HAL Id: hal-01403839
https://hal.archives-ouvertes.fr/hal-01403839v2
Submitted on 4 Jan 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
The emerging role of ECM crosslinking in T cell
mobility as a hallmark of immunosenescence in humans
Authors’ names and affiliations
Jean-Francois Moreau, Thomas Pradeu, Andrea Grignolio, Christine Nardini,
Filippo Castiglione, Paolo Tieri, Miriam Capri, Stefano Salvioli, Jean-Luc
Taupin, Paolo Garagnani, et al.
To cite this version:
Jean-Francois Moreau, Thomas Pradeu, Andrea Grignolio, Christine Nardini, Filippo Castiglione, et
al.. The emerging role of ECM crosslinking in T cell mobility as a hallmark of immunosenescence in
humans Authors’ names and affiliations. Ageing Research Reviews - ARR, Elsevier Masson, 2017, 35,
pp.322-335. �10.1016/j.arr.2016.11.005�. �hal-01403839v2�
ContentslistsavailableatScienceDirect
Ageing
Research
Reviews
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / a r r
Review
The
emerging
role
of
ECM
crosslinking
in
T
cell
mobility
as
a
hallmark
of
immunosenescence
in
humans
Jean-Francois
Moreau
a,g,∗,
Thomas
Pradeu
a,
Andrea
Grignolio
b,
Christine
Nardini
c,
Filippo
Castiglione
e,
Paolo
Tieri
e,
Miriam
Capri
d,
Stefano
Salvioli
d,
Jean-Luc
Taupin
f,
Paolo
Garagnani
d,
Claudio
Franceschi
daUniversityofBordeaux,CNRS-UMR5164,146rueLéoSaignat,33076Bordeaux,France bUniversityofRome“LaSapienza”,Rome,Italy
cPersonalgenomics,StradaleGrazie,Verona,Italy
dDepartmentofExperimental,DiagnosticandSpecialtyMedicine,InterdepartmentalCentre“L.Galvani”forBioinformatics,BiophysicsandBiocomplexity,
ViaSanGiacomo,12,UniversityofBologna,40126Bologna,Italy
eConsiglioNazionaledelleRicerche,IstitutoperleApplicazionidelCalcolo,Rome,Italy fUniversitéParis-Diderot,INSERMU1160,Paris,France
gCHUBordeaux,PlaceAmélieRaba-Léon,Bordeaux,France
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received1June2016
Receivedinrevisedform26October2016 Accepted7November2016
Availableonline19November2016 Keywords: Aging Immunosenescence Extracellularmatrix Mobility Immunecells
a
b
s
t
r
a
c
t
Immunosenescenceisthoughttoresultfromcellularagingandtoreflectexposuretoenvironmental stressorsandantigens,includingcytomegalovirus(CMV).However,notallofthefeaturesof immunose-nescenceareconsistent withthisview,and thishasledtotheemergenceof thesistertheoryof “inflammaging”.TherecentlydiscovereddiffusetissuedistributionofresidentmemoryTcells(TRM)
whichdon’trecirculate,callsthesetheoriesintoquestion.ThesecellsaccountformostTcellsresidingin barrierepitheliawhichsitinandtravelthroughtheextracellularmatrix(ECM).Withalmostallstudiesto datecarriedoutonperipheralblood,theage-relatedchangesoftheECMandtheirconsequencesforTcell mobility,whichiscrucialforthefunctionofthesecells,havebeenlargelyignored.Weproposeanupdate ofthetheoreticalframeworkofimmunosenescence,basedonanovelhypothesis:theincreasingstiffness andcross-linkingofthesenescentECMleadtoaprogressiveimmunodeficiencyduetoanage-related decreaseinTcellmobilityandeventuallythedeathofthesecells.Akeyelementofthismechanismis themechanicalstresstowhichthecellcytoplasmandnucleusaresubjectedduringpassagethroughthe ECM.Thishypothesisisbasedonan“evo-devo”perspectivebringingtogethersomemajorcharacteristics ofaging,tocreateasingleinterpretiveframeworkforimmunosenescence.
©2016TheAuthors.PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBYlicense (http://creativecommons.org/licenses/by/4.0/).
Contents
1. Introduction...323
2. Agingandimmunosenescence:currentknowledgeandthebiasesofpreviousstudiesbasedonblood...324
3. ThetightconnectionbetweenimmunecellsandtheECM...325
3.1. Mechanicalstressonthenucleus,thelargestandmostrigidcellcomponent...325
3.2. Lessonslearnedfromvarioushereditaryimmunedeficienciesinwhichcellmobilityisaltered...326
4. Immunosenescence,cellmobilityandage-relatedchangesintheECM:the“mesh”connection...327
4.1. ECMchangesovertime:how,whenandwhy?...327
4.2. ConsequencesofECMalterationswithage...327
4.3. ECMandtheC.elegansmodelofaging...328
∗ Correspondingauthor.Presentaddress:CNRS-UMR5164ImmunoConcept, Bor-deauxUniversity,146,rueLéoSaignat,33076BORDEAUXCedex,France.
E-mailaddress:jfmoreau@u-bordeaux.fr(J.-F.Moreau).
http://dx.doi.org/10.1016/j.arr.2016.11.005
4.4. ECMandthenakedmoleratmodelofaging...328
4.5. Hyaluronanscanalsobeinflammatory ... 328
4.6. ECM,mechanotransductionandthemobilityofimmunecells...328
5. ConsequencesofthelowermobilityofT-lymphocytesandtheirhigherdeathrate...330
5.1. Necrosis,apoptosis,pyroptosisandinflammasomeactivation...330
5.2. Tlymphocytedepletionanditslinktohomeostaticproliferationandautoimmunity...330
6. Conclusion...331
Conflictsofinterest ... 331
Acknowledgments...331
References...331
1. Introduction
Immunosenescence is defined asage-related changes in the immunesystem.Itisassociatedwithaprogressivedeterioration oftheabilitytomountimmuneresponsesandwithahigher mor-talityrateintheelderly.Immunosenescenceiscurrentlythoughtto dependonlifelongantigenload,leadingtothesenescenceofcellsin theimmunecompartment,withaprominentroleattributedtothe chronicanti-cytomegalovirus(anti-CMV)response.Thereseemsto beanincreasinguseofimmuneresourcesallocatedtotheanti-CMV responsewithaging,a processthat ultimatelyleadsto exhaus-tion.Thecauseremainsunclearandinhumansthefewstudies examiningthepresenceofviralreactivationintheblood,found itnegative.Moredataarethereforeneededinthefieldofhuman aginginordertoconcludeonthispoint(McVoyandAdler,1989; Stoweetal.,2007;PawelecandDerhovanessian,2011;Parryetal., 2016).TheroleofCMVinimmunosenescenceisclearlyimportant, but,ratherthanbeingdirectlycausal,canalsobeinterpretedas aconsequenceofmoregeneralage-relatedchangesinthe three-dimensionalmicroenvironmentinwhichmostimmunecellsare mobileandoperate,theECM.Immunologistshaveneglectedthe implications ofsuchchanges, partly becausemost of the stud-iescarriedoutonimmunosenescence,atleastuntilveryrecently, focusedonbloodbecauseitisthemostaccessiblesourceofcellsand biologicalfluidinhumans.Althoughofvalue,thesedata,leadtoan overestimatedqualitativeandquantitativeimportanceofthis com-partmentintheunderstandingoftheimmunesystemphysiology. TherecentdiscoveryofresidentmemoryTcells,orTRM,showed
immunesurveillancetobelargelylocaland,therefore,not read-ilyaccessiblethroughstudiesonblood[seeforreview(Carbone, 2015)].
Here, we argue that efforts to decipher immunosenescence mustconsiderboth bloodand theECM.TheTRM arelocatedin
theECM,and theknownbiochemicalandbiophysical modifica-tionstothismediumassociatedwithagingconsequentlyhampers localimmunesurveillancebythesecells.ECMproteinsand proteo-glycanshavewell-documentedrolesinscaffolding,buttheyalso haveaprofoundeffectoncellbehavior,throughinteractionswith secreted ligands or cell-transmembranereceptors, in particular integrins.We suggestthattheprogressiveand irreversible age-relatedchangesintheextracellularmatrixmayactuallyprovide aunifyingframeworkexplainingallthemolecularandcellular fea-turesofimmunosenescence.Thekeypointisthatfortheimmune cellstobefunctional,theymust befree torecirculate,navigate and restwithin theextracellular matrix, in tissues and organs. Thispoint is instrumental in tissue surveillance andprotection (Ariotti etal.,2012)evenin theabsenceofperipheral lympho-cytes(Steinbachetal.,2016).Wewillconsiderimmunosenescence withinthisframework,focusingontheadaptiveimmunesystem andTcellsinparticular,eventhoughthesecellsareneithertheonly onestobeaffectedduringagingnortheonlyonesconcernedwith mobility.
Wearguethatthemobilityofimmunecellsinnon-lymphoid tissuesis anecessary elementforeffective immunity.Alackof immune cell mobility, either intrinsic, as in hereditary defects affecting actin remodelingfor example as wewill seelater, or extrinsic,asinaging,resultsinanimpairmentofimmuneresponses. Nothree-dimensional(3D)modelofderegulatedcellmobilityhas everbeenproposedorexploredinthecontextof immunosenes-cence.Weshowherethatourhypothesisismoreconsistentwith theavailabledatathancurrentalternativetheories.Wehopethat thishypothesiswhichisbasedonreviewsoffieldsthathavenot hithertobeconnectedtogetherwillpromotefuturestudies,insilico andinvitro,tovalidatethistheoryexperimentally.The3Dmodel canreconcilemanyfeaturesofaging,suchasthealteredresponses tovaccination,whichisinessencebothamemoryandalocal pro-cess,anddysfunctionsofperipheraltolerance(autoimmunity).The chronicprocessofTcelldeathduetomechanicalstresswithinthe cross-linkedmeshoftheagedECMmayalsoaccountforactivation oftheinflammasome(IL1,IL18,NFB),leadingtoinflammaging, andtoastateofimmunedeficiencytypicalofagedsubjects.These twoelementstogetherunderliethephenomenonofviral reactiva-tion(atthebeginninglocalandultimatelysystemic)leadingtothe clonalamplificationofCD8+Tcellsandanincreaseinthe
propor-tionofmemoryTcellsfoundintheblood(Sylwesteretal.,2005; Nikolich-Zugich,2008;Fulopetal.,2013;Fulopetal.,2015).
AlargeamountofTcellsinthebodyaretissue-residentmemory Tcellsthatdon’trecirculate,asdemonstratedbythemostrecent studies(ThomeandFarber,2015;ParkandKupper,2015;Carbone, 2015;Steinertetal.,2015;FanandRudensky,2016).Physiological mobilityinECManditsimpactonTcellsurvivalanddifferentiation arethereforeoftheutmostimportance,includingforlocal anti-CMVdefense(ThomandOxenius,2016).Tcellsurvivalisimpaired in veryconstrained environments, astheforced passageof the cellsinsuchconstrainedconditionsleadstomultipledamageto theplasmamembraneandnucleus,potentiallyculminatingincell death(Denaisetal.,2016;Raabetal.,2016).Inflammasomesare activatedinresponsetoincrementalproductionofdangersignals comingeitherfrominsideoroutsidethecells(Ostanetal.,2015) andleadingtoproductionofIL1,IL18aswellastheactivationofthe NFBpathwaytypicalofinflammaging(FranceschiandCampisi, 2014).Furthermore,limited mobility decreasesthe numbersof themostneededTcellslocallypresentintissues,leadingto:(i) viralreactivationnotnecessarilydetectedinblood,duetoalackof properlocalimmunosurveillanceasshowninhereditaryimmune deficienciesresultinginseverelyimpairedlymphocytemobility; (ii)clonalexpansionofa verylimitedrangeofTcellsfollowing antiviralresponses;(iii)repertoirereductionduetohomeostatic forcesintheabsenceofthethymus,ashomeostasisispurelyabout maintainingcellnumbers,nottheirdiversity.Allthesefactorsare additionalfeaturestypicalofaging,mutuallyenhancedinavicious circlethat,wesuggest,ismediatedbyage-relatedECMdegradation andadirectconsequenceofimpairedlymphocytemobility.
