Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-777, 1978
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=da59c99e-b492-48f3-b564-98ba22d9577b
https://publications-cnrc.canada.ca/fra/voir/objet/?id=da59c99e-b492-48f3-b564-98ba22d9577b
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001768
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Mechanism of burning of fully-developed compartment fires
Harmathy, T. Z.
er
National Research
Conseil national
k
Council Canada
N21d
de recherches Canada
0 .7777
K
PUB
MECHANISM
OF BURNING OF
FULLY-DEVELOPED COMPARTMENT FIRES
sby T.Z. Harmathy
Reprinted, from
Combustion and Flame Vol. 31, No. 1, 1978 p. 265 273 d : : ! q DBR Paper No. 777 u p , ; 8 . - k&-J I Division of Building Research
SOMMAIRE
La croyance selon laquelle un manque d'air limite la vitesse de d6veloppement d'un incendie 2 ventilation contr816e est trPs
rhpandue. Apres examen critique, ce principe ne tient pas. D'autres m6canismes qui pourraient expliquer la faqon dont le dhbit de l'air dans un compartiment contrsle la vitesse de combustion (pyrolyse) d'un mathriau combustible cellulosique sont examinhs et un modsle plausible est prGsent6. L'hypothsse sugg6r6e est que l'oxydation de la surface carbonis6e est le lien qui explique le rapport observg entre le taux de pyrolyse et le taux d1entr6e d'air. Le modsle proposg permet de tirer des conclusions compatibles avec les connaissances actuelles sur les incendies I ventilation contr816e et ceux 2 surface de
combustible contrCl6e. Une tentative d'explication du m6canisme de combustion I 11int6rieur d'une enceinte de polym6res synthstiques non carbonisables est 6galement donn6e.
Mechanism
of Burning
of
Fully-Developed Compartment Fires
T. Z. HARMATHY
Fire Research Section, Division o f Building Research, National Research Council o f Canada, Ottawa, KIA OR6
It has been widely believed that in ventilation-controlled building fires a shortage of air limits the rate of burning. This concept is critically examined and proved untenable. Other possible mechanisms by which the flow rate of air into a compartment may control the rate of burning (pyrolysis) of cellulosic fuel are ex- amined and a plausible model presented. It is suggested that oxidation of surface char is the link by which the rate of pyrolysis is related to the rate of entry of air. The proposed model provides conclusions compat- ible with present knowledge of both ventilation-controlled and fuel-surface-controlled fires. It is emphasized, in conclusion, that the model is probably not applicable to compartments in which the fuel consists pre- dominantly of non-charring plastics.
INTRODUCTION combustion of volatiles (a series of gas-phase reactions), and (iii) the oxidation of the solid That the rate of burning of cellulosic fuel in fully- decomposition product, char (a heterogeneous developed compartment fires is, under a wide solid-gas reaction). On the other hand, what fire range of conditions, approximately proportional scientists refer to as rate o f burning is, in fact, to compartment ventilation [I, 2, 3, 4, 5, 61 the rate of loss of mass of the fuel associated was probably the first important discovery of fire primarily with the departure of the volatile decom- science. The explanation seemed quite straight- position products and, to a lesser degree, with the forward. The rate of entry of air, it was believed, oxidation of char. This has nothing to do with
limits the extent to which oxidation reactions can the rate of the dominant combustion process, approach the stoichiometric relations and, in the combustion of the volatiles, although this turn, limits the rate of heat evolution in a com- process is the chief source of heat evolution in partment. This view, apparently, still persists. the compartment. Clearly, it would be justified Several years ago the author [7] pointed out to replace the expression rate of burning by the that referring to poorly vented fires as "ventila- more appropriate (but still not fully accurate) tion-controlled" is indeed very apt, since the rate term rate of pyrolysis or rate of volatile produc- of entry of air literally controls, not limits, the tion.
