HAL Id: hal-02842704
https://hal.inrae.fr/hal-02842704
Submitted on 7 Jun 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Effets de fortes températures d’élevage sur le sexe ratio
de descendances issues de néomâles de Oreochromis
niloticus
Jean-François Baroiller, Alexis Fostier, Chantal Cauty, Xavier Rognon,
Bernard Jalabert
To cite this version:
Jean-François Baroiller, Alexis Fostier, Chantal Cauty, Xavier Rognon, Bernard Jalabert. Effets de
fortes températures d’élevage sur le sexe ratio de descendances issues de néomâles de Oreochromis
niloticus. The Third International Symposium on Tilapia in Aquaculture, 41, ICLARM, 574 p., 1996,
ICLARM Conference Proceedings, 971-8709-42-8. �hal-02842704�
246
Effects of High Rearlng Temperatures on the Sex Ratio
of Progeny from Sex Reversed Males
of
Oreochromls nllotlcus
J.F. BAROILLER•
Programme aquaculture et péche du Centre de coopération Internationale
en recherche agronomique pour le développement, Département
d'élevage et de médecine vétérinaire (C/RAD-EMVT)
BP 5095, 34033 Montpellier, Cedex /, France
Département piscicole de l'Institut des Savanes (IDESSA)
BP 621, Bouake 01, Côte d'ivoire
A. FOSTIER
C. CAUTY
Laboratoire de physiologie des poissons de /'Institut national
de la recherche agronomique (INRA)
Campus de Beaulieu, 35042 Rennes Cedex, France
X. ROGNON
(C/RAD-EMVT/IDESSA)
BP 621, Bouake 01. Côte d'ivoire
B. JALABERT
Laboratoire de physiologie des poissons de l'Institut national
de la recherche agronomique (INRA)
Campus de Beau/leu, 35042 Rennes Cedex, France
BAROILLER, J.F .. A. FOSTIER. C. CAUTY. X. ROGNON and B. JALABERT. 1996. Effects of high rearing temperatures on the sex ratio of progeny from sex reversed males of Oreochromis nllotlcus,
p. 246~256. ln R.S.V. Pullln, J. Lazard. M. Legendre, J.B. Amon Kothias and D. Pauly (eds.) The Third
International Symposium on Tilapia ln Aquaculture. ICLARM Conf. Proc. 41, 5 75 p.
Abstract
ln Oreochromis niloticus, hormonal sex reversai of an entlre progeny from crosses between a sex reversed XX male (produced by steroid treatment) and a normal XX female, resulted ln a progeny of sex reversed XX male siblings. The sex ratio of the progenies produced by single pair matings of 22 of these sex reversed males is only rarely all-female, as opposed to predictions of a monofactorial sex determination model. ln order to assess a potential thermosensitive differentiation, progenies from these sex reversed males were submitted to high temperatures for 21 days. Nlne to 13 day-old fry post-fertilization (PF) from 1 7 progenies were reared at high temperatures ranglng from 30 to 36°C and . to an average control temperature of 28°C. respectlvely. No slgnlflcant difference in survlval was observed between the two groups of Fry with 76.2 and 74.2% survival rates at 28 and 30-36°C, respectively. Ail survlvlng fry (an average of about 1 OO per batch) were sexed by histological examination of tissue squashes at 60-90 dPF. High temperatures shifted the contrai sex ratios from 0 to 91 %. As the low mortalities cannot account for these deviations, thermosensitivity is demonstrated in O. ni/of/eus.
•Current address: CIRAD-EMVT, Laboratoire de physiologie des poissons. INRA, Campus de Beaulieu, 35042 Rennes Cedex, France.
Introduction
ln controlled envlronments, the early and prollfic breeding of tilapias (Baroiller and Jalabert 1989) results in rapid over-population with a tendency to stunting. The artiflcial control of tilapia reproduc-tion is therefore essential to make its culture profitable. Since individual males show better growth rates than females (Pruglnln 1967: Hickling 1968; Hanson et al. 1983), three approaches have been suggested to produce all-male populations: ( 1) manual sexing by examination of the urogenital papilla (Hickling 1963; Guerrero 1975): (2) hybridization (reviews of Lovshin 1982; Majumdar and McAndrew 1983: Wohlfarth and Hulata 1983); and (3) hormonal sex reversai (reviews of Guerrero 1982; Hunter and Donaldson 1983; Pandian and Varadaraj 1987). Manual sexing by examination of the urogenital papilla (2.7-10% errors) results in the elimination of half of the population after two to three months (Baroiller and )alabert 1989). Al-though today, hormonal sex reversai is the most widely used of the three techniques because of its efficiency and reliability, it is often questioned. For example, the effects of the degradation products of synthetic steroids have not been studied sufficiently, especially for their ecological consequences. Hybridization using two parental species of culture interest like
Oreochromis niloticus
and
O. aureus
does not systematicallyproduce 100% males (Majumdar and McAndrew 1983; Wohlfarth and Wedekind 1991).