Wewilldevelopthisideaanditsconsequencesthroughaseries ofsteps.Wewillfirst(Section2)discussagingbyfocusing,in
par-ticular,ontheagingoftheimmunesystem(immunosenescence). Wewillrelatetheimportanceofimmunecellmobilitytothe mech-anismsunderlyingECMagingandcross-linking,whichincreasethe constraintsoncellmobility.
WewillhighlightthefunctionalconsequencesoflowerTcell mobilityandTcelldeath,throughwell-knownhereditaryimmune deficienciesresultinginimpairedTcellmobility,suchasDOCK8, Coronin-1,CDC42orPGM3deficiencies(Section3).
Wewillthen(Section4)associatetheimpairedmobilityofT cellswithECMaging.Finally(Section5),wewilldiscussthelikely specificconsequencesofthislackofmotilityandinducedcelldeath forestablishmentoftheimmunosenescencephenotype.
2. Agingandimmunosenescence:currentknowledgeand thebiasesofpreviousstudiesbasedonblood
Overthelast30years,considerableeffortshavebeenmadeto understandtherelationshipbetweenagingandthedeclineofthe immunesystemandthecontributionofimmunosenescencetothe phenotypesobservedinagingindividuals(Franceschietal.,2000; Franceschietal.,2000a;Salviolietal.,2006).Thesephenotypes includetheaccumulationofCD8+CD28−cells,CMVseropositivity,
andaninversionoftheCD4/CD8ratio,partoftheimmune risk profile(IRP)thatseemstopredictmortalityinpeopleovertheage of65(Hadrupetal.,2006).
Onekeyquestionconcernstheextenttowhichthymicmature lymphocyteoutputcontributestoTcellhomeostasis,and there-fore,theextenttowhich age-relatedchanges inthis organcan beconsideredtodriveTcellaging.Maintenance ofthenaiveT cellpoolishighlydependentonthymicoutputinagingmice.In humansitseemstobebasedmainlyontheperipheraldivisionof pre-existingTcells,inaphenomenonknownashomeostatic prolif-eration,asdemonstratedincasesofneonatalthymectomy(Johnson etal.,2012;Sauceetal.,2012;denBraberetal.,2012;Thomeand Farber,2015;vandenBroeketal.,2016).Theglobalrepertoireof naiveCD4+Tcellsremainsdiverseuntilninthdecadeoflife,when
thereseemstobeanincreaseincellturnover,rapidlyfollowed byrepertoirecontraction.AlossofthymicTcelloutputcan, there-fore,bequantitativelycompensatedbyhomeostaticproliferationin ordinaryconditions,withoutfurtherconsequencesduetothewide diversityoftherepertoire.However,homeostaticproliferation can-notcompensateforalossofTcelldiversity.Inelderlyindividuals witha continual, progressive,stochastic loss of T lymphocytes duetoanexternalcause,andcharacterizedbyacumulativeeffect overtime,homeostaticproliferationoftheremainingcells accel-eratesthelossofTcelldiversity,bydilutingoutexistingminority clones(GoronzyandWeyand,2005).Regardlessoftheactualage ofthepatient,advancedHIVinfection,characterizedbyamassive andcontinuouslossofTcells,seemstoreproducesomefeatures ofaging,withunderlyingimmunosenescenceandinflammaging (NixonandLanday,2010;ZapataandShaw,2014).Therefore,both in aging subjectsand in patientswith advanced HIV infection, immunosenescenceoftheadaptiveimmunesystemisnota sim-pledeteriorationoftheimmunesystem.Instead,itresultsfrom adynamicdriftunderthepressureofcontinuousexposuretoan antigenicloadand an increasingly limited capacity togenerate newTCR-bearingcells,leadingtotheaccumulationofmemoryT cellsandanage-associated declineinTcellrepertoirediversity (Yageretal.,2008).Notably,decreaseinnaiveTcelllevels, lead-ingtorepertoireshrinkage,hasalsobeenreportedinagingapes (Cicin-Sainetal.,2007).
Thecausalmechanismsunderlyingtheseadaptationshaveyet tobeidentified,butarealmostcertainlydiverse.However, home-ostatic proliferation to correct imbalances in the number of T cellsinvolvestherecognitionofself–determinantsbynaiveTcells
(Richards et al.,2016), whichmayconstitute animportantlink betweenagingandautoimmunity(Khiongetal.,2007).
Theenvironmentalcontextinwhichthecellsarefoundmust alsobeconsidered,inadditiontothereportedcell-autonomous defectsandstem-cellaging[seeforreview(Montecino-Rodriguez etal.,2013).Theimportanceofcellenvironment ishighlighted bytworemarkableexamples.Firstly,mouseCD4+Tcells
gener-ated fromhematopoietic stem cells (HSC) from old donorsare functionalinyoungbutnotinoldrecipients(Eatonetal.,2008). Secondly,changesintheepithelialcomponentofthethymus,the lymphopoieticorgan,haveshowntobecrucialfortheearly pro-gressivedecreaseinthymicoutputwithage(Hamazakietal.,2016; Youmetal.,2016).
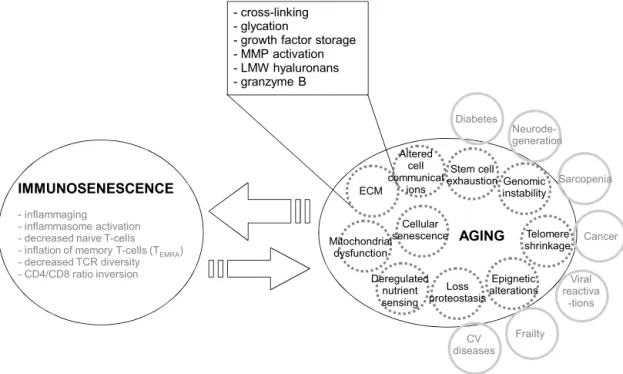
Immunosenescenceisalsoinfluenced bythegeneral mecha-nismsofagingoccurringinthebody,thoughthesemechanisms remain elusive (Grimm, 2015; Cohen, 2015). Severalhallmarks ofaginghavebeenidentified,allofwhichhaveprofounddirect or indirect effects on the immune system (Lopez-Otin et al., 2013;Kennedyetal.,2014).Thefirstmechanismofagingtobe identifiedwascellularsenescence,inwhichtelomereshortening limitsthenumberofreplicationcycles(Hayflickand Moorhead, 1961;Campisi,2013).SenescentcellsthathaveaccumulatedDNA damagehaveasenescence-associatedsecretoryphenotype (SASP), characterizedbytheproductionandsecretionoflargeamountsof proinflammatorycytokines,matrixmetalloproteinases(MMP)and othersolublemediators(vanDeursen,2014).Senescentcells accu-mulateinolderindividuals,andthisisthebasisof“inflammaging”, aconceptputforwardbyoneofus(CF)tostressthecloselinks betweenagingandchronicinflammation(Franceschietal.,2000b; Salviolietal.,2006;Franceschietal.,2007).Thestateofchronic inflammationthatisahallmarkofaginginhumansaccountsforthe comorbidities(Fig.1)andmortalityassociatedwithagingamong which atherosclerosis,osteoporosis,osteoarthritis, diabetes[see forreview(FranceschiandCampisi,2014;Kennedyetal.,2014)]. However,cellularsenescencealonecannotaccountfor immunose-nescence.
ThedecreaseinnaiveTcellsandtheincreaseinmemoryTcells canbothbeexplainedbyasustainedlossofcellsinacontextof chronicimmuneresponsesassociatedwithadecreaseinthymic output(Nikolich-Zugich,2008;Fulopetal.,2013).This immunod-eficiencywouldaccountforthestrongassociationbetweenCMV seropositivityandmortalityduetocardiovascularcausesobserved intheelderly(Savvaetal.,2013).ChronicCMVreplicationmaybe seenasanindirectconsequenceoftheslowdevelopmentofthis immunodeficiency,aslatentvirusesarereactivatedonceacertain thresholdofimmunodeficiencyisreachedasshownalsoinmouse models(Policetal.,2001).Fromthisstandpoint,CMVshouldnot beseenasthecausalagentofimmunosenescence,althoughwe acknowledgethisvirusandtheimmuneresponsetoit,contribute torepertoireshrinkageandinflammation(Fulopetal.,2013).
Crucially,thecurrentoverallviewofimmunosenescenceis par-tialbecausemost,ifnotall,studiesonaginginhumansarebasedon bloodsamples,forpracticalreasons.However,Tcellsintheblood aresubjectedtostrongselectionthroughtraffickingregulation[see forreview(ThomeandFarber,2015)].Thetissue-residentmemory Tcells(TRM)(Sathaliyawalaetal.,2013),whichhaveyettobe
stud-iedinagingresearch,areofparticularimportancehere(Gebhardt etal.,2011).Intissue,TRMaremorenumerousthanTcells
recircu-latingfromtheblood(Steinertetal.,2015),andtheymayremain withintissuesfortheentirelifetimeoftheindividual.Thistissue retentioniscontrolledbyCD69expressionandthedownregulation offactorspromotingtissueegress.Itisdevelopmentallyregulated throughexpressionofthespecifictranscriptionalregulatorsHobit andBlimp1(Mackayetal.,2016).Thelymphocytesresidentin tis-suesincludenotonlyTcells,butalsoNKT,orevenNKcellsinthe liver,reproducingthediversityofthesubpopulationsknowntobe
ECM
-cross-linking -glycation
-growth factor storage - MMP activation -LMW hyaluronans -granzyme B Diabetes Cancer Sarcopenia Viral reactiva -tions Frail
ty
CV diseases Neurode-generationAGING
Altered cell communications instabGenomicility
Telomere shrinkage
Epignetic alterations Loss
proteostasis Deregulated nutrient sensing Mitochondrial dysfunction Stem cell exhaustion Cellular senescence
IMMUN
OSENES
CENC
E
-inflammaging
-inflammasome activation - decreased naive T-cells -inflation of memory T-cells (TEMRA) - decreased TCR diversity -CD4/CD8 ratio inversion
Fig.1.Relationshipsofimmunosenescencewithagingmechanismsandcomorbidities.AgingmechanismsfollowtheninehallmarksofagingestablishedbyLopez-Otin (Lopez-Otinetal.,2013).Externalcirclesdepicttheco-morbiditiesassociatedwithagingwhichalsocooperatetomodulateagingphenotype.TheECManditsalterations linkedtoagingwillconstraintimmunecellmobilitywhileinducingcelldeath.ECMalterationisahallmarkofagingandthereforeacrucialprocesstobetterunderstandthe phenomenonofaging.FundamentalmechanismsassociatedwithECMagingarerepresentedintheexpandedbox,inrelationwiththealteredcellularcommunications.ECM servesnotonlyforthecellstomigratewithin,butalsoforgrowthfactorsstorageandreceptoranchorageasitisthecaseforintegrinsandCD44.
presentinbloodandbarriertissues(Gasteigeretal.,2015;Fanand Rudensky,2016).Tissue-residentlymphocyteshavebeenfoundin thegastrointestinaltract,lungs,skinandreproductivetract(Farber etal.,2013)[seeforreview (SchenkelandMasopust,2014)and (Clark,2015)]butalsointhebrain(Steinbachetal.,2016).Inmouse, theyhavebeenshowntobeakeyelementinimmunedefenses againstall microbes,includingCMV (Smith etal., 2015) (Thom etal.,2015).Inbothmiceandhumanscytomegalovirusinduces TRMparticularlyinmucosaltissueswhichareimportantviral
sanc-tuariesandentrysites(ThomandOxenius,2016).Theyseemto functionasanorgan-autonomousfirstlineofdefenseeveninthe absenceofcirculatingCD8+memoryTcells(Steinbachetal.,2016) showingthatrecirculationofthesecellsbetweenthebloodand thesetissue-compartmentsaredispensableforefficientorgan pro-tection.KnowninmousemodelofCMVinfection,virallatencyof CMV,canpromotesthecontinuous,low-levelrecruitmentof cir-culatingCMV-specificTcellstotheTRMpopulationofthesalivary
glandmaintainingapoolofTRMatthesiteofviralreplication(Smith etal.,2014),apointwhichremainstobeformallyproveninhuman aging.
