Activin signaling controls a wound-induced program
essential for regenerative patterning
by
Jennifer K. Cloutier
A.B. Honors, Human and Developmental Regenerative Biology (2013) Harvard University, Cambridge, MA
Submitted to the Department of Biology
In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY May 2020
© 2020 Jennifer K. Cloutier. All rights reserved.
The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in medium now
known or hereafter created.
Signature of Author ……….……… Department of Biology May 21, 2020 Certified By ……… Peter W. Reddien Professor of Biology Thesis Supervisor Accepted By……… Mary Gehring Associate Professor of Biology Co-Director, Committee for Graduate Students
Activin signaling controls a wound-induced program essential for regenerative patterning
By
Jennifer K. Cloutier
Submitted to the Department of Biology on May 21st, 2020 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology
ABSTRACT
A central problem in animal regeneration is how animals determine what body part to regenerate. Planarians are flatworms that can regenerate any missing body part, and are studied to identify mechanisms underlying regeneration. At transverse amputation planes, a poorly understood mechanism specifies regeneration of either a head or a tail. This head-versus-tail regeneration decision-making process is referred to as
regeneration polarity and has been studied for over a century to identify mechanisms that specify what to regenerate. The Wnt antagonist gene notum is induced within hours after injury robustly at anterior-facing wounds preferentially, where it specifies head regeneration. We report that Activin signaling is required for regeneration polarity, and the underlying asymmetric activation of notum preferentially at anterior-facing wounds. We propose Activin signaling is involved in regeneration-specific responses broadly in the animal kingdom.
Planarian patterning requires signaling from specific subsets of muscle cells. Furthermore, several of these subsets have been shown to express specific
transcription factors, for which inhibition results in specific patterning phenotypes. Muscle heterogeneity and function in regeneration can be further studied through optimized single-cell sequencing datasets. We report an improved 10x –based
planarian muscle cell scRNA-seq dataset that predicts novel transcription factors associated with muscle cell heterogeneity.
Thesis Supervisor: Peter W. Reddien Title: Professor of Biology
Acknowledgements
I would like to thank my thesis advisor Peter for guiding me every step of the way of this process. Your never-ending passion for science, and curiosity about the natural world have given me new perspective inside and outside of lab. I also immeasurably value your commitment as a mentor, and willingness to come to the bench and participate in experiments. It has been a privilege to learn from you both scientifically and personally. I would like to thank the members of my thesis committee, David Page and Adam Martin. I have valued their guidance in my PhD, and especially their willingness to ask me explicitly what my goals are and how they can be supported. I would like to thank Terry-Orr Weaver for serving on my thesis committee for the first two years of my PhD. I would also like to thank Amy Wagers for serving on my dissertation defense committee, and bringing a news perspective to its critical discussion.
I want to thank my HST MD, MIT Biology, and MD-PhD classmates and friends for teaching me so much about medicine, science, and life. The administrative teams of these programs have been an invaluable resource. I want to thank Nick Polizzi, Patty Cunningham, Betsey Walsh, Amy Cohen, Rick Mitchell, Matthew Frosch, and Loren Walensky for their support.
I can wholeheartedly say that the relationships I have made with my labmates, both new and old, are what have made this past five years both possible, and some of my most cherished memories. Lucila – you have been an incredible mentor to me, and I feel grateful to call you my friend. Amelie, Chris, Kate, Deniz, Conor – thank you for always listening to me when I get excited, and validating my excitement. Shannon, Aneesha, Cecilia, Hunter, Catherine, Tom, Chan, Zach, Tessa, Kwadwo, Travis, Olivia, Sam, Jack, Jia, Ashley, Isaac, Sarah, Kellie, and Omri – thank you for being positive forces in my life, and science. Lauren, I am so thankful to have met you, and that I have tricked you into thinking we are sometimes twins – truly I look up to you immensely, and deeply value our friendship.
For the past five years I have lived in a wonderful community called Quincy House as a Resident Tutor of Harvard College. My students and fellow tutors have pushed me to be a better person, and to maintain my youth even when I’m tired. This home away from home was only made possible by the guidance of Faculty Deans Lee and Deb Gehrke. Since my time as an undergraduate at Harvard College, they have been a constant presence of warmth, fun, encouragement. Fun Dean Deb, you are dearly missed. I have been so fortunate to have wonderful college friends, and proud to say we are as close as ever. Ethan has particularly supported me over the past five years (even trying to bring me groceries as I type this). I would also like to thank Sarah, Peggy, Elizabeth, Katherine, Chris, Anna, Rylie, Monika, Kim, Rachel, Xin, and Sandy for being the
maintain close relationships with my childhood friends Claire, Emily, Amy, Selina, Molly, Mariah (in no small part because of the wonders of the internet).
I want to thank my family for their support and unwavering belief in me. Nana, Papa, Nan, Grandpa, Aunt Debbie and Aunt Sandy – thank you for believing in me. I want to thank my Mom for encouraging me to be curious, creative, strong, and kind. I want to thank my Dad for always challenging me to do my best, and inspiring me to persevere through adversity. I want to thank my little brother Robbie inspiring me with his actions to be a more compassionate, empathetic, and brave.
Stephen, you have supported me in many more ways than you could ever know. Your love, willingness to be silly, humility, diligence and calming presence bring joy to every day I have spent with you. I can’t wait to see what is next for us!
TABLE OF CONTENTS CHAPTER 1: Introduction
Forward……….….. I. Basic Mechanisms of Patterning………..……… II. Planarian Patterning and Regeneration………...………….. III. TGF-β in Patterning and Regeneration………...……. Figures………...……. References………..……
CHAPTER 2: activin-2 is required for regeneration of polarity on the planarian anterior-posterior axis Abstract………..……… Introduction……….…..………. Results……….…. Discussion………..…….……. Figures………... Tables……...………...………...……. Materials and Methods…...………..……...….…… References………...…….
CHAPTER 3: 10x based single-cell RNA sequencing reveals cell type
heterogeneity and cell-type-specific expression of transcription factors in planarian muscle Abstract………..… Introduction……….………….. Results……….…….. Discussion……….……… Figures……… Materials and Methods……… References………
CHAPTER 4: Conclusions and Future Directions
I. Polarity Decisions in Planarian Regeneration…………..………... II. Activin-Follistatin Signaling in Planarians………...………. III. 10x-based scRNA-seq for further Interrogation of Cell Transcriptomes….…...…... Conclusions………...………... References……… 12 14 18 34 40 48 73 75 77 88 94 122 131 135 146 147 149 157 159 175 178 182 185 188 190 191
Chapter 1
Introduction
Contributions: The contents of this chapter were written by Jennifer K Cloutier with editing by members of the Reddien laboratory including Peter Reddien.
Foreword
The ability to replace missing tissues or parts of an animal is a fascinating and
incredible phenomenon termed regeneration. Remarkably, this ability to replace what is lost through injury is widespread and naturally occurring throughout the animal kingdom. From cnidarians to acoels to platyhelminthes to vertebrates, many organisms have some regenerative capacity – whether it be partial organ replacement or whole-body regeneration from a complete axis amputation. Despite this wealth of
organisms that regenerate, many traditional model organisms lack the regenerative capability found in other animals, including whole-body regeneration – making some aspects of regeneration difficult to study without utilization of new, emerging model systems. Planarians, which can perform whole-body regeneration, have emerged as a powerful model for study of regeneration.
This thesis aims to understand signaling required for head-tail polarity in planarian whole-body regeneration, as well as the molecular underpinnings of heterogeneity seen in planarian muscle. This introductory chapter will provide an overview of key concepts related to the topics presented in this thesis. First, I will present an overview of basic mechanisms of patterning in development. This section is intended to give context to the inductive and positional signaling seen in adult biology and regeneration presented later in the thesis. Next, I will describe the fundamentals of planarian biology and regeneration with a focus on patterning in regeneration.
