HAL Id: hal-02991781
https://hal.archives-ouvertes.fr/hal-02991781
Submitted on 27 Nov 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
tooth apatite
Nicolas Séon, Romain Amiot, Jérémy Martin, Mark Young, Heather
Middleton, François Fourel, Laurent Picot, Xavier Valentin, Christophe
Lécuyer
To cite this version:
Nicolas Séon, Romain Amiot, Jérémy Martin, Mark Young, Heather Middleton, et al.. Thermophys-iologies of Jurassic marine crocodylomorphs inferred from the oxygen isotope composition of their tooth apatite. Philosophical Transactions of the Royal Society B: Biological Sciences, Royal Society, The, 2020, 375 (1793), pp.20190139. �10.1098/rstb.2019.0139�. �hal-02991781�
For Review Only
Thermophysiologies of Jurassic marine crocodylomorphs inferred
from the oxygen isotope composition of their tooth apatite
Nicolas Séon1, Romain Amiot1,*, Jeremy E. Martin1, Mark T. Young2, Heather Middleton3,
François Fourel4, Laurent Picot5, Xavier Valentin6,7, Christophe Lécuyer1
1UMR 5276, Laboratoire de Géologie de Lyon, Terre, Planètes et Environnement, Université Claude Bernard Lyon 1/CNRS/École Normale Supérieure de Lyon, 69622 Villeurbanne Cedex, France; nicolasseon@orange.fr ; romain.amiot@univ-lyon1.fr; jeremy.martin@ens-lyon.fr; christophe.lécuyer@univ-lyon1.fr
2 School of GeoSciences, Grant Institute, University of Edinburgh, James Hutton Road, Edinburgh,
EH9 3FE, UK; mark.young@ed.ac.uk
3 16 Rodwell Road, Weymouth, Dorset, DT4 8QL, UK; heathermidd@yahoo.co.uk
4 Laboratoire d'Ecologie des Hydrosystèmes Naturels et Anthropisés, CNRS UMR 5023, Université
Claude Bernard Lyon 1, France; francois.fourel@univ-lyon1.fr
5 Paleospace, Avenue Jean Moulin, 14640 Villers sur mer, France; l.picot@paleospace-villers.fr 6 Laboratoire PALEVOPRIM, UMR 7262 CNRS INEE & University of Poitiers, France; xavier.valentin@univ-poitiers.fr
7 Palaios, Research Association, 86300 Valdivienne, France * Corresponding author
Keywords: Metriorhynchidae, Teleosauridae, Jurassic, Thermophysiology, Oxygen and carbon
isotopes, tooth apatite
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
SummaryTeleosauridae and Metriorhynchidae were thalattosuchian crocodylomorph clades that secondarily adapted to marine life and coexisted during the Middle to Late Jurassic. While
teleosaurid diversity collapsed at the end of the Jurassic, most likely as a result of a global cooling of the oceans and associated marine regressions, metriorhynchid diversity was largely unaffected, although the fossil record of Thalattosuchia is poor in the Cretaceous. In order to investigate the possible differences in thermophysiologies between these two thalattosuchian lineages, we analysed stable oxygen isotope compositions (expressed as δ18O values) of tooth apatite from metriorhynchid
and teleosaurid specimens. We then compared them to the δ18O values of coexisting
endo-homeothermic ichthyosaurs and plesiosaurs, as well as ecto-poïkilothermic chondrichthyans and osteichthyans. The distribution of δ18O values suggests that both teleosaurids and metriorhynchids
had body temperatures intermediate between those of typical ecto-poikilothermic vertebrates and warm-blooded ichthyosaurs and plesiosaurs, metriorhynchids being slightly warmer than
teleosaurids. We propose that metriorhynchids were able to raise their body temperature above that of the ambient environment by metabolic heat production, as endotherms do, but could not maintain a constant body temperature compared to fully homeothermic ichthyosaurs and plesiosaurs.
Teleosaurids, on the other hand, may have raised their body temperature by mouth-gape basking, as modern crocodilians do, and benefited from the thermal inertia of their large body mass to maintain their body temperature above ambient one. Endothermy in metriorhynchids might have been a byproduct of their ecological adaptations to active pelagic hunting, and it probably allowed them to survive the global cooling of the Late Jurassic, thus explaining the selective extinction affecting Thalattosuchia at the Jurassic-Cretaceous boundary.
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
IntroductionExtant crocodylians are archosaurs that rely on environmental sources of heat in order to
raise and maintain their body temperature by behavioural thermoregulation in a restricted range bracketed by “critical minimum” and “critical maximum” temperatures (Cowles and Bogert, 1944; Pough and Gans, 1982). Within this critical range, crocodylians tend to keep their body temperature within a narrower activity range from about 25°C to 40°C as determined empirically for a few extant species (see Markwick (1998) for a review). Consequently, the spatial and temporal
distribution of crocodylians is limited by the temperatures of their living environments and by their seasonal fluctuations. Due to their rather conservative growth morphology and their restricted latitudinal distribution today, and in the geologic record, extant and fossil representatives of the crown group have been used as climate proxies for more than a century (Berg, 1965; Crichton, 1825; Markwick, 1998; Owen, 1850). Based on observations of extant representatives of crown group Crocodylia Owen, 1842, Markwick (1998) proposed that the occurrence of fossil
representatives imply a living Mean Annual Air temperature (MAT) ≥14.2°C and a Coldest Month Mean temperature (CMM) ≥5.5°C. These minimum limits have been tentatively used to constrain the climatic environment of long-extinct crocodylomorphs (Amiot et al., 2011), as well as the more distantly related clade Choristodera (Tarduno et al., 1998).
The crocodylomorph clade Thalattosuchia became secondarily adapted to marine life during the Mesozoic, and is subdivided into the families Teleosauridae Saint-Hilaire, 1831 and
Metriorhynchidae Fitzinger, 1843. While teleosaurids retained a morphology reminiscent of typical semi-aquatic longirostrine crocodylomorphs, in having extensive osteoderm coverage and limbs adapted for terrestrial locomotion (Eudes-Deslongchamps, 1869), metriorhynchids had a more hydrodynamic body plan, with a hypocercal tail, and hydrofoil-like forelimbs (Fraas, 1902; Young et al., 2010). The bone histology of Callovian teleosaurids and metriorhynchids has been used to hypothesize that thalattosuchians were ectothermic (i.e. they rely on environmental sources of heat in order to raise their body temperature) and poikilothermic (i.e. their body temperature vary along
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
with that of their environment), with teleosaurids being capable of mouth-gape basking on shore, whereas metriorhynchids thermoregulated differently by staying close to the water surface (Hua and De Buffrénil, 1996). Hua and de Buffrénil (1996) did note that young metriorhynchids may have had a faster growth rate than wild extant crocodylians.
