HAL Id: hal-02378293
https://hal.archives-ouvertes.fr/hal-02378293
Submitted on 25 Nov 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution| 4.0 International License
exosome to the Ski complex in Arabidopsis
Heike Lange, Simon Ndecky, Carlos Gomez-Diaz, David Pflieger, Nicolas
Butel, Julie Zumsteg, Lauriane Kuhn, Christina Piermaria, Johana Chicher,
Michael Christie, et al.
To cite this version:
Heike Lange, Simon Ndecky, Carlos Gomez-Diaz, David Pflieger, Nicolas Butel, et al.. ARTICLE
RST1 and RIPR connect the cytosolic RNA exosome to the Ski complex in Arabidopsis. Nature
Communications, Nature Publishing Group, 2019, 10, pp.1-12. �10.1038/s41467-019-11807-4�.
�hal-02378293�
RST1 and RIPR connect the cytosolic RNA exosome
to the Ski complex in Arabidopsis
Heike Lange
1,5
, Simon Y.A. Ndecky
1
, Carlos Gomez-Diaz
1
, David P
flieger
1
, Nicolas Butel
2
, Julie Zumsteg
1
,
Lauriane Kuhn
3
, Christina Piermaria
3
, Johana Chicher
3
, Michael Christie
4
, Ezgi S. Karaaslan
4
,
Patricia L.M. Lang
4
, Detlef Weigel
4
, Hervé Vaucheret
2
, Philippe Hammann
3
& Dominique Gagliardi
1,5
The RNA exosome is a key 3’−5’ exoribonuclease with an evolutionarily conserved structure
and function. Its cytosolic functions require the co-factors SKI7 and the Ski complex. Here we
demonstrate by co-puri
fication experiments that the ARM-repeat protein RESURRECTION1
(RST1) and RST1 INTERACTING PROTEIN (RIPR) connect the cytosolic Arabidopsis RNA
exosome to the Ski complex. rst1 and ripr mutants accumulate RNA quality control siRNAs
(rqc-siRNAs) produced by the post-transcriptional gene silencing (PTGS) machinery when
mRNA degradation is compromised. The small RNA populations observed in rst1 and ripr
mutants are also detected in mutants lacking the RRP45B/CER7 core exosome subunit. Thus,
molecular and genetic evidence supports a physical and functional link between RST1, RIPR
and the RNA exosome. Our data reveal the existence of additional cytosolic exosome
co-factors besides the known Ski subunits. RST1 is not restricted to plants, as homologues with a
similar domain architecture but unknown function exist in animals, including humans.
https://doi.org/10.1038/s41467-019-11807-4
OPEN
1Institut de biologie moléculaire des plantes, CNRS, Université de Strasbourg, Strasbourg, France.2Institut Jean-Pierre Bourgin, INRA, AgroParisTech, CNRS,
Université Paris-Saclay, Versailles, France.3Plateforme protéomique Strasbourg Esplanade FR1589 du CNRS, Université de Strasbourg, Strasbourg, France.4Max
Planck Institute for Developmental Biology, Tübingen, Germany.5These authors jointly supervised this work: Heike Lange, Dominique Gagliardi. Correspondence and
requests for materials should be addressed to H.L. (email:hlange@unistra.fr) or to D.G. (email:dominique.gagliardi@ibmp-cnrs.unistra.fr)
123456789
T
he RNA exosome provides all eukaryotic cells with a key
3′−5′ exoribonucleolytic activity that participates in the
maturation of various non-coding RNAs and in the
degradation of both non-coding and coding RNAs (reviewed in
refs.
1–5). The RNA exosome consists of a core complex
com-posed of nine subunits (Exo9) to which the exoribonucleases
RRP6 and DIS3/RRP44 differentially associate within the
nucleolus, nucleoplasm or cytosol
6–8. Whilst the overall structure
and function of the RNA exosome is conserved, both the
com-position and enzymatic activities of exosome complexes vary
among organisms. For example, most non-plant Exo9s including
those in yeast and human are catalytically inactive
9, whereas plant
Exo9s have retained a phosphorolytic activity originating from its
prokaryotic ancestor
10. This unique phosphorolytic activity of
plant Exo9 acts in combination with the hydrolytic activities
provided by RRP6 and DIS3
10. Another exception among RNA
exosomes is the association of human Exo9 with functionally
distinct DIS3L and DIS3 proteins, only the latter of which is
conserved in yeast and plants
6,11.
In all eukaryotes investigated, the catalytic activities of the RNA
exosome are modulated by cofactors termed activator–adapter or
exosome targeting complexes. These complexes aid in the
recog-nition of specific types of RNA substrates and couple
exosome-mediated degradation to cellular processes, such as ribosome
biogenesis or mitosis
12–19. All exosome targeting complexes that
have been characterised to date contain an RNA helicase from the
MTR4/SKI2 family as a central component. In addition, exosome
targeting complexes typically comprise RNA-binding proteins,
non-canonical
poly(A)
polymerases
or
factors
mediating
protein–protein interactions. Most exosome targeting complexes
described to date are nuclear. They include the TRAMP
(TRF4-AIR1-MTR4 polyadenylation) complexes
20–22in both baker’s
yeast and humans, the human PAXT (polyA tail exosome
tar-geting) complex
23, the NEXT (nuclear exosome targeting)
com-plexes that differ slightly in humans and plants
24,25and the
MTREC (Mtr4-like 1 (Mtl1)-Red1-core) complex in
fission
yeast
26,27. These MTR4 containing complexes assist the exosome
in nuclear RNA surveillance by targeting various RNA substrates,
including precursors of ribosomal and other non-coding RNAs,
spurious transcripts generated by pervasive transcription and
untimely, superfluous or misprocessed mRNAs
21,24,25,27–32. In
remarkable contrast to the diversity of nuclear exosome cofactors,
a single conserved protein complex, the Superkiller (Ski) complex,
is known to assist the exosome in the cytosol. The Ski complex
consists
of
the
MTR4-related
RNA
helicase
SKI2,
the
tetratricopeptide-repeat protein SKI3 and two copies of the
WD40-repeat protein SKI8
33–35. Association of the Ski complex
with the exosome core complex requires an additional
protein, SKI7
36. Recent data revealed the functional conservation
of SKI7 across eukaryotes
37. In mammals and plants, SKI7 is
produced by alternative splicing from a single locus that encodes
also the HBS1 protein
37–39. HBS1 functions together with the
G-protein Dom34/PELOTA in No-Stop decay by releasing
ribo-somes stalled on RNAs lacking a stop codon
40–43. In the yeast
Saccharomyces cerevisiae, Ski7 and Hbs1 are closely related
para-logs. Therefore, it was for long inferred that yeast Ski7 mediates
the association of the exosome with the ribosome
[34,44. Recent
data now challenge this view by showing that the Ski2–Ski3–Ski8
complex can directly bind to ribosomes, while Ski7 is associated
with Exo9
35,45.