rate of burning; more exactly, it controls the rate- Although for some fuels (among them all determining link in a complex process hidden cellulosic materials) the nature of the decomposi- behind the simple word burning. tion reactions depends on the presence or absence What, in everyday language, is referred to as of oxygen in the atmosphere, the rate of pyrolysis ~ u r n i n g is, with cellulosic fuels, a process consisting is controlled largely by the rate of heat supply
3f three entirely different types of reaction: to the decomposing fuel. Consequently, the rate
3)
the pyrolysis of fuel into (roughly) 85% volatile of entry of air into the compartment can controlmd
15% solid decomposition products, (ii) the the rate of pyrolysis (the so-called rate of burning) Eopyright 01978 by The Combustion InstituteT. Z. HARMATHY
I
only in an indirect way: by being instrumentalin regulating the rate of heat supply to the fuel. Various mechanisms by which ventilation may control the rate of pyrolysis of cellulosic materials will be critically surveyed and a plausible model suggested. The mechanism of pyrolysis (burning) of non-charring plastics will also be hypothesized.
CRITICISM OF SOME SIMPLE CONCEPTS
Traditionally, the dependence of rate of burning on compartment ventilation has been explained by claiming that the rate of entry of air sets an upper limit to which oxidation reactions can approach the stoichiometric relations. That this view is untenable can be proved by analysing a multitude of compartment burn-out experiments. An equation developed by Thomas et al. (See Eq. (2i) in Ref. [5]) reveals that in fully developed, ventilation-controlled fires the ratio of the rate of entry of air to the rate of volatile production is approximately 5.41. The author [7] found an even higher ratio of rate of air flow to rate of volatile production, 6.13. In comparison, the stoichiometric air requirement for the volatile decomposition products of a "typical" wood is only about 4.19 kg air per kg volatilesl. Con- sidering that under fuel-surface-controlled condi- tions (to be discussed later) the ratio of air flow rate to rate of volatile production is necessarily higher than 5.41, one can state with fair certainty that the rate of entry of air is sufficient for the complete combustion of cellulosic materials within the boundaries of the compartment. In spite of this, the appearance of large flames outside the windows of burning compartments is common. One may now propose that it is probably the rate of combustion of the volatile decomposition products, not the degree of completeness of their combustion, that is controlled by the rate of
The actual air requirement for a burning wood is some- what larger than the given value because a lesser amount of char also oxidized simultaneously with the volatile decomposition products. But even if it is assumed that the char is consumed by oxidation as soon as it forms, the stoichiometric air requirement is still only 5.11 kg air/kg fuel [7], below the level provided by ventilation.
entry of air and drives the pyrolysis reactions2 by thermal feedback. Yet this suggestion will not stand scrutiny either. One must realize that the gaseous phase in a burning compartment does not consist of a well-stirred mixture of volatiles, air, and combustion products; in other words, that the conditions in it are far from ideal for gas- phase reactions. In fact, as d l be discussed later, the flame envelope fairly well divides the gas- filled space into two zones, one consisting mainly of air at virtually undepleted oxygen content, the other mainly of volatile decomposition products and combustion gases. Consequently, it is as a rule the rate of entrainment of air into the flame envelope, not the rate of entry of air into the compartment, that controls the rate of com- bustion of the volatiles and thereby possibly affects the decomposition of the fuel.
Admittedly, increased ventilation does create improved turbulence along the flame boundaries, enhancing the rate of air entrainment into the flames and, in turn, the rate of combustion of the volatiles. Yet any suggestion that the rate of combustion of the volatile decomposition products might have more than a marginal effect on t h e rate of pyrolysis of the fuel can be dismissed, for the following reasons.
The rate of combustion of the volatile decom position products has a decisive influence on t temperature of the gases and compartment boun aries during fire. As heat supply to the decom
posing fuel is expected to take place by hig temperature-dependent transfer processes, one
There are contradictory views on whether the pyrol of wood is primarily an endothermic or an exothe process. According to Roberts [8, 91, und atmospheric conditions the over-all heat of d tion is slightly on the exothermic side. At 1 peratures, however, up to about 320°C,
fuel "suitable" for the exothermic reactions, heat mus be supplied to its virgin core. In addition to that re quired to drive the low-temperature endothermi reactions, this heat also includes the heat of vapo tion of moisture, and the sensible heat required raising the temperature of the core through the range endothermic reactions. It is not contradictory, the fore, to say that the pyrolysis of wood, at least up to certain temperature, requires a steady supply of heat.