Given the crucial importance of TRM, any exploration of
immunosenescenceshouldtakeintoaccounttheECM,the envi-ronmentinwhichthesecellsarefound.Thisplacingofimmune systemphysiologyintocontextisofvitalimportance,butstillrarely (ifever),doneinstudiesonimmunosenescence.
3. ThetightconnectionbetweenimmunecellsandtheECM
3.1. Mechanicalstressonthenucleus,thelargestandmostrigid cellcomponent
Cellsmaybecarriedalonginamobilemedium,suchaslymph orblood,butherewewillexcludesuchpassivemobility,tofocus insteadontherequirementsfor theintrinsic motilityofTcells, particularlywithintheECM.The trans-endothelialmigration of lymphocytesisrelevantinthiscontext,becauseofthe
biochem-icalandbiophysicalnatureoftheECMinthevesselwall(Kohn etal.,2015).
We willdeal hereexclusivelywithT cells, butmany differ-ent cell types from both the adaptive and innate arms of the immunesystemaremotilewithintheECM.Neutrophils,for exam-ple,areprobablythemostmobileofallimmunecells,recirculating frequently and rapidly between the bone marrow, blood and then tissues. These cells display an age-related loss of migra-torycapacity,withpredictableconsequencesaging(Sapeyetal., 2014).Neutrophilsarehighlydeformableandcancrossporesonly microns in diameter (Rowat et al., 2013), due tothe flexibility oftheirnuclearmembrane,whichlackslamin-A,amoleculethat restrictsnucleardeformability,therebylimitingmigrationthrough constrictionsandtherateof3Dmigration(Haradaetal.,2014). Thereisadelicatebalancebetweenthemechanicalprotectionfrom ruptureaffordedbythepresenceoflamininthelamina,limitingcell motilityandnuclearplasticityallowingmovementsofcellsthrough mesh(GerlitzandBustin,2011).Unsurprisingly,laminopathies,an heterogeneousgroupofhereditarydiseasescausedbymutations ofthelamin-Agene,areoftencharacterizedbybothaccelerated agingandhighlevelsofinflammation(BurtnerandKennedy,2010). Tlymphocytesdisplaylamin-Aexpressionwhenactivated,butnot whenresting,possiblyreflectingdifferencesinmotilitybeforeand afteractivationdependingoncelllocationandfunction.Thefew studiesfocusingonthelymphocytecompartmentinlaminopathies have reportedmajor changesin T cellbehavior, due toaltered synapseformationandactivationprocesses,consistentwiththe hypothesisthatlamin-Aisrequiredforactivation(Rocha-Perugini andGonzález-Granado,2014).Lymphocytedevelopmental abnor-malitieshavealsobeenreportedinthelaminKOmodel(Haleetal., 2010),butarelationshipbetweenthequalityofimmuneresponses andthemobility ofimmunesystemcellshasyettobe demon-stratedinaffectedpatients.Matrixstiffness,lamin-Aproteinlevels inthenucleusandcellmobilityareknowntoberelated(Swiftetal., 2013),butthepotentialconsequencesoftheserelationshipsforthe immunesystemduringaginghavenotbeenexplored.
Ithasrecentlybeenshownthatmigratingmammaliancellsare susceptibletoruptureofthenuclearmembranewhensubjected tostrongmechanicalconstraints,suchaspassagethroughsmall pores(3mindiameter).Suchruptureswouldresultinamixing ofthenuclearandcytoplasmcontents.Majoreventsofthistype arefrequent(90%ofthecellsinvitro,accordingtoarecentstudy (Denaisetal.,2016)),butseemtoberapidlyrepaired(alongwith theDNAdouble-strandbreakstheycreate)byspecificmechanisms (Raabetal.,2016)(Denaisetal.,2016),andamongwhichautophagy orproteasomerolescouldbehypothesized.However,therepair mechanismsmaynotbealwayscompletelysuccessful,potentially leadingtocelldeath, or canceroustransformation (Hniszet al., 2016;Zhangetal.,2015).Thestresstowhichthenucleusis sub-jected,inadditiontocausingDNAstrandbreaks,alsoinducesmajor inflammatorypathways(IL6andNFB),potentiallyaccountingfor theinflammatorystatusassociatedwithagingandaddingto cur-rentknowledgeofcellsenescence(LeBerreetal.,2012;McGregor etal.,2016).Thenucleus appearstherefore astheplace where geneticinformationisstoredbutalsoasamechanicalsensor[see forreview(BustinandMisteli,2016).Asobservedforthenuclear envelope,thestressontheplasmamembraneanditsmaintenance probablyplayimportantrolesalsoinaging(Lauritzenetal.,2015). AsdiscussedinSection4below,themobilecellsoftheimmune systemhaveparticularlyhighlevelsofexposuretotheserisks. 3.2. Lessonslearnedfromvarioushereditaryimmunedeficiencies inwhichcellmobilityisaltered
DOCK8isaguaninenucleotideexchangefactor(GEF)that acti-vatessmallGTPases(Cotéand Vuori,2007),and alsoactsasan adaptorintheTLR9-MYD88signalingpathway(Jabaraetal.,2012). DOCK8controlscellcytoskeletalfunctions(secretion,cell interac-tions)andmigration,andisexpressedonlyincellsoftheimmune system.DOCK8mutationsresultinacombinedimmunodeficiency syndrome.DOCK8-deficientpatientshaverecurrentotitis,sinusitis, andpneumonia,recurrentS.aureusskininfections,H.simplexorH. zosterinfections,andpersistenthumanpapillomavirusinfections. Mostpatientshave severe atopy withanaphylaxis, and several developsquamous-cellcarcinomas.Biologically,somehavehigh serumIgElevelsorhypereosinophilia,otherspresentlowcounts ofTcells andB-cells,andlowserumIgM levelswhiletheirIgG antibodyresponsesarevariable(Zhangetal.,2009).Tcell acti-vation,survival,proliferation andprimingbydendriticcells are affected.Other cells, including dendritic and NK cells, are also crippled,resultinginpoorcellcytotoxicityandlowlevelsof antivi-ralcytokineproduction.Notably,DOCK8-deficientdendriticcells migratepoorlytothelymphnodes(Lambeetal.,2011;Randall et al., 2011). Microscopy observations of T cells from patients, migrating withinthe three dimensionsof the dermis microen-vironment in human skin biopsy samples, showed that these cells had abnormalelongated shapesand long migration times withinthemesh,phenotypesobservedinnormalcellsafterDOCK8 silencingwithsiRNA.Remarkably,DOCK8-deficientcellssenseand migratetowardaSDF-1chemokine(CXCL12)gradientnormallyin two-dimensionalandliquidenvironments.Moreover,in3D envi-ronments,butnotinliquidmedium,Tcellsfromnormalindividuals inwhichDOCK8issilencedinduceaspecificformofdeathknownas “cytothripsis”(Zhangetal.,2014a).Thistypeofcelldeathresults fromtheexertionofmechanicalforcesontheplasmacell mem-braneandthemorerigidnucleus,leadingtotearingoftheplasma membrane.The elongatedcellphenotypeleading todeathalso occurswhenTcellsmigratethroughpores,agarose,ICAM-coated orcollagen-coatedsurfacestowhichtheyadhere,demonstrating aclearrelationshipbetweenshapeandlocalconstraintson mobil-ity.Thus,theabnormalshapeanddeathofcellslackingDOCK8 areassociatedwithmovementconstraintsduetoaconfinedspace,
observedinthedermis,accountingforthephenotypeofpatients, withtheirhighfrequencyofskindiseases(Mouwetal.,2014).
DOCK8activatesCDC42,whichregulateslymphocyteshapeand cytoskeletalstructuresduringcellmovements,includingdendritic cellmigration(Haradaetal.,2012).CDC42thenactivatesseveral effectors,includingP21-activatedkinase(PAK)andthe Wiskott-Aldrich Syndrome Protein (WASP). Knockout of the small Rho GTPaseCDC42 reproduces someofthefeatures ofDOCK8 defi-ciency,whereasWASPlossfromTcellsdoesnot(Humblet-Baron etal.,2007).However,WASPdeficiencyisassociatedwith abnor-malimmuneresponses,reflectingthecomplexinterplaybetween theseproteinsintheorchestrationofcellmobility.
Similarly,Coronin-1(Coro1)deficiencyleadstoapronounced immunodeficiencyphenotyperesemblingthatofDOCK8-deficient patients(Fögeretal.,2006;Shiowetal.,2008;Hogquist,2008). Coro1 regulatesactin polymerization. Mutation of the CORO1A genecausesprofoundperipheralTcelllymphopenia,thoughtto beduetoaninabilityofTcellstomigrateoutofthethymusandto enterandleavelymphnodes.However,thesecellswerealsoshown tobegenerallylessmobileinthepresenceofthismutation.
TheimmunedefectinDOCK8-deficientindividualsprincipally concernsthemaintenanceoftheTRMcompartment,butinnormal
individuals,itcouldalsorelyonECMqualityandquantitywhichare specifictoeachtissue(Bonnansetal.,2014).AlterationstotheECM wouldmodifythemobilityofcellsthroughthismatrix,inasimilar mannertoDOCK8mutation.Inaddition,themobilityofimmune cellsisrequiredforcorrectactivationofTcellsandisapreliminary stepforcontactbetweenTcellsorTregcellsandDCsinsecondary lymphoidorgans(Sixt,2011;Kastenmülleretal.,2012;Hondaetal., 2014;Liuetal.,2015).ECMalterationsmayalsoaffectdiverse pro-cesses,includingtheformationofthethymicepithelium,which playsakeyroleinTcellproduction(Shenetal.,1994;Mouwetal., 2014).