The Ski complex is conserved in Arabidopsis thaliana
46, but
its physical association with the exosome core has not been
investigated yet. An initial experiment to affinity-capture factors
associated with the Arabidopsis exosome identified the
homo-logue of DIS3 and two nuclear RNA helicases, AtMTR4 and its
closely related homologue HEN2
25. In addition, Arabidopsis
Exo9 systematically co-purified with a 1840 amino acid
ARM-repeat protein of unknown molecular function named
RESUR-RECTION1 (RST1)
25. RST1 was originally identified in a
genetic screen for factors involved in the biosynthesis of
epi-cuticular waxes
47. Epicuticular waxes are a protective layer of
aliphatic very long-chain (VLC) hydrocarbons that cover the
outer surface of land plants
48. rst1 mutants have less wax on
floral stems than wild-type plants, and ~70% of the seeds
produced by rst1 mutants are shrunken due to aborted
embryogenesis
47. The molecular function of RST1 remains
unknown. Interestingly, one of the two RRP45 exosome core
subunits encoded in the Arabidopsis genome, named RRP45B or
CER7 (for ECERIFERUM 7) was also identified in a genetic
screen aimed at identifying enzymes or regulators of wax
bio-synthesis
49. The wax-deficient phenotype of rrp45b/cer7
mutants (cer7 from now on) is suppressed by mutations in genes
encoding RNA silencing factors, such as RDR1, RDR6, AGO1,
SGS3 and DCL4
50,51. This and the identification of small RNAs
accumulating in cer7 mutants revealed that the wax deficiency
observed in cer7 plants is due to post-transcriptional silencing of
CER3 mRNAs
50,51, encoding a protein that together with the
aldehyde decarbonylase CER1 catalyses the synthesis of VLC
alkanes from VLC acyl-CoAs
52,53. These results demonstrated
that the RNA exosome contributes to the degradation of the
CER3 mRNA and that the wax-deficient phenotype of cer7
mutants is a consequence of the established link between RNA
degradation and silencing pathways
50,51,54. Indeed, in plants,
the elimination of degradation intermediates such as uncapped
or RISC-cleaved mRNAs by 3′−5′ and 5′−3′ exoribonucleases
prevents
that
they
trigger
post-transcriptional
silencing
(PTGS)
55–60, a mechanism required for the destruction of
non-self RNAs originating from viruses or transgenes.
Here, we demonstrate by multiple reciprocal co-purification
assays coupled to mass spectrometry analyses that the Arabidopsis
exosome core complex Exo9 associates with the ARM-repeat
protein RST1, SKI7, another protein that we named RIPR (for
RST1 interacting protein) and the Ski complex. Our data show
that RST1 and RIPR suppress the silencing of transgenes as well
as the production of secondary siRNAs from endogenous
exo-some targets such as RISC-cleaved transcripts and certain
endo-genous mRNAs which are prone to PTGS. Those mRNAs include
the CER3 mRNAs explaining that the rst1 and ripr mutants share
the cer7 wax-deficiency phenotype. Taken together, our
bio-chemical and genetic data establish RST1 and RIPR as cofactors
of the cytoplasmic exosome and the Ski complex in plants.
Results
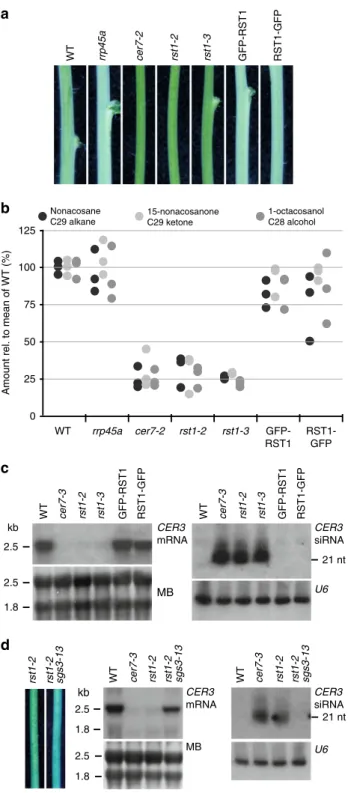
Wax de
ficiency in rst1 mutants is caused by CER3 silencing. To
investigate whether the wax deficiency of rst1 plants is linked to
compromised degradation of the CER3 mRNA as reported in cer7
mutants
49, we compared the stems of wild-type and mutant
plants grown under identical conditions. Due to the light
reflecting properties of the wax crystals that cover the outer
cuticle, stems of wild-type Arabidopsis plants appear whitish (or
bluish in cold light, Fig.
1
a). Consistent with previous reports,
plants lacking the exosome core subunit RRP45A have whitish
stems signifying intact wax biosynthesis
49. By contrast, plants
with T-DNAs inserted in the RRP45B/CER7 (AT3G60500) and
RST1 (AT3G27670) loci have glossy green stems indicating wax
deficiency
47,49(Fig.
1
a). Gas chromatography followed by mass
spectrometry (GC-MS) analysis of extracts obtained from the
stem surface confirmed that the amounts of the VLC derivatives
nonacosane, 15-nonacosanone and 1-octacosanol, three major
components of epicuticular stem wax in Arabidopsis, were
simi-larly reduced in cer7 and in rst1 mutants (Fig.
1
b). Ectopic
expression of RST1 fused to GFP in rst1-3 plants restored the
biosynthesis of nonacosane, 15-nonacosanone and 1-octacosanol
and resulted in wild-type-like whitish stems (Fig.
1
a, b). Previous
studies established that the wax deficiency of cer7 mutants is
due to post-transcriptional silencing of the mRNA encoding
CER3
49–51,54, a subunit of a VLC alkane-forming complex
52,53.
Indeed, RNA blots revealed a severe reduction of the CER3
mRNA and an accumulation of CER3-derived small RNAs in
both cer7 and rst1 mutants (Fig.
1
c). Mutating the PTGS factor
SUPPRESSOR OF GENE SILENCING 3 (SGS3) in rst1-2 plants
abolished the production of CER3-derived small RNAs, restored
wild-type levels of the CER3 mRNA and allowed the production
of epicuticular wax as demonstrated in the rst1 sgs3 double
mutant (Fig.
1
d). These results show that the wax-deficient
phenotype of rst1 mutants is caused by silencing of the CER3
mRNA, as reported for cer7 mutants.
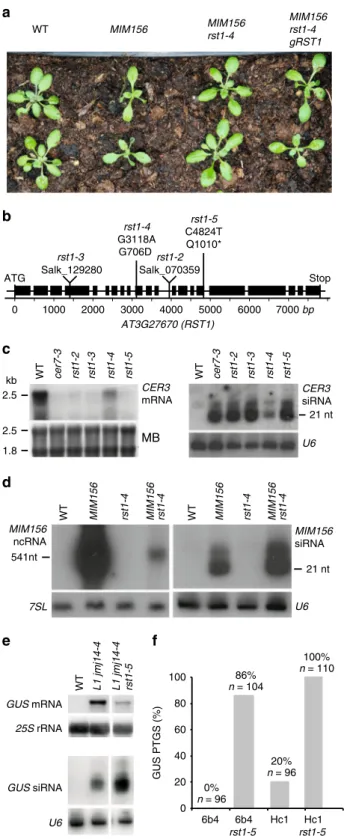
RST1 is a suppressor of transgene silencing. Two independent
genetic screens identified rst1 point mutations as suppressors of
silencing. The
first screen aimed to identify mutations
suppres-sing the phenotype of MIM156 plants. MIM156 plants express an
artificial non-coding RNA with an uncleavable miRNA 156
recognition site
61. Ectopic expression of this miRNA target
mimicry (MIM) construct reduces both levels and activity of
endogenous miR156 and leads to a characteristic phenotype with
spoon-shaped cotyledons, prematurely serrated rosette leaves and
a reduced leaf initiation rate during vegetative growth (Fig.