led to conclude that the rate of pyrolysis is very sensitive to the prevailing temperatures and, in turn, to the rate of gas-phase reactions. This con- clusion is, however, not confirmed by observation. (The lack of sensitivity of the rate of burning to the compartment temperature can be observed, for example, by comparing in the report of the CIB cooperative program [ l o ] corresponding values of rate of burning (p. 37-38) and tempera- ture (p. 4748.) This fact was brought up earlier by the author [ l l ] as one of the reasons for questioning the assumed role of thermal feedback in compartment fires [12].) The implicit recogni- tion that the rate of pyrolysis of cellulosic fuel shows very little dependence on temperature conditions made it possible for many laboratories to conduct experimental studies of the rate of compartment burning without extensive instru- mentation.
It is quite obvious by now that the gas-phase oxidation reactions cannot play a decisive role in the mechanism by which compartment ventila- tion controls the rate of pyrolysis of the fuel. According to the model suggested by the author [7] a few years ago, it is the oxidation of the sur- face char over the decomposing fuel that is instru- mental in controlling pyrolysis by ventilation. Unfortunately, this model was not described in sufficient detail and apparently escaped the atten- tion of subsequent investigators of the subject of fully developed compartment fires. It seems desirable, therefore, to present the model with more detailed support.
SUGGESTED MECHANISM
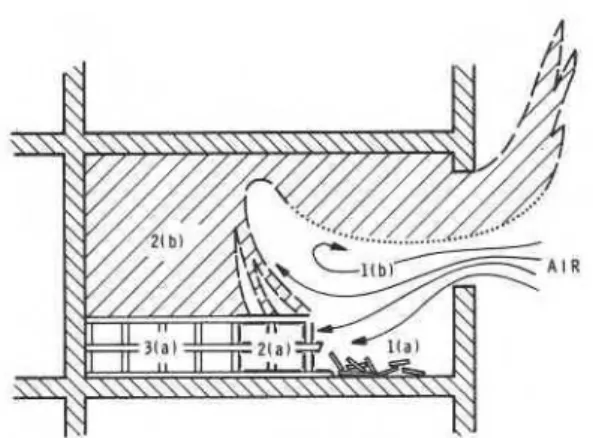
Figure 1 is a schematic illustration of a burning compartment at an advanced stage of fire (assumed to be ventilation-controlled). The furniture is modelled as a loosely-packed wood pile uniformly distributed on the floor. Part of the pile, that near the window, has already been reduced to ember. The lower regions of the compartment are divided into three zones. Zone l(a) contains the glowing remains of the collapsed fuel network. Zone 2(a), which will be referred to as the zone of intense pyrolysis, contains a still erect segment of the fuel network and is characterized by the fact that part
of the surface of the decomposing fuel is covered with oxidizing char (shown in heavier lines). Finally, zone 3(a) contains the part of the fuel network that is in an early stage of decomposition. The gas-filled space above the fuel network is divided into two zones. Zone l(b) is occupied mainly by air; zone 2(b) consists of a mixture of volatile decomposition products and combustion gases. These two zones are separated by the flame envelope (shown by dashed and dotted lines). Air moves rather freely over the collapsed fuel network in zone l(a). As the rate of oxidation of the ember is relatively low, the bulk of the air passes by this zone without significant depletion of its oxygen content. Then, as the air flow reaches zone 2(a), it separates into two streams. One penetrates the fuel network t o feed oxidation of the surface char and limited combustion of the volatile decomposition products within the fuel network. The other air stream rises with the bulk of the volatiles released by the fuel and part of it becomes entrained in the flame envelope3. Zone 3(a) receives scarcely any oxygen and, therefore, lacks combustion-induced heat sources. A slow decomposition of fuel takes place under the effect of thermal feedback (mainly by radiation) from the flame envelope and from the hot gases and compartment boundaries.