Martin et al. (2014) analysed the diversity of marine crocodylomorphs through the Mesozoic and Paleogene, and observed a significant correlation between Sea Surface Temperatures (SST) and generic diversity within four lineages, including the teleosaurids. Teleosaurids, at least European representatives, experienced a diversity crash at the end of the Jurassic during a global cooling of Tethyan waters (Lécuyer et al., 2003b), but according to Fanti et al. (2016) they may have
continued to survive until at least the Hauterivian along the southern coast of the Tethys Sea. Metriorhynchids however, appear to have experienced an explosive radiation during the Callovian, surviving through the Late Jurassic until the Aptian (Chiarenza et al., 2015). When metriorhynchids became extinct is currently unclear. Young et al. (2010) hypothesized a two-step extinction for
Metriorhynchidae, first a diversity crash at the end Jurassic, then a final extinction during the cold
icehouse interval of the Valanginian (Pucéat et al., 2003). However, recent re-evaluations of
Cretaceous fossils and updated phylogenetic studies have disproved both steps of this hypothesis,
with post-Valanginian metriorhynchid specimens known and no fewer than four metriorhynchid
lineages crossed the Jurassic-Cretaceous boundary (Chiarenza et al., 2015; Ősi et al., 2018; Young
et al., 2014a). The global thalattosuchian fossil record however is still poor, the known diversity of this clade is still heavily biased by the European rock record, as well as global marine record sampling biases for specific time spans (e.g. Aalenian, Oxfordian, Early Cretaceous). Regardless of the geologic megabiases, there is still a conspicuous diversity mismatch within Thalattosuchia that raised the question whether metriorhynchids evolved a distinct thermoregulatory strategy, such as endothermic capabilities that would explain their diversity and survival under cool SSTs (Martin et al., 2014). 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
In order to investigate the thermophysiology of metriorhynchids, we analysed the oxygen isotope composition of the enamel phosphate of their teeth (δ18O
p). Indeed, the δ18Op value of
vertebrate apatite (the mineral constituting bone, teeth and some fish scales) depends on the animal’s body water δ18O
bw value, as well as its body temperature (Kohn, 1996; Longinelli and
Nuti, 1973a; Luz et al., 1984). For air breathing vertebrates, the body water has a δ18O
bw value
controlled by oxygen input coming from drinking water, food and inhaled oxygen (through metabolic water production), as well as oxygen loss as water vapour through transcutaneous evaporation, sweat, exhaled vapour, and liquid water in urine and feces, some of these losses being associated with oxygen isotope fractionation (Kohn, 1996; Langlois et al., 2003). A fractionation equation that relates the apatite phosphate δ18O
p value to that of body water and body temperature
(Tb) can be adapted from the phosphate-water temperature scale previously established by
Longinelly and Nuti (1973a) and recently updated by Lécuyer et al. (2013). Such equation has proven to be valid for a large range of invertebrate and vertebrate bioapatites (Kolodny et al., 1983; Lécuyer et al., 1996; Longinelli and Nuti, 1973b, 1973a):
Tb (°C) = 117.4 – 4.5 (δ18Op - δ18Obw) (1)
This relationship is commonly used to reconstitute past SSTs based on fish apatite as most of them have a body temperature similar to that of their surrounding water, and a body water δ18O
bw
value equal to ambient one (Dera et al., 2009; Lécuyer et al., 2003b; Picard et al., 1998; Pucéat et al., 2003). Air-breathing vertebrates have a body water 18O-enriched relative to environmental
water, the magnitude of which depends on the amount of body water loss through exhaled H2Ovapor
and transcutaneous evaporation. Direct measurements have shown that terrestrial mammals and birds have body water from about 4‰ to 7‰ 18O-enriched relative to their drinking water
(Lazzerini et al., 2016; Longinelli, 1984; Wolf et al., 2013) whereas the body waters of semi aquatic
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
to aquatic crocodylians, turtles and birds are about 2‰ to 3‰ 18O-enriched relative to their drinking
water (Amiot et al., 2007; Barrick et al., 1999; Lazzerini et al., 2016).
In this study we estimate the body temperatures of metriorhynchid and teleosaurid
thalattosuchians recovered from five Jurassic localities in England and France. By comparing Tb of
thalattosuchians to those of associated ichthyosaurs or plesiosaurs, as well as to their ambient SST, we show that metriorhynchids may have evolved some degree of endothermic-like
thermophysiology, whereas teleosaurids retained a more typical ecto-poikilothermy.
Materials and methods Sample collection
We analysed eighty-eight fossil teeth of Jurassic fish and marine reptiles for their oxygen isotope composition of phosphate (δ18O
p), as well as for their oxygen (δ18Oc) and carbon (δ13Cc)
isotope composition of apatite carbonate. The first English locality is Smallmouth Sands, Dorset (Figure 1), from the lower part of the Kimmeridge Clay Formation dated as lower Kimmeridgian (Young et al., 2014b). The second English locality is the Oxford Clay Formation Peterborough clay pits, a world-famous fossiliferous collection of sites, dated to the middle Callovian, which has yielded a rich marine fauna along with terrestrial elements (Martill and Hudson, 1991). The first French locality is “Les Vaches Noires” of Normandy, dated as upper Callovian, where the “Marnes de Dives” Formation crops out, consisting of marls where a rich fauna composed of marine and terrestrial elements have been recovered (Lebrun and Courville, 2013). The second French locality is the excavation of “Les Lourdines” near the eponymous quarry, dated as middle Callovian, which
is composed of white limestone (calcaire des Lourdines) where a marine fauna and some terrestrial plants have been recovered (Barale et al., 1974). Finally, the locality of Cintheaux dated as
Bathonian consists of the “Pierre de Caen” limestone that has yielded a marine fauna with some terrestrial elements (Rioult, 1963). Studied tooth specimens include those of metriorhynchid and teleosaurid thalattosuchians, ichthyosaurs, plesiosaurs, as well as of chondrichthyans and
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
osteichthyans (Supplementary table 1). When possible, tooth enamel was sampled using a
spherical diamond-tipped drill bit. For smaller teeth, the bulk enamel and dentin were ground using an agate mortar and pestle.
Analytical techniques
Oxygen isotope analysis of biogenic apatite phosphate
Apatite powders have been treated following the wet chemistry protocol described by Crowson et al. (1991) and slightly modified by Lécuyer et al. (1993). This protocol consists in the isolation of phosphate (PO43-) from apatite as silver phosphate (Ag3PO4) crystals using acid
dissolution and anion-exchange resin. For each sample, 20-30 mg of enamel powder was dissolved in 2 mL of 2 M HF. The CaF2 residue was separated by centrifugation and the solution was
neutralized by adding 2.2 mL of 2 M KOH. Amberlite™ IRN 78 anion-exchange resin beads were added to the solution to isolate the PO43- ions. After 24 hours, the solution was removed, the resin
was rinced with deionized water and eluted with 27.5 mL of 0.5 M NH4NO3. After 4 hours, 0.5 mL
of NH4OH and 15 mL of an ammoniacal solution of AgNO3 were added and the solutions were
placed in a thermostated bath at 70 °C for 7 hours allowing for the precipitation of Ag3PO4 crystals.
Oxygen isotope compositions were measured using a high temperature elemental analyzer interfaced in continuous flow mode to an isotopic ratio mass spectrometer (Fourel et al., 2011) at the Plateforme d’Ecologie Isotopique du Laboratoire d’Ecologie des Hydrosystèmes Naturels et Anthropisés (LEHNA – UMR5023, Université Claude Bernard Lyon 1). For each sample, 5
aliquots of 300 μg of Ag3PO4 were mixed with 300 μg of pure graphite powder loaded in silver foil
capsules. Pyrolysis was performed at 1450 °C using a varioPYROcubeTM Elemental Analyzer
(Elementar GmbH) interfaced in Continuous Flow mode with an IsoprimeTM Isotopic Ratio Mass
Spectrometer (Elementar UK Ltd). Measurements have been calibrated against silver phosphate precipitated from the NBS120c (natural Miocene phosphorite from Florida), as well as against the NBS127 (Barium sulfate precipitated using seawater from Monterey Bay, California, USA). The
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
value of NBS120c was fixed at 21.7‰ (V-SMOW) according to Lécuyer et al. (1993), and that of NBS127 set at the certified value of 9.3‰ (V-SMOW; (Halas and Szaran, 2001; Hut, 1987)) for correction of instrumental mass fractionation during CO isotopic analysis. Silver phosphate precipitated from standard NBS120c along with the silver phosphate samples derived from fossil bioapatites was repeatedly analyzed (δ18O
p = 21.72±0.22‰, n = 20) to ensure that no fractionation
occurred during the wet chemistry. Data are reported as δ18O values with respect to V-SMOW (in
‰ δ units).
Oxygen and carbon isotope analysis of biogenic apatite carbonate
In order to remove potential organic contaminants as well as secondarily precipitated calcite, about 10 mg of apatite powder was pre-treated following the protocol of Koch et al. (1997).