A fourth, intraspecific approach was suggested by Yamamoto (1969) and has been used for the past ten years. This technique consists in producing homogametic male and female broodfish which in turn produce all-male progenies. ln species like
O. aureus,
where the male is homogametlc (ZZ), feminization followed by an analysis of the sex ratio of theprogenies from the females produced by single pair matings are sufficient steps to produce such individuals (Hammerman and Avtallon 1979; Jensen and Shelton 1979). ln species like O.
niloticus,
where the male is heterogametic, an additional step is requlred to produce a new, viable and fertile YY genotype (Baroiller 1988b; Baroiller and jalabert 1989; Scott et al. 1989; Varadaraj 1989).However, in most of these intra- and interspecific studies, some sex ratios are incompatible with a simple monofactorial sex determination, XX/XY or ZZ/ZW depending on the tilapia species (reviews of Mairet al. 1991a, 1991b; Wohlfarth and Wedekind 1991 ). To explain these unexpected sex ratios, two other models were suggested: one suggests combining two alleles from an autosomal locus and two of the three sex chromosomes (Avtalion and Hammerman 1978; Hammerman and Avtalion 1979); the other suggests a polygenic model (Majumdar and McAndrew 1983; Mairet al. 1987). However, these two theories cannot ex-plain all the results found in the literature (Avtalion and Don 1990: Wohlfarth and Wedekind 1991).
However, in several species of inver-tebrates (Bacci 1965: Charnov and Bull
t 977) and lower vertebrates (Conover 1984: Adkins-Regan 1987: Dournon et al. 1990), environmental factors can determine the phenotypic sex, independently of the genotypic sex determined durlng fertillzation. Temperature is the major factor in the environmental sex determination of a majority of lower vertebrates (Bull 1983). ln fish, the use of various hormone treatments inducing functional inversions artificially has shown a high level of plasticity in gonadal differentiation (Yamamoto 1969; Hunter and Donaldson 1983). Moreover, environmental factors (social and thermal) are known to influ-ence sex determination in hermaphrodites (Harrington 196 7, 1968, 1971; Reinboth
248
1975; Bruslé and Bruslé 1983; Chan and Yeung 1983). lt is only in the last ten years that thermodependent sex determination has been identified in a gonochoristic fish (Conover and Kynard 1981; Conover 1984: Conover and fleisher 1986; Conover and Heins 1987 a, 1987b). ln species where heterogamety cannot simply account for the varied sex ratios observed, environ-mental factors could be involved (Conover and fleisher 1986: Chourrout 1988). Sibling sex reversed males of O.
niloticuswith
a majority of progenies showing unexpected sex ratios, based on a classic monofactorial mode!, were recently produced (Baroiller, this vol.). Studies on the effect of high rearing temperatures on the sex ratio were consequently conducted on this type of progeny.Materlals and Methods
Animais
Seven sex reversed males produced by hormone treatment using a family of fry of O.
niloticus
of the "Bouake strain" (Baroiller et al., in press) were identified by their all-female individual progeny. One of these sex reversed males was bred again and a sex reversai treatment was applied to its entire progeny (Baroiller, this vol.). Nine of the sex reversed males thus produced were selected for breeding and placed individually with normal females at a sex ratio of 4: 1 in 400-1 aquaria. Most of the sex reversed males originating from the same stock produced theoretically unexpected males (Baroiller, this vol.). One of these males (male 03) naturally originating from a sex reversed male was used as a broodfish in the present experiment. The water temperature was kept at 2 7°C using a thermoregulatlng device. Each animal was identified by a tag inserted in its dorsal musculature. Reproduction was detected by the onsetof maternai mouthbrooding behavior (eggs then fry) which is characterized by the dilation of the female's mouth. As soon as this particular characteristic was observed, ail other individuals were removed leaving the female to incubate in the aquarium. Five days after hatching, I.e., nine days post-fertilization (dPF), fry were removed from the mother's mouth. Each progeny, identified by its fertilization date and the tags on the parents, was di-vided into two to five equal batches of at least 100 individuals. Each batch was reared separately in 200-1 aquaria. The water in the aquaria was filtered, aerated and thermoregulated. Batches for exposure to high temperatures were placed in small 0.5-1 plastic containers containing an air stone. These containers were left afloat in the aquaria for a few hours for temperature adjustment before releasing the fry which remained 21 days in the thermoregulated aquarium. This period corresponds to the optimum treatment duration to produce ail-male populations by hormonal sex reversai with a natural androgen, 11 B-hydroxyandrostenedione ( 11 B-OH~4) (Baroiller and Toguyeni, this vol.). Fry were fed ad libitum six times a day and seven days a week using first-feeding salmonid feed (Aqualim) distrib-uted by an automatic feeder during the
12-hour photoperiod. Seventeen sex reversed male progenies were used in the present study. After treatment, batches of one month-old fry were stocked indi-vidually in externat 1 .5-m3
tanks where they were fed ad libitum six times a day and six days a week until sexing at 60-90 dPF.
Jdentlflcatlon oF the Phenotyplc Sex At a minimal age of two to three months, when the histological characteristics of the male and female differentiation are already established (Baroiller 1988a, 1988b), ail fry from each batch were
dissected and their gonads examined by simple squash, using a t 25X microscope. The presence of previtellogenic or vitellogenic oocytes and the lobular configuration revealed the ovary and the testis, respectively, and consequently the phenotypic sex.