This model claims that the pyrolysis of fuel is driven, to a great extent, by the heat generated by oxidation of the char covered surfaces of the network and that therefore zone 2(a) is by far the largest source of volatile production. (The heat of combustion of the char is about twice that of the volatile decomposition products.) The important part that the oxidation of char plays in the process of pyrolysis can be established by both observa-
It has been observed [5, 131 that air entrainment is very poor along the horizontal frame envelope (shown by dotted line in Fig. I), which represents the lower boundary of the mixture of volatiles and combustion products as it moves under the ceiling towards the window. On the other hand, entrainment is quite vigorous along the boundaries of the vertical column of volatiles (shown by dashed line). Consequently, the height of the compartment has a much more pro- nounced effect on the rate of gakphase reactions than have its other dimensions.
T. Z. HARMATHY tions and special experimental techniques. In
crib burning experiments, spots of glowing char appear soon after ignition, starting at the lower edges of the outer sticks, and gradually extend over the entire crib surface. That oxidation does indeed contribute to the glow of char, was con- firmed recently by the author [14] by studying the effect of forced ventilation on the rate of burning (pyrolysis) of cribs of wood and various plastics. While the rate of burning of non-charring platics was found t o be virtually unaffected by the rate of air flow across the crib, that of wood and charring plastics increased markedly (up to a maxi- mum) with ventilation. This finding reflects the known response of the oxidation of carbon to the flow rate of oxidizer [15]. The important role of the across-the-crib ventilation in the burning of wood cribs was noted earlier by McCarter and Broido [16] who also observed the lack of response of the burning process to thermal feedback from the flames.
The low thermal conductivity of the surface char offers partial explanation to the relative insensitivity of the rate of pyrolysis to the temper- ature prevailing in the compartment (and, in turn, to the rate of gas-phase reactions). Along those surfaces where the char does not take part in oxidation, it insulates the undecomposed core of the fuel from heat transfer by radiation. Where oxidation takes place, the temperature of the char surface usually exceeds that of the environ- ment, so that the role of the hot environment is only a passive one; it "blankets" the glowing surface and thus allows a large portion of the heat of oxidation to be transferred to the fuel core.
Friedman noted [17] that the fuel configura- tions benefiting most from this blanketing effect are usually those that lose most heat when it is absent. On account of this, and because of the observed insensitivity of crib fire pyrolysis rates to prevailing temperature conditions, one is led to conclude that the rate of pyrolysis of fuel does not depend substantially on either the geometry of the fuel network or the rate of combustion of the volatile decomposition products in the compartment. (Further confirmation of the former claim comes from the work of Butcher
et al., experimenting with different crib arrange- ments [18] and with actual furniture [19]
.
Some degree of dependence of the rate of burning (py- rolysis) on the porosity of the fuel bed is, how- ever, suggested by the work of Nilsson [20] and Thomas and Nilsson [2 1 ] ).As the volatiles are gradually depleted in the zone of intense pyrolysis, the fuel network (furni- ture in general) collapses and an adjacent section of the network becomes exposed to the flush of inflowing air. In this way the zone of intense pyrolysis slowly moves from the window to the back of the compartment. The existence of such zonal decomposition of fuel has been confirmed by observations [ l o , 221.
As discussed in the introduction, the rate of production of volatile decomposition p r ~ d u c t . ~ U,, is somewhat different from what is commonly referred to as rate of burning, namely the rate of loss of mass of the fuel, R(=dC/dt). It has been shown [7] that the relation between the two is approximately as follows:
Because, according t o the model, the oxidation of char is the chief source of the heat that drives the decomposition reactions, it seems reasonable to assume that the rate of volatile production is proportional to the part of the'surface area of the decomposing fuel that is at any particular time covered by oxidizing char5, A, ; i.e ., that
See Nomenclature.
Experiments performed by burning cross-cribs of wood (for example, Refs. [23] and [24] and some synthetic polymers [14] revealed that the rate of decomposition of a batch of fuel is constant for a substantial part of the process, in spite of the fact that the total surface area of the cribs gradually decreases. Consequently, in any equation expressing proportionality between the rate of pyrolysis and the surface area of the fuel or any portion of it, that area must be regarded as constant. It is convenient to relate such areas to the prefire condi- tions. It is incorrect to assume, as some authors do, that the rate of decompostion decreases in proportion to the fuel surface and, therefore, that ventilation-controlled fires may change at some stage of the process into fuel- surface-controlled fires.