2
a).
MIM156 rst1-4 plants were recovered from an EMS-treated
population of MIM156 plants that had been visually screened for
restoration of normal growth and development. MIM156 rst1-4
plants display the spoon-shaped cotyledons of the parental line,
but wild-type-like leaf initiation rates and rosette leaf serration.
We mapped the suppressor mutation by whole-genome
sequen-cing to the RST1 gene and specifically the G3118A mutation
causing a G706D amino acid change (Fig.
2
b). Expressing a
genomic RST1 construct in MIM156 rst1-4 plants restored the
MIM156 phenotype confirming that the rst1-4 mutation was
responsible for the suppressor effect (Fig.
2
a). We then tested the
accumulation of the CER3 mRNA and CER3-derived siRNAs in
this novel rst1 allele. As compared with the T-DNA insertion
alleles rst1-2 and rst1-3, rst1-4 mutants had residual levels of the
full-length CER3 mRNA and lower levels of CER3-derived
siR-NAs, indicating that rst1-4 is a weak allele (Fig.
2
c). Next, we
analysed the accumulation of both the full-length MIM156
non-coding RNA and MIM156-derived siRNAs by RNA blots. This
experiment revealed low levels of MIM156-derived siRNAs in the
parental MIM156 line indicating that the MIM156 transcript is
spontaneously targeted by PTGS, as often observed with highly
expressed transgenes. Compared with the parental line, MIM156
rst1-4 plants had reduced levels of the full-length MIM156
tran-script, but accumulated increased levels of MIM156-derived
siRNAs (Fig.
2
d). The increased accumulation of these siRNAs in
the MIM156 rst1-4 suggests that RST1 restricts the production of
MIM156-derived siRNAs, which prevents complete destruction of
the full-length transcript by PTGS.
a
1.8b
WT rrp45a cer7-2 rst1-2 rst1-3 GFP-RST1 RST1-GFP cer7-3 rst1-2 rst1-2 sgs3-13 cer7-3 rst1-2 rst1-2 sgs3-13 MBd
rst1-2 rst1-2 sgs3-13 kb 2.5 2.5 1.8 U6 WT WT 21 nt CER3 mRNA CER3 siRNA 2.5 1.8 2.5 WT cer7-3 rst1-2 rst1-3 GFP-RST1 RST1-GFP cer7-3 rst1-2 rst1-3 GFP-RST1 RST1-GFP MB U6c
kb WT 21 nt CER3 mRNA CER3 siRNA rrp45a cer7-2 rst1-2 rst1-3 GFP-RST1 RST1-GFP WT 0 25 50 75 100 125Amount rel. to mean of WT (%)
Nonacosane C29 alkane 1-octacosanol C28 alcohol 15-nonacosanone C29 ketone
Fig. 1 The wax-deficient phenotype of rst1 mutants is caused by silencing of the CER3 gene.a Inflorescence stems of Arabidopsis plants of the indicated genotypes. GFP-RST1 and RST1-GFP are rst1-3 plants expressing RST1 fused to GFP. The whitish appearance of wild-type stems is due to a layer of the epicuticular wax deposited on the stem surface, while wax-deficient stems appear green and glossy. To better visualise the difference between normal and glossy stems, the white balance of the photograph was set to cold light (3800 K), which accounts for the bluish appearance of the picture. b Relative amounts of major stem wax compounds extracted from Arabidopsis stem sections.c Levels of CER3 mRNA and CER3-derived siRNAs in WT, cer7 and rst1 mutants. The total RNA extracted from stem samples of the indicated genotypes was separated by denaturing agarose (left) or polyacrylamide (right) electrophoresis, transferred to membranes and hybridised with a probe specific to CER3. The methylene blue (MB) stained membrane and hybridisation with a probe specific to U6 snRNA are shown as loading controls, respectively.d Mutating SGS3 restores the wax phenotype of rst1 mutants. Stem sections from rst1-2 and rst1-2 sgs3-13 plants are shown on the left. RNA blots show full-length CER3 mRNA (mid), and CER3-derived siRNAs (right) in RNA samples extracted from stems of the indicated genotypes. The source data are available at [https://doi.org/ 10.6084/m9.figshare.c.4483406]
The second screen directly aimed at identifying factors
affecting the post-transcriptional silencing of the 35Sprom:GUS
transgene in the reporter line L1 jmj14-4
62. This screen identified
rst1-5, a C4824T mutation in RST1 resulting in a truncation of the
RST1 protein (Q1010*) (Fig.
2
b). Compared with the L1 jmj14-4
parental line, L1 jmj14-4 rst1-5 plants had decreased levels of GUS
mRNA and increased levels of GUS-derived siRNA (Fig.
2
e). This
result resembled the effects of rst1-4 on the accumulation of
MIM156 transcript and MIM156-derived siRNAs (Fig.
2
d).
Backcrossing L1 jmj14-4 rst1-5 to wild-type yielded rst1-5 plants,
which showed a pronounced accumulation of CER3-derived
siRNA similar to rst1-2 and rst1-3 (Fig.
2
c). To further
demonstrate that rst1-5 enhances PTGS, we introduced the
rst1-5 mutation into the well-established reporter lines 6b4 and
Hc1
57(Fig.
2
f). These lines harbour the same 35S:GUS transgene
as the L1 jmj14-4 line, but inserted at different locations in the
Arabidopsis genome. In a wild-type background, line 6b4 does not
trigger sense transgene PTGS (PTGS), while line Hc1 triggers
S-PTGS in 20% of the population (Fig.
2
f). In genetic backgrounds
having impaired RNA degradation, both Hc1 and 6b4 lines
trigger S-PTGS at increased frequencies, which provides a
quantitative readout
25,55–57,59,63. 86% of the 6b4 rst1-5 plants
and 100% of the Hc1 rst1-5 plants triggered silencing of the 35S:
GUS reporter (Fig.
2
f). This result confirmed that RST1 functions
as a suppressor of S-PTGS comparable with other proteins
involved in RNA degradation
25,55–57,59,63. Of note, the role of
RST1 as S-PTGS suppressor is also supported by an independent
study published during the reviewing process of our paper
64.
RST1 co-purifies with the exosome, SKI7 and RIPR. To
examine the intracellular distribution of RST1, we used a rst1-3
mutant line expressing RST1 proteins fused to GFP at its N- or
C-terminus. Both fusion proteins were functional as they rescued the
wax deficiency of rst1-3 (Fig.