Thevariousdegreesoflymphopeniaobservedatdifferentsites inthebody(spleen,skin,etc.)inDOCK8-deficientpatients prob-ablyresultfromacombinationoffactorsdifferinginmagnitude betweenpatients.Lymphopeniainbloodandtissuesisassociated withpoorer controloverlatentviruses,in turntriggeringacute antigen-drivenclonalamplificationandinflationoftheTEMRA
com-partment.In thelong term,lymphopeniamaybe compensated by homeostatic proliferation and/or thymic output, depending on the age of the patient, but with a change in their respec-tivefrequencies.Indeed,CD8+Tcells thatareCD57+(Brenchley et al., 2003), CD57+/CCR7−/CD27− (Papagno et al., 2004), or
CD45RA+/CCR7−/CD27−/CD28−(Ruferetal.,2003;Romeroetal., 2007)display the greatest expansion in vivo, as demonstrated byTCR excision circle(TREC) quantification ortelomerelength measurement,but thesecells donot proliferate invitro follow-ingTCR-mediatedstimulation.DOCK8-deficientCD8+Tcellsubsets
havehigherproportionsofCD57+CD27−CD28− cellsinboththe
memoryandTEMRAcellsubsets,withnaivecellsdisplaying
unusu-allyhighlevelsofCD95expression(Randalletal.,2011).These features are similar to those observed in young HIV-infected patients(BoassoandShearer,2008;ZapataandShaw,2014)and intheelderly(Vescovinietal.,2014).
Actindynamicsandcelllongevityareknowntobelinkedin yeast,in aged miceand humans(Fögeret al.,2006; Brockand Chrest,1993).YeastswithslowactindynamicsaccumulateF-actin, releaseROSandhavehigherrateofcelldeath.Conversely, increas-ingactindynamicsinnormalcells canincreaselifespanby65% (Gourlayetal.,2004).Actindynamicsanditsregulationtherefore profoundlyaffectmanyaspectsoflymphocytelifeandsurvival,as notedsometimeagoforTlymphocytes(BrockandChrest,1993). Allthesestudiesconcentratedonintrinsicdefectsofcelldynamic butextrinsicfactorsshouldalsobeconsidered.
Inthisview,abnormalitiesofcertaintypesofglycosylationdue toautosomalrecessivephosphoglucomutase3(PGM3)mutations (Zhangetal.,2014b)havealsorecentlybeendescribed.Affected patientspresentasyndromeresemblingDOCK8deficiency,with atopy, immune deficiency, autoimmunity and neurocognitive impairment,suggestingapossibledecreaseincellmobilityinthese patientstoo,potentiallyduetochangesintheextracellularmatrix witheffectsoncellmigration.
4. Immunosenescence,cellmobilityandage-related changesintheECM:the“mesh”connection
TheECMisanacellular3Dstructurecomposedoftissue-specific combinationsofalargenumberoffibrillarproteinssuchas col-lagens,proteoglycans,andglycoproteins(Hynes,2009).Collagen fibersmaintaintheshapeofthetissues,astheyareinextensible, butflexibleandstrong.Collagensarethemostabundantproteins intheECM(Bella,2016)Thereare28differentformsofcollagen, belongingtoeightclassesthatdifferbiochemicallyinthenatureof theiraggregatedformsandspeciescomposition.
Fibroblast-matrix interactions have long been known to be importantinaging(Baileyetal.,1998;Varanietal.,2006).These interactionsarecurrentlythefocusofintenseresearchin devel-opmentandcancerbiology.Inaging,stiffeningofthejointsand ofthevasculartreeinthekidney,retinaandheartareobserved, togetherwithchangesinbasalmembranepropertiesdueto pro-foundalterationstocollagenstructureandmetabolism,through thecross-linkingoffibers,inparticular.Moreover,therateof col-lagensynthesis isalsoaffected.It graduallyslowsdownduring childhood,reachingaplateauinadultsandthendecreaseinmost tissuesintheelderly.
Incancer,cross-linkingandsubsequentstiffeningoftheECM around the tumor seems to be a prerequisite for transformed cellinvasivenessandimportantlyfortheprotectionofthesecells againstimmunesystemcontrol(Leventaletal.,2009).ECM alter-ationsprobablyalsopromotecelltransformation(Seoetal.,2015). Cellularintegrins,whichbindtotheECM,providecancercellswith thepositivesignalsrequiredfortumorprogression(Chenetal., 2015).Thissituationresemblesthatdescribedforstemcells,the fateofwhichisalsolargelydeterminedbyECMinteractions(Guilak etal.,2009).
4.1. ECMchangesovertime:how,whenandwhy?
Thecross-linking theoryofaging datesfromthelate 1950s. According tothis theory, proteins, in particular collagens, lose theirfunctionsfollowingexcessivecross-linkingduetoreaction withaldehydemetabolites[see(Baileyetal.,1998)].Two differ-entmechanismsdrivethechangesinthemechanicalproperties of collagen with age. The first involves the specific enzymatic cross-linking of lysine or hydroxylysine, and is fundamental todevelopment.With age,a second,non-specific, cross-linking mechanismoccurs.Thismechanisminvolvesthenon-enzymatic chemicalreactionofprotein,peptides,aminoacids,nucleicacids, andlipidswithglucose,fructose,ascorbicacidorpentose(Selland Monnier,1989),inaprocessknownasglycation(Maillard reac-tion),togenerateadvancedglycationendproducts(AGEs)(Sjöberg and Bulterijs,2009).Glucosepane isthemostabundanttype of protein cross-link identified to date in vivo. It is found in the extracellularmatrix,whereitparticipatesincollagencross-linking. By increasing collagenstiffness and limiting porosity size, glu-cosepanecross-linksmayhavesignificantimplicationsforseveral age-related diseases, including cardiovascular disease,diabetes, andosteoporosis(Monnieretal.,2014;Boger,2015;Draghicietal., 2015).ProteinturnoverisanimportantdeterminantofAGE
accu-mulationinproteinsand,therefore,oftheirdegreeofcross-linking (GaggarandWeathington,2016).Collagenshaveaverylong half-life(117years forcartilage,15yearsfor skin),resultinginhigh andcumulativeratesofglycatedproductaccumulationintheECM (Verzijletal.,2000).Thisaccumulationisacceleratedby hyper-glycemiaindiabeticpatients,andthisisthoughttobethemajor causeof higher morbidityand mortalityin thesepatients. Dia-betic patientshave impairedtissue repair mechanismsand are knowntobepronetoskininfections.Theprevalenceofdiabetes increaseswithage,potentiallyworseningagingoutcomesoverall. Glycationisthoughttooccurmostlyintheextracellular environ-ment,butproteinswithincellsmayalsobespecificallyglycated. Thisisthecaseforvimentin,whichseemstobeahighlysensitive targetforchemicalglycation,butwithahighturnover,likely lim-itingtherelevanceofthisfactorinourdiscussion(Kueperetal., 2007).Thisobservationis,however,ofinterestwhenconsidered togetherwiththoseforlamins,asbothmoleculesplaykeyrolesin nuclearenvelopebiology.Inaddition,glycatedcollagenscan oxi-dizelipids,generatingmoleculessuchasmalondialdehyde,which hasa longhalf-lifeand diffusesaway toreactwithproteinsor nucleicacids,therebymodifyingtheirbiologicalproperties. Rele-vanttocellmobility,invitrotreatmentwithmethylglyoxal,another oxidizingagent,hasbeenreportedtodecrease celladhesionto matricesby70–90%(Bailey,2001).
Proteoglycansareanotherabundantcomponentofthe connec-tivematrix involved inthe age-relatedchanges tothephysical propertiesoftissues.Throughtheirelectriccharge,these compo-nentsof theECMare alsoimportantfor thebindingof growth factors,suchasIGF1,totheirscaffolds(Parkeretal.,1998)andfor thereleaseofIL1alphafollowingECMmodificationbygranzymeB (McElhaneyetal.,2012).Decorin,themainproteoglycaninskin, regulates collagen matrix assembly. This protein is distributed along collagen fibrils and the decorin glycoaminoglycan (GAG) chain controlsthedistance betweenthesefibrils. Reducing the lengthofdecorinGAGchainsreducesthedistancebetween colla-genfibrils,decreasingmeshporosity,asobservedinaging(Bailey, 2001).
4.2. ConsequencesofECMalterationswithage
Changesto“mesh”porosityduetocross-linkingoralterationsin relativecollagenspeciescompositionwouldbeexpectedto mod-ifycellmobilityprofoundlyintheECM.Thischangeinmobility wouldparticularlyaffecttheimmunecells,althoughmodifications areexpectedtobebothlocation-dependentduetovariable com-positionsofECMindistincttissues(Groulxetal.,2011;Soretetal., 2015;Hallmannetal.,2015)andcell-dependent,dueto variabil-ityintheadaptationofnucleusstiffnesstotheenvironment(Wolf etal.,2013)(Swiftetal.,2013).
Inmice,lowlevelsofgrowthhormoneproductionduetoan embryonicpituitaryglanddefectresultintheproductionofmice onethirdthesizeofnormalmice,butwitha40%higherlifespan (Flurkeyetal.,2001).Interestingly,collagencross-linkinglevelsin thetailwerefoundtobeonlyonethirdofthoseinnormalmice, whichsuggeststhatacomplexinterplaybetweenpituitarygland andECMexists.Asshownbythenakedmoleratmodelofagingseen below,onelinkisembodiedbyCD44signaling(Tianetal.,2013). Inthisregard,ageddwarfmicehaveCD4+ andCD8+ memoryT
celllevels(CD44+)similartothoseseeninyoungcontrolanimals,
andmuchlowerthanthoseinagedcontrolmice(Flurkeyetal., 2001).Furthermore,verysignificantdifferencesareobservedinfive othertestsprobingtheimmunestatusoftheseanimals, support-ingtheconclusionthatinthesemice,thehigherlifeexpectancy andthebetterimmunestatusthanwild-typemice,arecorrelated withdifferencesintheECM.However,norelationshiphasyetbeen experimentallyconfirmedinthisfield.
InEcuador,agroupofhumanswithlifelongIGF-1deficiency causedbyaGHreceptor(GHR)mutation(Laronsyndrome)have beenshowntobemuchmoresensitivetoinsulinthanage-and BMI-matchedcontrolrelatives,despitehavingahighpercentage ofbodyfat.Noneoftheseindividualswerediabetic,whereas6%of theirunaffectedrelativeswerediabetic,andonlyoneofthe20 indi-vidualswithGHRdeficiencydiedfromcancer,whereas20%oftheir relativesdiedfromthisdisease(Guevara-AguirreandRosenbloom, 2015).Interestingly,theoffspringofonecentenarianwasfoundto havelowlevelsofcirculatingIGF1bioactivity,inverselycorrelated withinsulinresistance(Vitaleetal.,2012).
Takenaltogether,thesedataareconsistentwitharoleforthe IGF-1pathwayinaging,butthisrolemaybeatleastpartlyindirect, andshouldconsiderthepossibilityofECMalterations.
4.3. ECMandtheC.elegansmodelofaging
In nematodes, mutations preventing insulin/IGF1 signaling, suchasdaf-2mutations,doublelifespan.Removalofthegermline precursorcellsalsoextendswormlifespan60%,probablyby alter-ingendocrinesignaling.Thesetwoeffectsareadditive,resulting inaquadruplingoflifespan.Bymanipulatingtheexpressionofa fewgenesfromtheinsulin/IGF1axislifespancanbeincreasedby afactorofsix,withnoapparentlossofhealthoractivity( Arantes-Oliveira,2003).Aboutadozenpathwaysareknowntobeimportant inaging,butmatrixremodelinghasbeenidentifiedasanessential signatureoflongevityinallspeciestested,includingnematodes, leadingtotheconclusionthatthepromotionof ECM conserva-tionishighlybeneficial(Ewaldetal.,2014)andcouldserveasan additionaltargetinthecontrolofaging.