Powders were washed with a 2% NaOCl solution to remove organic matter, then rinsed five times with double deionized water and air-dried at 40 °C for 24 hours. 0.1 M acetic acid was then added and left for 24 hours, after which the powder was again rinsed five times with double deionized water and then air-dried at 40 °C for 24 hours. The powder/solution ratio was kept constant at 0.04 g mL-1 for both treatments. Stable isotope compositions of carbonate oxygen and carbon were
carried out at the Plateforme d’Ecologie Isotopique du Laboratoire d’Ecologie des Hydrosystèmes Naturels et Anthropisés (LEHNA – UMR5023). The measurement were performed using an isoFLOW system connected on line in continuous flow mode to a precisION mass spectrometer (Elementar UK Ltd). For each sample, two aliquots of 2 mg of pretreated apatite powder were loaded in LABCO Exetainer® 3.7 mL soda glass vials, round bottomed with exetainers caps (LABCO UK Ltd) and were reacted with anhydrous phosphoric acid. The reaction took place at 90°C in a temperature regulated sample tray. The CO2 gas generated during the acid digestion of the
carbonate sample was then transferred to the mass spectrometer via the centrION interface. A calibrated CO2 gas was used as a monitoring gas. Typical reproducibilites are 0.05‰ and 0.07‰
respectively for δ13C and δ18O measurements. For tooth apatite, the acid fractionation factor α
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
(CO2-apatite carbonate) of 1.00773 determined for the NBS120c phosphate rock reference material
has been selected (Passey et al., 2007). Calibrated material used is Carrara Marble (δ18O
V-PDB =
-1.84‰; δ13C
V-PDB = +2.03‰; (Fourel et al., 2016) NBS18 ( δ18OV-PDB = -23.2‰; δ13CV-PDB =
-5.01‰) and NBS120c (δ18O
V-PDB = -1.13‰; δ13CV-PDB = -6.27‰; (Passey et al., 2007) Isotopic
compositions are quoted in the standard δ notation relative to V-SMOW for oxygen and V-PDB for carbon.
Results
Oxygen isotope compositions of apatite phosphate (δ18O
p), apatite carbonate (δ18Oc) and
carbon isotope compositions of apatite carbonate (δ13C
c) are reported in Supplementary table 1
along with published δ18O
p values of teeth and bones of marine vertebrates (Anderson et al., 1994;
Bernard et al., 2010; Billon-Bruyat et al., 2005). Analysed teeth have δ18O
p values ranging from
18.3‰ to 21.8‰ V-SMOW, δ18O
c values ranging from 24.1‰ to 28.3‰ V-SMOW and δ13CC
values ranging from -11.2‰ to 7.7‰ V-PDB. At the three sites of Smallmouth Sands,
Peterborough and Les Vaches Noires, ichthyosaurs and plesiosaurs have slightly lower mean δ18O P
values than those of co-occurring thalattosuchians (Supplementary table 2). For each locality, SST has been calculated from the δ18O
p values of fish using equation 1
(Supplementary table 2) considering that Tb ≈ Tsw, and that δ18Obw ≈ δ18Osw (Kolodny et al.,
1983). Because average seawater δ18O
sw value may have varied between -1‰ and 0‰ V-SMOW
depending on the amount of seawater stored as polar ice (Shackleton and Kennett, 1975), an average value of -0.5‰ was arbitrarily selected for temperature calculation, keeping in mind that the associated error in temperature calculation is about 2.3°C (based on the slope of 4.5 of equation 1).
The body temperature of studied marine reptiles have also been estimated using equation 1, and assuming a seawater-body water 18O-enrichement of 2‰, a general enrichment observed
among semi-aquatic and aquatic air breathing vertebrates, including extant crocodylomorphs
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
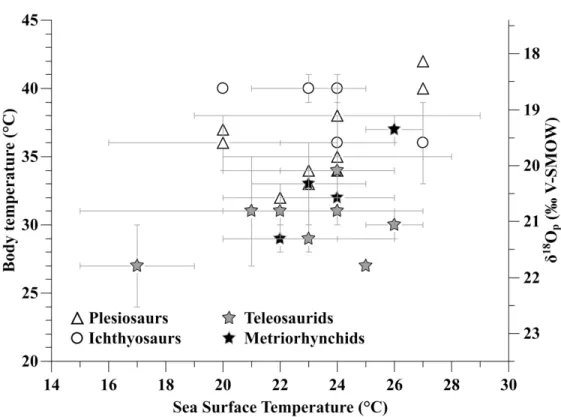
(Amiot et al., 2007; Barrick et al., 1999; Lazzerini et al., 2016). While ichthyosaurs and plesiosaurs have a body temperature within the 32-40°C range compatible with their known endo-homeothermy (Bernard et al., 2010), teleosaurids have lower Tb ranging from 27°C to 31°C, and metriorhynchids
show intermediate body temperatures ranging from 29°C to 37°C (Supplementary table 2).
Discussion
Original preservation of the stable isotope compositions
Before discussing the thermophysiological significance of the oxygen and carbon isotope compositions of vertebrate apatites, pristine preservation of the isotopic record needs to be assessed. Indeed, biotic and abiotic processes leading to the decomposition, burial and fossilization of living organisms may alter the original isotopic composition of bioapatite through processes of secondary precipitation, ion adsorption or dissolution-recrystallization of bioapatite (Blake et al., 1997; Kolodny et al., 1996; Lécuyer et al., 2003a; Trueman et al., 2003; Zazzo et al., 2004a, 2004b). Although no method can definitely demonstrate whether the original isotope compositions have been kept, several ways to assess the preservation state of the isotopic record have been proposed (Fricke et al., 1998; Iacumin et al., 1996; Kolodny et al., 1996; Lécuyer et al., 2003a; Pucéat et al., 2004; Tütken et al., 2008; Zazzo et al., 2004b). In modern skeletal tissues of vertebrates, carbonate and phosphate precipitate close to equilibrium with body water, so the δ18O
p and δ18Oc values are
positively correlated. Because isotopic exchange rates between carbonate-water and phosphate-water are significantly different, re-equilibration of both compounds during diagenesis is not expected and altered enamel should show isotopic shifts from the empirical δ18O
p–δ18Oc line.
Therefore, it is expected that the distribution of pristine δ18O values of fish and reptile tooth enamel
should follow a line with a slope close to unity mimicking those established between the δ18O c and
δ18O
p values of modern mammals (Bryant et al., 1996; Chenery et al., 2012; Iacumin et al., 1996;
Lécuyer et al., 2010; Zazzo et al., 2004b). Despite the narrow range in the distribution of oxygen isotope compositions (Figure 2), both δ18O
c and δ18Op values fall within the range observed in
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
extant and fossil marine vertebrates (Kolodny and Luz, 1991; Lécuyer et al., 2003b; Vennemann et al., 2001). This correlation shows that the oxygen isotope compositions of structural carbonate in both tooth and bone apatites have preserved to a certain degree its original record.
A clue to the primary preservation of stable carbon isotope composition of apatite carbonate is the systematic and significant difference in δ13C
c values between fish and marine reptiles (Figure 3). Air breathing reptiles have δ13C
c values mainly reflecting that of their diets with an isotope
fractionation that depends on their digestive physiology (Passey et al., 2005), as expected. In aquatic environments, the relationship between fish carbonate and diet δ13C values is complicated
as a substantial amount of the carbon may be derived from dissolved inorganic carbon (DIC) of their ambient water (McConnaughey et al., 1997; Thorrold et al., 1997) with a higher δ13C value
(Santos et al., 2011). Finally, the weight percentage of carbonate in analysed fossil apatites
(Supplementary table 1) lies within the expected biological range of modern vertebrate apatites of 2-13% (Brudevold and Soremark, 1967; Rink and Schwarcz, 1995; Vennemann et al., 2001). From these lines of evidence, we can assume that oxygen isotope compositions of apatite phosphate and carbonate have kept at least a significant part of their original information, and might be interpreted in terms of seawater temperature and thermophysiology.