Results
The mean survival rate (5) at the time of sexing did not differ significantly between the contrai batches reared at temperatures of 26-29°C (S = 76.2% for 1,879 sexed fry) and the batches subjected to hlgher temperatures ranging from 30 to 36°C (S = 74.2% for 2,880 fry). These survivais produced batches of an average of 1
OO
individuals at the time of sexing. For three families offry MXX9 x FXX10, MXX9 x FXX 11 and MXX7 x FXX7, two contrai batches were reared at a mean temperature of 28°C (Tables 1 and 2). No significant difference was observed in the sex ratio among batches of the same family. Seventeen families of fry, each divided into several batches, were reared at two temperatures: 20 batches served as contrais (temperatures of 26-29°C); and 32 batches were exposed to higher rearing tempera-tures ranging from 30 to 36°C (Table 3). Compared to con trois, 16 of these familles showed different sex ratios in at least one of the experimental batches (Tables 1 and 2, Fig. 1). These deviations in sex ratios cannot be attributed to differential mortalities related to high temperatures. The low mortality rate observed in most batches does not sufficiently explain these large deviations. ln the XX17 x XXl 1 cross especially, the seven and six deaths observed in the contrai batch and in the batch exposed to 36°C, respectively, cannot explain the difference of 91% of males between the two populations of 99 and 1OO
fry previously identified. The sex ratios of progenies from sex reversedmales of
O.
niloticuswere
therefore directly influenced by the high rearing tempera-tures.No slgniflcant difference in sex ratios was observed between the batches exposed to 31 °C and the contrai batch (Table 3). Among the sex ratios of six batches exposed to 32-33°C, l'ive ofthem were significantly biased towards the males compared to the percentages observed in the contrai batches exposed to 2 7°C (Table 3). Between 34 and 36°C, 20 batches out of 23 showed significant changes in the percentage of males as compared to thelr respective contrai batches. For tem-peratures ;::; 32°C, proportions of males in experimental populations increased from 0 to 91 % as compared to the contrai· sex ratios.
The intensity of response was neither directly proportional to the temperature applied, nor to the percentage of males produced at the contrai temperature. Progenies differed significantly in their sensitivity to temperature, both vis-à-vis an effective minimal temperature and in the extent of deviation in sex ratios in identical culture conditions. ln experiments using the same temperature of 36°C, changing male sex ratios (14.1 to 91 %) were observed depending on the progenies (Tables 1 and 2). Strong paternal (XX9 x XX16/XX18 x XX.16) and maternai (XX17 x XX15/XX17 x XX11) influences were observed on the thermosensltivity of the progenies (Table 3). However, the proportion of males increased with the temperature in nearly ail experimental batches.
Out of ail progenies used, only one family showed no deviation in sex ratio regardless of the temperature used. ln contrast to ail other experiments, temperature treatments were applied to fry from the XX4 x XXS cross only after 15 dPF instead of 9-13 dPF. This result probably reflects a critical period of thermosensltivity. Beyond certain stages of differentiation,
250
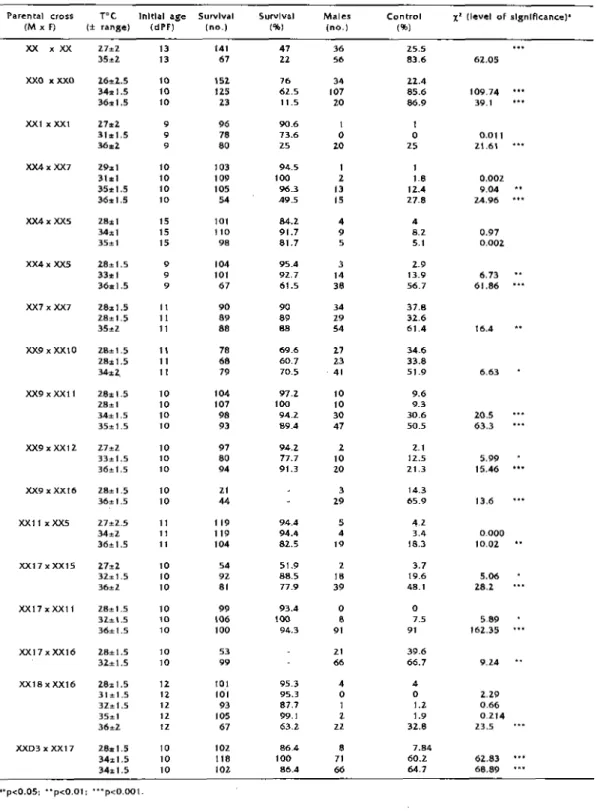
Table 1_ Characterlstlcs of Oreochromls nJJotlcus fry reare:d at dlfferent te-mperatures: for detalls of parental crosses, see text.