BURNING O F COMPARTMENT FIRES
Fig. 1. Illustration of zonal burning of cellulosic fuel in fully developed, ventilation-controlled compartment fires.
The experimental finding that R (and with it U,,) is approximately constant for a substantial part of the period of the fully developed fire implies, by virtue of Eq. (2), the approximate constancy of A,. As
all
oxidizing char surfaces that are effective in volatile production are located within the zone of intense pyrolysis (Fig. I), A, can be regarded as proportional to the surface area of the fuel in this zone,In turn, Afz can be expressed in a formal way as some portion of the total free surface area of the fuel network prior t o fire, A f , as
where a is a variable factor suspected t o be a func- tion of the flow rate of air, U,, and the complexity of the fuel network.
The task is now to find a logical expression for a . It seems reasonable to assume that the penetra- tion of air into the fuel network (and with it a) is directly proportional to the flow rate of air and inversely proportional to some variable characteristic of the complexity of the network. By selecting the free surface area of the fuel network for the latter variable, a can be formu- lated as
By combining Eqs. (1) to (5) one obtains the following equation for the rate of loss of the mass of fuel (which, unlike Uv, is a conveniently meas- urable quantity):
Thus the described model of compartment burning and subsequent reasoning confirm that the so- called rate of burning is proportional to the ventilation of the compartment. What makes the model and this line of reasoning even more con- vincing is the fact that (although applied t o ventilation-controlled fires only) they point to the existence of fuel-surface-controlled fires as well.
It is obvious from Eq. (4) that a cannot be larger than 1; in other words, that the zone of intense pyrolysis cannot be larger than the entire compartment. From this it follows that Eq. (5) is applicable only as long as &/Af
<
1 /C3 and that a should be defined as 1 whenever Ua/Af>
l/C3. On the other hand, the meaning of the condition a = 1 is that the entire fuel network in the com- partment is simultaneously involved in the decom- position process. For this situation, after com- bining Eqs. (1) to (4) (with a = I), one obtainsThis equation states that if the rate of entry of air into the compartment is large in relation to the total free surface area of the fuel (i.e., U,/Af
>
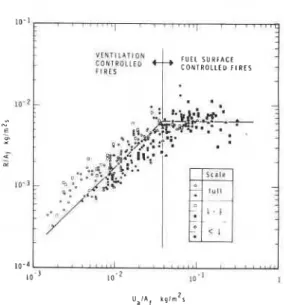
1 /C3), the so-called rate of burning will be propor- tional to the free surface area of the fuel. This is the kind of fire that is referred to as fuel-surface- controlled.The results of over 250 full-scale and reduced- scale compartment burn tests, reported in ten publications (cited in Ref. [7]), are plotted in Fig. 2 in a normalized form suggested by the described model of fully-developed compartment fires. In spite of the significant spread of the experimental points, the existence of two distinct regimes, one ventilation-controlled and the other fuel-surface-controlled, is clearly recognizable.
Fig. 2. Burning behavior of cellulosic fuel in compartment.
(The spread of the points is imputable to three factors: (i) the poor reproducibility of the char- acteristics of fully-developed compartment fires even under controlled laboratory conditions [25, 261, (ii) the deliberate neglect in the de- scribed model of a host of variables which de- monstrably have effect on the rate of burning (pyrolysis) at a lower significance level, and (iii) the lack in many reports of sufficient dimensional details concerning the fuel, which compelled the author to estimate the free surface area either from a general description of the fire load or-in the case of fuel-surface-controlled fires-from the duration of fully-developed fire with the aid of Eq. (1
11.1
The "best" values of the constants C1C2 and C3 have been assessed from Fig. 2. They are: C1C2 = 0.00578 and C3 = 26.22. With these values Eqs. (6) and (7) can be re-written in the following explicit forms:
where the dimension of the constant 0.0062 is kg/m2s, and the first equation obviously relates
T. Z. HARMATHY
to ventilation-controlled, the second to fuel- surface-controlled fires.