Body temperature reconstruction
In a previous study of marine reptile thermophysiology, Bernard et al. (2010) interpreted the oxygen isotope differences between marine reptiles and coexisting fish from a large range of water temperatures (estimated from 14°C to 34°C) in terms of Tb differences. They concluded that the
three lineages Ichthyosauria, Plesiosauria and Mosasauridae were most likely endothermic and homeothermic marine reptiles, although for mosasaurids this was later debated (Harrell Jr et al., 2016; Motani, 2010). The method used in Bernard et al. (2010) cannot clearly identify the
thermophysology of thalattosuchians (Figure 4). Indeed, the available sample-set of thalattosuchian specimens is restricted to five localities with a narrow range of low paleolatitudes from about 29°N
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
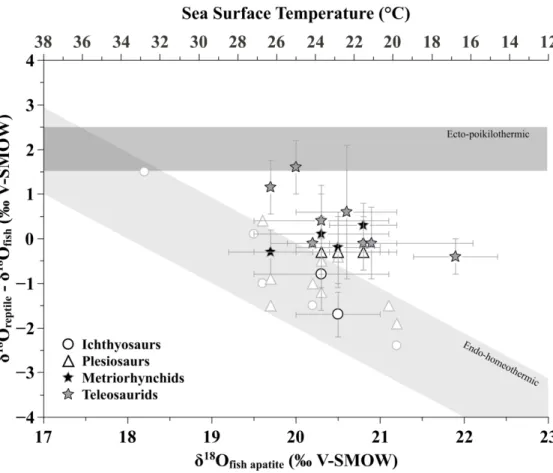
to 36°N (Supplementary table 2; palaeolatitudes calculated using the online application of van Hinsbergen et al. (2015)), as well as a narrow range of estimated paleotemperatures from 22±2°C to 27±2°C. Consequently, the sampled sites do not allow the two strategies of thermophysiology to be clearly distinguished. Most metriorhynchids and teleosaurids show intermediate values between the expected range for endotherms and ectotherms, with the exception of the metriorhynchids from the middle Callovian of Les Lourdines which fall within the expected range of endotherms, and one teleosaurid from the Bathonian of Cintheaux, which has a typical ectotherm signature (Figure 4).
Using equation 1 and assuming that thalattosuchians have a δ18O
bw value of about 2‰ more
positive than their ambient seawater, both metriorhynchids and teleosaurids have a body temperature above their environmental one, metriorhynchids being slightly warmer than teleosaurids in average (Supplementary table 2; Figure 5). However, thalattosuchian Tb are systematically below the calculated ones of co-occurring ichthyosaurs and plesiosaurs for which the body temperature was calculated from the same equation 1 and using a body water δ18O
bw value
similar to that of thalattosuchians.
An endothermic-like thermophysiology interpreted from metriorhynchid δ18O
p values seems
likely according to their known morphology and ecology as active pelagic predators (Andrade et al., 2010; Fernández and Gasparini, 2000; Hua and De Buffrénil, 1996; Massare, 1987; Young et al., 2013, 2012). Active predation would require elevated metabolic rates compatible with an
endothermic thermophysiology. This seems plausible considering the large suite of evidence for an endothermic ancestral condition for Archosauria, and a reversal within the crocodylomorph lineage to an ectothermic state coming from different fields of biology, including developmental biology (Seymour et al., 2004), physiology (Farmer and Sanders, 2010), anatomy (Summers, 2005),
palaeohistology (de Ricqlès et al., 2008), and phylogenetic signal extraction (Legendre et al., 2016). Endothermy within metriorhynchids might have been inherited from their archosaur ancestors, and “reactivated” along with the acquisition of morphological adaptations to active pelagic predation. However, body temperature regulation seems to have been limited as observed Tb vary with varying
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
seawater temperature (Figure 5). Limited thermoregulatory capacities would be compatible with the restricted range of paleolatitudinal occurrences compared to fully endo-homeothermic
ichthyosaurs and plesiosaurs having been found from equatorial to polar seas (Bardet et al., 2014). Interpretation of the oxygen isotope composition of teleosaurid apatite in terms of body temperature may be strongly biased by their possible semi-aquatic and eurhyaline ecology, some species having been found in estuarine or freshwater environments (Buffetaut, 1982; Martin et al., 2019, 2016; Wilberg et al., 2019). As most teleosaurids retained a typical semi-aquatic
crocodylomorph morphology (external mandibular fenestrae, extensive osteoderm cover and limbs adapted to terrestrial locomotion), it is hypothesized that they were ectothermic and poikilothermic ambush predators spending most of their time motionless, and mouth-gape basking like modern crocodylians. However, during the late Kimmeridgian-early Tithonian there is evidence for a subclade of teleosaurids that became more pelagic (Foffa et al., 2019). The oxygen isotope composition of teleosaurid apatite indicates that they kept a body temperature lower than that of ichthyosaurs and plesiosaurs, but close to those of metriorhynchids. However, more estuarine living environments can be characterized by more negative δ18O values as a result of the mixing between
seawater and river waters having negative δ18O values such as in the case of the San Francisco Bay
(Ingram et al., 1996). In this example, waters from the Sacramento river having δ18O values
ranging from about -12‰ to -10‰ mix with seawater of 0‰ and results in estuarine waters that
can have δ18O values of -3‰ to -10‰. Keeping this in mind, then the calculated body temperature
of teleosaurids living in estuarine environments would be lower, and their apatite δ18O values would
fit within the expected range of ectotherms. Moreover, the possible semi-aquatic lifestyle of
teleosaurids could at least partly account for their elevated δ18O
p values as a result of body water
loss through transcutaneous evaporation. In the studied localities where teleosaurid specimens were found, fossil remains of continental vertebrates and plants have been found, indicating a proximity to landmasses and a possible estuarine origin of teleosaurids. Gigantothermy and behavioural thermoregulation may also account for the calculated body temperatures of teleosaurids close to
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
those of metriorhynchids. Today, large marine crocodylians, such as ~1000 kg adult individuals of the saltwater crocodile (Crocodylus porosus), are able to raise their body temperature well above ambient temperatures through mouth-gape basking behaviours and can retain this elevation by the thermal inertia of their large body size (Grigg et al., 1998; Seebacher et al., 1999). Large
teleosaurids may have used a similar behavioural thermoregulation as extant marine crocodiles (C.
porosus) and would have raised their body temperatures close to that of Jurassic metriorhynchids
and maintained it within a narrow range. This would explain the apparent tendency of
teleosaurid-fish isotope difference to parallel that of endo-homeothermic ichthyosaurs and plesiosaurs (Figure
4). Most metriorhynchid specimens were of smaller body-size than teleosaurids (Young et al., 2016, 2011), and metriorhynchids would have been unable to mouth-gape bask onshore (Young et al., 2010).
Based on the available isotopic dataset, it seems likely that teleosaurids retained a typical ecto-poikilothermic thermophysiology in agreement with their morphology and ecology, whereas metriorhynchids may have been endothermic, being able to raise their body temperature above an ambient one, and close to that of other warm-blooded marine reptiles. However, metriorhynchids could not have achieved efficient thermoregulation as suggested from their varying body
temperature along with varying SST.
Concluding remarks
The possible difference in thermophysiologies between metriorhynchids and teleosaurids inferred from their stable oxygen isotope composition of apatite can at least partly explain the peculiar thalattosuchian biodiversity pattern of the Jurassic and Early Cretaceous (Martin et al., 2014; Young et al., 2014a). Teleosaurid diversity crashed at the end of the Jurassic, a time of global marine temperature decline, probably as a result of the temperature change or the global regression affecting their ecological niches (Bardet et al., 2014). Endothermy may have helped
metriorhynchids to cope with global cooling, and due to their pelagic ecology, they might not have
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
been as affected by the marine regressions as teleosaurids. This may explain their success across the Jurassic-Cretaceous boundary (Chiarenza et al., 2015; Tennant et al., 2017; Young et al., 2014a). At this point, we cannot only speculate when metriorhynchids became extinction, or why. All we can mention is that Jurassic metriorhynchids had an imperfect endothermic and poikilothermic
thermophysiology. We hope future studies will investigate whether the Late Jurassic pelagic subclade of teleosaurids began to develop endothermic capabilities, and test whether Cretaceous metriorhynchids, the most marine adapted of all thalattosuchians (Hua et al., 2000; Young et al., 2010), evolved toward enhanced thermoregulatory abilities.