Planarians have been a model organism used in regenerative studies for over 100 years, and have emerged in the modern molecular era as a model for these
functional patterning roles have been applied to this signaling pathway, it is
understudied relative to other major signaling pathways (i.e., Wnt signaling) found in planarians – giving the opportunity to further unlock the mechanisms of whole-body regeneration through functional insights into TGF-β signaling.
I. Basic Mechanisms of Patterning
Patterning is the process by which cells adopt region-appropriate identity and spatial arrangements relative to one another. In order to pattern tissue, organisms utilize several mechanisms of propagating fate-regulating information in a position-dependent manner. Cell-cell communication and signaling pathways such as TGF-β, Wnt, Notch, Hh, and Fgf are utilized in development and regeneration to provide this information. Cells can signal through physical touch (i.e., across gap junctions), autocrine (cell signals to itself), paracrine (cell signals to nearby cell), or endocrine (cell signals to distant cell) signaling dependent on pathway mechanics. In order to better understand individual patterning mechanisms, it is important to define the identity of an involved signal and where it is coming from, along with other properties that would tune the signal and its environment. Two well studied mechanisms of disseminating patterning information are morphogen gradients and embryonic organizers described in detail below.
Morphogens
In 1917 D’Arcy Thompson proposed that mathematical principles that govern inorganic matter could also be used to understand biological phenomena – famously including principles of self-organization (1, 2). In 1940 Conrad H. Waddington presented the idea that chemical substances he called ‘evocators’ diffuse through developing tissues in order to influence developmental decisions (3). Over a decade later in 1952, Alan Turing – inspired by these two ideas - coined the word ‘morphogen’ as chemical substances that diffuse through and react with tissues in a developing embryo to
influence pattern (4). Turing’s goal was to create a mathematical model that could explain the creation of repeated patterns in space – such as in a developing embryo. His model described a reaction-diffusion system where pattern would be derived from diffusion of a short-range activator and a long-range inhibitor leading to oscillatory patterning. He then suggested that tuning this system could result in the myriad of patterns seen throughout biology (Figure 1.1A). Repeating patterns derived from homogenous fields of cells such as mammalian hair follicle placement (5, 6) can be modeled reasonably well by reaction-diffusion systems. Furthermore, functional genetic studies have unveiled one such activator-inhibitor pair demonstrating properties of a reaction-diffusion system as Wnt and Dkk4 in murine hair follicle placement (7). However, in the modern era, it has been shown that many systems are far more complex such as those that require branching morphogenesis (8), and it has been difficult to identify particular functional inhibitor/activator pairs that satisfy this model.
Noting the vast intricacies seen in patterns in nature, Lewis Wolpert proposed that the morphogen model be expanded to take into account many environmental inputs. He proposed that ‘positional information’ drove pattern formation based of the fact that many environmental cues could be received and interpreted at any given position (9, 10). This thinking laid out in its simplest form was presented as the ‘French Flag Model’ (9). In this model, a morphogen displays graded concentration across an axis and cells derive their identity based upon their position in that axis. Cell fates
cannot be intermediate, and thus are thresholded across the axis giving the appearance of several distinct bands of different cell fates – akin to the bands of color on a French flag (Figure 1.1B). In Drosophila embryos, a simple anterior-posterior (AP) gradient of
bicoid has been seen to determine the identity of body segments across the axis by activating anterior gap genes such as hunchback to promote anterior segment formation (11). Still, the complexity of tissue patterning must take into account other factors such as initial cell composition, tissue shape, complex regulatory networks, and mechanical forces in order to accurately describe what drives pattern formation in all systems. For example, interactions between the targets cells of a morphogen can have major roles in determining the resultant pattern.
Embryonic Organizers
An “organizer” is a focal group of cells that instructs tissue by providing signals that are independent of tissue context, and that can promote particular fates and pattern (12, 13). Early experimental forays into this concept used tissue grafts to explore the effects of moving tissues to ectopic locations. Two closely related species with different
pigmentation patterns were used to distinguish the origin of resulting structures (14). This technique was termed heteroplastic transplantation (14). For example, in 1909, Ethel Browne transplanted anterior tissue from one hydra to another of different
pigment, and saw induction of a new axis made entirely of host tissue (15). Whether this tissue graft was an organizer would require more functional characterization, however its influence on surrounding tissues demonstrated clear inductive properties. Induction is the ability of particular cells to influence choices of other cells, and competence describes the ability of a given tissue to respond to inductive signals (3, 16). Organizers should have near invariant signaling capability independent of tissue location, however a given tissue may or may not be competent to respond to these signals (12).
The classic experiment that propagated the idea ‘organizers’ came from embryonic transplantation experiments from the laboratory of Hans Spemann, and performed by Hilde Mangold (13). Mangold grafted the dorsal lip of the blastopore of a Xenopus laevis embryo to ventral side of a different embryo. Mangold noted that the graft gave rise to mostly notochord and induced dorsalization in surrounding tissues including ectopic somite and central nervous system formation (17). Remarkably, this graft induced a secondary axis in the embryo resulting in conjoined twins with perfectly aligned axes (17) (Figure 1.1C). After the tragic death of Hilde Mangold, Spemann went on to use heteroplastic transplantation to show that the dorsal lip of the blastopore was the only region of the developing embryo that was not competent to be induced by surrounding cell fates when transplanted – that it was almost invariant to its
environmental context, and always gave rise to dorsal tissues (13, 18). The dorsal lip of the blastopore was thus named the Spemann-Mangold Organizer for its ability to induce fate and pattern in new tissue contexts resulting in an ectopic axis (13).
After this discovery, it took some time to identify the molecular players of the Spemann organizer. Dorsal and ventral signals have been identified that regulate the formation and activity of the organizer include components of major pathways like Wnt and TGF-β signaling (13). The homeobox gene goosecoid was identified as both marking the Spemann-Mangold organizer and having the ability to induce organizer formation upon mRNA injection (19). Over time other structures have been identified that have organizer-like properties including ZPA and AER regions in limb development, notochord, floor plate and roof plate in post gastrulation embryology, the
II. Planarian Patterning and Regeneration
The phylum Platyhelminthes contains species of freshwater flatworms known as
planarians that demonstrate a remarkable ability regenerate. Regeneration involves the ability to replace tissue composition and form from remaining body fragments after amputation (21). First known to be described as an obscure species that could
regenerate its form by Prussian zoologist Peter Simon Pallas in 1774, planarians have fascinated scientists for centuries (22, 23). In the 1800s scientists like Thomas Hunt Morgan and Harriett Randolph furthered this knowledge through systematic surgical studies and cellular observations (24, 25). The marvel of this phylum was punctuated by facts such as planarians could regenerate from a 1/279th of their body’s original size (26). In order to accomplish the re-establishment of form and replacement of loss tissues, planarians require a cellular source for generating replacement tissues, as well as patterning mechanisms capable of adapting to diverse injuries.
Planarians are widely abundant throughout the world, and have great diversity – ranging from terrestrial to freshwater habitats (27). At the turn of the 21st century,
research on these animals was reinvigorated by advances that allow probing the cellular and molecular underpinnings of regeneration (21). The freshwater planarian Schmidtea mediterranea is of particular interest to this field as these animals can perform whole-body regeneration from almost any amputation, including head regeneration, and a wealth of molecular resources exist for this species (28). S. mediterranea are amenable to robust gene expression inhibition through RNA interference (RNAi), allowing for functional studies of regenerative mechanisms (29). A clonal population of this species was transported from a single well in Spain in the early 2000s, and disseminated to
laboratories all over the world. This species has therefore been used for a plurality of modern experimentation (30).