ThemolecularroleofECMinthepreventionofagingremains tobeunderstood,butdiversemechanismsappeartobeinvolved. Thesemechanismsmayberelatedtoresistancetooxidativestress ormayoperateattheinterfacebetweenseveralsignaling path-ways,includingthoseinvolvingCD44(Tianetal.,2013;Pontaetal., 2003),TGFbeta,boundIGF1,andintegrins.Theymayalsorelateto themechanicalrelationshipsbetweenthenucleusandtheECMas pointedoutbefore.TheabilityoftheECMtobindgrowthfactors isanotherkeyaspectthatcouldbemodifiedforresearchpurposes (Martinoetal.,2014).Inthisregards,parabiosisexperimentshave shownthatthetransferofbloodfroma youngmousetoanold mouseincreasesbraincellgrowth,promotesbrainplasticity, mem-oryformationandtherepairofdamagedspinalcord,andreverses theage-relatedthickeningoftheheartwalls.Theserejuvenation processesmayreflectareversalofthedegradationofECM func-tioninagedindividuals,includingthequenchingofROSandAGE, decreasesinECMcross-linking,andthereplenishmentoftheECM withgrowthfactors,suchasIGF1(Conboyetal.,2005;Loffredo etal.,2013;Villedaetal.,2014;Elabdetal.,2014;Scudellari,2015). Theeffectsprobablydifferbetweentissues,reflectingdifferencesin ECMcompositionandinterestinglyalsolinkedtothedistinctrates ofagingnotedfordifferentorgans(Ceveninietal.,2008).
Suchtreatmentwouldalsoreversethedeclineinimmunestatus associatedwithaging,leadingtoadecreaseininflammaging,the replenishmentofnaivematureTcellsandhematopoieticstemcells, andanabolitionoflatentvirusreactivation,buttheseeffectshave yettobedemonstratedexperimentally(ConboyandRando,2012). 4.4. ECMandthenakedmoleratmodelofaging
Thenakedmolerathasanexceptionallylonglifespan,atover30 years,muchlongerthanthefouryearsforrelatedmousespeciesof similarsize.Furthermore,nocaseofcancerhaseverbeenreported inthisspecies,despitemanyyearsofobservationofnakedmole ratcolonies.Thisremarkableresistancetocancerseemstobedue tothesecretionbyfibroblastsoflargeamountsofanECM
com-ponent,thehigh-molecularmassmoleculehyaluronan(HMMH), duetohighlevelsofsynthesisandlowlevelsofcatabolism.The hyaluronansynthaseofthemoleratdiffersfromthoseof13other speciestestedbytwoaminoacidsinthecatalyticdomain(N178S andN301S).Oneofthesedifferencesconcernsanasparagine(N) residuetotallyconservedinallotherspeciestested.Thesefindings shouldledtoasearchforpolymorphismsofthehyaluronan syn-thasegeneinhumansthatmightbeassociatedwithcentenarians (Tianetal.,2013).Theskin,heart,brainandkidneyofnakedmole ratsarehighlyenrichedinHMMH.Thedisruptionofsignaling path-ways,inducingthemalignanttransformationofmousefibroblasts (H-RASandSV40),doesnotleadtothetransformationofnaked moleratfibroblasts.However,theeliminationofhyaluronan over-production,byknockingdownexpressionofagenerequiredforits synthesisoroverexpressinggenerequiredforitscatabolism, ren-derstheresistantcellssusceptibletomalignanttransformations andleadstotumorformationinmice.Thisremarkablephenotype seemstoinvolvesignalingthroughthehyaluronanreceptorCD44. TheintracytoplasmicpartofCD44interactswithNF2,which par-ticipatesinapathwaymediatingcontactinhibition.Inaddition,the affinityofCD44tohyaluronaninnakedmoleratcellsistwicethatin mouseorhumancells.TRMdoexpressCD44,whichisahallmarkof
memoryTlymphocytes,raisingthepossibilitythatthehyaluronan effectmayalsobemediatedpartlybyimmunecells.
However,tothebestofourknowledge,nostudieshaveyetbeen carriedoutonthenakedmolerateimmunesystem,with investi-gatorsinsteadfocusingincell-intrinsiccluestocancerresistance ratherthan onextrinsicfactors,suchas theimmunesystemin relationtoECM.
4.5. Hyaluronanscanalsobeinflammatory
Hyaluronandegradationproductsatinjurysitescanstimulate theexpression ofinflammatory genes byvarious immune cells (Jiangetal.,2007).CD44seemstoberequiredfortheclearance ofhyaluronandegradationproductsinlunginjuryand transplan-tation,in which hyaluronan clearance maybeimpaired bythe absenceofdraininglymphvesselsinthegraft,resultingin persis-tentinflammationandrejection(JiangandNicolls,2014;Maltzman etal.,2015).Intype1diabetes,autoimmuneinsulitisisassociated withtheislet-specificdepositionofhyaluronan,whereasthe inhi-bitionofhyaluronansynthesispreventsthediseaseinmice(Nagy etal.,2015).HyaluronanfragmentsusebothToll-likereceptor(TLR) 2 and TLR4 tostimulate theexpression of inflammatory genes in macrophages(Scheibneret al.,2006).Low-molecularweight hyaluronanfragmentsandingeneraldegradationsproductsofECM (matrikines)arethereforecandidatesforadirectrolein inflammag-ing,mediateddirectlyorindirectlyasDAMPsthroughtheimmune system(Evankoetal.,2012;GaggarandWeathington,2016).
ECMalterations mayhaveindirectpro-inflammatory effects, bydisrupting theinteractionwithcellintegrins responsiblefor connectingthecellsurfacetotheactinnetwork.Interestingly,in dendriticcells,theabsenceofbeta2-integrin-mediated cytoskele-talorganizationleadstomembranecompartmentalizationandan absenceofassociationoftheGM-CSFreceptorwithactin, result-inginhigherlevelsofsignalingviathis receptorandconferring amigratorymaturationphenotypeondendriticcells, leadingto theTh1primingofnaiveTcellsandanhigherneutrophilsurvival (Morrisonetal.,2014).
4.6. ECM,mechanotransductionandthemobilityofimmunecells Besidesthemechanicalstressofthenucleusmentionedbefore, cells can be sensitive toECM ageing through others pathways importantforcellmigration(Friedletal.,2011).Themechanism by which cells sense ECM stiffness is called
mechanotransduc-Loss of T-cells/ Lymphopenia
Inflammasome activation
-Hereditary defects of cell mobility -HIV infections
- Immunosupressive regimens -Chemotherapeutics -Ageing Homeostatic proliferation of T-cells TCM Decreased mobility in tissue
Viral reactivations CMV, EBV, etc.
Autoimmunity
-inflammaging
- decrease of naive T-cell compartment -inflation of memory T-cells TEMRA - decreased TCR diversity -increase of T-cells TCM
-CD4/CD8 ration inversion linked to antiviral responsesTCM
Immunosenesce
nce in blood
ECM al
terat
ions
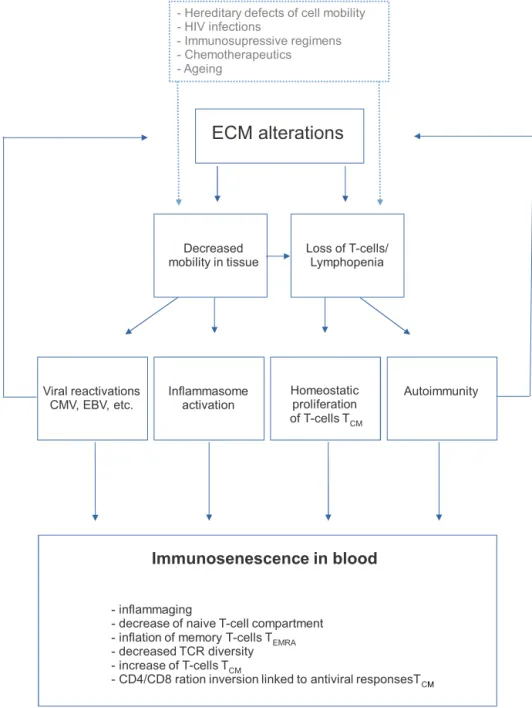
Fig.2. Roleofextracellularmatrixalterationsinimmunosenescence.TheincreaseinECMcross-linkingwithagingplacesconstraintsonthemobilityofimmunecells, accountingforthephenotypeassociatedwithaging.Othersituationsoftenencounteredinclinicalpracticemayalsoleadtothisphenotype(hereditarydefectsofcell mobility,andTcelldepletionasinHIVinfection,immunosuppressivetreatmentsorchemotherapy).
tion(Iskratschetal.,2014).Mechanotransductionplaysakeyrole inadjustingECMmechanicstocellbehaviororfunction,mostly through integrins (Humphrey et al., 2014).For this reason, 2D invitroexperimentalsettingsarenotentirelyrepresentativeof3D situationsinvivo,asreportedinpreviousstudies(Harunagaand Yamada,2011;Hortonetal.,2016).Mechanotransductionisalsoa potenttriggerofepithelialmesenchymaltransition(EMT)(Nelson andBissell,2006;BissellandHines,2011;ArendtandKuperwasser, 2015).Italsoplaysawidelyacceptedandstudiedrolein develop-ment(EMTtype1)(Dupontetal.,2011;Halderetal.,2012),(Piccolo, 2012;HeisenbergandBellaiche,2013;Porazinskietal.,2015).It hasbeencloselylinkedtotheprogressionofcancerstometastasis (EMTtype3)andimplicatedincancerinitiation(Seoetal.,2015; ArendtandKuperwasser,2015;BissellandHines,2011),butrarely associatedwithwoundhealing(EMTtype2),andimmunology.
Immunecellshaveanumberofspecificfeaturesofimportance in this context, and theirintrinsic mobility is closely linkedto
surveillance,asillustratedbythedescriptionsof immunodeficien-ciesprovidedabove.LifeonEarthbeganwithsinglecells,some of which much later,grouped togetherand evolvedinto meta-zoans(DaviesandLineweaver,2011).Inmulticellularity,thereis aneedforcellstoanchorthemselvestogethertoachieve mechan-icalcoherence.Wecanstillseeevidenceofthestepsleadingto thedevelopmentofcomplexmulticellularindividuals from sin-glecells, in intermediateforms, fromChlamydomonas toVolvox (Kirk,2005)(SheltonandMichod,2014).Fromthismodel, exper-imentaldatashowthatECMplaysastrikingroleinthisprocess (HallmannandKirk,2000).Noinformationabouttheroleofthe ECMinimmunesystembiologyisavailable,withtheexception ofsecondarylymphoidorganphysiology,whichisnotconsidered here(Kastenmülleretal.,2012).Mobilecellsmightthereforebe expected to have evolved specific mechanismsmodulating the consequencesofanchoragewithintheECM.Anunderstandingof thesemechanisms would greatlyimprove theway we seeand
understandimmunityandthepathophysiologyofmanydiseases, includingautoimmune(Sofatetal.,2015)andinfectiousdiseases andofcourseaging.
Insummary,themechanismsofECMcross-linkinginagingare wellestablished,buttheireffectsonimmunecellmobilityinthe bodyremainlargelyunknown.Givenrecentfindingsformemory residentTcells andthefunctionalimportanceofcelltrafficking betweenlymphnodes,bloodand,aboveall,withintissues,we sug-gestthatalinkbetweenthesetwoaspectscouldaccountforthe featuresassociatedwithimmunosenescence.