Acknowledgments
The authors would like to thank the Hunterian Museum of Glasgow, Mr and Mrs Pennetier for providing material from England and France and Gilles Cuny for his constructive comments. MTY is financially supported by a Leverhulme Trust Research Project grant (RPG-2017-167).
References
Amiot, R., Lécuyer, C., Escarguel, G., Billon-Bruyat, J.-P., Buffetaut, E., Langlois, C., Martin, S., Martineau, F., Mazin, J.-M., 2007. Oxygen isotope fractionation between crocodilian phosphate and water. Palaeogeogr. Palaeoclimatol. Palaeoecol. 243, 412–420.
https://doi.org/10.1016/j.palaeo.2006.08.013
Amiot, R., Wang, Xu, Zhou, Z., Wang, Xiaolin, Buffetaut, E., Lécuyer, C., Ding, Z., Fluteau, F., Hibino, T., Kusuhashi, N., Mo, J., Suteethorn, V., Wang, Y., Xu, X., Zhang, F., 2011. Oxygen isotopes of East Asian dinosaurs reveal exceptionally cold Early Cretaceous Climates. Proc. Natl. Acad. Sci. 108, 5179–5183. https://doi.org/10.1073/pnas.1011369108 Anderson, T.F., Popp, B.N., Williams, A.C., Ho, L.-Z., Hudson, J.D., 1994. The stable isotopic
records of fossils from the Peterborough Member, Oxford Clay Formation (Jurassic), UK: palaeoenvironmental implications. J. Geol. Soc. 151, 125–138.
https://doi.org/10.1144/gsjgs.151.1.0125
Andrade, M.B. de, Young, M.T., Desojo, J.B., Brusatte, S.L., 2010. The evolution of extreme hypercarnivory in Metriorhynchidae (Mesoeucrocodylia: Thalattosuchia) based on evidence from microscopic denticle morphology. J. Vertebr. Paleontol. 30, 1451–1465.
https://doi.org/10.1080/02724634.2010.501442
Barale, G., Cariou, E., Radureau, G., 1974. Etude biostratigraphique et paléobotanique des gisements de calcaire blanc callovien au Nord de Poitiers. Geobios 7, 43–69. https://doi.org/10.1016/S0016-6995(74)80018-5
Bardet, N., Falconnet, J., Fischer, V., Houssaye, A., Jouve, S., Suberbiola, X.P., Perez-García, A., Rage, J.-C., Vincent, P., 2014. Mesozoic marine reptile palaeobiogeography in response to drifting plates. Gondwana Res. 26, 869–887. https://doi.org/10.1016/j.gr.2014.05.005
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Barrick, R.E., Fischer, A.G., Showers, W.J., 1999. Oxygen isotopes from turtle bone: applications for terrestrial paleoclimates? Palaios 14, 186–191. https://doi.org/10.2307/3515374
Berg, D.E., 1965. Krokodile als Klimazeugen. Geol. Rundsch. 54, 328–333.
Bernard, A., Lécuyer, C., Vincent, P., Amiot, R., Bardet, N., Buffetaut, E., Fourel, F., Martineau, F., Mazin, J.-M., Prieur, A., 2010. Regulation of body temperature by some Mesozoic marine reptiles. Science 328, 1379–1382. https://doi.org/10.1126/science.1187443
Billon-Bruyat, J.-P., Lécuyer, C., Martineau, F., Mazin, J.-M., 2005. Oxygen isotope compositions of Late Jurassic vertebrate remains from lithographic limestones of western Europe:
implications for the ecology of fish, turtles, and crocodilians. Palaeogeogr. Palaeoclimatol. Palaeoecol. 216, 359–375.
Blake, R.E., O’Neil, J.R., Garcia, G.A., 1997. Oxygen isotope systematics of biologically mediated reactions of phosphate: I. Microbial degradation of organophosphorus compounds.
Geochim. Cosmochim. Acta 61, 4411–4422.
Brudevold, F., Soremark, R., 1967. Chemistry of the mineral phase of enamel, in: Mills, A. (Ed.), Structural and Chemical Organization of Teeth, Volume 2. Elsevier, Amsterdam, pp. 247– 277.
Bryant, D.J., Koch, P.L., Froelich, P.N., Showers, W.J., Genna, B.J., 1996. Oxygen isotope
partitioning between phosphate and carbonate in mammalian apatite. Geochim. Cosmochim. Acta 60, 5145–5148.
Buffetaut, E., 1982. Radiation évolutive, paléoécologie et biogéographie des crocodiliens mésosuchiens, Mémoires de la Société géologique de France. Nouvelle série. Société Géologique de France, Paris.
Chenery, C.A., Pashley, V., Lamb, A.L., Sloane, H.J., Evans, J.A., 2012. The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite. Rapid Commun. Mass Spectrom. 26, 309–319.
Chiarenza, A.A., Foffa, D., Young, M.T., Insacco, G., Cau, A., Carnevale, G., Catanzariti, R., 2015. The youngest record of metriorhynchid crocodylomorphs, with implications for the
extinction of Thalattosuchia. Cretac. Res. 56, 608–616. https://doi.org/10.1016/j.cretres.2015.07.001
Cowles, R.B., Bogert, C.M., 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. Hist. 83, 261–296.
Crichton, A., 1825. On the Climate of the Antediluvian World and Its Independence of Solar Influence: And on the Formation of Granite. Ann. Philos. 9, 207–217.
Crowson, R.A., Showers, W.J., Wright, E.K., Hoering, T.C., 1991. Preparation of phosphate samples for oxygen isotope analysis. Anal. Chem. 63, 2397–2400.
https://doi.org/10.1021/ac00020a038
de Ricqlès, A., Padian, K., Knoll, F., Horner, J.R., 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a “phylogenetic signal” in bone histology, in: Annales de Paléontologie. Elsevier, pp. 57–76. https://doi.org/10.1016/j.annpal.2008.03.002
Dera, G., Pucéat, E., Pellenard, P., Neige, P., Delsate, D., Joachimski, M.M., Reisberg, L.,
Martinez, M., 2009. Water mass exchange and variations in seawater temperature in the NW Tethys during the Early Jurassic: evidence from neodymium and oxygen isotopes of fish teeth and belemnites. Earth Planet. Sci. Lett. 286, 198–207.
https://doi.org/10.1016/j.epsl.2009.06.027
Eudes-Deslongchamps, E., 1869. Notes Paléontologiques. Le Blanc Hardel & Savy, Caen and Paris. Fanti, F., Miyashita, T., Cantelli, L., Mnasri, F., Dridi, J., Contessi, M., Cau, A., 2016. The largest
thalattosuchian (Crocodylomorpha) supports teleosaurid survival across the Jurassic-Cretaceous boundary. Cretac. Res. 61, 263–274.
https://doi.org/10.1016/j.cretres.2015.11.011
Farmer, C.G., Sanders, K., 2010. Unidirectional airflow in the lungs of alligators. Science 327, 338–340. https://doi.org/10.1126/science.1180219 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Fernández, M., Gasparini, Z., 2000. Salt glands in a Tithonian metriorhynchid crocodyliform and their physiological significance. Lethaia 33, 269–276.
https://doi.org/10.1080/002411600750053835
Fitzinger, L., 1843. Systema Reptilium, Fasciculus Primus, Amblyglossae. Braumüller et Seidel, Vienna.