Planarians are bilaterally symmetric and possess a body plan with three primary axes; anterior-posterior (AP), medial-lateral (ML) and dorsal-ventral (DV) (31) (Figure 1.2A). Other important anatomical regions include head-tip and tail-tip, termed anterior- and posterior-poles respectively, as well as the dorsal-ventral boundary. Planarian anatomy consists of an outer epidermal layer covering a multi-layered body-wall
musculature (32). The space encapsulated by the body-wall muscle consists of the gut, nervous system, parenchymal cells, protonephridia, phagocytic cells, other muscle derivatives, and stem cells (termed neoblasts) (33, 34). Planarians possess a bi-lobed cephalic ganglia (brain) at their anterior end, and two ventral nerve cords running along their AP axis which taken together form the central nervous system (CNS). The
planarian Schmidtea mediterranea possesses two anterior, negatively phototactic eyes, and a muscularized mid-body pharynx. This pharynx connects into the planarian gut via a small connection termed the esophagus, and exits a ventral hole in the planarian body termed the mouth. Planarian pharynges can move in and out of the planarian body during periods of ingestion of food or excretion of waste. The planarian epidermis is ciliated on the ventral side of animals to allow for animal gliding in water. Planarians are also covered in a thick protective layer of mucus. This anatomical complexity is
reconstituted with incredibly high fidelity in regeneration.
Planarian regeneration has historically been subdivided into two phenomenon termed: “epimorphosis” and “morphallaxis” (35). While there has been some debate as to the terms’ meaning (36-39), broadly speaking epimorphosis involves blastema
formation through cellular proliferation at a wound site, whereas morphallaxis involves regenerative changes that take place away from the wound (21). For example,
planarians restructure their body proportions long after missing tissues are replaced following amputation. This process of morphallaxis may also be required outside of an injury context as planarians can change dramatically in size dependent on intake of food. The species Schmidtea mediterranea can range in size from ~1mm to over 1 cm depending on nutrient ingestion (34, 40). Despite this range of sizes, anatomical proportions are maintained such that planarians of different sizes appear as scaled-down or –up versions of their counterparts.
The planarian, Schmidtea mediterranea, has both a sexual and asexual strain. The sexual strain is hermaphroditic and reproduces through cross-fertilization. As Platyhelminthes, planarians are part of the Sprialia superphylum, so named for the prevalence of spiral cleavage patterns in embryogenesis broadly represented in this clade. Planarian embryos, by contrast, undergo anarchic cleavage of their blastomeres during development (41, 42); the mechanisms of which remain poorly characterized. The asexual strain reproduces through an obligate regenerative mechanism. In order to accomplish this without incurring random injury, the planarian will anchor its body to an underlying surface and contract its body until a tail fragment ‘fissions.’ This fission fragment will then go on to regenerate an entirely new properly patterned planarian within two weeks. With robust regeneration so commonly occurring in these species, planarians have developed a cellular toolkit for regenerative success; including a pluripotent cellular source and GPS-like coordinate system for patterning.
Current Techniques in Planarian Biology
Over the past two decades, many modern experimental tools have been adapted for use in planarians. Planarian transcriptomes (43, 44) and a genome assembly (45) have been determined. These resources have been used to facilitate RNA sequencing
approaches, including single cell-RNA sequencing technologies (46-48). Planarians have been shown to be amenable to many RNA sequencing techniques such as bulk sequencing, Smart-Seq2, and Drop-seq (47, 49). Recently the genome has been used to decipher Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq) data (50), with further advancements in chromatin biology in sight. Planarians are readily fixed and amenable to a host of tissue labeling and histological techniques (51). Their small size enables whole-mount labeling, for example in the case of RNA-probe-based fluorescence in situ hybridization (52). Functional studies were made possible by the application of RNAi to planarians (29, 53).Double-stranded RNA (dsRNA) can rapidly disseminate throughout the planarian body by simply feeding it to the animals. Planarian cycling cells can be tagged with the thymidine analog bromodeoxyuridine (BrdU) (54), selectively eliminated with irradiation (55), sorted based on DNA content using FACs (56), and transplanted (57). Some antibodies have been developed or adapted to planarians for immunostaining (58-62), western blots (63), chromatin
immunoprecipitation (chIP) (64-66), and fluorescence activated cell sorting (FACS) (67). Technical challenges in the field include the development of genome editing, live
Clonogenic Pluripotent Neoblasts as the Cellular Source for Regeneration
Planarian neoblasts were originally defined as ‘undifferentiated, embryonic-like cells residing in the adult parenchyma’ by Harriet Randolph who was observing the cellular morphology of Lumbriculus annelids (25, 68). Neoblasts are cells with large nuclei and scant cytoplasm found throughout the planarian body length save for the head-tip and pharynx, and comprise about ~20-30% of the cells in the animal (69, 70). Neoblasts are the only cycling cells in the planarian and their progeny go on to form all the cells in this animal; thus they are described as stem cells. Evidence for this first came from
utilization of irradiation, which eliminates cycling cells. Following irradiation, planarians both lost neoblasts and could no longer survive or regenerate (55, 70-74). Furthermore, neoblasts are the only cells to take up the cycling cell label BrdU in short pulses (54). Since neoblasts are the only cells with varying DNA content, they can be prospectively isolated by fluorescent assisted cell sorting (FACS) based on size, granularity and 2C or 4C DNA content (56). Definitive evidence that a single neoblast is capable of
pluripotency came from single cell transplants into an irradiated host – where a single neoblast could rescue whole animals devoid of cycling cells post lethal irradiation (57, 75).
Neoblasts express a unifying set of genes including homologs of known germline-associated genes (piwi, vasa, nanos) (76, 77), and stem cell maintenance genes (78-84). One such gene, encoding the PIWI protein SMEDWI-2, has been shown to regulate planarian stem cells (85). Upon inhibition of smedwi-2 planarians display ventral curling, head regression, lesions and eventual death by lysis after 10-14 days (85). This phenotype is consistent with what is seen upon lethal irradiation. Neoblasts,
however, are now appreciated to have abundant heterogeneity as well (46, 86, 87). Specialized neoblasts are cycling cells that express neoblast specific transcripts as well as fate specific transcription factors (75, 86, 87). In fact, most neoblasts are now
considered to be specialized (46). Recently, one class of neoblasts were prospectively isolated by antibody-based FACS for a tetraspanin (TSPAN) protein and these cells were pluripotent in transplantations (67). From previous sequencing datasets, these cells were seen to have neural lineage specific gene expression demonstrating potential specialization to the neural lineage (46).
Planarian Anterior-Posterior Regeneration
The Wnt pathway is a developmental signaling pathway that is critical to embryonic patterning and cell proliferation. In brief, canonical Wnt signaling occurs when a Wnt ligand binds a Frizzled receptor complex (88-90). The downstream effect of this binding event is to sequester the catenin destruction complex to prevent degradation of β-catenin (91). When the transcription factor β-β-catenin is not destroyed, it can translocate to the nucleus and interact with TCF/Lef-family transcription factors to activate Wnt target gene expression (92-94). Planarians robustly express many Wnt pathway and Wnt antagonist homologs. Many of these genes are expressed in transcriptional gradients along the AP axis and act as functional regulators of AP patterning (95-97) (Figure 1.2B). In fact, it has been hypothesized that across evolution animals
preferentially express Wnt pathway-promoting components in the posterior and Wnt pathway inhibitors in the anterior (98). This Wnt gradient, from posterior to anterior, is
hypothesized to broadly inform AP patterning in both embryonic and adult tissues across the Metazoa (98).