1
), and showed a diffuse cytoplasmic
distribution in root cells of stable Arabidopsis transformants
similar to the cytoplasmic marker protein PAB2 (Fig.
3
).
There-fore, we conclude that RST1 is a cytoplasmic protein. The diffuse
cytoplasmic localisation of RTS1 is in agreement with a recent
independent study
65. In our previous experiments, RST1
co-purified with Exo9 using the core exosome subunit RRP41 as
bait
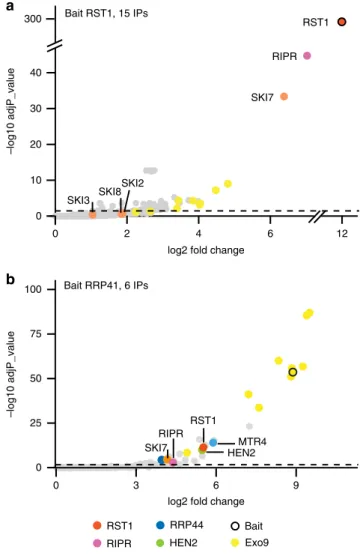
25. To verify the association of RST1 with Exo9, we used
GFP-RST1 or GFP-RST1-GFP as baits for immunoprecipitation (IP)
experiments followed by LC-MS/MS analyses (15 IPs). Indeed,
amongst the proteins that were enriched in RST1 IPs were the
nine canonical subunits of Exo9: CSL4, MTR3, RRP4, RRP40A,
RRP41, RRP42, RRP43, RRP45B/CER7 and RRP46 (Fig.
4
a;
Supplementary Data 1). By contrast, we did not detect HEN2 or
MTR4, the two main cofactors of nucleoplasmic or nucleolar
exosomes, respectively. Noteworthy, RST1 co-purified with the
cytoplasmic protein encoded by AT5G10630, the mRNA of which
is alternatively spliced to produce either HBS1 or SKI7 proteins.
As compared with the HBS1 mRNA, the SKI7 mRNA contains an
additional exon encoding the putative exosome interaction
0 20 40 60 80 100 6b4 6b4 rst1-5 Hc1 Hc1 rst1-5 0% n = 96 20% n = 96 86% n = 104 100% n = 110 GUS PTGS (%)
a
b
f
U6 7SL WT MIM156 rst1-4 MIM156 rst1-4 MIM156 siRNA MIM156 rst1-4 MIM156 rst1-4 WT WT MIM156 MIM156 rst1-4 MIM156 rst1-4 gRST1 rst1-2 Salk_070359 rst1-3 Salk_129280 rst1-4 G3118A G706D rst1-5 C4824T Q1010* 0 1000 2000 3000 4000 5000 6000 7000 bp Stop ATG AT3G27670 (RST1) WT cer7-3 rst1-2 rst1-3 rst1-4 rst1-5c
d
541nt 21 nt MIM156 ncRNAe
WT L1 jmj14-4 L1 jmj14-4 rst1-5 GUS mRNA GUS siRNA 25S rRNA U6 2.5 1.8 2.5 MB U6 kb 21 nt CER3 mRNA CER3 siRNA WT cer7-3 rst1-2 rst1-3 rst1-4 rst1-5Fig. 2 RST1 suppresses silencing of transgenes. a The rst1-4 mutation suppresses the developmental phenotype induced by a MIM156 transgene. b Diagram of the AT3G27670 gene encoding the RST1 protein. Boxes represent exons, lines represent introns. Triangles indicate the position of the T-DNA insertions in rst1-2 and rst1-3 lines. Vertical lines indicate the point mutations in rst1-4 and rst1-5.c rst1-4 is a weak allele. Accumulation of the CER3 mRNA (left) and CER3-derived siRNAs (right) in wild-type (WT), cer7-3 and the four rst1 alleles used in this study shown by RNA blots hybridised with a probe specific to CER3. The methylene blue stain of the membrane (MB) and hybridisation to U6 snRNA are shown as loading controls.d RNA blots showing the accumulation of the full-length MIM156 ncRNA and MIM156-derived siRNAs visualised by hybridisation with a probe specific to the IPS1 backbone of the MIM156 transgene. 7SL RNA and U6 snRNA are shown as loading controls.e RNA blots showing that the rst1-5 mutation results in reduced levels of the GUS mRNA and increased levels of GUS-derived siRNAs in the L1 jmj14-4 background. 25S rRNA and U6 snRNA are shown as loading controls.f The rst1-5 mutation increases S-PTGS frequency in both 6b4 and Hc1 reporter lines. The barplot shows the proportion of plants with silenced GUS expression in the indicated genotypes. The source data are available at [https://doi.org/10.6084/m9. figshare.c.4483406]
domain of the Arabidopsis SKI7 protein
37,39. Inspection of the
peptides detected in the RST1 IP revealed the presence of peptides
specific to the SKI7 splice isoform (Supplementary Fig. 1). This
and the fact that SKI7 proteins are bound to yeast and human
exosome complexes indicate that the AT5G10630 gene product
which co-purified with RST1 is indeed SKI7 rather than HBS1.
The three core proteins of the cytoplasmic Ski complex SKI2, SKI3
and SKI8 were not significantly enriched (Fig.
4
a). Together, these
IP results support the exclusively cytoplasmic localisation of RST1
and confirm its interaction with the Exo9 core complex.
Fur-thermore, a protein of unknown function encoded by AT5G44150
and that we termed RIPR for RST1 INTERACTING PROTEIN
was the most enriched protein in all RST1 IPs (Fig.
4
a).
Because our previous purifications of Arabidopsis exosome
complexes with tagged RRP41 as bait were analysed using an
older and less sensitive mass spectrometer
25, we repeated the
experiment using the same experimental settings as we used for
the RST1 IPs (6 IPs, Fig.
4
b; Supplementary Data 2). This new
experiment confirmed the previously reported co-purification of
the conserved exoribonuclease RRP44 and the two nuclear RNA
helicases MTR4 and HEN2 with Exo9 and reproduced the
co-purification of RST1. In addition, the new experiment revealed a
significant enrichment of both RIPR and SKI7 in the RRP41 IPs.
Peptides specific to the alternative subunit RRP45A were
present in the RRP41 IPs, but absent when RST1 was used as bait,
suggesting that RST1 may preferentially interact with CER7 (aka
RRP45B)-containing exosome complexes. To test this hypothesis,
we stably expressed GFP-tagged RRP45A and RRP45B/CER7 in
Arabidopsis. Indeed, both RST1 and SKI7 were significantly
enriched with CER7 as bait (Fig.
5
; Supplementary Data 3).
RRP45A co-purified with the nucleoplasmic RNA helicase HEN2,
which confirmed the association of HEN2 with Exo9-RRP45A
previously observed in the reciprocal IP
27. The high number of
experiments (15 IPs) was also sufficient to detect the
exoribonu-clease RRP44, which is consistently poorly enriched in IPs of the
Arabidopsis Exo9
10,25(also seen in Fig.
4
). We cannot formally
rule out that the failure to detect RST1 and SKI7 with
RRP45A-GFP is due to technical reasons. However, our data suggest that
RST1 and SKI7 are principally associated with RRP45B/CER7.