Foffa, D., Johnson, M.M., Young, M.T., Steel, L., Brusatte, S.L., 2019. Revision of the Late
Jurassic deep-water teleosauroid crocodylomorph Teleosaurus megarhinus Hulke, 1871 and evidence of pelagic adaptations in Teleosauroidea. PeerJ 7, e6646.
https://doi.org/10.7717/peerj.6646
Fourel, F., Martineau, F., Lécuyer, C., Kupka, H.-J., Lange, L., Ojeimi, C., Seed, M., 2011. 18O/16O
ratio measurements of inorganic and organic materials by elemental analysis–pyrolysis– isotope ratio mass spectrometry continuous-flow techniques. Rapid Commun. Mass Spectrom. 25, 2691–2696. https://doi.org/10.1002/rcm.5056
Fourel, F., Martineau, F., Tóth, E.E., Görög, A., Escarguel, G., Lécuyer, C., 2016. Carbon and oxygen isotope variability among foraminifera and ostracod carbonated shells. Ann. Univ. Mariae Curie-Sklodowska Sect. AAA–Physica 70, 133–156.
Fraas, E., 1902. Die Meer-Krocodilier (Thalattosuchia) des oberen Jura unter specieller Berücksichtigung von Dacosaurus und Geosaurus. Palaeontographica 49, 1–72.
Fricke, H.C., Clyde, W.C., O’Neil, J.R., Gingerich, P.D., 1998. Evidence for rapid climate change in North America during the latest Paleocene thermal maximum: oxygen isotope
compositions of biogenic phosphate from the Bighorn Basin (Wyoming). Earth Planet. Sci. Lett. 160, 193–208.
Grigg, G.C., Seebacherd, F., Beard, L.A., Morris, D., 1998. Thermal relations of large crocodiles,
Crocodylus porosus, free-ranging in a naturalistic situation. Proc. R. Soc. Lond. B Biol. Sci.
265, 1793–1799. https://doi.org/10.1098/rspb.1998.0504
Halas, S., Szaran, J., 2001. Improved thermal decomposition of sulfates to SO2 and mass
spectrometric determination of δ34S of IAEA SO-5, IAEA SO-6 and NBS-127 sulfate
standards. Rapid Commun. Mass Spectrom. 15, 1618–1620. https://doi.org/10.1002/rcm.416 Harrell Jr, T.L., Pérez-Huerta, A., Suarez, C.A., 2016. Endothermic mosasaurs? Possible
thermoregulation of Late Cretaceous mosasaurs (Reptilia, Squamata) indicated by stable oxygen isotopes in fossil bioapatite in comparison with coeval marine fish and pelagic seabirds. Palaeontology 59, 351–363. https://doi.org/10.1111/pala.12240
Hua, S., De Buffrénil, V., 1996. Bone histology as a clue in the interpretation of functional
adaptations in the Thalattosuchia (Reptilia, Crocodylia). J. Vertebr. Paleontol. 16, 703–717. https://doi.org/10.1080/02724634.1996.10011359
Hua, S., Vignaud, P., Atrops, F., Clément, A., 2000. Enaliosuchus macrospondylus Koken, 1883 (Crocodylia, Metriorhynchidae) du Valanginien de Barret-le-Bas (Hautes Alpes, France): un cas unique de remontée des narines externs parmi les crocodiliens. Géobios 33, 467–474. https://doi.org/10.1016/S0016-6995(00)80080-7
Hut, G., 1987. Consultants’ group meeting on stable isotope reference samples for geochemical and hydrological investigations. accessible at :
http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/18/075/18075746.pdf 1– 43.
Iacumin, P., Bocherens, H., Mariotti, A., Longinelli, A., 1996. Oxygen isotope analyses of co-existing carbonate and phosphate in biogenic apatite: a way to monitor diagenetic alteration of bone phosphate? Earth Planet. Sci. Lett. 142, 1–6.
Ingram, B.L., Conrad, M.E., Ingle, J.C., 1996. Stable isotope and salinity systematics in estuarine waters and carbonates: San Francisco Bay. Geochim. Cosmochim. Acta 60, 455–467. https://doi.org/10.1016/0016-7037(95)00398-3
Koch, P.L., Tuross, N., Fogel, M.L., 1997. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J. Archaeol. Sci. 24, 417–429.
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Kohn, M.J., 1996. Predicting animal δ18O: Accounting for diet and physiological adaptation.
Geochim. Cosmochim. Acta 60, 4811–4829. https://doi.org/10.1016/S0016-7037(96)00240-2
Kolodny, Y., Luz, B., 1991. Oxygen isotopes in phosphate of fossil fish — Devonian to recent, in: Taylor, H.P., O’Neil, J.R., Kaplan, I.R. (Eds.), Stable Isotope Geochemistry: A Tribute to Samuel Epstein. Geochemical Society, University Park, pp. 105–119.
Kolodny, Y., Luz, B., Navon, O., 1983. Oxygen isotope variations in phosphate of biogenic apatites, I. Fish bone apatite-rechecking the rules of the game. Earth Planet. Sci. Lett. 64, 398–404. https://doi.org/10.1016/0012-821X(83)90100-0
Kolodny, Y., Luz, B., Sander, M., Clemens, W.A., 1996. Dinosaur bones: fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 126, 161–171.
Langlois, C., Simon, L., Lécuyer, C., 2003. Box-modeling of bone and tooth phosphate oxygen isotope compositions as a function of environmental and physiological parameters. Isotopes Environ. Health Stud. 39, 259–272. https://doi.org/10.1080/10256010310001621146
Lazzerini, N., Lécuyer, C., Amiot, R., Angst, D., Buffetaut, E., Fourel, F., Daux, V., Betancort, J.F., Sánchez Marco, A., Lomoschitz, A., 2016. Oxygen isotope fractionation between bird eggshell calcite and body water: application to fossil eggs from Lanzarote (Canary Islands). Sci. Nat. 103, 81. https://doi.org/10.1007/s00114-016-1404-x
Lebrun, P., Courville, P., 2013. Le Jurassique des falaises des Vaches-noires. Fossiles Hors serie 4, 16–33.
Lécuyer, C., Amiot, R., Touzeau, A., Trotter, J., 2013. Calibration of the phosphate δ18O
thermometer with carbonate–water oxygen isotope fractionation equations. Chem. Geol. 347, 217–226. https://doi.org/10.1016/j.chemgeo.2013.03.008
Lécuyer, C., Balter, V., Martineau, F., Fourel, F., Bernard, A., Amiot, R., Gardien, V., Otero, O., Legendre, S., Panczer, G., 2010. Oxygen isotope fractionation between apatite-bound carbonate and water determined from controlled experiments with synthetic apatites precipitated at 10–37°C. Geochim. Cosmochim. Acta 74, 2072–2081.
https://doi.org/10.1016/j.gca.2009.12.024
Lécuyer, C., Bogey, C., Garcia, J.-P., Grandjean, P., Barrat, J.A., Floquet, M., Bardet, N., Pereda-Superbiola, X., 2003a. Stable isotope composition and rare earth element content of vertebrate remains from the Late Cretaceous of northern Spain (Laño): did the
environmental record survive? Palaeogeogr. Palaeoclimatol. Palaeoecol. 193, 457–471. Lécuyer, C., Grandjean, P., Emig, C., 1996. Determination of oxygen isotope fractionation between
water and phosphate from living lingulids: Potential application to palaeoenvironmental studies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 126, 101–108.