Planarians have two genes encoding β-catenin proteins known as β-catenin-1 and β-catenin-2 (95, 99-101). β-catenin-1 participates in canonical Wnt signaling,
whereas β-catenin-2 has been shown to physically interact with junctional proteins such as cadherins (101). β-catenin-1 is required for maintenance of posterior identity (95, 99, 100). Upon β-catenin-1 inhibition, posterior tissue identity is lost and ectopic heads form. Head formation does not require amputation and occurs solely at the dorsal-ventral boundary, with the first ectopic head always forming at the original tail-tip. This process of replacement of tail tissue with head tissue eventually leads to animals becoming radially hypercephalized (95, 99, 100). Antibody-labeling studies detected a β-catenin-1 protein gradient along the planarian AP axis – explaining the graded
activation of Wnt signaling expected from functional studies in planarians (62, 63). This requirement for Wnt signaling suppression in head formation has been key to
understanding the regenerative capacities of planarians across evolution. Although many if not all planarian species are capable of regenerating from a whole axis amputation, some planarians are incapable of head regeneration (102-104). Remarkably this head regeneration can be rescued by lowering the Wnt signaling environment through β-catenin inhibition, allowing previously incapable species to regenerate heads (105-107).
There have been nine Wnt ligand homologs identified in Schmidtea
mediterranea. A plurality of these wnts are expressed in a gradient highest in the
small AP stripe at the planarian tail tip (108) (Figure 1.2C). It is also robustly expressed after wounding close to the wound face at all tested wounds (108). Inhibition of wnt1 results in ectopic head formation at posterior-facing wounds. Irradiation blocks the new tail tip expression (posterior pole) of wnt1 after tail amputation but does not block wound-induced wnt1 expression and does not block initial activation of the posteriorly expressed wntP-2. Irradiated wnt1 RNAi head fragments failed to activate wntP-2 at the posterior-facing wound, consistent with a model that wound-induced wnt1 is required for the initiation of posterior PCG expression (108). Additionally, inhibition of one of several genes that encodes a frizzled protein, fz1/2/7, can result in ectopic posterior heads at low frequency (63).
Multiple genes that encode Wnt inhibitors are also expressed in gradients in planarians, with a bias to anterior expression. Secreted frizzled related proteins (sFRPs) are thought to inhibit Wnt signaling by acting as decoy receptors for Wnt ligands (109-111). sFRP-1 is expressed in the planarian head tip (95, 99, 100). Notum is a deacylase that acts to cleave a palmitoleate moiety on Wnts required for binding to Frizzleds (89, 112). notum is notably expressed in the planarian head tip, brain, and preferentially at anterior-facing wounds (113). notum RNAi causes tails to regenerate in the place of heads at anterior-facing wounds, indicating notum is required for specification of head regeneration. notum RNAi in uninjured animals can cause anteriorization within the head, including ectopic anterior formation of eyes and anteriorization of the brain commissure. (113).
Wnt signaling can also affect intra-organ AP patterning. wntA (also known as wnt11-6, or wnt4) is expressed preferentially in the posterior of the planarian brains (96,
114). Upon wntA inhibition, the planarian brain increases in size and elongates posteriorly (96, 114, 115). Eye shape has also been shown to be affected by Wnt signaling; for example, β-catenin-1 and TCF-2 inhibition has been shown to elongated eye shape on the AP axis (116, 117). A truncated form of β-catenin (β-catenin-4) is believed to act as a negative regulator of canonical Wnt signaling and can affect planarian eye shape through inhibiting proper photoreceptor differentiation (117).
Hedgehog (Hh) signaling has also been implicated in AP patterning, but only in amputated animals that have undergone axial regeneration (118-120). Low Hedgehog signaling (inhibition of hh) has been shown to produce a blunted tail, whereas high Hedgehog signaling (inhibition of the inhibitory receptor ptc) causes ectopic anterior tails to form (118). Hedgehog signaling has also been shown to modulate levels of wound-induced wnt1 expression, which may explain the aberrant AP patterning in regeneration that occurs following Hh pathway perturbation (118).
Another family of genes encoding broadly conserved FGF Receptor-like proteins termed FGFRLs, has been identified as playing a prominent role in planarian AP
patterning (115, 121-123). The FGFRLs contain the extracellular domains and transmembrane domains expected of an FGF Receptor (FGFR), but lack the intracellular kinase domain (115, 121, 124). The signaling capacity and associated signaling pathway components of these proteins remain unknown. The founding member of this group of genes, nou-darake (ndk), means "brains everywhere" in
Japanese (121). Upon inhibition of ndk, which is expressed in anterior planarian muscle and broadly through the planarian brain, the brain elongates posteriorly (115, 121).
the original eyes (121). Other members of the ndk family are named nou-darake-likes (ndls) and are expressed in muscle in anterior and mid-body-enriched gradients (115, 124).
Two FGFRL-Wnt circuits have been uncovered, which regulate head and mid-body patterning (115, 123). ndk, together with fz5-8/4 and wntA regulate the AP pattern of the head, with inhibition of each of these genes causing posterior brain expansion and ectopic eye formation (115). In a second set, the combined inhibition of ndl-3, wntP-2, and/or ptk7 (kinase-dead Wnt co-receptor) (125) causes mid-body posterior
expansion including duplication of the mouth and pharynx (123). The midbody/tail distinction is also regulated by the circuitry of Wnt signaling. A broadly conserved Wnt signaling transcriptional target, the transcription factor-encoding sp5 gene acts in the tail to inhibit the expression of ndl-3 and ptk-7, as well as other mid-body genes (126).
Upregulation of the Wnt pathway through the action of pathway components evi/wntless (127) (Wnt secretion) (128-130), teashirt (116, 131) (Wnt transcriptional target) (132), and disheveled-1 (133) (disruption of the β-catenin destruction complex) (134), promotes posterior fates or ‘tail-ness.’ By contrast downregulation of the Wnt pathway through the action of negative regulatory Wnt pathway components APC (99) and axin (135) (which encode components of the β-catenin destruction complex) (91) promotes anterior fates or ‘head-ness.’ FGFRLs function to promote anterior and mid-body boundaries (115, 123) and Hedgehog signaling is required for re-establishment of AP polarity in regeneration (118).
Medial-lateral and Dorsal-ventral Axis Patterning
Although much less work has been done on medial-lateral (ML) and dorsal-ventral (DV) axis patterning in planarians, key findings have revealed a few overarching regulatory pathways. The Slit/Robo pathway regulates ML patterning. For example, when the midline-expressed slit gene is inhibited, midline collapse is seen including the
emergence of cyclopia in regeneration (97, 136). wnt5 is expressed laterally and causes midline expansion following inhibition. For instance, ectopic lateral eyes, brain lobes, and duplicated nerve cords are observed in wnt5 RNAi animals (97, 127). wnt5 and slit have also been shown to have reciprocal expression domains that regulate one
another, such that when one is inhibited the other expands (97). Finally, the posteriorly expressed wnt11-2 gene is required for proper midline intestine branch patterning in the tail (97).
The Bmp pathway regulates DV patterning (137-139). bmp4 is expressed at the planarian dorsal-midline and is required for dorsal patterning (137-139). Upon bmp4 RNAi, animals become ventralized, including brain duplication and the ability to locomote on their newly ciliated dorsal side in long-term RNAi conditions (137-139). This ventralization process has been shown to involve the loss of differentiation of dorsal fates and failed inhibition of ventral fates in the dorsal neoblast compartment (140). bmp4 also is important for midline patterning, with its inhibition causing
duplicated medial eyes and an indented midline blastema in regeneration (137-139). Modulators of the BMP pathway have also been shown to change DV patterning: RNAi of admp (141) (TGF-β ligand that potentiates Bmp) causes ventralization, whereas RNAi of noggin-1 and noggin-2 (142-144) (bmp inhibitors) causes dorsalization.