The difference in the interactome of CER7 and RRP45A cannot
be explained by different intracellular localisation of the baits,
because both CER7-GFP and RRP45A-GFP were present in the
nuclear and cytoplasmic compartments (Supplementary Fig. 2).
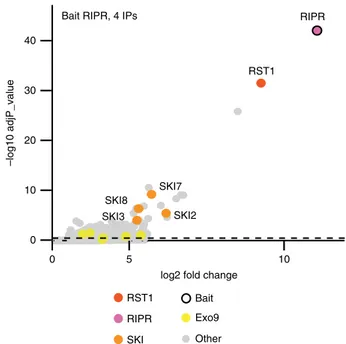
In order to confirm the physical association of RIPR with
RST1, we used RIPR with GFP-tags at either the N-terminal or
the C-terminal ends as bait in co-purification experiments (4 IPs,
Fig.
6
; Supplementary Data 4). RST1 was the most enriched
protein in RIPR IPs. The nine subunits of the exosome were also
detected, but were less enriched than in the IPs with RST1 as bait
(compare Figs.
4
,
6
). By contrast, SKI7 as well as the three
components of the Ski complex SKI2, SKI3 and SKI8 were
amongst the most significantly enriched proteins co-purifying
with RIPR (Fig.
6
).
Altogether, the multiple reciprocal IPs confirm the interaction
of RST1 with CER7-containing exosome core complexes and
identify SKI7 and RIPR as additional binding partners of both
RST1 and Exo9. Furthermore, our data indicate that RST1 binds
Exo9 and SKI7, while RIPR binds to RST1-SKI7 and the Ski
complex.
Loss of RIPR function phenocopies
rst1 mutants. RIPR is
conserved amongst
flowering plants but has no clear sequence
homologues in mosses, green algae or outside of the green lineage.
RIPR is a 356 amino acid protein that lacks obvious functional
domains and motifs or sequence homologies to known proteins.
Confocal microscopy of Arabidopsis roots stably expressing
RIPR-GFP fusion proteins revealed a diffuse cytoplasmic
dis-tribution (Fig.
7
a) similar to the intracellular distribution of RST1
tRFP-PAB2 RST1-GFP
GFP-RST1 RST1-GFP
Fig. 3 RST1 is a cytosolic protein. Confocal microscopy of root tips from Arabidopsis rst1-3 plants expressing GFP-RST1 and RST1-GFP fusion proteins under the control of the constitutive UBIQUITIN 10 promoter. Poly(A)-binding protein 2 at its N-terminus fused to tRFP (tRFP-PAB2) and expressed under the control of its own promoter is shown as cytosolic marker. Scale bars are 10µm. The source data are available at [https://doi. org/10.6084/m9.figshare.c.4483406] RST1 SKI RIPR Exo9 RRP44 Other HEN2 Bait MTR4 40 300 0 10 20 30 0 2 4 6 12 –log10 adjP_value RST1 SKI7 RIPR Bait RST1, 15 IPs –log10 adjP_value Bait RRP41, 6 IPs SKI7 RST1 RIPR
log2 fold change SKI2 SKI3 SKI8 0 25 50 75 100 0 3 6 9 MTR4 HEN2
a
b
log2 fold change
Fig. 4 RST1 co-purifies with the exosome, SKI7 and RIPR. Volcano plots show the enrichment of proteins co-purified with GFP-tagged RST1 (a) or RRP41 (b) as compared with control IPs. Y- and X-axis display adjusted p-values and fold changes, respectively. The dashed line indicates the threshold above which proteins are significantly enriched (adjP < 0.05). The source data are available in Supplementary Data 1 and 2
(Fig.
3
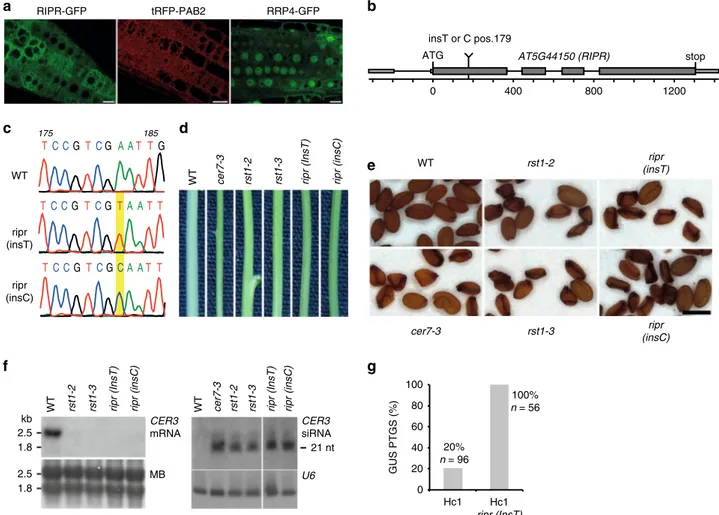
). Because T-DNA insertion mutants in the AT5G44150
locus were not available in Arabidopsis stock centres, we obtained
two independent mutants using a CRISPR-Cas9 strategy. The
mutants, named ripr(insT) and ripr(insC), had single T or C
nucleotides inserted at position 179, creating premature stop
codons 60 and 65 amino acids after the start codon, respectively
(Fig.
7
b, c; Supplementary Fig. 3). Interestingly, ripr(insT) and
ripr(insC) mutant plants have glossy green stems resembling the
stems of rst1 and cer7 plants (Fig.
7
d). Moreover, about 70% of
the seeds produced by ripr(insT) and ripr(insC) plants were
shrunken, similar to the proportion of unviable seeds produced
by rst1-2, rst1-3 or cer7-3 plants (Fig.
7
e; Supplementary Fig. 4)
47.
RNA blots confirmed that the wax-deficient phenotype of ripr
(insT) and ripr(insC) is due to the accumulation of CER3-derived
small RNAs and silencing of the CER3 mRNA (Fig.
7
f). Those
results demonstrate that RIPR can, like RST1, suppress the
pro-duction of small RNAs from the CER3 locus. Finally, 56/56 Hc1
ripr(insT) plants triggered silencing of the GUS PTGS reporter
(Fig.
7
g), demonstrating that RIPR also supresses transgene
silencing. Taken together, loss of RIPR induced very similar
physiological and molecular phenotypes as loss of RST1 or the
exosome subunit CER7.
mRNA-derived small RNAs accumulate in
cer7, ripr and rst1.
The association of RST1 and RIPR with the exosome complex
and the fact that loss of RST1 or RIPR phenocopies the cer7
mutation suggested that both RST1 and RIPR are involved in the
exosome-mediated degradation of the CER3 mRNA before it can
become a template for the production of CER3-derived small
RNAs. To identify other common targets of RST1, RIPR and the
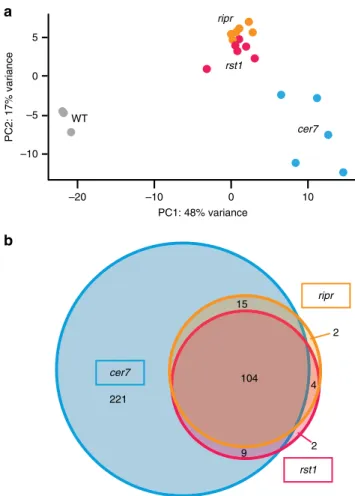
exosome complex, we analysed small RNA libraries prepared
from wild-type plants and from cer7, rst1 and ripr mutants
(Fig.