Pare-rital cross r·c lnl!lal age Survlval Survlval Male-s Control x' (level of slgnlflcance)'
(MX F) (± range) (dPF) (no_) (%) (no.) (%)
XX X XX Z7±2 13 141 47 36 25.5 35±2 13 67 22 56 83.6 62.05 XXO xXXO 26;,2.5 10 152 76 34 22.4 34: l.5 10 125 62.5 107 85.6 109.74 36:1.5 10 23 11.5 20 86.9 39.1 XX1 xXXl 27:2 9 96 90.6 1 l 31:1.5 9 78 73.6 0 0 0.011 36±2 9 80 25 2.0 25 21.61 XX4xXX7 Z9=l 10 103 94.5 l 1 31±1 10 109 100 2 1.8 0.002 35., l.5 10 105 96.3 13 12.4 9.04 36: l.5 10 54 49_5 15 27.8 24.96 XX4xXX5 28±1 15 101 84.2 4 4 34±1 15 110 91.7 9 8.2 0.97 35±1 15 98 81.7 5 5.1 0.002 XX4x XX5 28±1.5 9 104 95_4 3 2.9 33.,1 9 101 92.7 14 13_9 6.73 36±1.5 9 67 61.5 38 56.7 61.86 XX7 X XX7 Z8=l.5 Il 90 90 34 37.8 28±1.5 ll 89 89 29 32.6 35"'2 11 88 88 54 61.4 16.4 XX9xXXl0 28±1.5 11 78 69.6 27 34.6 28±1.5 ll 68 60.7 23 33.8 34±2, ll 79 70.5 '41 51.9 6.63 XX9xXX11 :Z8±1.5 10 104 97.2 10 9.6 28±1 10 107 100 10 9_3 34± 1.5 10 98 94.2 30 30.6 20.5 35±1.5 10 93 89.4 47 50.5 63.3 XX9xXX12 27±2 10 97 94.2 2 Z-1 33± 1.5 10 80 77.7 10 12.5 5.99 36±1.5 10 94 91.3 zo 21.3 15.46 XX9 X XX16 28±1.5 10 21 3 14.3 36±1.5 10 44 29 65.9 13.6 XX1lxXXS 27±2-5 11 119 94_4 5 4.2 34±2 11 119 94.4 4 3.4 0.000 36± l.5 11 104 82.S 19 18.3 10.02 XX17xXX15 27±2 10 54 51.9 2 3.7 32= 1.5 10 92 88.5 IB 19.6 5.06 36±2 10 81 77_9 39 48.1 28.2 XXl7xXXl1 28±1.5 10 99 93.4 0 0 3L±L5 10 106 100 8 7.5 5.89 36±1.5 10 100 94.3 91 91 162.35 XXl7xXXl6 28±1.5 10 53 21 39.6 32±1.S 10 99 66 66.7 9_24 XX18xXX16 28± l.5 12 101 95_3 4 4 31 ±1.5 1Z 101 95.3 0 0 2.29 3Z±1.5 12 93 87.7 1 1.2 0.66 35±1 12 105 99.1 l 1.9 0.214 36±2 lZ 67 63.2 zz 32.8 23.5 XXD3 x XXl 7 :Z8±l.5 10 102 86.4 8 7.84 34±1.5 10 118 100 71 60.2 62.83 34± l.5 10 102 86.4 66 64.7 68.89 '"p<0.05: .. p<0.01: ... p<0.001.
IOO M 36 M
T
T
eo
•
'"
•
î
"S ll4E
60 36î
0
298
.e
28 c 40•
~ 'Ol.
T
~ 2034T
XXI XK4 XXII XXl7 XX9 XX7 XXII xxo
Sel< reverse<! males
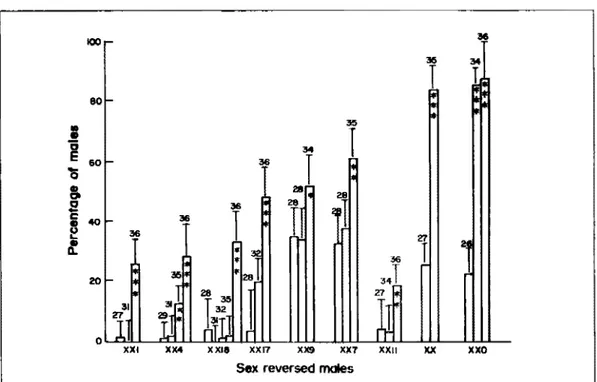
Fig. 1. Effects of temperature {Z7-36°C) on the percentages of males in the progeny of Oreodlromis
nllotlcus from various parental crosses: for details on parental crosses, see Table 1 and text; scale bars represent standard errors and asterisks represent levels of slgnlflcant dlfferences of the hlgh temperature from lower temperatures ln that group (•p<0.05: .. p<0.01; ... p<0.001).
Table Z. Sex ratio responses in batches of
Oreochromis niloticus fry reared at different temperatures.
Temperatures No. batches No. significant
{oC) tested responses
31 3 0 3Z 4 3 33
z
z
34 7 6 35 6 4 36 10 10the gonadal sex may no longer be influenced by external factors such as temperature.
The progeny from the XX
t
7 x XX 11 cross, with a sex ratio corresponding to the ex-pected sex ratio following a simple genetic model in a se.x reversed male ofO. niloticus,
Table 3. Parental effect on the sex ratios of
Oreochromls n/lotlcus fry reared at different temperatures: for details on parental crosses, see text.