Wound Response in Planarian Regeneration
One of the hallmarks of planarian regeneration is the wound response. This response includes three major components: upregulation of mitosis (59), upregulation of
apoptosis (61), and wound-induced gene expression (47, 145), over two phases: the generic wound response (~0-12 hrs post amputation) (47) and the missing tissue response (~12-48 hrs post amputation) (59) (Figure 1.3A). In brief, wounding causes a widespread mitotic burst (59), local apoptotic response (61), and wound-induced gene expression (47, 145) in the first ~12 hours at ~all wounds. If the wound removes tissue (59), the missing tissue response is triggered and includes a second local (i.e., close to the wound site) mitotic peak (59), widespread apoptosis (61), and sustained wound-induced gene expression (47, 145).
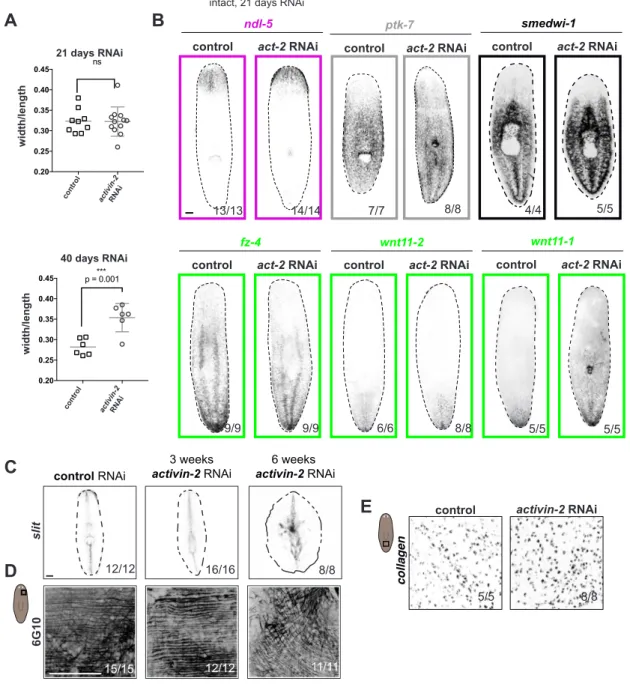
Although the mechanistic trigger of the wound response remains unknown, the wound-induced TGF-β pathway inhibitor follistatin has been shown to be required for the missing tissue response (146). Upon inhibition of follistatin, animals fail to form blastemas and fail to regenerate on a typical timescale following amputation (146-148). This phenotype can be suppressed by simultaneous inhibition of follistatin and the TGF-β ligands activin-1 and activin-2 (146, 148). Upon inspection, the generic wound
response occurs as expected in follistatin inhibited animals, but the missing tissue response fails to occur independent of wound geometry (146). Surprisingly, these hallmarks of regeneration are dispensable for regeneration from wounds with missing tissue – regeneration occurs over a longer timescale with new tissues forming
independent of a blastema (147). One exception is the failure to regenerate heads following follistatin RNAi in an amputation plane location-dependent manner (147).
follistatin inhibited animals lose the ability to regenerate a head when amputations are made in a Wnt high environment (i.e., tail) (147). Evidence indicates that this is a consequence of an inappropriately high level of wound-induced wnt1 seen in the
generic wound response of these RNAi animals (147, 149). Consistent with this model, the failure of follistatin RNAi animals to regenerate a head can be suppressed by wnt1 inhibition (147).
Muscle as the Source for Patterning Information
Planarian muscle is composed of mononucleate fibers that contain long projections when isolated by cell maceration (34, 150, 151). Planarian muscle includes many layers with clear anatomical distinctions, specific gene expression profiles, and the expression of unique fate specific transcription factors. Body wall muscle encircles the planarian parenchyma and sits underneath the external epidermis (149, 152). Body-wall muscle consists of three anatomically defined, concentric layers; longitudinal fibers (running parallel to AP axis and specified by myoD), diagonal fibers (fate specific transcription factors unknown), and circular fibers (running parallel to the ML axis, specified by nkx1-1) from outer-most to inner-most (149, 153, 154) (Figure 1.3B). Planarian body wall muscle expresses components characteristic of both striated and smooth muscle in other organisms, including troponin I, troponin T, ttn (striated), and calponin (smooth) (152). Planarians also contain internal muscle subsets all specified by the fate specific transcription factor foxF-1 (49). foxF-1+ muscle is comprised of fibers that run along the DV axis (DV fibers; medially specified by gata4/5/6-2, and laterally by nk4), and muscle associated with the planarian intestine (specified by gata4/5/6-3) (49). Furthermore, two
signaling centers each comprised of a cluster of muscle cells exist at the head tip and tail tip, respectively, termed the anterior pole and posterior pole. The anterior pole expresses signals including the Wnt inhibitor notum and these cells are specified by expression of foxD (20, 113, 155, 156). The posterior pole also expresses signals including wnt1 and is specified by islet and pitx (108, 157-159). Upon visualization by electron microscopy, planarian muscle fibers look to contain Z-discs attached to thick and thin filaments typical of contractile muscle (150, 160, 161). Planarian muscle fibers can also be visualized by immunostaining (6G10, PHRED-1, and V5277 antibodies) (60, 149, 162), however, this labeling does not extend to the cell nucleus making it difficult to correlate a given fiber with the perinuclear RNA expression of secreted proteins.
Just as a pluripotent cellular source must exist in every animal fragment that regenerates, patterning information must be disseminated to every new fragment. In principle, this would require the mechanisms of patterning information to be encoded in any given fragment that can regenerate from many wound geometries. It was noted that many components, including ligands and secreted inhibitors, belonging to the
developmental signaling pathways implicated in planarian pattern were often co-expressed in a specific sub-epidermal layer (163). Upon inspection, it was shown that this layer of cells was planarian muscle. It has also been shown that when these developmental pathway-related genes are expressed in the wound response, they are preferentially expressed in muscle (163). The term position control gene or PCG was coined to refer to any gene that 1) was regionally expressed along a body axis, and 2) either showed a patterning RNAi phenotype or is predicted to be part of a pathway with a known planarian patterning phenotype when perturbed (i.e., Wnt, Bmp). PCGs are
heavily enriched in planarian muscle (163). Upon amputation, PCGs rescale to
accommodate the new tissue size within 48 hours after injury (163). Interestingly, some of this rescaling can dynamically occur within pre-existing muscle cells in an amputated fragment, as is well demonstrated by the expression of genes encoding certain Wnt ligands (i.e., wnt2 and wntP-2) (163). Further experimentation showed that planarian muscle secretes the majority of the planarian extracellular matrix (ECM), solidifying planarian muscle as having three distinct roles: contraction for motility, secretion of ECM for cellular support, and expression of patterning information (152).
There is abundant functional evidence for planarian muscle acting as the major source of patterning information in these animals. Upon RNAi of different lineage specific transcription factors that specify muscle, planarians fail to pattern tissues correctly in regeneration. Interestingly, some phenotypes are unique to the depletion of a given fibre type. Planarians lacking an anterior pole (inhibition of foxD) either
regenerate with cyclopia or fail to regenerate a head (155, 156). Planarians lacking a posterior pole (inhibition of islet or pitx) fail to regenerate a tail (157-159). Planarians lacking circular fibers (inhibition of nkx1-1) widen and display split blastemas resulting in laterally duplicated heads and tails (149). DV muscle is required for ML patterning, and intestinal muscle as well as medial DV muscle is required for proper intestinal
morphology (49).