Lécuyer, C., Grandjean, P., O’Neil, J.R., Cappetta, H., Martineau, F., 1993. Thermal excursions in the ocean at the Cretaceous-Tertiary boundary (northern Morocco): δ18O record of
phosphatic fish debris. Palaeogeogr. Palaeoclimatol. Palaeoecol. 105, 235–243. https://doi.org/10.1016/0031-0182(93)90085-W
Lécuyer, C., Picard, S., Garcia, J.-P., Sheppard, S.M.., Grandjean, P., Dromart, G., 2003b. Thermal evolution of Tethyan surface waters during the Middle-Late Jurassic: Evidence from δ18O
values of marine fish teeth. Paleoceanography 18, 1076–1091. https://doi.org/10.1029/2002PA000863
Legendre, L.J., Guénard, G., Botha-Brink, J., Cubo, J., 2016. Palaeohistological evidence for ancestral high metabolic rate in archosaurs. Syst. Biol. 65, 989–996.
https://doi.org/10.1093/sysbio/syw033
Longinelli, A., 1984. Oxygen isotopes in mammal bone phosphate: A new tool for
paleohydrological and paleoclimatological research? Geochim. Cosmochim. Acta 48, 385– 390. https://doi.org/10.1016/0016-7037(84)90259-X
Longinelli, A., Nuti, S., 1973a. Revised phosphate-water isotopic temperature scale. Earth Planet. Sci. Lett. 19, 373–376. https://doi.org/10.1016/0012-821X(73)90088-5
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Longinelli, A., Nuti, S., 1973b. Oxygen isotope measurements of phosphate from fish teeth and bones. Earth Planet. Sci. Lett. 20, 337–340. https://doi.org/10.1016/0012-821X(73)90007-1 Luz, B., Kolodny, Y., Horowitz, M., 1984. Fractionation of oxygen isotopes between mammalian
bone-phosphate and environmental drinking water. Geochim. Cosmochim. Acta 48, 1689– 1693. https://doi.org/10.1016/0016-7037(84)90338-7
Markwick, P.J., 1998. Fossil crocodilians as indicators of Late Cretaceous and Cenozoic climates: implications for using palaeontological data in reconstructing palaeoclimate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 137, 205–271. https://doi.org/10.1016/S0031-0182(97)00108-9 Martill, D.M., Hudson, J.D. (Eds.), 1991. Fossils of the Oxford Clay, Palaeontological Association
Field guide to fossils. The Palaeontological Association, London.
Martin, J.E., Amiot, R., Lécuyer, C., Benton, M.J., 2014. Sea surface temperature contribute to marine crocodilian evolution. Nat. Commun. 5, 1–7. https://doi.org/10.1038/ncomms5658 Martin, J.E., Deesri, U., Liard, R., Wattanapituksakul, A., Suteethorn, S., Lauprasert, K., Tong, H.,
Buffetaut, E., Suteethorn, V., Suan, G., Telouk, P., Balter, V., 2016. Strontium isotopes and the long-term residency of thalattosuchians in the freshwater environment. Paleobiology 42, 143–156.
Martin, J.E., Suteethorn, S., Lauprasert, K., Tong, H., Buffetaut, E., Liard, R., Salaviale, C., Deesri, U., Suteethorn, V., Claude, J., 2019. A new freshwater teleosaurid from the Jurassic of northeastern Thailand. J. Vertebr. Paleontol. e1549059.
https://doi.org/10.1080/02724634.2018.1549059
Massare, J.A., 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. J. Vertebr. Paleontol. 7, 121–137. https://doi.org/10.1080/02724634.1987.10011647
McConnaughey, T.A., Burdett, J., Whelan, J.F., Paull, C.K., 1997. Carbon isotopes in biological carbonates: respiration and photosynthesis. Geochim. Cosmochim. Acta 61, 611–622. Motani, R., 2010. Warm-blooded “sea dragons”? Science 328, 1361–1362.
Ősi, A., Young, M.T., Galácz, A., Rabi, M., 2018. A new large-bodied thalattosuchian
crocodyliform from the Lower Jurassic (Toarcian) of Hungary, with further evidence of the mosaic acquisition of marine adaptations in Metriorhynchoidea. PeerJ 6, e4668.
https://doi.org/10.7717/peerj.4668
Owen, R., 1850. On the fossil crocodilia of England. Edinb. New Philos. J. 49, 248–250. Owen, R., 1842. Report on British Fossil Reptiles. Part II. Rep. Br. Assoc. Adv. Sci. Plymouth
Meet. 1841 60–240.
Passey, B.H., Cerling, T.E., Levin, N.E., 2007. Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Commun. Mass Spectrom. 21, 2853–2859.
Picard, S., Garcia, J.-P., Lécuyer, C., Sheppard, S.M.F., Cappetta, H., Emig, C., 1998. d18O values of coexisting brachiopods and fish: Temperature differences and estimates of paleo–water depths. Geology 26, 975–978.
Pough, F.H., Gans, C., 1982. The vocabulary of reptilian thermoregulation, in: Gans, C. (Ed.), Biology of the Reptilia Vol. 12. Physiology, C. Physiological Ecology. Academic Press, London, pp. 17–23.
Pross, J., Link, E., Ruf, M., Aigner, T., 2006. Delineating sequence stratigraphic patterns in deeper ramp carbonates: Quantitative palynofacies data from the Upper Jurassic (Kimmeridgian) of southwest Germany. J. Sediment. Res. 76, 524–538. https://doi.org/10.2110/jsr.2006.031 Pucéat, E., Lécuyer, C., Sheppard, S.M.., Dromart, G., Reboulet, S., Grandjean, P., 2003. Thermal
evolution of Cretaceous Tethyan marine waters inferred from oxygen isotope composition of fish tooth enamels. Paleoceanography 18, 1029. https://doi.org/10.1029/2002PA000823 Pucéat, E., Reynard, B., Lécuyer, C., 2004. Can crystallinity be used to determine the degree of
chemical alteration of biogenic apatites? Chem. Geol. 205, 83–97.
Rink, W.J., Schwarcz, H.P., 1995. Tests for diagenesis in tooth enamel: ESR dating signals and carbonate contents. J. Archaeol. Sci. 22, 251–255.
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Rioult, M., 1963. Le Calcaire de Caen, dépôt de rivage du Bathonien normand. Bull. Société Linn. Normandie 3, 119–141.
Saint-Hilaire, G., 1831. Recherches sur de grands sauriens trouvés à l’état fossile aux confins maritimes de la Basse-Normandie, attribués d’abord au crocodile, puis déterminés sous les noms de Teleosaurus et Steneosaurus. Mém. Académie Sci. 12, 1–138.
Santos, G.M., Ferguson, J., Acaylar, K., Johnson, K.R., Griffin, S., Druffel, E., 2011. Δ14C and δ13C
of seawater DIC as tracers of coastal upwelling: a 5-year time series from Southern California. Radiocarbon 53, 669–677.
Seebacher, F., Grigg, G.C., Beard, L.A., 1999. Crocodiles as dinosaurs: behavioural
thermoregulation in very large ectotherms leads to high and stable body temperatures. J. Exp. Biol. 202, 77–86.
Seymour, R.S., Bennett-Stamper, C.L., Johnston, S.D., Carrier, D.R., Grigg, G.C., 2004. Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol.
Biochem. Zool. 77, 1051–1067.
Shackleton, N.J., Kennett, J.P., 1975. Paleotemperature History of the Cenozoic and the Initiation of Antarctic Glaciation: Oxygen and Carbon Isotope Analyses in DSDP Sites 277, 279 and 281. DSDP Initial Rep. - Deep Sea Drill. Proj. 29, 743–756.
https://doi.org/10.2973/dsdp.proc.29.117.1975
Summers, A.P., 2005. Evolution: warm-hearted crocs. Nature 434, 833. https://doi.org/10.1038/434833a
Tarduno, J.A., Brinkman, D.B., Renne, P.R., Cottrell, R.D., Scher, H., Castillo, P., 1998. Evidence for extreme climatic warmth from Late Cretaceous Arctic vertebrates. Science 282, 2241– 2244.
Tennant, J.P., Mannion, P.D., Upchurch, P., Sutton, M.D., Price, G.D., 2017. Biotic and
environmental dynamics through the L ate J urassic–E arly C retaceous transition: evidence for protracted faunal and ecological turnover. Biol. Rev. 92, 776–814.
https://doi.org/10.1111/brv.12255
Thorrold, S.R., Campana, S.E., Jones, C.M., Swart, P.K., 1997. Factors determining δ13C and δ18O
fractionation in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 61, 2909– 2919.
Trueman, C., Chenery, C., Eberth, D.A., Spiro, B., 2003. Diagenetic effects on the oxygen isotope composition of bones of dinosaurs and other vertebrates recovered from terrestrial and marine sediments. J. Geol. Soc. 160, 895–901.