5. ConsequencesofthelowermobilityofT-lymphocytes andtheirhigherdeathrate
5.1. Necrosis,apoptosis,pyroptosisandinflammasomeactivation The preservation or loss of membrane integrity in dying cells determines whether cell death is inflammatory (Wallach etal.,2016).Someofcellcomponentsleakingoutofcells have beenidentifiedasdamage-associatedmolecularpatterns(DAMPs). These components,together withpathogen-associated molecu-larpatterns(PAMPs),constitutethegeneric“dangersignals”that, accordingtothedangertheory(Matzinger,2002),aresensedby dendriticcells, leadingtoanupregulationoftheirexpressionof costimulatorymolecules on theantigen-presenting cell surface (PradeuandCooper,2012;Konoetal.,2014)necessaryfornaive Tcellactivation.Conversely,membraneintegrityismaintainedfor awhileduringearlyapoptosis,beforecaspase-mediated fragmen-tation.Thisallowsthemacrophagestoengulfandclearthedanger signals,thus preventinginadequateactivation of Tcells (Green etal.,2009).However,iftoomanyapoptoticcellsareproduced, overwhelmingtheclearancecapacityofthemacrophages,orifthis capacityisdecreasedforsomereason,thenapoptoticcellsmight notbeclearedrapidlyenough,resultingintheleakingofapoptotic bodies.ThesebodieswouldreleaseDAMPs,resultinginahighly inflammatoryenvironment.Fromourhypothesis,wecanpredict thatthesetwosituationswouldoccurinsynergyoverlong peri-odsduringaging,asECMremodelingwouldseverelyimpairthe mobilityoflymphocytesandmacrophages,ultimatelyleadingto thedeathofthesecellsinsituinresponsetomechanicalstress. Othertriggersmayalsobeimportant.Inparticular,HighMobility GroupBox1(HMGB1)isanuclearproteinreleasedbynecroticcells thatpromotescytokinereleasebyinteractinginflammatorycell recruitmentviaTLR4andCXCL12cellmigration(Schiraldietal., 2012).Thisandotherexamples(Limetal.,2015;Vacchellietal., 2015)demonstratethatinflammationmaybecloselyconnected toimpairedmobility,potentiallyleadingtotheestablishmentofa viciouscircleinthecontextsofagingandECMmodifications.
Furthermore,thereleaseofIL1betainducesahighly inflamma-toryformofcelldeathknownaspyroptosis,whichhasrecently beenshowntoaccountforthemassiveTcelllossand inflamma-torystatusofHIVpatients(Doitshetal.,2014).Theinnatemolecular partoftheimmunesystemsensestheseotherwisehiddencell com-ponents(Davisetal.,2011;LamkanfiandDixit,2012), whereas cells,suchasmacrophages,drivea viciouscirclebyresponding toIL1andfurtherdegradingtheECMleadingto“macroph-aging” (Franceschietal.,2000b).IL1betaandIL18areprototypical inflam-matorycytokinessecretedfollowingcytoplasmiccleavageofthe correspondingproproteinsbycaspase-1activatedfollowing poly-merisationofinflammasome. Thetranscriptionalactivation and expressionoftheseproproteinsandofinflammasomecomponents followssignalingthroughTLRandcytokinesindiversecells[for detailssee(Martinonetal.,2009)].IL1isthensecreted,activating signalingviaitsreceptor,throughtheNF-Bpathway,and trig-geringtheinflammatoryprogramintargetcells(Mathewsetal.,
2008).Sixtypesofinflammasomeshavebeendescribedinhumans, eachessentiallyspecificforanarrayofPAMPsorDAMPs,mostof whichareabundantmoleculeswithimportantfunctions,enabling theinflammasometosensecellularinjuries.Forexample,ATP,RNA, DNA,cholesteroldepositionandcrystalsareknowntoactivatethe NLRP3inflammasome,whichplaysamajorrolein atherosclero-sis(Zhengetal.,2014).Interestingly,theinflammasomehasalso beenreportedtosenseactindynamics,whichisessentialforthe detectionofintracellularpathogens(Kimetal.,2015).Asdescribed above,thelow-molecularweightproductsofhyaluronan break-down,aresultofECMinjury,bindtoTLR2,inducingproductionof pro-IL1andpro-IL18(Scheibneretal.,2006).Inflammasomeshave alsobeenshowntoacceleratethedeclineofthymicfunction(Youm etal.,2012).Insummary,thisarrayofobservationsshowsthetight intricacywhichexistsbetweenECMandinflammation,pledging foritsconsiderationinimmunosenescenceandaging.
Withinthisinflammatoryframework,thehighlevelsofIL6 con-sistentlyobservedinthebloodoftheelderlymaydirectlyreflect thenuclearstressresultingfromtheECMremodeling.Indeed,the magnitudeofnucleardeformationisrelatedtoexpressionlevelsfor aspecificarrayofgenes,themosttranscribedofwhicharehistones H4(A-D)andH3F,butalsoIL6(LeBerreetal.,2012).Nuclear enve-loperupturehasbeenshowntocauseDNAbreakageandrepairthat mightcontributetotheDNAdamageresponse(Zhangetal.,2015; Raabetal.,2016),butexchangesofmaterialbetweenthecytoplasm andnucleusmightalsoprovideasourceofinternalDAMPsdirectly sensedalongthesevariouspathways.
Overall,theubiquitousECMmodificationsassociatedwith col-lagenglycationandcross-linkingareprobablydirectlyorindirectly followedbyaseriesofeventsleadingtothechronicproductionof highlypotentinflammatorycytokines,underlyingthe inflammag-inganditsconsequencesseenintheelderly.
5.2. Tlymphocytedepletionanditslinktohomeostatic proliferationandautoimmunity
ChronicTcelllossinducesthreehighlyregulatedprocessesof Tcellreplenishmentinmammalswhichare:1)thematureTcell egressfromthethymus,2)theclonalamplificationofcellsengaged inanimmuneresponse,and3)thehomeostaticproliferationofT cells.
Intheelderly,asthymicfunctionisabsent,Tcellcompartment replenishmentisdependentexclusivelyonhomeostatic prolifer-ation.Inthisprocess,existingTcellsproliferateintheabsenceof exogenousantigen,duetotheirintrinsicself-recognition proper-tiesresultingfromtheirpreviouspositiveandnegativeselection in thethymus(Vrisekoopet al.,2008; den Braber etal., 2012; Johnson etal., 2012).Homeostatic proliferation may,therefore, alsobelinkedtothedevelopmentofauto-immunity(Goronzyand Weyand,2012)astheTcellrepertoireisbuiltonaprincipleof basicbutlimitedrecognitionofself(Mason,1998),knownas auto-reaction[see(Pradeu,2012)].
Innormaladults,thisbasalautoreactivestatedoesnotleadto auto-immunediseasesbecauseofseveralmechanisms,collectively called“peripheraltolerance”, butmostly involvingregulatoryT cells,whichinhibiteffectorTcellfunctionandwhichhavebeen showntoaccumulatewithage(Sharmaetal.,2007).
Most of our insight into T cell dynamics replenishment originatesfromanalysesofTcellreconstitutionintheblood fol-lowingperipherallymphopenia,asobservedduringHIVinfection, chemotherapy totreat cancer, transplantation and aging. Lym-phopenia is known to break tolerance (Jones et al., 2013), as highlightedbyreportsforhematopoieticstemcelltransplantation (Matsuokaetal.,2010).Intheseconditions,theTcellswiththe highestaffinityforMHCplusself-peptidesproliferatefasterthan
thosewithaloweraffinityleadingtodysregulatedimmunesystem activation.
Inmousemodels,homeostaticproliferationafterlymphopenia alsoinducesthespontaneousproliferationofnaiveandmemory Tcells butwithlittleauto-immunity(LeCampionet al.,2009), becauseoftheconcomitantexpansionofTregulatorycells(Tregs) tocontrolthis phenomenon(Piccaetal.,2006).However,ifthe expansionsofthesetwopopulationsweretobedissociated,then transientauto-immunedisordersarise.Thisiswhatisobservedin theimmunereconstitutioninflammatorysyndrome(IRIS),found inHIV-infectedpatientswithlowCD4+Tcellcountsgivenhighly activeantiretroviraltherapy(Shelburneetal.,2005)orinNODmice (LeCampionetal.,2009).
Tregsinteractwithdendriticcellsinthelymphnodes,inwhich theysuppresseffectorTcellpriming,subsequentlymigratingto non-lymphoidtissues,inwhichtheysuppresseffectorTcell func-tionslocally.ThesuppressionexertedbyTregsisnotspecifictothe antigen;itis,instead,highlydependentoncolocalizationwiththe effectorTcellstobesuppressed(Antunesetal.,2008).Tregsmigrate rapidlyfromthebloodtositesofinflammation,highlightingtheir strongdependenceonanormalmigratorycapabilitytomediate theirsuppressivefunction.Changesin theirmigration capacity, duetotheECMalterationsknowntooccurinaging,crippletheir regulatoryfunctions,leadingtohigherlevelsof auto-immunity. Tregsuppressivefunctionis,thus,highlydependentoneffective migrationmechanisms,whichmaybedisruptedbyECMalteration, therebyexacerbatinginflammatoryprocessesandpartly account-ingforage-relatedauto-immunity.
6. Conclusion
Tcellsarehighlymobilecellswithfunctionsinimmunitythat arehighlydependentontheirabilitytomigrateparticularlyfor thoseresidingintissues.WeargueherethatchangestoTcell migra-tioncapacityduetowell-characterizedECMchangesduringaging mayplayakeyroleintheagingprocess,bycripplinginteractions betweenimmune cells and preventingtheirtrafficking (Fig.2). Studiesofhereditaryimmunodeficienciesinvolvingalackof effi-cientactinremodelinghaveshownthatTcelllossresultsfromthe deathofmigratorycells.InadditiontotheconsequencesofTcell deathforinflammation,theprogressivedepletionofTcellsleads toviralreactivation(herpesvirus)andtriggersmechanismsofT cellreplenishmentthatmayleadtosomedegreeofautoimmunity. Thesemechanismsprovideinformation abouttheconsequences ofECMremodelinginfundamentalimmunologyaswellassome explanationfor immunosenescence,but theymayalsoserve as appropriatetreatmenttargets.Earlyin2015,twostudies convinc-inglyshowedthatprovidingthehostwithTcellsagainsttumorsin thecontextofascaffoldmatrixcreatedafavorableenvironmentfor thegenerationofeffectivehumoralandcellularimmuneresponses totumorantigens(Stephanetal.,2015;WeberandMulé,2015). Thisobservationreflectsalsotheexistenceoftertiaryectopic lym-phoidorgans,insynovialtissuefromrheumatoidarthritispatients forexample(Weyandetal.,2003),demonstratinghereagain,the three-dimensionalnatureofimmunity.
Conflictsofinterest
Noneoftheauthorshaveanyconflictofinteresttodeclare.
Acknowledgments
We would like to thank Maria Mamani-Matsuda, Myriam Capone, JenniferHoward, LynnChiu and Maureen O’Malleyfor helpfuldiscussionsandrevisionofthemanuscript.
ThomasPradeureceivedfundingfromtheEuropeanResearch Council(ERC)undertheEuropeanUnion’sHorizon2020research andinnovationprogram−grantagreementno.637647-IDEM.
References
Antunes,I.,Tolaini,M.,Kissenpfennig,A.,Iwashiro,M.,Kuribayashi,K.,Malissen,B., Hasenkrug,K.,Kassiotis,G.,2008.Retrovirus-Specificityofregulatorytcellsis neitherpresentnorrequiredinpreventingretrovirus-inducedbonemarrow immunepathology.Immunity29,782–794,http://dx.doi.org/10.1016/j. immuni.2008.09.016.