Some authors prefer to express rate of burning in terms of the so-called ventilation parameter, A,h1I2 (where A , is the window area and h the window height) instead of the rate of entry of air. If it is assumed that (i) at the onset of fully developed fire the window panes are broken and the full window area is available for ventilation, (ii) the fire compartment is perfectly sealed from other parts of the building, and (iii) no forced ventilation is used, the following equation describes the relation between the rate of entry of air and the ventilation parameter [7] :
where pa is the density of air entering the com- partment, and g is acceleration due to gravity. As the duration of the period of fully devel- oped fire, T, roughly coincides with the period of
production of volatile decomposition products, it depends on the portion of the total amount of fuel in the compartment that becomes volatilized in the decomposition process and on the rate of volatile production. It has been estimated [7] that, on the average, 87.2 per cent of the mass of fuel prior t o fire, G o , will turn into volatile decom- position products. Thus, utilizing Eq. (I), the duration of the fully developed period of fire can be expressed as
After substituting the appropriate expressions for R from Eqs. (6a) and (7a), one obtains, for ventilation-controlled fires,
for fuel-surface-controlled fires,
BURNING O F COMPARTMENT FIRES
In Eq. (1 1) the definition of the specific surface of fuel has been utilized:
It must be emphasized that derivation of Eqs. [6a], [7a], [ l o ] , and [ l l ] without the need for heat balance considerations was made possible by a peculiarity of cellulosic fuels-the observation that their pyrolysis is driven to a great extent by the heat produced by oxidation of their surface char layer and consequently does not depend on the rate of combustion of the volatile decomposi- tion products in the compartment, at least not to the same extent as does pyrolysis of most other solid fuels. One should remember that what has been described in this section is only a model. In view of the poor reproducibility of fully-devel- oped compartment fires,'this model describes the rate of burning (pyrolysis) and the duration of fire in terms of three variables,
U,,
A f , and G o ,and deliberately neglects those variables which can influence the process of burning at a lower significance level only. That the conclusions are reasonably accurate has been proved by compari- son with a multitude of experimental findings derived from full-scale and reduced-scale burn-out tests (see Fig. 2) performed on rooms of more or less conventional dimensions. It remains to be seen whether they are also applicable t o compartments of markedly different shape, dimension, and pos- sibly other fuel arrays with much greater or lower fraction of fuel surface area exposed to room radiation.
COMPARTMENT FIRES INVOLVING PLASTICS Recent experiments performed by the author [14] have indicated that the mechanism of burning of piles of char-forming plastics is analo- gous to that of cellulosic materials. It seems pos- sible, therefore, that Eqs. [6a], [7a], [ l o ] and [ I I ] , perhaps with different constants, can satis- factorily describe the rate of burning (pyrolysis) and duration of compartment fires involving char- forming plastics also.
Unfortunately, most of the important syn- thetic polymers pyrolyze without leaving behind carbon-rich solid decomposition products. Obviously, the mechanism of burning described earlier is not applicable to such materials. As they are not covered with a heat-producing oxidiz- ing char layer, the pyrolysis of these materials must rely entirely on the heat produced by com- bustion of the gaseous decomposition products within the fuel network and on thermal feedback from the flames, hot gases, and compartment boundaries. Consequently, it is unlikely that their rate of pyrolysis can be predicted with sufficient accuracy without an analysis of the heat balance over the compartment.
There are other significant differences between cellulosics and non-charring plastics. The stoichio- metric air requirement is almost three times as high for many of the latter materials as for the volatiles of cellulosic materials. In addition, many (those of high B-numbers [ I S ] ) pyrolyze much faster than wood or paper under similar conditions. Consequently, the rate of entry of air may be much less than is needed for the complete com- bustion of the pyrolysis products and a substan- tial part of the gasified fuel must, perforce, burn outside the compartment. This fact is not, how- ever, expected to influence significantly the rate of heat evolution inside the compartment where rate of combustion of volatiles depends primarily on rate of entrainment of air into the flame envelope.