8
a). This analysis identified more than 300 mRNAs that gave
rise to small RNAs in cer7 (Supplementary Data 5), including
five
of the six mRNAs that were previously shown to undergo
silen-cing in absence of the RRP45B/CER7 exosome subunit
51. Many
of the loci that generate small RNAs in cer7 mutants have
pre-viously been shown to produce siRNAs in ski2 single mutants,
ski2 xrn4 double mutants or in the decapping mutants dcp2 and
vcs
57,58,60, and/or are known or predicted targets of miRNAs
(Supplementary Data 5). Because these siRNAs are produced
from protein-coding genes in RNA degradation mutants, they
have been termed ct-siRNAs (coding-transcript siRNAs) or
rqc-siRNAs (RNA quality control rqc-siRNAs)
57,58,60. About one-third of
the loci that generated rqc-siRNA in cer7 mutants produced
significant amounts of rqc-siRNAs in rst1 and ripr mutants as
well, while only very few loci were specifically observed in only
ripr or rst1 (Fig.
8
b). The observation of quasi identical
popula-tions of small RNAs in rst1 and ripr and the fact that almost all
loci affected by rst1 or ripr are also affected in cer7 strongly
support the conclusion that RST1 and RIPR are required for the
degradation of at least a subset of cytoplasmic exosome targets.
Discussion
This study identifies RST1 and RIPR as two previously unknown
cofactors which support the function of the cytoplasmic RNA
exosome in Arabidopsis. Three lines of evidence back our
con-clusion. Firstly, RST1 and RIPR are physically associated with the
0 10 20 40
0 2 4 6 10
log2 fold change
–log10 adjP_value 0 25 50 75 100 125 10 5 0
log2 fold change
–log10 adjP_value RRP45A,14 IPs CER7, 6 IPs SKI7RST1 RIPR RST1 SKI RIPR Exo9 RRP44 Other HEN2 Bait MTR4 HEN2 RRP44 MTR4
Fig. 5 RST1 and RIPR are bound to CER7-containing exosomes. Volcano plots show the enrichment of proteins co-purified with GFP-tagged RRP45B/CER7 (a) or RRP45A (b) as compared with control IPs. Y- and X-axis display adjusted p-value and fold change, respectively. The dashed line indicates the threshold above which proteins are significantly enriched (adjP< 0.05). The source data are available in Supplementary Data 3
log2 fold change RST1 SKI7 SKI2 SKI8 SKI3 0 10 20 30 40 10 5 0 –log10 adjP_value RIPR Bait RIPR, 4 IPs
RST1 SKI
RIPR Exo9 Other Bait
Fig. 6 RIPR co-immunoprecipitates RST1, SKI7 and the Ski complex. The Volcano plot shows the enrichment of proteins co-purified with GFP-tagged RIPR as compared with control IPs. Y- and X-axis display adjusted p-value and fold change, respectively. The dashed line indicates the threshold above which proteins are significantly enriched. The source data are available in Supplementary Data 4
exosome core complex and the Ski complex, respectively, both of
which act together in the degradation of cytoplasmic RNAs.
Secondly, both RST1 and RIPR suppress the silencing of
trans-genic reporters similar to almost all known main RNA
degrada-tion factors including proteins involved in decapping, the 5′−3′
exoribonucleases XRN3 and XRN4, and both subunits of Exo9
and Exo9 cofactors involved in 3′−5′ RNA degradation
25,55–57,59.
Thirdly, loss-of-function mutations of either RST1, RIPR or the
exosome subunit CER7 lead to the accumulation of illegitimate
siRNAs generated from endogenous protein-coding genes many
of which have been previously found to produce siRNAs in ski2,
xrn4 ski2, or in mutants of the decapping complex
57,58,60,66.
The current hypothesis for the production of rqc-siRNAs
is that mRNA degradation intermediates, such as decapped,
deadenylation or cleaved mRNAs, including the fragments
pro-duced by RISC, must be rapidly eliminated to avoid that
they serve as substrates for the synthesis of double-stranded
RNA by endogenous RNA-dependent RNA polymerases and
SGS3
55–58,60,67. Likely, the largely redundant functions of the
cytoplasmic 5′−3′ exoribonuclease XRN4, the 3′−5′
exoribonu-clease DIS3L2/SOV and the cytoplasmic exosome together with
the Ski complex ensure the rapid elimination of mRNAs after
their degradation has been initiated by decapping, deadenylation
or RISC-mediated cleavage, thus preventing the production of
rqc-siRNAs. Vice versa, accumulation of rqc-siRNAs indicates
impaired RNA degradation. The fact that similar rqc-siRNA
profiles are observed in cer7, rst1 and ripr mutants indicates that
CER7, RST1 and RIPR contribute to the degradation of an
overlapping set of mRNA targets.
Due to a natural variation, the Col-0 accession that is used as
wild-type here and in most other studies investigating RNA
degradation in plants lacks a fully functional DIS3L2/SOV 3′−5′
exoribonuclease and can therefore be regarded as a sov
mutant
66,68. Compared with plants expressing a functional SOV
homologue, Col-0 does not accumulate rqc-siRNAs (except
siR-NAs derived from the AT2G01008 mRNA)
66, perhaps because
most of its RNA substrates can also be degraded by the
cyto-plasmic exosome. Therefore, we cannot exclude that the
RRP4-GFP RIPR-GFP tRFP-PAB2
a
0 400 800 1200 AT5G44150 (RIPR) ATG stop insT or C pos.179b
WT cer7-3 rst1-2 rst1-3 ripr (InsT) ripr (insC)
185 TC CGTCG A AT TG 175 TC CG TCGTA AT T TC CGTCGCA AT T
d
c
WT cer7-3 rst1-2 rst1-3 ripr (insT) ripr (insC)e
0 20 40 60 80 100 Hc1 Hc1 ripr (InsT) 20% n = 96 100% n = 56 GUS PTGS (%)g
2.5 1.8 2.5 1.8WT rst1-2 rst1-3 ripr (InsT) ripr (insC) WT cer7-3 rst1-2 rst1-3 ripr (InsT) ripr (insC)
MB CER3 siRNA U6 CER3 mRNA kb
f
21 nt WT ripr (insT) ripr (insC)Fig. 7 Loss of RIPR function phenocopies rst1 mutants. a Confocal microscopy of plants expressing RIPR-GFP, the cytosolic marker tRFP-PAB2 and a GFP-tagged version of the exosome subunit RRP4. Scale bars are 20µm. The source data are available at [https://doi.org/10.6084/m9.figshare.c.4483406]. b Scheme of the AT5G44150 gene encoding the RIPR protein. Small and large boxes represent exons in the UTRs and CDS, respectively, lines represent introns. The single insertion of a T or C nucleotide at position 179 (from the ATG) results in a frameshift, which creates a premature stop codon. c Electropherograms of the genomic DNA sequence surrounding the relevant position of the AT5G44150 locus in wild-type, ripr(insT) and ripr(insC) plants. d Stem sections from wild-type (WT), cer7, rst1 and ripr plants. e cer7-3, rst1 and ripr mutants produce similar proportions of non-viable seeds. Scale bar is 0.5 mm. Please also see the uncropped pictures provided in Supplementary Fig. 4.f Northern blots showing the downregulation of the CER3 mRNA (left) and the upregulation of CER3-derived siRNAs (right) in ripr mutants. Loading controls show the methylene blue stained membrane (MB, left) and the hybridisation with a probe specific to U6 snRNA (right). The uncropped blots can be seen in Supplementary Fig. 5, and are also available at [https://doi. org/10.6084/m9.figshare.c.4483406].g RIPR is a silencing suppressor. The barplot shows the percentage of plants that spontaneously trigger silencing of the Hc1 35S prom:GUS S-PTGS reporter
accumulation of rqc-siRNAs in cer7, rst1 and ripr is only observed
because these mutants simultaneously lack SOV. However, the
fact that the wax-deficient phenotype caused by the production of
CER3-derived siRNAs is also observed in Landsberg and C24
accessions, both of which possess a functional SOV protein,
implies that SOV and the cytoplasmic RNA exosome are not fully
redundant.