Arantes-Oliveira,N.,2003.Healthyanimalswithextremelongevity.Science302,
http://dx.doi.org/10.1126/science.1089169,611–611.
Arendt,L.M.,Kuperwasser,C.,2015.Workingstiff:howobesityboostscancerrisk. Sci.Transl.Med.7,http://dx.doi.org/10.1126/scitranslmed.aac9446, 301fs34–301fs34.
Ariotti,S.,Beltman,J.B.,Chodaczek,G.,Hoekstra,M.E.,vanBeek,A.E.,
Gomez-Eerland,R.,Ritsma,L.,vanRheenen,J.,Marée,A.F.M.,Zal,T.,deBoer, R.J.,Haanen,J.B.A.G.,Schumacher,T.N.,2012.Tissue-residentmemoryCD8+T cellscontinuouslypatrolskinepitheliatoquicklyrecognizelocalantigen.Proc. Natl.Acad.Sci.109,19739–19744,http://dx.doi.org/10.1073/pnas.
1208927109.
Bailey,A.J.,Paul,R.G.,Knott,L.,1998.Mechanismsofmaturationandageingof collagen.Mech.AgeingDev.106,1–56, http://dx.doi.org/10.1016/S0047-6374(98)00119-5.
Bailey,A.J.,2001.Molecularmechanismsofageinginconnectivetissues.Mech. AgeingDev.122,735–755,http://dx.doi.org/10.1016/S0047-6374(01)00225-1. Bella,J.,2016.Collagenstructure:newtricksfromaveryolddog.Biochem.J.473,
1001–1025,http://dx.doi.org/10.1042/BJ20151169.
Bissell,M.J.,Hines,W.C.,2011.Whydon’twegetmorecancer?Aproposedroleof themicroenvironmentinrestrainingcancerprogression.Nat.Med.17, 320–329,http://dx.doi.org/10.1038/nm.2328.
Boasso,A.,Shearer,G.M.,2008.Chronicinnateimmuneactivationasacauseof HIV-1immunopathogenesis.Clin.Immunol.126,235–242,http://dx.doi.org/ 10.1016/j.clim.2007.08.015.
Boger,D.L.,2015.Whensugarisnotsosweet.Science350,275–276,http://dx.doi. org/10.1126/science.aad3298.
Bonnans,C.,Chou,J.,Werb,Z.,2014.Remodellingtheextracellularmatrixin developmentanddisease.Nat.Rev.Mol.CellBiol.15,786–801,http://dx.doi. org/10.1038/nrm3904.
Brenchley,J.M.,Karandikar,N.J.,Betts,M.R.,Ambrozak,D.R.,Hill,B.J.,Crotty,L.E., Casazza,J.P.,Kuruppu,J.,Migueles,S.A.,Connors,M.,Roederer,M.,Douek,D.C., Koup,R.A.,2003.ExpressionofCD57definesreplicativesenescenceand antigen-inducedapoptoticdeathofCD8+Tcells.Blood101,2711–2720,http:// dx.doi.org/10.1182/blood-2002-07-2103.
Brock,M.A.,Chrest,F.,1993.Differentialregulationofactinpolymerization followingactivationofrestingTlymphocytesfromyoungandagedmice.J. Cell.Physiol.157,367–378,http://dx.doi.org/10.1002/jcp.1041570221. Burtner,C.R.,Kennedy,B.K.,2010.Progeriasyndromesandageing:whatisthe
connection?Nat.Rev.Mol.CellBiol.11,567–578,http://dx.doi.org/10.1038/ nrm2944.
Bustin,M.,Misteli,T.,2016.Nongeneticfunctionsofthegenome.Science352, 671–678,http://dx.doi.org/10.1126/science.aad6933.
Campisi,J.,2013.Aging,cellularsenescence,andcancer.Annu.Rev.Physiol.75, 685–705,http://dx.doi.org/10.1146/annurev-physiol-030212-183653. Carbone,F.R.,2015.Tissue-residentmemorytcellsandfixedimmunesurveillance
innonlymphoidorgans.J.Immunol.195,17–22,http://dx.doi.org/10.4049/ jimmunol.1500515.
Cevenini,E.,Invidia,L.,Lescai,F.,Salvioli,S.,Tieri,P.,Castellani,G.,Franceschi,C., 2008.Humanmodelsofagingandlongevity.ExpertOpin.Biol.Ther.8, 1393–1405,http://dx.doi.org/10.1517/14712598.8.9.1393.
Chen,Y.,Terajima,M.,Yang,Y.,Sun,L.,Ahn,Y.-H.,Pankova,D.,Puperi,D.S., Watanabe,T.,Kim,M.P.,Blackmon,S.H.,Rodriguez,J.,Liu,H.,Behrens,C., Wistuba,I.I.,Minelli,R.,Scott,K.L.,Sanchez-Adams,J.,Guilak,F.,Pati,D., Thilaganathan,N.,Burns,A.R.,Creighton,C.J.,Martinez,E.D.,Zal,T.,
Grande-Allen,K.J.,Yamauchi,M.,Kurie,J.M.,2015.Lysylhydroxylase2induces acollagencross-linkswitchintumorstroma.J.Clin.Invest.125,1147–1162,
http://dx.doi.org/10.1172/JCI74725.
Cicin-Sain,L.,Messaoudi,I.,Park,B.,Currier,N.,Planer,S.,Fischer,M.,Tackitt,S., Nikolich-ˇZugich,D.,Legasse,A.,Axthelm,M.K.,Picker,L.J.,Mori,M.,
Nikolich-ˇZugich,J.,2007.DramaticincreaseinnaïveTcellturnoverislinkedto lossofnaïveTcellsfromoldprimates.Proc.Natl.Acad.Sci.104,19960–19965,
http://dx.doi.org/10.1073/pnas.0705905104.
Clark,R.A.,2015.ResidentmemoryTcellsinhumanhealthanddisease.Sci.Transl. Med.7,http://dx.doi.org/10.1126/scitranslmed.3010641,269rv1–269rv1. Cohen,J.,2015.Death-defyingexperiments.Science350,1186–1187,http://dx.doi.
org/10.1126/science.350.6265.1186.
Conboy,I.M.,Rando,T.A.,2012.Heterochronicparabiosisforthestudyofthe effectsofagingonstemcellsandtheirniches.CellCycle11,2260–2267,http:// dx.doi.org/10.4161/cc.20437.
Conboy,I.M.,Conboy,M.J.,Wagers,A.J.,Girma,E.R.,Weissman,I.L.,Rando,T.A., 2005.Rejuvenationofagedprogenitorcellsbyexposuretoayoungsystemic environment.Nature433,760–764,http://dx.doi.org/10.1038/nature03260.
Coté,J.-F.,Vuori,K.,2007.GEFwhat?Dock180andrelatedproteinshelpRacto polarizecellsinnewways.TrendsCellBiol.17,383–393,http://dx.doi.org/10. 1016/j.tcb.2007.05.001.
Davies,P.C.W.,Lineweaver,C.H.,2011.CancertumorsasMetazoa1.0:tapping genesofancientancestors.Phys.Biol.8,15001,http://dx.doi.org/10.1088/ 1478-3975/8/1/015001.
Davis,B.K.,Wen,H.,Ting,J.P.-Y.,2011.TheinflammasomeNLRsinimmunity, inflammation,andassociateddiseases.Annu.Rev.Immunol.29,707–735,
http://dx.doi.org/10.1146/annurev-immunol-031210-101405.
Denais,C.M.,Gilbert,R.M.,Isermann,P.,McGregor,A.L.,teLindert,M.,Weigelin,B., Davidson,P.M.,Friedl,P.,Wolf,K.,Lammerding,J.,2016.Nuclearenvelope ruptureandrepairduringcancercellmigration.Science352,353–358,http:// dx.doi.org/10.1126/science.aad7297.
denBraber,I.,Mugwagwa,T.,Vrisekoop,N.,Westera,L.,Mögling,R.,Bregjede Boer,A.,Willems,N.,Schrijver,E.H.R.,Spierenburg,G.,Gaiser,K.,Mul,E.,Otto, S.A.,Ruiter,A.F.C.,Ackermans,M.T.,Miedema,F.,Borghans,J.A.M.,deBoer,R.J., Tesselaar,K.,2012.MaintenanceofperipheralnaiveTcellsissustainedby thymusoutputinmicebutnothumans.Immunity36,288–297,http://dx.doi. org/10.1016/j.immuni.2012.02.006.
Doitsh,G.,Galloway,N.L.K.,Geng,X.,Yang,Z.,Monroe,K.M.,Zepeda,O.,Hunt,P.W., Hatano,H.,Sowinski,S.,Mu ˜noz-Arias,I.,Greene,W.C.,2014.Celldeathby pyroptosisdrivesCD4T-celldepletioninHIV-1infection.Nature505, 509–514,http://dx.doi.org/10.1038/nature12940.
Draghici,C.,Wang,T.,Spiegel,D.A.,2015.Concisetotalsynthesisofglucosepane. Science350,294–298,http://dx.doi.org/10.1126/science.aac9655.
Dupont,S.,Morsut,L.,Aragona,M.,Enzo,E.,Giulitti,S.,Cordenonsi,M.,Zanconato, F.,Digabel,J.L.,Forcato,M.,Bicciato,S.,Elvassore,N.,Piccolo,S.,2011.Roleof YAP/TAZinmechanotransduction.Nature474,179–183,http://dx.doi.org/10. 1038/nature10137.
Eaton,S.M.,Maue,A.C.,Swain,S.L.,Haynes,L.,2008.Bonemarrowprecursorcells fromagedmicegenerateCD4tcellsthatfunctionwellinprimaryandmemory responses.J.Immunol.181,4825–4831,http://dx.doi.org/10.4049/jimmunol. 181.7.4825.
Elabd,C.,Cousin,W.,Upadhyayula,P.,Chen,R.Y.,Chooljian,M.S.,Li,J.,Kung,S., Jiang,K.P.,Conboy,I.M.,2014.Oxytocinisanage-specificcirculatinghormone thatisnecessaryformusclemaintenanceandregeneration.Nat.Commun.5, 4082,http://dx.doi.org/10.1038/ncomms5082.
Evanko,S.P.,Potter-Perigo,S.,Bollyky,P.L.,Nepom,G.T.,Wight,T.N.,2012. HyaluronanandversicaninthecontrolofhumanT-lymphocyteadhesionand migration.MatrixBiol.31,90–100,http://dx.doi.org/10.1016/j.matbio.2011.10. 004.
Ewald,C.Y.,Landis,J.N.,Abate,J.P.,Murphy,C.T.,Blackwell,T.K.,2014.
Dauer-independentinsulin/IGF-1-signallingimplicatescollagenremodelling inlongevity.Nature519,97–101,http://dx.doi.org/10.1038/nature14021. Föger,N.,Rangell,L.,Danilenko,D.M.,Chan,A.C.,2006.Requirementforcoronin1
intlymphocytetraffickingandcellularhomeostasis.Science313,839–842,
http://dx.doi.org/10.1126/science.1130563.
Fan,X.,Rudensky,A.Y.,2016.Hallmarksoftissue-residentlymphocytes.Cell164, 1198–1211,http://dx.doi.org/10.1016/j.cell.2016.02.048.
Farber,D.L.,Yudanin,N.A.,Restifo,N.P.,2013.HumanmemoryTcells:generation, compartmentalizationandhomeostasis.Nat.Rev.Immunol.14,24–35,http:// dx.doi.org/10.1038/nri3567.