Lacking sufficient experimental information one can only speculate on the probable course of fires involving non-charring plastics. As heat production by combustion of the volatile decom- position products within the fuel network is confined to zone 2(a), which, in addition, is the recipient of strong thermal feedback from the vertical section of the flame envelope, one can expect that the general pattern of burning will not be greatly different from that shown in Fig. 1. Nevertheless for non-charring plastics (unlike cellulosic fuel), under the effect of high radiant heat fluxes originating from the hot gases above and from the compartment boundaries, partici- pation of zone 3(a) in volatile production is also expected to be substantial.
T. Z. HARMATHY With regard to rate of pyrolysis, R , the fol-
lowing two a priori arguments can be advanced: (I) Because the intensity of heat supply to the surface of the decomposing fuel strongly depends on temperature conditions in the compartment, the thermal properties and total area of the com- partment lining, which have a bearing on temper- ature, must also have a strong effect on R.
(2) Because the rate of heat evolution in the compartment relies on the rate of air entrainment into the flame envelope and the efficiency of entrainment improves as the ceiling height in- creases, R is expected to depend more on height than on other dimensions of the compartment. Observations of films of compartment fires involving plastics and recent studies of liquid fuel fires by Bullen [27] seem to indicate that for fuels susceptible to fast pyrolysis a new type of burning mechanism may become effective. Fires involving such materials may become "pyrolysis- controlled." To understand this kind of mech- anism, assume that after a period of quasi-steady- state burning the rate of pyrolysis in the com- partment increases. The increased volatile produc- tion will gradually build up the layer of com- bustible gases that floats under the ceiling in the direction of the window. Unable t o come in con- tact with air in sufficient quantities, they leave the compartment unburned. As they leave, these gases narrow down that portion of the window opening available for entry of air. Since both the amount of air and the space available for air entrainment are decreased, the rate of heat evolu- tion in the compartment drops off and with it, temperature. Lower temperatures will naturally result in reduction of the rate of heat supply to the fuel surface and, in turn, in a trend towards restoration of the earlier level of rate of pyrolysis. There is a superficial similarity between pyroly- sis-controlled fires of plastics and ventilation- controlled fires of cellulosic materials. In both the rate of pyrolysis seems t o be independent of, or only weakly dependent on, the total fire load and specific surface of the fuel. Yet there are basic differences. The most important is that in pyroly- sis-controlled fires temperature is an essential link between pyrolysis and ventilation, whereas in ventilation-controlled fires the relation between pyrolysis and ventilation is direct.
CONCLUSION
The conventional concept of ventilation-controlled fires, according t o which shortage of air in a com- partment limits the rate of combustion of fuel, has been shown to be untenable. Alternative mechanisms by which the rate of entry of air might control the rate of pyrolysis of cellulosic materials have been examined and a plausible model suggested. This model has been proved to result in conclusions compatible with current knowledge concerning rate of pyrolysis in both ventilation-controlled and fuel-surface-controlled fires. It is pointed out in conclusion that the suggested model will probably not be applicable to non-charring plastics whose pyrolysis relies on the heat produced by combustion of the volatile decomposition products.
This paper is a contribution from the Division o f Building Research, National Research Council of Canada, and is published with the approval of the Director o f the Division.
NOMENCLATURE
A area, m2
C constant, various dimensions g acceleration due to gravity, 9.8 m/s2
G total mass of fuel, kg
h height of window, m
R rate of loss of the mass of fuel (so-called rate of burning), kg/s
t time, s
U
mass flow rate; mass rate of production, kg/sa factor, defined by Eq.
(9,
dimensionlessp density, kg/m3
7 duration of fully developed fire, s
cp specific surface of fuel, m2/kg Subscripts
a of air
c of surface covered with char
f of fuel
o prior to fire
v
of volatile decomposition productsw of the window
BURNING O F COMPARTMENT FIRES
REFERENCES
Fujita, K., Characteristics of Fire Inside a Non- combustible Room and Prevention of Fire Damage, Building Research Institute, Japan, Report 2(h).
Kawagoe, K., Fire Behaviour in Rooms, Building Research Institute, Japan, Report 27, 1958.
Simms, D. L., Hird, and Wraight, H. G. H., The temperature and duration of fires, Part I. Some Experiments with Models with Restricted Venti- lation, JFRO, Fire Research Note No. 412, 1960.