It is important to note that both the loci concerned and the
levels of siRNAs from a given loci vary among plants of the same
genotype and grown under identical conditions. Not each of the
loci that give rise to rqc-siRNA undergoes silencing, i.e., full
suppression of its expression
66. Yet, PTGS is obviously
con-sistently triggered for certain Arabidopsis mRNAs. For instance,
compromising 3′−5′ degradation by the exosome (and in the
absence of SOV in the Col-0 accession) leads to silencing of the
CER3 and few other mRNAs, while distinct loci appear to be
more sensitive to impaired decapping or 5′−3′ decay
57,58,60.
Interestingly, many of the mRNAs that reproducibly generate
small RNAs in RNA degradation mutants, including the CER3
mRNA, have actually no sequence homology to miRNAs
(Sup-plementary Data 5). It is therefore tempting to speculate that
mRNAs prone to PTGS possess common intrinsic features that
trigger the recruitment of SGS3 and RDR6. One of the currently
discussed propositions is that highly expressed mRNAs are more
likely to generate aberrant RNAs than moderately or low
expressed ones
60. In addition, certain mRNAs prone to RNA
silencing may be cleaved by off-targeted RISC, or are perhaps
substrates of other endonucleases. Alternatively, secondary
structures or strong association to proteins may impede
degra-dation by at least one of the otherwise largely redundant 5′−3′
and 3′−5′ degradation pathways and could explain why some
mRNAs are more likely to become substrate for RNA-dependent
RNA polymerases than mRNAs which are efficiently degraded
from both directions. Yet, about 30% of the rqc-siRNAs
gen-erating loci in rst1 and ripr are known or predicted miRNA
targets (Supplementary Data 5). Hence, at least for those, the
initial substrate for RDR6-dependent siRNA production could be
a RISC-cleaved mRNA fragment. Since miRNAs and AGO1, the
main effectors of RISC, are associated with polysomes
69,70, 5′
cleavage products that could be generated by RISC on polysomes
resemble truncated mRNAs without a stop codon and without a
polyA tail. Therefore, we can presume that RST1 and RIPR,
together with the Ski complex and the RNA exosome, participate
in the elimination of no-stop RNA. The notion that 5′
RISC-cleaved fragments that fail to be degraded by RST1–RIPR–SKI
and the exosome become a substrate for the production of small
RNAs
fits well with the observation that the full-length cleavage
fragments of only 10–20% of the Arabidopsis miRNA targets can
be detected in the non-stop decay mutant pelota
67. Of note, this
study and ours used the Col-0 accession, which does not express a
functional SOV/DIS3L2
68. Therefore, the respective contribution
of the exosome and the SOV/DIS3L2 pathways to prevent the
production of siRNAs from 5′ fragments of RISC-cleaved mRNAs
remains to be specifically addressed.
Our data also have important implications on the physical
organisation of the cytoplasmic RNA exosome and the Ski
complex. In Arabidopsis and closely related species, two genes
encode the exosome core subunit RRP45. Arabidopsis RRP45A
and RRP45B/CER7 share 88% identity over their
first 300 amino
acids. Both subunits are located in cytosolic and nuclear
com-partments, and are at least in stable transformants, similarly
enriched in nucleoli. Interestingly, both RST1 and RIPR only
associated with RRP45B/CER7-containing exosomes, while no
peptide of RST1 was detected in any of the 14 experiments that
we performed using RRP45A as bait. Instead,
RRP45A-containing
exosomes
preferentially
co-purified with the
nuclear RNA helicase HEN2, in line with previous results
obtained using HEN2 as bait
25. Our results indicate that RST1
preferentially associates with the CER7-containing version of
the Arabidopsis exosome. Compared with RRP45A, CER7
pos-sesses an extra C-terminal domain of 135 amino acids, which
may be important for the recruitment of RST1. However, a
previous study suggested that these extra amino acids are
dis-pensable for the function of CER7 in the degradation of the
CER3 mRNA
49. Moreover, the ectopic expression of RRP45 in
cer7 mutants rescued their wax-deficient phenotype
49. A
pos-sible explanation is that the cer7-3 allele might be a knockdown
rather than a knockout mutant and still express residual
amounts of CER7, which may be sufficient to promote the
degradation of CER3 mRNAs when elevated levels of
RRP45-containing exosomes take over other functions such as nuclear
RNA surveillance. We can also speculate that overexpression of
RRP45A allows a weak interaction with RST1 that is below the
detection level in our IPs with RRP45A as bait. An alternative
scenario could be that the physical interaction between RST1
and Exo9 is not essentially required for the function of both
proteins in the turnover of the CER3 mRNA.
The observation that RST1 is amongst the most enriched
proteins captured with either RRP41 or CER7 as bait suggests that
RST1 is associated with the exosome core complex. The strong
enrichment of both RIPR and SKI7 with RST1 as bait and the
15
b
a
9 4 2 221 2 104 ripr rst1 cer7 rst1 ripr WT cer7 –10 –5 0 5 –20 –10 0 10 PC2: 17% variance PC1: 48% varianceFig. 8 Loss of RST1 or RIPR results in the accumulation of small RNAs that are also produced in cer7 mutantsa Multidimensional scaling plot illustrating global variance and similarities between the 21/22 nt small RNA populations detected in the replicates of WT, cer7, rst1 and ripr.b Venn diagram showing that rst1 and ripr mutants accumulate quasi identical populations of small RNAs almost all of which are also detected in cer7 mutants. The source data are available in Supplementary Data 5
observation that the Ski complex purifies mainly with RIPR
suggests that RST1 and RIPR link the exosome to the Ski complex
in plants. Future experiments will address the possibility that
RIPR may be required to link the Ski complex with the core
exosome while RST1 could stabilise the binding of Exo9 and
SKI7. Other interesting possibilities are that RST1 and/or RIPR
affect the recognition of target RNAs or the recruitment of the
exosome to ribosomes. Yet, we
find only a few ribosomal proteins
enriched in individual IPs (Supplementary Data 1–4, or explore
the interactive volcano blots available on
figshare [
https://doi.org/
10.6084/m9.figshare.c.4483406
]. Hence, we do not detect the
association of the exosome to the ribosome that was observed in
yeast
34,35,45. Whether this has a technical basis or truly reflects a
poor association of Exo9–RST1–RIPR–Ski complex with
ribo-somes remains to be investigated.