Planarians lacking longitudinal fibers (inhibition of myoD) are a particularly interesting case study. During normal tissue turnover, nonamputated animals lacking longitudinal fibers elongate along their AP axis. These animals fail to rescale PCGs and fail to regenerate upon amputation. Upon further assessment, it was shown that these
animals also fail to mount a missing tissue response. follistatin, the gene previously reported to be required for the missing tissue response, is specifically expressed in longitudinal fibers when wound induced. Furthermore, activin-1 has been shown to rescue the effects of myoD RNAi early following RNAi initiation, before the animals elongate. In this study, it was also shown that certain genes characteristic of the wound response like the head promoting notum also display wound-induced expression
specific to longitudinal fibers, whereas others like the tail promoting wnt1 are expressed more broadly. As notum is only expressed at anterior-facing wounds, these results place an emphasis on longitudinal muscle biology in understanding how the polarity decision of a given cell can affect the overall patterning of an organism (149).
III. TGF-β in Patterning and Regeneration
TGF-β signaling involves multiple pathways that are prominently studied in
developmental biology. Canonical signaling occurs in a paracrine/autocrine fashion when a secreted TGF-β ligand binds to a receptor complex: a type-II receptor dimer that recruits two type-1 receptors upon ligand binding (164-168). Type-II receptors then auto-phosphorylate and phosphorylate type-I receptors, which in turn activate R-SMAD proteins by phosphorylation (169-171). This phosphorylation event then causes a conformational change in the R-SMAD, causing it to dissociate from the receptor and allow binding to the co-SMAD, SMAD-4 (171). The SMAD/co-SMAD complex then translocates to the nucleus to activate specific transcription (171) (Figure 1.4A). TGF-β ligands are composed of hetero- and homodimers requiring catalytic or basic cleavage of two initial pro-proteins (164, 172). A common defining feature of the mature, carboxy-region of these proteins is seven cysteines closely grouped to form a rigid, tertiary structure called a cysteine knot (173, 174). This region is highly conserved and often used for phylogenetic analysis. The signaling can be split into two major sub-categories divided based on ligand identity: 1) BMP, AMH, ADMP, some GDFs, and 2) TGF-β, Activins, Nodal, Myostatin (GDF-8), some other GDFs. These sub-categories signal through different R-SMADs: group 1 ligands signal using SMAD-1/5/9 and group 2 ligands using SMAD-2/3 (175). TGF-β signaling is found in species throughout the animal kingdom. The last common ancestor of the metazoa is predicted to have encoded genes for BMP2/4, 3-10, ADMP, Activin, and Myostatin (176) (Figure 1.4B). Some TGF-β superfamily ligands have been the focus of many in-depth developmental
studies, while for others relatively little is known. A brief description of some of the canonical roles different ligands play in development is outlined below.
BMP in Development
In the fly Drosophila melanogaster, decapentaplegic (dpp) is the best studied member of the TGF-β super family and is a homolog of bmp-2/4 (177, 178). dpp is dorsal-promoting in the fly embryo; loss of dpp expression leads to ventralization that is
embryonic lethal (179, 180). Other animals also possess a well characterized embryonic Bmp dorsal-ventral signaling gradient – from dorsal in protostomes (including
Drosophila and planarians) and from ventral in chordates (including chick and Xenopus) (181). The fact that the orientation of BMP expression (and its inhibitors) on the DV axis is reversed across species is hypothesized to be due to an ancient inversion of the axis during the evolution of deuterostomes (182).
During fly development, dpp is required for mesoderm segmentation across the DV axis (183, 184). Dorsal epidermal dpp promotes the expression of the Homeobox gene tinman, which is required for the formation of visceral and cardiac mesoderm (185, 186). dpp also serves an important function in delivering patterning information from a segmented organ (i.e., somites) to nearby unsegmented tissue (i.e., epithelium) to ensure overall morphological divisions across all tissue types in an organ. This process is particularly well characterized in gut development, where dpp expression is controlled by the gene ubx in the pre-segmented visceral mesoderm to pattern the underlying unsegmented intestinal epithelia by varying expression of the homeotic gene labial (181, 187, 188). Intra-organ size and patterning dependent on dpp also includes
appendages formed from imaginal discs where dpp can be expressed in a horizontal stripe (178, 189).
Nodal in Development
The Nodal ligand is conserved in deuterostomes including in chordates and
echinoderms such as sea urchins, but is only found in select protostomes such as snails (190, 191). Nodal is an example of a TGF-β ligand that has been co-opted for specific processes in deuterostomes (190). Nodal signals through Smad-2/3 (192). Nodal signaling plays key roles in mesendoderm induction and left-right asymmetry in development (193).
The history of investigation of Nodal helps elucidate some of the challenges in understanding the roles of individual TGF-β pathway components (193). Work on Nodal in Xenopus and zebrafish has also helped clarify the role of Activin ligands, in particular the lack thereof, in early embryogenesis and mesoderm induction (193). Prior to the discovery of Nodal, TGF-β ligands including Activins and Vg1 were thought to serve as inducers of mesoderm in the early embryo (194). This conception came from studies where the Activin receptors were modified to no longer be able to signal in the early embryo (195-197). However, curiously, Activin null mice had no problem gastrulating or grossly forming mesoderm (198, 199). The cloning and inhibition of nodal gave a clear explanation that Nodal in fact signalled through Activin receptors to specifically induce mesendoderm in vertebrates – and furthermore that Activin itself was not shown to have a clear role in the early embryo (193, 200-202).
Activin, Myostatin, and Follistatin in Development and Regeneration
Activin, Myostatin (also known as GDF-8), GDF-11 (also known as BMP-11), and TGF-β-1 are closely related TGF-β ligands known to signal through Smad-2/3 and to be antagonized by the inhibitory ligand Follistatin (203, 204). This group of ligands and their signaling pathway has been identified as a negative regulator of muscle growth (205). This discussion will focus on Activin and Myostatin as they are established as ancient and widespread amongst the Bilateria (176, 191). Many of these ligands are dispensable for embryonic viability, and instead appear to be utilized for specific biology found in adult tissues.
Activins were first purified from gonads as proteins that could stimulate FSH (follicle stimulating hormone) biosynthesis and release from the mammalian pituitary (206, 207). Activins have since then been identified to perform a myriad of functions in adult biology. In the fly, they have been studied as paracrine signals regulating diverse processes like tissue metabolism, size, and cell cycle (208). In mouse, activin and follistatin are both transcriptionally induced in dermal wounding (209-211). They also have a functional role in wound healing, where Activin inhibited mice display delayed wound healing (212). Follistatin inhibited mice display quickened wound healing with hypertrophic, fibrous scarring (213). In mice, Activin A is not required for development as Acitvin A null (Inhba-/-) mice survive to birth – but does have a role in later gonadal maturation, and maxofacial morphology (198). More broadly, there is no clear role for Activin signaling embryonic development (193). Activin also has the ability to negatively regulate muscle mass in adult mice (204). Despite these diverse roles, much remains to
be discovered about the overall basic principles governing the repeated use of Activin as a signaling molecule in adult tissues.