Tütken, T., Vennemann, T.W., Pfretzschner, H.U., 2008. Early diagenesis of bone and tooth apatite in fluvial and marine settings: Constraints from combined oxygen isotope, nitrogen and REE analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 266, 254–268.
van Hinsbergen, D.J., de Groot, L.V., van Schaik, S.J., Spakman, W., Bijl, P.K., Sluijs, A., Langereis, C.G., Brinkhuis, H., 2015. A paleolatitude calculator for paleoclimate studies. PloS One 10, e0126946. https://doi.org/10.1371/journal.pone.0126946
Vennemann, T.W., Hegner, E., Cliff, G., Benz, G.W., 2001. Isotopic composition of recent shark teeth as a proxy for environmental conditions. Geochim. Cosmochim. Acta 65, 1583–1599. Wilberg, E.W., Turner, A.H., Brochu, C.A., 2019. Evolutionary structure and timing of major
habitat shifts in Crocodylomorpha. Sci. Rep. 9, 514. https://doi.org/10.1038/s41598-018-36795-1
Wolf, N., Newsome, S.D., Fogel, M.L., Del Rio, C.M., 2013. The relationship between drinking water and the hydrogen and oxygen stable isotope values of tissues in Japanese Quail (Cortunix japonica). The Auk 130, 323–330. https://doi.org/10.1525/auk.2013.12075 Young, M.T., Bell, M.A., De Andrade, M.B., Brusatte, S.L., 2011. Body size estimation and
evolution in metriorhynchid crocodylomorphs: implications for species diversification and niche partitioning. Zool. J. Linn. Soc. 163, 1199–1216. https://doi.org/10.1111/j.1096-3642.2011.00734.x 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Young, M.T., Brusatte, S.L., Beatty, B.L., De Andrade, M.B., Desojo, J.B., 2012. Tooth-on-tooth interlocking occlusion suggests macrophagy in the Mesozoic marine crocodylomorph
Dakosaurus. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 295, 1147–1158.
https://doi.org/10.1002/ar.22491
Young, M.T., Brusatte, S.L., Ruta, M., de Andrade, M.B., 2010. The evolution of
Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometrics, analysis of disparity, and biomechanics. Zool. J. Linn. Soc. 158, 801–859. https://doi.org/10.1111/j.1096-3642.2009.00571.x
Young, M.T., de Andrade, M.B., Brusatte, S.L., Sakamoto, M., Liston, J., 2013. The oldest known metriorhynchid super-predator: a new genus and species from the Middle Jurassic of England, with implications for serration and mandibular evolution in predacious clades. J. Syst. Palaeontol. 11, 475–513. https://doi.org/10.1080/14772019.2012.704948
Young, M.T., de Andrade, M.B., Cornée, J.-J., Steel, L., Foffa, D., 2014a. Re-description of a putative Early Cretaceous “teleosaurid” from France, with implications for the survival of metriorhynchids and teleosaurids across the Jurassic-Cretaceous Boundary. Ann. Paléontol. 100, 165–174. https://doi.org/10.1016/j.annpal.2014.01.002
Young, M.T., Rabi, M., Bell, M.A., Foffa, D., Steel, L., Sachs, S., Peyer, K., 2016. Big-headed marine crocodyliforms and why we must be cautious when using extant species as body length proxies for long-extinct relatives. Palaeontol. Electron. 19, 1–14.
https://doi.org/10.26879/648
Young, M.T., Steel, L., Middleton, H., 2014b. Evidence of the metriorhynchid crocodylomorph genus Geosaurus in the Lower Kimmeridge Clay Formation (Late Jurassic) of England. Hist. Biol. 26, 551–555. https://doi.org/10.1080/08912963.2013.801468
Zazzo, A., Lécuyer, C., Mariotti, A., 2004a. Experimentally-controlled carbon and oxygen isotope exchange between bioapatites and water under inorganic and microbially-mediated
conditions. Geochim. Cosmochim. Acta 68, 1–12.
Zazzo, A., Lécuyer, C., Sheppard, S.M.F., Grandjean, P., Mariotti, A., 2004b. Diagenesis and the reconstruction of paleoenvironments: A method to restore original δ18O values of carbonate
and phosphate from fossil tooth enamel. Geochim. Cosmochim. Acta 68, 2245–2258. https://doi.org/10.1016/j.gca.2003.11.009 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Figure CaptionFigure 1: Palaeogeography of Northwestern Europe during the Middle-Late Jurassic (modified
from Pross et al. (2006). The red stars show the studied localities. 1: Peterboroug; 2: Smallmouth Sands; 3: Les Vaches Noires; 4: Les Lourdines excavation; 5: Cintheaux.
Figure 2: Oxygen isotope compositions of tooth phosphate reported against their corresponding
oxygen isotope composition of structural carbonate, as well as published values of modern and fossil fish for comparison (Kolodny and Luz, 1991; Lécuyer et al., 2003b; Vennemann et al., 2001). The dashed line with a slope a=1 illustrates the correlation between oxygen isotope composition of phosphate and carbonate.
Figure 3: Carbon isotope compositions of apatite carbonates (δ13C
c) from fish and marine reptile
samples. For each locality, the sizable difference between fish and coexisting marine reptiles δ13C c
values is considered as evidence for primary preservation of the stable isotope compositions of
studied specimens.
Figure 4: Model variation of the differences in the δ18O
p values of tooth phosphate between marine
reptiles and fish against the variation of the δ18O
p values of fish teeth, assuming (1) an ectothermic
and poikilothermic reptile [body water δ18O
bw values 2‰ enriched relative to a seawater value and
body temperature (T) equal seawater temperature]; (2) an endothermic reptile with body
temperature ranging from 35°C to 39°C and body water 2‰ enriched relative to a seawater value ranging from -1‰ to 0‰ (modified from (Bernard et al., 2010). For comparison, metriorhynchoids and teleosaurids values are reported, along with newly measured (black border) and published (grey border) ichthyosaurs and plesiosaurs values.
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Figure 5: Estimated body temperature of marine reptiles (left axis) and corresponding δ18O
p values
(right axis) are plotted against their environmental sea surface temperature estimated from fish δ18O p values. 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
For Review Only
Figure 1: Palaeogeography of Northwestern Europe during the Middle-Late Jurassic (modified from Pross et al. (2006). The red stars show the studied localities. 1: Peterboroug; 2: Smallmouth Sands; 3: Les Vaches
Noires; 4: Les Lourdines excavation; 5: Cintheaux.
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Figure 2: Oxygen isotope compositions of tooth phosphate reported against their corresponding oxygen isotope composition of structural carbonate, as well as published values of modern and fossil fish for comparison (Kolodny and Luz, 1991; Lécuyer et al., 2003b; Vennemann et al., 2001). The dashed line with
a slope a=1 illustrates the correlation between oxygen isotope composition of phosphate and carbonate. 297x196mm (300 x 300 DPI) 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Figure 3: Carbon isotope compositions of apatite carbonates (δ13Cc) from fish and marine reptile samples. For each locality, the sizable difference between fish and coexisting marine reptiles δ13Cc values is considered as evidence for primary preservation of the stable isotope compositions of studied specimens.
188x143mm (300 x 300 DPI) 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Figure 4: Model variation of the differences in the δ18Op values of tooth phosphate between marine reptiles and fish against the variation of the δ18Op values of fish teeth, assuming (1) an ectothermic and poikilothermic reptile [body water δ18Obw values 2‰ enriched relative to a seawater value and body temperature (T) equal seawater temperature]; (2) an endothermic reptile with body temperature ranging
from 35°C to 39°C and body water 2‰ enriched relative to a seawater value ranging from -1‰ to 0‰ (modified from (Bernard et al., 2010). For comparison, metriorhynchoids and teleosaurids values are
reported, along with newly measured (black border) and published (grey border) ichthyosaurs and plesiosaurs values. 192x158mm (300 x 300 DPI) 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56
For Review Only
Figure 5: Estimated body temperature of marine reptiles (left axis) and corresponding δ18Op values (right axis) are plotted against their environmental sea surface temperature estimated from fish δ18Op values.
213x154mm (300 x 300 DPI) 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56