Gross, D., and Robertson, A. F., Experimental fires in Enclosures, 10th Symposium (International)
on Combustion, The Combustion Institute, 1965,
p. 931.
Thomas, P. H., Heselden, A. J. M., and Law, M.,
Fully-Developed Compartment Fires; Two Kinds of Behaviour, JFRO, Fire Res. Techn. Paper No.
18, 1967.
Magnusson, S. E., and Thelandersson, S.,Tempera- ture-Time Curves of Complete Process of Fire De- velopment, Acta Polytechnica Scandinavia, Civil Engineering and Building Construction Series, No.
65, Stockholm, 1970.
Harmathy, T. Z., A New Look at Compartment Fires, Parts I and 11, Fire Technology 8, 196, 326
(1972).
Roberts, A. F., and Clough, G., Thermal Decom- position of Wood in an Inert Atmosphere, 9th
Symposium (International) on Combustion, The
Combustion Institute, 1963, p. 158.
Roberts, A. F., The Heat of Reaction During the Pyrolysis of Wood, Combust. Flame 17, 79 (1971).
Thomas, P. H., and Heselden, A. J. M., Interna- tional Co-operative Research Program of Conseil International du Bitiment.Fully-Developed Fires in Single Compartments; Comprehensive Analysis of Results, JFRO, Fire Research Note No. 923, Aug.
1972.
Harmathy, T. Z., The Role of Thermal Feedback in Compartment Fires, Fire Technology 11, 48 (1975).
Thomas, P. H., Old and New Looks at Compart- ment Fires, Fire Technology 11,42 (1975).
Hinkley, P. L., Wraight, H. G. H., and Theobald, C. R., The Contribution of Flames Under Ceilings to Fire Spread of Compartments, Part I., Incom- bustible Ceilings, JFRO. Fire Research Note No.
712,1968.
Harmathy, T. Z., Experimental Study of the Effect of Ventilation on the Burning of Piles of Solid Fuels
Combust. Flame 31, 259-264 (1978) (preceding
article).
Spalding, D. B., Convective Mass Transfer, Edward Arnold Ltd., London, 1963.
McCarter, R. J. and Broido, A., Radiative and Convective Energy From Wood Crib Fires, Pyro- dynamics 2,65 (1965).
Friedman, R., Behavior of fires in compartments, International Symposium, Fire Safety o f Combus-
tible Materials, University of Edinburgh, October
1975.
Butcher, E. G., Bedford, G. K., and FardelI, P. J., Further Experiments on Temperatures Reached by Steel in Buildings, Proceedings of Symposium at Fire Research Station, Jan. 1967, JFRO, 1968, p.
1.
Butcher, E. G., Clark, J. J., and Bedford, G. K., A Fire Test in which Furniture was the Fuel, JFRO,
Fire Research Note No. 695, 1968.
Nilsson, L., The Effect of Porosity and Air Flow Factor on the Rate of Burning of Fires in Enclosed Spaces, Swedish Natl. Bldg. Res. Inst., Report R.
22, 1971.
'Thomas, P. H. and Nilsson, L., Fully Developed Compartment Fires: New Correlations of Burning Rates, JFRO, Fire Research Note No. 979, 1973.
Heselden, A. J. M., Some Fires in a Single
om-
partment with Independent Variation of Fuel Surface Area and Thickness, JFRO, Fire Research Note No. 469, 1961.Gross, D., Experiments on the Burning of Cross Piles of Wood, J. Research, NITS, 66C(2), 99 (1962).
O'Dogherty, M. J., , and Young, R. A., Miscel- laneous Experiments on the Burning of Wooden Cribs, JFRO, Fire Research Note No. 548, 1964.
Lie, T. T., private communication.
Babrauskas, V. and Williamson, R. B., Post- Flashover Compartment Fires, University of Cali- fornia, Berkeley, Rept. No. UBC FRC 75-1, 1975,
p. 60.
Bullen, M. L., A Combined Overall and Surface Energy Balance for Fully-Developed Ventilation- Controlled Liquid Fuel Fires in Compartments,