Myostatin, also known as GDF-8, was first identified as a negative regulator of muscle mass in mammals (214, 215). Myostatin mutants display both hypertrophy and hyperplasia of muscle famously coined as the “double-muscle phenotype” (214). This phenotype is at least in part a consequence of the regulation of the muscle-specific transcription factor myoD (216, 217). Myostatin is itself almost exclusively expressed in the muscle lineage in mice (214). Given human cases of myostatin-regulated muscle hypertrophy, it has long been viewed as a possible therapeutic target for muscle wasting diseases such as muscular dystrophies and spinal muscular atrophies (218). However, with little therapeutic benefit seen in landmark trials, researchers began to wonder whether underlying biology in patients could explain this discrepancy. In recent years, it has been shown that patients with muscle wasting disease, may already downregulate myostatin endogenously – while other related ligands such as Activin-A and GDF-11 (embryonic null is lethal, negative regulator of muscle mass in adults) are still expressed at high enough levels to be potential targets for further inhibition (219). In addition, recent evidence demonstrates that Activin-A regulates muscle mass more prominently than Myostatin in primates, placing the spotlight on other, previously less considered regulators of muscle mass for therapeutic benefit (220).
In Platyhelminthes, there is a clade of ligands that are phylogenetically related to both Myostatin and Activins, however it is difficult to determine whether they are most related to either ligand (i.e., they are categorized as Activin-Myostatin-like) (176). In Schmidtea mediterranea three such ligands have been identified, termed inhibin-1,
activin-1, and activin-2. A functional relationship has also been established between follistatin and activin-1/2 in this system where Follistatin is predicted to inhibit Activin (146, 148). In summary, this group of TGF-β ligands are seen to act in adult biology, wounding, and muscle biology across many phyla.
Figure 1.1
One morphogen
Morphogen gradient
‘French Flag’ Cell Fate Distribution
One short-range activator and
one long-range inhibitor
Activator/Inhibitor ‘oscillating’ gradients ‘Reaction Diffusion’ Repeated Pattern A B C
Site of the transplant Dorsal lip of the blastopore
Dorsal Ventral
Animal
Vegetal
Figure 1.1: Models of patterning involve morphogens and organizers in development
(A) Schematic of the ‘Reaction Diffusion’ model of patterning. One short-range activator (blue, left) interacts with one long-range inhibitor (red, left). The effect of these two substances on one another is depicted by positive and negative genetic arrows (Left). These interactions are predicted to result in an oscillatory distribution of these
substances across a one-dimensional axis (Middle). Repeated patterns result from the distribution of these two substances (Right).
(B) Schematic of the ‘French Flag’ model of patterning. One morphogen (blue, left) is a physical substance distributed in a gradient (middle) across a one-dimensional axis. This axis is subdivided based on a threshold level of morphogen presence (middle), to form a cell fate distribution resembling the distribution of color seen on a French Flag. (C) Cartoon depicting a historical experiment used to establish the Spemann-Mangold organizer. The dorsal lip of the blastopore (also known as the Spemann-Mangold organizer) is transplanted from one frog embryo the ventral side of another. When the host embryo develops, it forms an ectopic axis. The resulting organism is fused on the ventral side. Dorsal lip of the blastopore (pink), transplantation site (pink sphere), and axes (gray) are denoted.
wnt-low
wnt-high
intact 0 hpa 16 hpa 48 hpa 12 dpa
16 hpa
notum
wnt1
Figure 1.2
Dorsal view Ventral view
eyes pharynx mouth Anterior Posterior Dorsal-Ventral Plane Dorsal Ventral intestine nerve cord pharynx
neoblasts intestine CNS muscle
A C sFRP-1 sFRP-2 wnt11-1 fz4-3/4 wntP-2, axin2, wntless fz5/8-4 ptk7 fz-4-1/2 fz5/8-3 wnt2 wnt1, wnt11-2 ndl-1 ndk ndl-5 ndl-4 ndl-2 Wnt signaling Nodarake related ndl-3 B kal1 Dorsal-Ventral Plane Dorsal enriched Ventral enriched Medial enriched Lateral enriched slit bmp4 nlg8 bmp4 wnt5 netrin-3 wnt5 netrin-3 nog1
Figure 1.2: Planarian gene expression is spatially defined and can be regenerated after injury
(A) Cartoon schematics demonstrating planarian body axes, anatomy, and example tissue types. Dorsal view and ventral view of the planarian anterior-posterior (AP) axis demonstrating placement of eyes, pharynx, and mouth (labels)(Right). Cartoon of a midbody dorsal-ventral (DV) section schematizes internal anatomy including midline pharynx, as well as bilaterally symmetric ventral nerve cords and intestinal branches (Middle). Example classes of planarian tissues are cartooned including neoblasts (stem cells), intestine, CNS, and muscle (Right).
(B) Schematics of the location of muscle enriched position control genes (PCGs) and muscle regionalized genes (mRGs) for all three major body axes. Left shows AP axis focusing on Wnt signalling components and noudarake related genes. Right shows a subset of DV and ML specific genes on a projected onto a midbody DV section. Adapted from Scimone et al, 2016, and Reddien et al, 2018 (33, 116).
(C) Cartoons of planarian regeneration from an AP axis amputation. At 16 hours post amputation (hpa), animals express wound-induced wnt1 (green) at all wounds and wound-induced notum preferentially at anterior-facing wounds (Left). Intact planarians express a transcriptional gradient of wnts along the AP axis where the posterior is wnt high and the anterior is wnt low. In regeneration, tail fragments express wound induced genes by 16 hpa, rescale the wnt gradient and form a new anterior pole by 48 hpa, and fully regenerate by 12 days post amputation (dpa). anterior poles are cartooned at head tips (pink), and wnt1+ posterior poles are cartooned at tail tips (green). Scalpel is shown is blue.
Time Post Injury W ound-Induced Gene Expression Cell division Cell death
Missing Tissue Response Generic Wound Response
Incision Injury without missing tissue Amputation Injury with missing tissue
Longitudinal body-wall muscle fibers Fiber orientation myoD RNAi No regeneration nkx1-1 RNAi Split heads Fiber orientation
Circular body-wall muscle fibers Body-wall muscle fibers
Figure 1.3
A
Figure 1.3: The wound response and musculature are required for patterning in planarian regeneration
(A) Schematic of the planarian wound response. The amplitude of wound-induced gene expression, cell division, and cell death are graphically represented over time post injury (Left). These graphs are split into two phases corresponding to the generic wound response (purple), and the missing tissue response (light blue). Example injuries are schematized (Right) to demonstrate injuries that cause missing tissue (amputation) versus those that do not (incision). The amplitude seen on the graph during the missing tissue response phase will not occur only an injury has missing tissue.
(B) Cartoon showing the three layers of body wall muscle fibers; circular (outer), diagonal, longitudinal (inner) (Left). Cartoon isolating longitudinal body-wall muscle fibers to demonstrate their orientation running along the AP axis. When longitudinal FSTF myoD is inhibited, animals lose longitudinal fibers in tissue turnover and fail to regenerate (Middle). Cartoon isolating circular body-wall muscle fibers to demonstrate their orientation running along the ML axis. When longitudinal FSTF nkx1-1 is inhibited, animals lose circular fibers in tissue turnover and animals fail to regenerate (Right).
