Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-1263, 1984
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=3cdc768b-eb1d-4253-b924-ac529213498f https://publications-cnrc.canada.ca/fra/voir/objet/?id=3cdc768b-eb1d-4253-b924-ac529213498f
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001729
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Burning, pyrolysis, combustion and char-oxidation: need for clarifying
terminology
Ser THl
N21d
no.
1263
C. 2
National Research
Conseil national
BE4943
I
*
Council Canada
de
recherches Canada
-- - -A
BURNING, PYROLYSIS, COMBUSTION AND CHAR-OXIDATION: NEED FOR CLARl FY ING TERMINOLOGY
by T.Z. Harmathy
ANALYZED
Reprinted from Fire and Materials Vol. 8, No. 4, 1984 p. 224
-
226DBR Paper No. 1263
Division of Building Research
Price $1.00 OTTAWA N R C
-
C I S T IWDG.
R S .
L I B R A R Y
I
05-
05-
3
0
j
B I ~ L I O T H ~ Q U E
Rech. Dbtim.
-
-
t C 1 S T L NRCC 24171SHORT COMMUNICATION
Burning, Pyrolysis, Combustion and Char-
oxidation
Need for Clarifying Terminology
T.
Z.
HamathyDivision of Building Research, National Research Council Canada, Montreal Road, Bldg M-59, Ottawa, Canada KIA OR6
The vague meaning of the word 'burning' bas long created communication ditlicolties among 6re scientists, and has led to unwarranted conclusions. It is suggested that the word 'burning' be used as an umbrella term, covering up to three dissimilar types of reactions: pyrolysis or gasification, (gas-phase) combustion, and char-oxidation.
Researchers often find that language does not offer In the case of charring solids, the process popularly
words suitable for their ideas. They can do one of two referred to as combustion is, in fact, a complex process
things: coin new words or reshape the meaning of consisting of three completely dissimilar types of reac- existing words. The latter alternative is not without tions which usually develop concurrently:
danger, for the original connotation of the word will linger on and may confuse the uninitiated reader. Fire science is one of those areas plagued by the lack of agreed terminology. The problem is sometimes so serious that communication between scientists be- comes all but impossible. The word 'combustion' is particularly troublesome. In everyday usage, it is re- garded as SynGii4lilO'iS with '5'aing'. (In fact, the verbal form of the word 'combust' is still not regarded as fully legitimate; its substitution by the verb 'bum' is almost compulsory.) 'Combustion' is thought of as the consumption by fire of a material, whether solid, li- quid, or gas, and is pictured as a process accompanied by flames. Flame, however, is a region of gas-phase reactions; solids and liquids can undergo flaming com- bustion only after being converted into gases. The conversion may take place by (1) vaporization (or sublimation), or (2) pyrolyis (thermal decomposition). Liquids and solids of simple molecular structures vap- orize (or sublime); polymeric materials (usually solids) pyrolyze. (The relatively small group of polymeric liquids will be left out of consideration in this article.)
Conversion can occur only if the material is first
(1) Pyrolysis: a series of endothermic (in the sense mentioned before) reactions;
(2) (Flaming) combustion of some or all of the gase-
ous decomposition products: homogeneous (gas with gas) reactions, usually exothermic; and (3) Oxidation of char: heterogeneous (solid with gas)
reactions, strongly exothermic.
It has been implicitly suggested1 that the following terminology be adopted:
(1) Let the word 'burning' be used as an umbrella term, denoting the overall process that results in the consumption of a fuel, irrespective of the specific reactions involved.
(2) Let the word 'combustion' be used in the strict sense to denote flaming (gas-phase) combustion: the homogeneous, exothermic reactions of some or all of the gaseous decomposition products that' take place in the flame.
(3) Let the heterogeneous, exothermic reaction of the char with the oxygen in the air be referred to as 'char-oxidation' .
