release Dendrimers” Liberate All End Groups upon a Single Triggering Event in the Dendritic Core,
Angew Chem Int Ed Engl, 2003, 42, 4490-4494, c) M. L. Szalai,
R. M. Kevwitch, D. V. McGrath, Geometric Disassembly of Dendrimers: Dendritic Amplification J Am Chem Soc, 2003,
125, 15688-15689, d) N. Fomina, C. McFearin, M. Sermsakdi, O.
Edigin, A. Almutairi, UV and Near-IR Triggered Release from Polymeric Nanoparticles, J Am Chem Soc, 2010, 132, 9540-9542.
27. P. Liu, B. Li, C. Zhan, F. Zeng, S. Wu, A two-photon-activated prodrug for therapy and drug release monitoring, Journal of
Materials Chemistry B, 2017, 5, 7538-7546.
28. P. T. Wong, S. Tang, J. Cannon, J. Mukherjee, D. Isham, K. Gam, M. Payne, S. A. Yanik, J. R. Baker Jr., S. K. Choi, A Thioacetal Photocage Designed for Dual Release: Application in the Quantitation of Therapeutic Release by Synchronous Reporter Decaging, ChemBioChem, 2017, 18, 126-135.
29. T. Lammers, S. Aime, W. E. Hennink, G. Storm, F. Kiessling, Theranostic Nanomedicine, Accounts of Chemical Research, 2011, 44, 1029-1038.
30. a) A. D. Turner, S. V. Pizzo, G. W. Rozakis, N. A. Porter, Photochemical activation of acylated alpha-thrombin, J Am
Chem Soc, 1987, 109, 1274-1275, b) A. D. Turner, S. V. Pizzo,
G. Rozakis, N. A. Porter, Photoreactivation of irreversibly inhibited serine proteinases, J Am Chem Soc, 1988, 110, 244-250, c) P. M. Koenigs, B. C. Faust, N. A. Porter, Photochemistry of enzyme-bound cinnamoyl derivatives, J Am Chem Soc, 1993, 115, 9371-9379.
31. a) N. Gagey, P. Neveu, C. Benbrahim, B. Goetz, I. Aujard, J.-B. Baudin, L. Jullien, Two-Photon Uncaging with Fluorescence Reporting: Evaluation of the o-Hydroxycinnamic Platform, J
Am Chem Soc, 2007, 129, 9986-9998, b) N. Gagey, P. Neveu, L.
Jullien, Two-Photon Uncaging with the Efficient 3,5-Dibromo-2,4-dihydroxycinnamic Caging Group, Angew Chem
Int Ed Engl, 2007, 46, 2467-2469.
32. X.-Y. Duan, B.-C. Zhai, Q.-H. Song, Water-soluble o-hydroxycinnamate as an efficient photoremovable protecting group of alcohols with fluorescence reporting, Photochemical
& Photobiological Sciences, 2012, 11, 593-598.
33. A. Paul, R. Mengji, O. A. Chandy, S. Nandi, M. Bera, A. Jana, A. Anoop, N. D. P. Singh, ESIPT-induced fluorescent o-hydroxycinnamate: a self-monitoring phototrigger for prompt image-guided uncaging of alcohols, Organic & Biomolecular
Chemistry, 2017, 15, 8544-8552.
34. A. Paul, M. Bera, P. Gupta, N. D. P. Singh, o-Hydroxycinnamate for sequential photouncaging of two different functional groups and its application in releasing cosmeceuticals, Organic & Biomolecular Chemistry, 2019, 17, 7689-7693. 35. a) S. Kitani, K. Sugawara, K. Tsutsumi, T. Morimoto, K.
Kakiuchi, Synthesis and characterization of thiochromone S,S-dioxides as new photolabile protecting groups, Chemical
Communications, 2008, (18), 2103-2105, b) Y. Zhang, H.
Tanimoto, Y. Nishiyama, T. Morimoto, K. Kakiuchi, Novel Photolabile Protecting Group for Phosphate Compounds, Synlett, 2012, 2012, 367-370, c) Y. Zhang, H. Zhang, C. Ma, J. Li, Y. Nishiyama, H. Tanimoto, T. Morimoto, K. Kakiuchi,
Study of the Paternò–Büchi type photolabile protecting group and application to various acids, Tetrahedron Lett., 2016, 57, 5179–5184, d) Hikage, S.; Sasaki, Y.; Hisai, T.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K., Synthesis of novel caged antisense oligonucleotides with fluorescence property, Journal of Photochemistry and Photobiology A: Chemistry, 2016, 331, 175-183.
36. C. Ma, Y. Zhang, H. Zhang, J. Li, Y. Nishiyama, H. Tanimoto, T. Morimoto, K. Kakiuchi, Synthesis and Photochemistry of a New Photolabile Protecting Group for Propargylic Alcohols,
Synlett, 2017, 28 (05), 560-564.
37. S. Hikage, Y. Nishiyama, Y. Sasaki, H. Tanimoto, T. Morimoto and K. Kakiuchi, Quantitative Photodeprotection Assessment of Caged Resveratrol by Fluorescence Measurement, ACS
Omega, 2017, 2, 2300-2307.
38. J. A. Peterson, C. Wijesooriya, E. J. Gehrmann, K. M. Mahoney, P. P. Goswami, T. R. Albright, A. Syed, A. S. Dutton, E. A. Smith, A. H. Winter, Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light, J Am Chem
Soc, 2018, 140, 7343-7346.
39. S. Barman, S. K. Mukhopadhyay, S. Biswas, S. Nandi, M. Gangopadhyay, S. Dey, A. Anoop, N. D. Pradeep Singh, A p-Hydroxyphenacyl–Benzothiazole–Chlorambucil Conjugate as a Real-Time-Monitoring Drug-Delivery System Assisted by Excited-State Intramolecular Proton Transfer, Angew Chem
Int Ed Engl, 2016, 55, 4194-4198.
40. a) S. Biswas, J. Das, S. Barman, B. Rao Pinninti, T. K. Maiti, N. D. P. Singh, Environment Activatable Nanoprodrug: Two-Step Surveillance in the Anticancer Drug Release, ACS Applied
Materials & Interfaces, 2017, 9, 28180-28184, b) Y. Venkatesh,
J. Das, A. Chaudhuri, A. Karmakar, T. K. Maiti, N. D. P. Singh, Light triggered uncaging of hydrogen sulfide (H2S) with real-time monitoring, Chemical Communications, 2018, 54, 3106-3109.
41. E. Abou Nakad, F. Bolze, A. Specht, o -Nitrobenzyl photoremovable groups with fluorescence uncaging reporting properties, Organic & Biomolecular Chemistry, 2018, 16, 6115-6122.
42. R. Durand-de Cuttoli, P. S. Chauhan, A. Pétriz Reyes, P. Faure, A. Mourot, G. C. R. Ellis-Davies, Optofluidic Control of Rodent Learning Using Cloaked Caged Glutamate, Proceedings of the
National Academy of Sciences, 2020, 117, 6831-6835.
43. A. Bardhan, A. Deiters, Development of photolabile protecting groups and their application to the optochemical control of cell signaling, Current Opinion in Structural Biology, 2019, 57, 164-175.
‡ Footnotes relating to the main text should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.