%Males
Parental Control at 36°C Dlfference•
cross Mx f {%) XX4xXX5 2.9 56.7 53.8 XX4xXX7 1 27.8 26.8** XX9 xXX12 2.1 21.3 19.2 XX9xXX16 14.3 65.9 51.6 ... XX17xXX15 3.7 48.1 44.4 XX17xXX11 0 91 91* .. XX9 xXX16 14.3 65.9 51.6 XX18xXX16 4 32.8 28.s·· ... p<0.01; ... p<0.001.
also
showed
a thetmosensitive differentiation. Similarly, the progeny of an unexpected male (male 03) from a cross between a sex reversed male and a normal female also showed a thermosensitive differentiation.zsz
Discussion
Very few studies have analyzed the environmental factors possibly involved in the sex determination of fish. Most studies have focused exdusively on her-maphrodite species. ln these species, the influence of environmental fac.tors on natural sex reversai has been demonstrated (reviews of Reinboth 1975; Chan and Yeung 1983). ln
RJvulus marmorâtusespecially,
low temperatures produced primary males (Harrington 196 7, 1968) and variations in the photoperiod resulted in the pro-duction of secondary males (Harrington
1971). ln gonochoristic species, only the studies on
Menidia menidia.
(Conover and Kynard 1981 : Conover 1984; Conover and Fleisher 1986; Conover and Heins 1987 a, 1987b) clearly demonstrated the effect of externat factors. Based on samples taken from the wild. a species of the same genus.M.
peninsulae,
may also . showthermosensitivity (Middaugh and Hem-mer 1987). Studies on viviparous species such as
Poeciliopsis /udda
suggest an en-vi ronmental influence on sex determina-tion. However, the deviations observed could be due to the effects of temperature (Sullivan and Schultz 1986), or of pH (Rubin 1985) on the physiology of the maternai parent, and might not reflect a direct effect on the embryo .. ln the rainbow trout( Oncorhynchus mykiss),
heat shocks ofzs
or Z9°C or longer exposures to 23°C do not significantly modify the sex ratio (Van den Hurk and Lambert 1982). ln tilapias, potential thermosensitivity has been studied in three species. lnO. aureus,
O.nlloticus
and their hybrids, low survivais (:::;; 26%) and the smalt average quantity of fry (23-28) produced in batches ex-posed to extreme temperatures ( 19.5 and 3Z0C) prevented differential mortality frombeing exduded as causal factor (Mair et al. 1990). In the same study, the sex ra-tios of two batches of
O. mossa.mblcus
exposed to 19-Z0°C differed signifîcantly
from the sex ratios of their contrais, with a bias towards the male sex. However, the survivais (10.6 and 35.3%) and the number of fry ( 18 and 41, respectively) from batches exposed to low tempera-ture did not lead to obvious conclusions.
ln view of the re\atively high survivais and numbers of fry used in the present study, the hypothesis of a differential mortality cannot explain the deviations observed in the sex ratio of progenies from sex reversed males of O.
niloticus.
Temperature may therefore have an effect on sex determination in O.
niloticus.
the proportions of males within a single family are, independent of the rearing temperature (26~36"C), under high paternal and maternai influences. At 34"C and above, a clear environmental effect on the sex ratio shows thermosensitive differentia-tion in progenies from sex reversed males of O.niloticus.
lnM. menidia,
the effect of temperature on the sex ratio is also under strong paternal influence: some prog-enies do not seem to be affected by the rearing temperatures; significant male and female percentages are observed at ex-treme temperatures (Conover and Heins1987b). Sex determination in
M. menidia.
is considered to be an intermediate step between an entirely genetic determina~ tian and an excfusively environmental de-termination (Conover and Heins 1987b). ln reptiles showing environmental sex de~ termination, an insignificant genetic base is found only in narrow ranges (sometimes only
z
°C) around the temperature thresholds and only one sex is produced at extreme temperatures (Bull et al. 1982); sex determination in such situations thus depends entirely on the environment.ln O.