Flurkey,K.,Papaconstantinou,J.,Miller,R.A.,Harrison,D.E.,2001.Lifespan extensionanddelayedimmuneandcollagenaginginmutantmicewith defectsingrowthhormoneproduction.Proc.Natl.Acad.Sci.98,6736–6741,
http://dx.doi.org/10.1073/pnas.111158898.
Franceschi,C.,Campisi,J.,2014.Chronicinflammation(Inflammaging)andits potentialcontributiontoage-associateddiseases.J.Gerontol.A.Biol.Sci.Med. Sci.69,S4–S9,http://dx.doi.org/10.1093/gerona/glu057.
Franceschi,C.,Valensin,S.,Bonafè,M.,Paolisso,G.,Yashin,A.,Monti,D.,De Benedictis,G.,2000.Thenetworkandtheremodelingtheoriesofaging: historicalbackgroundandnewperspectives.Biol.Aging35,879–896,http:// dx.doi.org/10.1016/S0531-5565(00)00172-8.
Franceschi,C.,Bonafè,M.,Valensin,S.,2000a.Humanimmunosenescence:the prevailingofinnateimmunity,thefailingofclonotypicimmunity,andthe fillingofimmunologicalspace.Vaccine18,1717–1720,http://dx.doi.org/10. 1016/S0264-410X(99)00513-7.
Franceschi,C.,Bonafè,M.,Valensin,M.,Olivieri,S.,DeLuca,F.,Ottaviani,M.,De Benedictis,E.,2000b.Inflamm-aging:anevolutionaryperspectiveon immunosenescence.Ann.N.Y.Acad.Sci.908,244–254,http://dx.doi.org/10. 1111/j.1749-6632.2000.tb06651.x.
Franceschi,C.,Bezrukov,V.,Blanché,H.,Bolund,L.,Christensen,K.,Benedictis,G.D., Deiana,L.,Gonos,E.,Hervonen,A.,Yang,H.,Jeune,B.,Kirkwood,T.B.L., Kristensen,P.,Leon,A.,Pelicci,P.G.,Peltonen,L.,Poulain,M.,Rea,I.M.,Remacle, J.,Robine,J.M.,Schreiber,S.,Sikora,E.,Slagboom,P.E.,Spazzafumo,P.E.,Stazi, M.A.,Toussaint,O.,Vaupel,J.W.,2007.Geneticsofhealthyagingineurope. Ann.N.Y.Acad.Sci.1100,21–45,http://dx.doi.org/10.1196/annals.1395.003. Friedl,P.,Wolf,K.,Lammerding,J.,2011.Nuclearmechanicsduringcellmigration.
CellStruct.Dyn.23,55–64,http://dx.doi.org/10.1016/j.ceb.2010.10.015. Fulop,T.,Larbi,A.,Pawelec,G.,2013.Humantcellagingandtheimpactof
persistentviralinfections.Front.Immunol.4,1–9,http://dx.doi.org/10.3389/ fimmu.2013.00271.
Fulop,T.,Dupuis,G.,Baehl,S.,LePage,A.,Bourgade,K.,Frost,E.,Witkowski,J.M., Pawelec,G.,Larbi,A.,Cunnane,S.,2015.Frominflamm-agingto
immune-paralysis:aslipperyslopeduringagingforimmune-adaptation. Biogerontology,http://dx.doi.org/10.1007/s10522-015-9615-7.
Gaggar,A.,Weathington,N.,2016.Bioactiveextracellularmatrixfragmentsinlung healthanddisease.J.Clin.Invest.126,3176–3184,http://dx.doi.org/10.1172/ JCI83147.
Gasteiger,G.,Fan,X.,Dikiy,S.,Lee,S.Y.,Rudensky,A.Y.,2015.Tissueresidencyof innatelymphoidcellsinlymphoidandnonlymphoidorgans.Science350, 981–985,http://dx.doi.org/10.1126/science.aac9593.
Gebhardt,T.,Whitney,P.G.,Zaid,A.,Mackay,L.K.,Brooks,A.G.,Heath,W.R., Carbone,F.R.,Mueller,S.N.,2011.Differentpatternsofperipheralmigrationby memoryCD4+andCD8+Tcells.Nature477,216–219,http://dx.doi.org/10. 1038/nature10339.
Gerlitz,G.,Bustin,M.,2011.Theroleofchromatinstructureincellmigration. TrendsCellBiol.21,6–11,http://dx.doi.org/10.1016/j.tcb.2010.09.002. Goronzy,J.J.,Weyand,C.M.,2005.Tcelldevelopmentandreceptordiversityduring
aging.Curr.Opin.Immunol.17,468–475,http://dx.doi.org/10.1016/j.coi.2005. 07.020.
Goronzy,J.J.,Weyand,C.M.,2012.Immuneagingandautoimmunity.Cell.Mol.Life Sci.69,1615–1623,http://dx.doi.org/10.1007/s00018-012-0970-0.
Gourlay,C.W.,Carpp,L.N.,Timpson,P.,Winder,S.J.,Ayscough,K.R.,2004.Arolefor theactincytoskeletonincelldeathandaginginyeast.J.CellBiol.164, 803–809,http://dx.doi.org/10.1083/jcb.200310148.
Green,D.R.,Ferguson,T.,Zitvogel,L.,Kroemer,G.,2009.Immunogenicand tolerogeniccelldeath.Nat.Rev.Immunol.9,353–363,http://dx.doi.org/10. 1038/nri2545.
Grimm,D.,2015.Whyweoutliveourpets.Science350,1182–1185,http://dx.doi. org/10.1126/science.350.6265.1182.
Groulx,J.-F.,Gagné,D.,Benoit,Y.D.,Martel,D.,Basora,N.,Beaulieu,J.-F.,2011. CollagenVIisabasementmembranecomponentthatregulatesepithelial cell-fibronectininteractions.MatrixBiol.30,195–206,http://dx.doi.org/10. 1016/j.matbio.2011.03.002.
Guevara-Aguirre,J.,Rosenbloom,A.L.,2015.Obesity,diabetesandcancer:insight intotherelationshipfromacohortwithgrowthhormonereceptordeficiency. Diabetologia58,37–42,http://dx.doi.org/10.1007/s00125-014-3397-3. Guilak,F.,Cohen,D.M.,Estes,B.T.,Gimble,J.M.,Liedtke,W.,Chen,C.S.,2009.
Controlofstemcellfatebyphysicalinteractionswiththeextracellularmatrix. CellStemCell5,17–26,http://dx.doi.org/10.1016/j.stem.2009.06.016. Hadrup,S.R.,Strindhall,J.,Køllgaard,T.,Seremet,T.,Johansson,B.,Pawelec,G.,thor
Straten,P.,Wikby,A.,2006.LongitudinalstudiesofclonallyexpandedCD8T cellsrevealarepertoireshrinkagepredictingmortalityandanincreased numberofdysfunctionalcytomegalovirus-specificTcellsintheveryelderly.J. Immunol.176,2645–2653.
Halder,G.,Dupont,S.,Piccolo,S.,2012.Transductionofmechanicaland cytoskeletalcuesbyYAPandTAZ.Nat.Rev.Mol.CellBiol.13,591–600,http:// dx.doi.org/10.1038/nrm3416.
Hale,J.S.,Frock,R.L.,Mamman,S.A.,Fink,P.J.,Kennedy,B.K.,2010.Cell-extrinsic defectivelymphocytedevelopmentinlmna-/-mice.PLoSOne5,e10127,
http://dx.doi.org/10.1371/journal.pone.0010127.
Hallmann,A.,Kirk,D.L.,2000.ThedevelopmentallyregulatedECMglycoprotein ISGplaysanessentialroleinorganizingtheECMandorientingthecellsof Volvox.J.CellSci.113,4605–4617.
Hallmann,R.,Zhang,X.,DiRusso,J.,Li,L.,Song,J.,Hannocks,M.-J.,Sorokin,L.,2015. Theregulationofimmunecelltraffickingbytheextracellularmatrix.Cell Adhes.Migr.36,54–61,http://dx.doi.org/10.1016/j.ceb.2015.06.006. Hamazaki,Y.,Sekai,M.,Minato,N.,2016.Medullarythymicepithelialstemcells:
roleinthymicepithelialcellmaintenanceandthymicinvolution.Immunol. Rev.271,38–55,http://dx.doi.org/10.1111/imr.12412.
Harada,Y.,Tanaka,Y.,Terasawa,M.,Pieczyk,M.,Habiro,K.,Katakai,T.,
Hanawa-Suetsugu,K.,Kukimoto-Niino,M.,Nishizaki,T.,Shirouzu,M.,Duan,X., Uruno,T.,Nishikimi,A.,Sanematsu,F.,Yokoyama,S.,Stein,J.V.,Kinashi,T., Fukui,Y.,2012.DOCK8isaCdc42activatorcriticalforinterstitialdendriticcell migrationduringimmuneresponses.Blood119,4451–4461,http://dx.doi.org/ 10.1182/blood-2012-01-407098.
Harada,T.,Swift,J.,Irianto,J.,Shin,J.-W.,Spinler,K.R.,Athirasala,A.,Diegmiller,R., Dingal,P.C.D.P.,Ivanovska,I.L.,Discher,D.E.,2014.Nuclearlaminstiffnessisa barrierto3Dmigration,butsoftnesscanlimitsurvival.J.CellBiol.204, 669–682,http://dx.doi.org/10.1083/jcb.201308029.
Harunaga,J.S.,Yamada,K.M.,2011.Cell-matrixadhesionsin3D.MatrixBiol.30, 363–368,http://dx.doi.org/10.1016/j.matbio.2011.06.001.
Hayflick,L.,Moorhead,P.,1961.Theserialcultivationofhumandiploidcellstrains. Exp.CellRes.25,585–621.
Heisenberg,C.-P.,Bellaiche,Y.,2013.Forcesintissuemorphogenesisand patterning.Cell153,948–962,http://dx.doi.org/10.1016/j.cell.2013.05.008. Hnisz,D.,Weintraub,A.S.,Day,D.S.,Valton,A.-L.,Bak,R.O.,Li,C.H.,Goldmann,J.,
Lajoie,B.R.,Fan,Z.P.,Sigova,A.A.,Reddy,J.,Borges-Rivera,D.,Lee,T.I.,Jaenisch, R.,Porteus,M.H.,Dekker,J.,Young,R.A.,2016.Activationofproto-oncogenes bydisruptionofchromosomeneighborhoods.Science351,1454–1458,http:// dx.doi.org/10.1126/science.aad9024.
Hogquist,K.A.,2008.Immunodeficiency:whenTcellsarestuckathome.Nat. Immunol.9,1207–1208,http://dx.doi.org/10.1038/ni1108-1207.
Honda,T.,Egen,J.G.,Lämmermann,T.,Kastenmüller,W.,Torabi-Parizi,P.,Germain, R.N.,2014.Tuningofantigensensitivitybytcellreceptor-dependentnegative feedbackcontrolstcelleffectorfunctionininflamedtissues.Immunity40, 235–247,http://dx.doi.org/10.1016/j.immuni.2013.11.017.
Horton,E.R.,Astudillo,P.,Humphries,M.J.,Humphries,J.D.,2016.
Mechanosensitivityofintegrinadhesioncomplexes:roleoftheconsensus adhesome.Exp.CellRes.343,7–13,http://dx.doi.org/10.1016/j.yexcr.2015.10. 025.