I heated up to an appropriate temperature level. This
The three component processes, pyrolysis, (gas-
I
preheating is usually tacitly included in the conversion phase) combustion and char-oxidation, are stronglyProcess. When taken in this loose sense, practically all interrelated. Pyrolysis is the process that produces
conversion processes can be looked upon as endother- gaseous fuel for combustion and solid fuel for char-
mic (heat-absorbing). oxidation. If it stops, the burning process will stop.
Vaporization is full conversion of a liquid into gase- Since pyrolysis (in the looser sense) is normally a
ous phase. Full version may also take place in the heat-consuming process, it can proceed only if heat is
pyrol~sis of polymers. With many kinds of polymers, continuously supplied to the solid. The heat may origi-
, however, the conversion into gases is not complete; a nate from
solid residue of high carbon content, char, is left
behind. Polymeric solids can, therefore, be divided (1) External sources,
into two broad categories: non-charring and charring (2) The flame,
solids. (With some polymeric solids minute amounts of (3) The oxidizing char.
char may form which, however, do not tend to ac- In the absence of external heat sources, pyrolysis is
cumulate. Such solids are regarded as non-charring.) contingent on the heat evolved in either the (gas-
CCC-0308-0501/84/0008-0224$01.50
NEED FOR CLAlUFYING TERMINOLOGY phase) combustion or the char-oxidation, or both.
(Gas-phase) combustion and char-oxidation can be looked upon as the driving reactions for the burning process.
'Thermal feedback' is a term often used to denote heat transfer from the flame to the solid. The transfer mechanism is radiation (predominantly) and convec- tion. The mechanism of heat transfer from the oxidiz- ing char to the virgin solid is conduction.
With non-charring polymeric solids, and simple sol- ids and liquids, the conversion of the material into gases is complete; no carbonaceous residue is left behind. The 'burning' process thus consists of two reactions: 'gasification' of the material (a term mean- ing non-charring pyrolysis, vaporization or sublima- tion), and (gas-phase) combustion. Naturally, with gaseous materials 'combustion' is 'burning'. Only with gases are the two words synonymous.
Table 1 shows conceivable component reactions for typical burning processes. With solids and liquids, gasification or pyrolysis is a necessary component of the overall burning process. With charring solids, as mentioned earlier, the driving reaction may be either (gas-phase) combustion (case A in Table 1) or char- oxidation (case B) or both (case C). Since char- oxidation is, as a rule, a stronger driving reaction than (gas-phase) combustion, case A is of a lesser practical significance. Case B, burning without (gas-phase) com- bustion, is the process referred to as 'smoldering'.
Table 1. Reactioiw covered by the term 'borning'
Polymeric solids Simple Gasse
Non- solids and Charring charring liquids Processes A B C
Pyrolysis or gasification J J J J J
(Gas-phase) combustion J J v ' J J
Char-oxidation J J
There are substantial differences in the burning characteristics of charring and non-charring solids. These are with respect to
(1) The effect of geometry of the solid, (2) Thermal feedback, and
(3) Ventilation.
THE
EFFECT
OF GEOMETRY OFTHE
SOLID
Charring solids (in the absence of external heat sources) cannot sustain burning in a pool configura- tion. (A pool configuration is a plane facing upwards.) If the solid is ignited, a char layer will build up on the surface. This layer will block the thermal feedback from the flame and quell the pyrolysis, and with it the supply of gaseous decomposition products to the flame. In other configurations, however, the inhibition of pyrolysis by the char may not be so effective, and sustained burning is possible. Figure 1 illustrates a
A I R
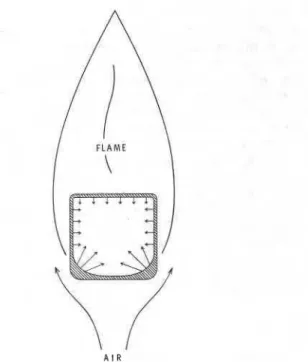
Figure 1. Burning of a char-forming solid.
horizontal square wood bar at an early stage of burn- ing. The gaseous pyrolysis products rise and undergo combustion above and by the sides of the bar. The charred lower corners are left accessible to an air- stream drawn up by the flame and will oxidize. Part of the heat of char-oxidation (which, per unit mass, is usually higher than the heat of combustion of the gaseous pyrolysis products) penetrates the virgin sub- stratum. If this heat flux is large enough to at least make up for the heat loss caused by the blockage of heat transmission from the flame by the thickening char cover along the top and side surfaces of the bar, sustained pyrolysis, and with it sustained burning, is ensured.