niloticus,
the environmentalintluence on sex determination can operate during a specific critical period of thermosensitivity: between 9 and 13 dPF. Beyond this range, sex dlfferentiation is probably irreversible, at least vis-à-vis ex-ternat factors, and would then correspond
to the genotype. A Z 1-day thermal treat-ment yields 91
%
maximum devlations if initiated before the 1 Sth dPF. Additional experiments are, however, necessary to determine accurately the duration of the critical period. Histologically, two basic events in oogenesis occur between 20 and 35 dPF at 27°C: oogonia proliferate be-tween 20 and 28, dPF followed by the first prophase between ZS to 35 dPF, or 756 to 945 degrees x days (Baroiller 1988a and b). During the same period, the highly progressive multiplication of the somatic and spermatogonla occurs in the testis (Baroiller 1988a and b). The chronology of these events depends on the rearing temperature and seems to be more accurately determined by a number of degrees x days than by an absolute age (this study). During a Z 1-day treatment beginning 10 dPF, high temperatures are applied before and at the onset of these events. ln M.menidia,
sensîtivity totem-per ature also occurs du ring a predse crltlcal period at the end of which gonadal sex differentiation takes place. The time of occurrence and the duration of this perlod depend upon the temperature (Conover and Kynard 1981 ; Conover and Heinst
987b). The same occurs ln turtles (Yntema 1979; Pi eau and Dorizzi 1981) and in alligators (Ferguson and Joanen 1982.).The c:hronological characteristics of this period as determined in the present study correspond, as in reptiles (Gutzke and Chymiy 1988) to the period of sensitiv-ity to hormonal treatment in the same spe-ci es (Barolller and Toguyeni, this vol.). ln
O. nllotlcus,
hormonal sex reversai treat-ments using an androgen identifled in vitro and testis-speciftc at the early stages of its ontogenesis (Baroiller 1988a and b) can be used to determine a period of hormonal sensltlvlty: to be efficient, the treatment with 1 t B-OH.::i4 should begin before t 5 dPF (Baroiller and Toguyeni, this vol.) and should last 21 days. However,in thermosensitive turtle species such as
Emys orbicularis,
production of somester-oids (estrogens), normally regulated via the genotype, would become thermodependent beyond certain critical temperature thresholds (Oorizzi et al. 1991). A thermosensitive factor may be involved in the regulation of the synthesis of enzymes that are specific to the estrogen production (Pieau et al. 1987). The level of these steroids would then determine the phenotypk sex. Such pattern may exist in most species with thermodependent sex determination (Zarborskl et al. 1988). Recent studies of
O. nlloticus
show that this type of environmental sex determination is found in progenles from normal males and females (Baroiller et al .. unpublished data).ln O.
niloticus,
sensitivity to tempera-ture similar to that observed inM.
men/dia
is described in the present study. The 34°C thermal threshold for thermosensitivity can be experienced by tilaplas in the wild as well as in culture conditions (Denzer 1968: Philippart and Ruwet t 982). This characteristic in sex determlnation could explain part of the unexpected results described in the literature. Environmen-tal sex determination could be far more common than initially believed (Conover 1984; Zaborski et al.
t
988). ln the platyflsh(Xlphophorus maculatus)
in which geneticdetermination is well-establlshed, unex-pected sex ratios have also been observed, induding some within a population. Long and complex studies have shown autosomal interactions in species possessing several sex-specifîc markers (Kallman 1984). ln view ofthese experi-mental difficulties, these analyses calinot be reasonably conducted on ail species presenting unexplained sex ratios. The present study suggests that a potential thermosensitivity in the species studied should first be investigated before under-takîng further studies.
254
Acknowledgements
We would like to thank D. Chourrout for reviewing the manuscript and for hls valuable suggestions. This study was conducted under the Groupement de coopération scientifique sur les bases biologiques de l'aquaculture (GCS/ BBA) by the "Aquaculture tropicale" study group.
References
Adkins-Regan, E. 1987. Hormones and sexual
dlfferentiation, p. 1-29. ln D.O. Norris and
R.E. Jones (eds.) Hormones and reproduc-tion in fishes, amphibians and reptiles. Ple-num Press. New York and London.
Avtallon, R.R. and l.S. Hammerman. 1978. Sex
determination in Sarotherodon (tilapia).
1. Introduction to a theory of autosomal
influence. Bamidgeh 30:110-115.
Avtalion, R.R. and
J.
Don. 1990. Sexdetermin-ing genes in tilapia: a model of genetic recombination emerging from sex ratio results of three generatlons of diploid gynogenetic
Oreochromis aureus. J. Fish Biol. 37:167-173.
Bacci, G. t 965. Sex determination. Pergamon Press,
Oxford, New York. 306 p.
Baroiller, J.F. 1988a. Etude corrélêe de l'apparition
des critères morphologiques de la
différenciation de la gonade et de ses
potentialités stéro1dogènes chez. Oreodiromls
niloticus. Université Pierre et Marie Curie, Paris VI. 89 p. Ph.D. dissertation.
Baroiller. J.F. 1988b. Etude des processus
morphologiques et endocrinologiques de la différenciation naturelle du sexe chez.
Oreochromis nf/otlcus. appliquée à la pro-duction de populations monosexes : inti'!rêts
et perspectives, p. 256-266. ln G.R.
Bernacsek and H. Powles (eds.) Aqua-culture systems research ln Afrlca. Proceed-ings of a Workshop ln Bouake. Côté d'ivoire, 14-17 November 1988. International
De-velopment Research Centre, Ottawa,
Canada.
Baroiller. J.F. and B. Jalabert. 1989. Contribu-tion of research in reproductive physiol-ogy to the culture of tilapias. Aquat. Liv-ing Resour. 2:105-116.
Barolller, J.F .. X. Rognon, V.C. Yapi and A. Cissé. Genetie characteriz.ation and sex
deter-mination in tilapia ln Côte d'ivoire. ln A.E.
E.knath, B.A. Acosta and R.S.V. Pullln (eds.) Present progress. future directions and needs
in l'ish genetics research ln Asia, Pacifie and Afrlca. ICLARM Conf. Proc. 38. (ln press.)
Bruslé, J. and S. Bruslé. 1983. La gonadogenèse des
poissons. Reprod. Nutr. Dév. 23(3):453-491.