Figure 1.4
Metazoan Common Ancestor
Non-vertebrate Deuterostomes Chordata Spiralia Ecdysozoa Non-bilaterians ADMP, BMP2/4, BMP3, BMP 5-8, BMP 9/10, Activin, Myostatin Nodal, Maverick Lefty GDF9 Maverick ALP BMP3 Nodal GDF 1/3 Gains of a Ligand Loss of a Ligand A B P SMAD 2/3 SMAD 4 P P SMAD 2/3 P SMAD 4 SMAD 2/3 target gene P P target gene Bmp4 Activin P SMAD 1/5/8 SMAD 4 SMAD 1/5/8 P SMAD 4 SMAD 1/5/8 Activin Myostatin Nodal Vg-1 TGFβ ALP BMP 2/4 BMP 5-8 ADMP AMH GDF6 Follistatin Noggin Chordin Receptor complex Type 1 (ALK 4/5/7/Baboon)
Type 2 (ActR2/Punt)
Receptor complex Type 1 (ALK1/2/3/6/Tkv/Sax)
Figure 1.4: The TGFβ superfamily defines a signaling pathway that is widespread throughout evolution
(A) Schematic of TGFβ signaling pathway. TGFβ signaling ligands bind to a Type II receptor homodimer in the absence of extracellular inhibitors. A receptor complex forms from two Type I and two Type II TGFβ receptor. Type II receptors then phosphorylate Type I receptors, which in turn phosphorylate R-SMADS. R-SMADS associate with SMAD 4, allowing for translocation to the nucleus and activation of the transcription of target genes. The TGFβ signaling pathway is divided here between ligands that signal using different R-SMADS; SMAD 2/3 (Left), and SMAD 1/5/8 (Right).
(B) Gains and losses of TGFβ superfamily ligands mapped onto a phylogenetic tree of the animal kingdom (Metazoa). Ligands gained at a branch point are shown in green, and ligands lost at a branch point are shown in red. Relevant superphyla are labeled at the node, and a representative species is cartooned above the node. Data supporting this tree is adapted from Kenny et al, 2014 (175).
References 1. E. Heller, E. Fuchs, Tissue patterning and cellular mechanics. J Cell Biol 211, 219-231 (2015). 2. J. A. Thomson, On Growth and Form. Nature 100, 21-22 (1917). 3. J. B. S. Haldane, Organisers and Genes. Nature 146, 413-413 (1940). 4. A. M. Turing, The chemical basis of morphogenesis. 1953. Bull Math Biol 52, 153-197; discussion 119-152 (1990). 5. B. N. Nagorcka, J. R. Mooney, The role of a reaction--diffusion system in the formation of hair fibres. J Theor Biol 98, 575-607 (1982). 6. B. N. Nagorcka, J. R. Mooney, The role of a reaction-diffusion system in the initiation of primary hair follicles. J Theor Biol 114, 243-272 (1985). 7. S. Sick, S. Reinker, J. Timmer, T. Schlake, WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314, 1447-1450 (2006). 8. D. Iber, D. Menshykau, The control of branching morphogenesis. Open Biol 3, 130088 (2013). 9. L. Wolpert, Positional information and the spatial pattern of cellular differentiation. Journal of theoretical biology 25, 1-47 (1969). 10. L. Wolpert, Positional information revisited. Development 107 Suppl, 3-12 (1989). 11. W. Driever, C. Nusslein-Volhard, The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54, 95-104 (1988). 12. C. Anderson, C. D. Stern, in Current Topics in Developmental Biology, P. M. Wassarman, Ed. (Academic Press, 2016), vol. 117, pp. 435-454.
13. E. M. De Robertis, Spemann's organizer and the self-regulation of embryonic fields. Mech Dev 126, 925-941 (2009). 14. R. G. Harrison, Experimentelle Untersuchungen Über die Entwicklung der Sinnesorgane der Seitenlinie bei den Ampkibien. Archiv für mikroskopische Anatomie 63, 35-149 (1903). 15. C. A. Browne, Adulteration and the Condition of Analytical Chemistry among the Ancients. Science 29, 455-458 (1909). 16. J. B. Gurdon, Embryonic induction--molecular prospects. Development 99, 285-306 (1987). 17. H. Spemann, H. Mangold, über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Archiv für mikroskopische Anatomie und Entwicklungsmechanik 100, 599-638 (1924). 18. H. Spemann, Embryonic development and induction. (Yale University Press, New Haven, 1938). 19. K. W. Cho, B. Blumberg, H. Steinbeisser, E. M. De Robertis, Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67, 1111-1120 (1991). 20. I. M. Oderberg, D. J. Li, M. L. Scimone, M. A. Gavino, P. W. Reddien, Landmarks in Existing Tissue at Wounds Are Utilized to Generate Pattern in Regenerating Tissue. Curr Biol 27, 733-742 (2017). 21. P. W. Reddien, A. Sánchez Alvarado, Fundamentals of planarian regeneration. Ann. Rev. Cell Dev. Bio. 20, 725-757 (2004).
22. P. S. Pallas, Miscellanea zoologica, quibus novae imprimis atque obscurae animalium species describuntur et observationibus iconibusque illustrantur... (Hagae Comitum, apud Pterum van Cleef, 1766). 23. J. G. Dalyell, Observations on Some Interesting Phenomena in Animal Physiology, Exhibited by Several Species of Planariae. Illustrated by Coloured Figures of Living Animals. (Edinburgh, 1814). 24. T. H. Morgan, Regeneration in Planaria maculata. Science 7, 196-197 (1898). 25. H. Randolph, Observations and experiments on regeneration in planarians. Arch. Entw. Mech. Org. 5, 352-372 (1897). 26. T. H. Morgan, Experimental studies of the regeneration of Planaria maculata. Arch. Entw. Mech. Org. 7, 364-397 (1898). 27. M. Vila-Farre, C. R. J, The Ecology of Freshwater Planarians. Methods Mol Biol 1774, 173-205 (2018). 28. A. Sánchez Alvarado, P. A. Newmark, The use of planarians to dissect the molecular basis of metazoan regeneration. Wound Rep. and Regen. 6, 413-420 (1998). 29. A. Sánchez Alvarado, P. A. Newmark, Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. 96, 5049-5054 (1999). 30. P. A. Newmark, A. Sánchez Alvarado, Not your father's planarian: a classic model enters the era of functional genomics. Nature Reviews Genetics 3, 210-219 (2002). 31. P. W. Reddien, The Cellular and Molecular Basis for Planarian Regeneration. Cell 175, 327-345 (2018).
32. L. H. Hyman, The Invertebrates: Platyhelminthes and Rhynchocoela The acoelomate bilateria. (McGraw-Hill Book Company Inc., New York, 1951), vol. II. 33. K. J. Pedersen, Cytological studies on the planarian neoblast. Z. Zellf. 50, 799-817 (1959). 34. J. Baguñà, R. Romero, Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia 84, 181-194 (1981). 35. T. H. Morgan, Regeneration. (The Macmillan Company, New York, 1901), pp. 316. 36. E. Saló, J. Baguñà, Regeneration and pattern formation in planarians. I. The pattern of mitosis in anterior and posterior regeneration in Dugesia (G) tigrina, and a new proposal for blastema formation. J. Embryol. Exp. Morphol. 83, 63-80 (1984). 37. B. Galliot, Signaling molecules in regenerating hydra. BioEssays 19, 37-46 (1997). 38. H. Ito, Y. Saito, K. Watanabe, H. Orii, Epimorphic regeneration of the distal part of the planarian pharynx. Dev. Genes Evol. 211, 2-9 (2001). 39. K. Agata, T. Tanaka, C. Kobayashi, K. Kato, Y. Saitoh, Intercalary regeneration in planarians. Dev Dyn 226, 308-316 (2003). 40. A. Thommen et al., Body size-dependent energy storage causes Kleiber's law scaling of the metabolic rate in planarians. Elife 8, (2019). 41. E. L. Davies et al., Embryonic origin of adult stem cells required for tissue homeostasis and regeneration. Elife 6, (2017). 42. J. M. Martin-Duran, E. Amaya, R. Romero, Germ layer specification and axial patterning in the embryonic development of the freshwater planarian Schmidtea polychroa. Dev Biol 340, 145-158 (2010).