Clearly, in the absence of char build-up, non- charring solids can sustain burning in a pool configura- tion.
THE
EFFECT OFTHERMAL FEEDBACK
With non-chamng solids, pyrolysis is controlled (in the absence of external heat sources) by thermal feedback from the flame. Consequently, their rate of burning depends strongly on the radiation potential of the flame, as determined by such factors as the heat of (gas-phase) combustion and the luminosity of the flame. In contrast, with charring materials char- oxidation is the dominant driving reaction; the role of thermal feedback (and, in turn, the radiation potential of the flame) is of secondary importance.
EFFECT OF VENTILATION
Because of the importance of char-oxidation as a driving reaction, the rate of burning of charring solids
depends strongly on the flow rate of air past their
(charred) surfaces. In a specially designed series of experiments the author demonstrated2 the marked influence of ventilation on the burning of charring solids, and the absence of such influence on non- charring solids.
Lack of clear terminology has long hindered the progress of fire science. It was, no doubt, one of the main reasons for the misconception surrounding the mechanism of ventilation control in fully de- veloped compartment fires involving cellulosics (char- ring solids). An observed proportionality between ven- tilation and 'burning' (as measured by the rate of mass loss) was thought of as reflecting a limitation imposed on the rate of 'combustion' of the fuel by the limited flow rate of air into the compartment. That the solid fuel is not directly available for combustion was over- looked. In a strict sense, the observation should be
stated as follows: there is a proportionality between the air flow rate into the compartment and the rate of loss of mass of the fuel. But the loss of mass is caused mainly by pyrolysis (more exactly, by the departure of gaseous pyrolysis products and, to a lesser degree, by char-oxidation), and since pyrolysis is a thermally acti- vated process, its rate cannot directly depend on air flow rate. Consequently, ventilation control must be looked upon as control of char-oxidation, the driving reaction for the burning process, by the air flow pas- * sing by the (charred) surfaces of the solid fuel.' This interpretation of ventilation control has led to a coher- ent theory concerning the burning of cellulosics in compartment fires.
This example shows that using clearly defined ter- minology is not only a linguistic requirement bearing on the clarity of communication, but an important first step in solving scientific problems.
REFERENCES
1. T. Z. Harmathy, Effect of the nature of fuel on the charac- tion on the burning of piles of solid fuels, Combustion and
teristics of fully developed compartment fires, Fire and Flame 31. 259 (1978).
Materials 3, 49 (1979).
2. T. Z. Harmathy, Experimental study on the effect of ventila- Received 22 August 1983; accepted 2 December 1983
T h i s p a p e r , w h i l e b e i n g d i s t r i b u t e d i n r e p r i n t f o r m by t h e D i v i s i o n of B u i l d i n g R e s e a r c h , r e m a i n s t h e c o p y r i g h t of t h e o r i g i n a l p u b l i s h e r . It s h o u l d n o t b e r e p r o d u c e d i n whole o r i n p a r t w i t h o u t t h e p e r m i s s i o n of t h e p u b l i s h e r . A l i s t of a l l p u b l i c a t i o n s a v a i l a b l e f r o m t h e D i v i s i o n may be o b t a i n e d by w r i t i n g t o t h e P u b l i c a t i o n s S e c t i o n , D i v i s i o n o f B u i l d i n g R e s e a r c h , N a t i o n a l R e s e a r c h C o u n c i l of C a n a d a , O t t a w a , O n t a r i o , KlA 0R6.