Bull, J.J. 1983. Evolution of sex determining
mecha-nîsms. Benjamîn/Cummlngs Publ. Co. lnc .. Menlo Park, Californîa.
Bull, j.J .. R.C. Vogt and M.G. Bulmer. 1982.
Her-itability of sex ratios in turtles wfth envi-ronmental sex determination. Evolution 36:333-341.
Chan, S.T.H. and W.S.B. Yeung. 1983. Sex con-trai and sex reversai in fish under natural
conditions. ln W.S. Haar, D.J. Randall and
LM. Donaldson (eds.) Fish physlology. Aca-demic Press, New York. Vol. 9(8): 1 71-222.
Charnov, E.L. and
J.J.
Bull. 1977. When ls sexenvlronmentally determined? Nature
2.66:828-830.
Chourrout, D. t 988. Revue sur le déterminisme
génétique du sexe des poissons téléostéens.
Bull. Soc. Zoo!. Fr. 113(2): 123-144.
Conover, D.O. 1984. Adaptative signlflcance of temperature dependent sex determl-natlon ·ln a flsh. Am. Nat. 123:297-313. Conover, D.O. and B.E. Kynard. 1981.
Environ-mental sex determînatîon: Interaction of temperature and genotype în .;i fish. Sci-ence 2.13:577-579.
Conover, D.0. and M. Fleisher. 1986. Tempera-ture sensitive period of sex determination in Men/dia menidia. Can.
J.
Fish. Aquat. Sel. 43:514-520.Conover. D.0. and S. Helns. 1987a. Adaptive
variation ln environmental and genetic sex de-termînation in a flsh. Nature 326(61 12):496-498.
Conover, D.O. and S. Heins. t 987b. The
envl-ronmental and genetic components of sex
ratio in Menidia menidia (Pisces:
Atherinidae). Copel.;i 3:732-743.
Denzer, H.W. 1968. Studies on the physlology
of young tilapia. ln Proceedings of the World
Symposium on Warm-water Pond Culture. Vol.4. FAO Flsh. Rep. 44:357-366.
Dorlul. M., M.T. Mignot, A. Guichard, G. Desvages and C. Pieau. 1991. lnvolvement of oes-trogens in sexual dlfferentiation of gonads as a function of temperature in turtles. Dif-ferentlatlon 4 7 :9-17.
Dournon, C., C. Houlllon and C. Pleau. 1990.
Temperature sex reversai in amphibians and reptiles. !nt. J. Dev. Biol. 34:81-92.. Ferguson, M.W.J. and T. Joanen. 1982.
Tempera-ture of egg Incubation determlnes sex ln
Alligator mississlplensls. Nature 296:850-853. Guerrero. R.D. 1975. Use of androgens for the
production of ail-male Tilapia aurea
(Stelndachner). Trans. Am. Fish. Soc.
Guerrero, R.D. 1982. Control of tilapla reproduction, p. 309-316. ln R.S.V. Pullin and R.H. Lowe-McConnell (eds.) The biology and culture of tilapias. ICLARM Conf. Proc. 7, 360 p. Gutzke, W.H.N. and O.B. Chymiy. 1988. Sensitive
perlods durlng embryogeny for hormonally lnduced sex determination in turtles. Gen. Comp. Endocrinol. 71 :265-267.
Hammerman, l.S. and R.R. Avtallon. 1979. Sex determination in Sarotherodon (tilapia). Part Il. The sex ratio as a tool for the de-termlnation of genotype - a model of autosomal and gonosomal influence. Theor. Appl. Genet. 55:177-187.
Hanson, T.R., R.O. Smltherman, W.L. Shelton and R.A. Dunham. 1983. Growth comparisons of monosex tilapia produced by separatlon of sexes, hybridization and sex reversai, p. 570-579. ln L. Fishelson and Z. Yaron (comps.) Proceedings of the Flrst International Sym-posium on Tilapia in Aquaculture. Tel Aviv University. Tel Avlv. Israel.
Harrington, R.W. )r. 1967. Environmentally con-trolled Induction of primary male gonochorists from eggs of the self-fertilii.-ing hermaphroditic fish, Rivulus marmoratus
Poey. Biol. Bull. 132:174-199.
Harrington, R. W. Jr. 1968. Delimitation of the thermolabile phenocritical period of sex determioation and dlfferentiation in the ontogeny of the normally hermaphroditic flsh, Rlvulu:;; marmoratus Poey. Physlol. Zool. 41:447-460.
Harrington. R. W. Jr. 1971. How ecologlcal and genetlc factors lnteract to determlne when self-fertillzlng hermaphrodites of Rlvulus marmoratus change into functlonal sec-ondary males. with a reappraisal of the modes of intersexuallty among fishes. Copeia 3:389-432.
Hickling, C.F. 1963. The cultlvatlon of tilapia. Sei. Am. 208(5):143-152.
Hickling, C.F. 1968. fish hybridization. FAO Fish. Rep. 44:1-11.
Hunter, G.A. and E.M. Oonaldson. 1983. Hor-monal sex control and its application to flsh culture. p. 223-303. ln W.S. Hoar. O.). Randall and E.M. Oonaldson (eds.) Flsh physiology. Vol. 9(8). Academic Press, New York.
Jensen, G.L. and W.L. Shelton. 1979. Effects of estrogens on Tilapia aurea: Implications for produètion of monosex genetic male tllapia. Aquaculture 16:233-242.
Kallman. K.O. 1984. A new look at sex determi-nation in poeclllld flshes, p. 95-171. ln B.). Turner (ed.) Evolutlonary genetics of fishes.
Plenum Press, New York. 636 p.
Lovshin, L.L. 1982. Tilapia hybrldlzation, p. 279-308. lnR.S.V. Pullin and R.H. Lowe-McConnell
(eds.) The blology and culture of tllaplas. ICLARM Conf.- Proc. 7, 360 p.
Malr, G.C., ).A. Beardmore and 0.0.F. Skibinski. 1990. Experimental evldence for environ-mental sex determinatlon ln Oreochromis
species, p. 555-558. ln R. Hlrano and 1. Hanyu (eds.) The Second Aslan Fisheries Forum. Asian Fisheries Society, Manlla, Philippines. 991 p.
Mair, G.C., O.). Penman. A. Scott, D.O.F. Sklbinski and J.A. Beardmore. 1987. Hormonal sex re-versai and the mechanlsms of sex determlna-tion in Oreochromls, p. 301-312. ln K. Tlews (ed.) Selection, hybridizatlon and genetic en-gineering in aquaculture. Vol. 2. Heeneman, Berlin.
Mair. G.C., A. Scott, O.). Penman, ).A. Beardmore and 0.0.F. Skibinski. 1991a. 1. Sex reversai, gynogenesls and triploidy in O. nllotlcus (L.) Theor. Appl. Genet. 82: 144- 1 52.
Malr. G.C., A. Scott, D.J. Penman, D.O.F. Skibinski and ).A. Beardmore. 1991 b. Il. Sex reversai, hybridii.ation, gynogenesls and triploidy in
O. aureus Steindachner. Theor. Appl. Genet. 82: 153-160.
Majumdar. K.O. and B.). McAndrew. 1983. Sex ratios from lnterspecific crosses wlthln the tilapias, p. 261-269. ln L. Fishelson and Z. Yaron (comps.) Proceedings of the Inter-national Symposium on Tilapia in Aqua-culture. Tel Aviv University. Tel Aviv, Israel. Middaugh. O.P. and M.). Hemmer. 1987.
Influ-ence of envlronmental temperature on sex ratios ln the tldewater silverslde, Menidia peninsu/ae (Plsces: Atherinidae). Copeia 1987(4):958-964.
Pandian. T.). and K. Varadaraj. 1987. Techniques to regulate sex ratio and breeding in tllapia. Curr. Sei. 56(8):337-343.
Philippart, ).Cl. and ).Cl. Ruwet. 1982. Ecology and distribution of tilaplas. p. 15-59. ln R.S.V. Pullin and R.H. Lowe-McConnell (eds.) The biology and culture of tilapias. ICLARM Conf. Proc. 7, 360 p.
Pleau. C. and M. Dorii.i.i. 1981. Oetermination of temperature sensitive stages for sexual differentlatlon of the gonads in embryos of the turtle Emys orblcularis L. ). Morphol.
170:373-382.
Pieau. C .. M. Dorizzi and G. Desvages. 1987. Une hypothèse sur l'implication des hormones oestrogènes dans la différenciation sexuelle des gonades chei. les amphibiens, les rep-tiles et les oiseaux. ln •Association des physiologistes", Cinquante-cinquième réunion. Bordeaux. ). Physiol. 30A: (abstract). Pruginin, ). 196 7. Report to the Govemment of Uganda
on the experimental fish culture project in Uganda, 1965-1966. UNPD/FAO Rep. NOTA 2446.
,..,
;
I"
International Center for Living Aquatic
Resources Management Centre de recherches
era
oceanologiques.
)
I
r";f~~~31
L'lnstitut fran!;ais de recherche scientifique
pour Ie developpement en cooperation
With the cooperation of
c!
Centre de cooperation internationale en recherche agronomique pour Ie developpelTlent
Cooperation francaise
'fbA
Centre technique
de cooperation agricole et rurale '
;
So-, =.
11.::1
~- - . -
-~
~-Tl;IÉ THIRD INTERNATIONAL SYMPOSIUM
ON TILAPIA IN AQUACULTURE
lnte(n;ttl<intll Cootcr fot L.i'IÎf"Q Aquatic
ResourcN Management
r.,;5;.::1
L' Mafltut frun~Î$ d• r«t'M:f'çhc $<:Ï4ntlflqu•
pour le dëveloppement en COOl)êl"aU<W\
1
EHTERED IN NÂGA ]
Edited
by
R
.
S.V. P)lll.IN
J.LAZill
1\1. LEGENDRE
J.B. AMON
KOTHJAS
D.PAULY
Tnutslarlnn.• byC.
LHOMMF.-BlNUDlN
1996
•
~trG Cie coopération JMtrnttionMç Ct'I teeh•n:he
agro1'omlque pour le df}w)foppemenl
With
the ç
oop
er
ation
of
Centre technique