Interestingly, a recent study in yeast identified Ska1 as a
protein that impedes the association of the yeast Ski–Exo9
complex with the ribosome
45. Similar to RIPR in Arabidopsis,
Ska1 affinity captured the Ski complex. But unlike RST1 or RIPR,
the Ska1–Ski complex is not required for the degradation of
coding regions, and instead, has a specific function in the
elim-ination of RNAs devoid of ribosomes such as 3′ UTRs or long
non-coding RNAs. Apparently, overexpressing of Ska1
out-competes the association of the Ski complex with ribosomes,
suggesting that the association the Ski complex with either Ska1
or the ribosome is mutually exclusive
45. Of note, sequence
homologues of Ska1 seem to be restricted to S. cerevisiae and
some closely related fungi, although proteins with similar
func-tions may exist in other species.
RIPR seems to be conserved in
flowering plants but is absent
from the genomes of mosses and green algae, suggesting a relative
recent evolutionary origin. By contrast, RST1 is deeply conserved
in the green lineage. Moreover, a single ARM-repeat protein
comprising the same domain of unknown function DUF3037
(IPR022542) as RST1 is conserved in humans, across all metazoa
and in ancient amoebozoa such as Dictyostelium, but is
appar-ently absent from modern fungi (PTHR16212 protein family).
The human DUF3037 protein KIAA1797 was named Focadhesin,
because its GFP fusion protein has been detected in focal
adhe-sion points of astrocytoma cells
71. Interestingly, a recently
gen-erated high-throughput data set monitoring the migration of
proteins in sucrose gradients with or without RNase treatment
detected Focadhesin as a putative component of an
RNA-dependent complex
72[
http://r-deep.dkfz.de/
]. More work is
needed to fully understand the molecular function of human
Focadhesin. It will be interesting to investigate whether
Focad-hesin is also associated with the function of the RNA exosome in
animals.
Methods
Plant material. Plants were grown on soil or in vitro on the Murashige and Skoog medium supplemented with 0.5% sucrose at 20 °C in 16 h light and 8 h darkness. All plants were of the Col-0 accession, which served as wild-type in all experiments. The T-DNA insertion lines cer7-2 (Salk_003100), cer7-3 (GK_089C02), rrp45a (GK_665D02), sgs3-13 (Salk_039005) and rst1-2 (Salk_070359), rst1-3
(Salk_129280) have been described in refs.49,50and47, respectively. The rst1-5 and rst1-4 alleles are EMS alleles identified during this study. Starting point for the identification of rst-5 was the EMS mutagenesis of the line L1 jmj14-4 line, in which PTGS of the 35Sprom:GUS transgene inserted at the L1 locus is partially impaired by the jmj14-4 mutation62. The rst1-4 mutant was identified following EMS treatment of MIM15661. EMS mutagenesis of seeds was performed as described in ref.73. Mutations were identified by mapping-by-sequencing using pooled F2 plants exhibiting the phenotype of interest. Sequencing libraries prepared with the Illumina TruSeq DNA Sample Preparation Kit were 10-plexed (Illumina adapters Set A) perflow-cell lane and sequenced on an Illumina HiSEquation 2000 instrument to obtain at least tenfold genome coverage. The SHOREmap technique was used to identify SNPs and mapping intervals. The EMS mutants were back-crossed to Col-0 to remove the MIM156 transgene (rst1-4) or the jmj14-4 mutation
and the L1 35Sprom:GUS reporter (rst1-5). Presence or absence of the transgenes and mutations were confirmed by PCR genotyping.
PTGS analysis. Hc1 rst1-5, Hc1 ripr(insT) and 6b4 rst1-5 plants were obtained by crosses. S-PTGS frequencies were assessed by GUS activity assays. Briefly, 0.5-1 µg of soluble proteins extracted from inflorescence leaves were incubated with 150 µl of 2 mM 4-methyl-umbelliferym-β-glucuronide, 29 mM Na2HPO4, 21 mM NaH2PO4,
10 mM EDTA. Fluorescence was determined at 15 s intervals for 30 min at 37 °C with a Fluoroscan Ascent 2.6. The GUS activity corresponds to the slope of the curve. Typically, GUS activity in non-silenced 6b4 and Hc1is >500 FLUO min−1µg−1. Plants are considered silenced if GUS activity is <50 FLUO min−1µg−1.
CRISPR-Cas9 editing ofAT5G44150. The target site at position + 179 from the ATG of the AT5G44150 gene was selected using the CRISPR plant webtool [http:// www.genome.arizona.edu/crispr/CRISPRsearch.html]. No off-targets were pre-dicted for the guide RNA TCATACCGATCCCAATT’CGA targeting the com-plementary strand at Chr5:17764907-17764927. Hundred picomoles of the oligonucleotides 5′-ATTGTCATACCGATCCCAATTCGA-3′ and 5′-AAACTCG AATTGGGATCGGTATGAC-3′ were phosphorylated for 30 min at 37 °C using 1 mM ATP and 1 unit polynucleotide kinase (NEB) in the buffer supplied by the manufacturer and then hybridised in a thermocycler (5 min 95 °C, cooling rate 5 °C/min, 5 min 25 °C). Hundred femtomoles of hybridised oligonucleotides were ligated overnight at 18 °C to 10 ng of Aar1-digested and dephosphorylated vector pKI1.1 R74. An aliquot of the ligation mixture was transformed in TOP10 E. coli cells (Invitrogen). The correct insertion of the guide RNA in the vector was con-firmed by Sanger sequencing before plasmids were introduced in Agrobacterium tumefaciens strain GV3101 for the transformation of Col-0 plants byfloral dip. pKI1.1R’s T-DNA confers a red fluorescence protein expressed under the seed-specific OLEO1 promoter. Fluorescent T1 seeds were selected using an epifluorescence-equipped binocular. Plants were genotyped by high-resolution melting using the precision melt supermix (Biorad) in a Roche Lightcyler 480 and further confirmed by Sanger sequencing. Plants carrying an insertion at the AT5G44150 target site were selfed, and RFP-negative T2 seeds devoid of the Cas9-containing T-DNA were selected for outgrowth. Two independent T2 plants homozygous for the insertion of a single T or C at position+ 179 were selected for further characterisation.
Expression of GFP-tagged fusion proteins. RRP41-GFP lines were made with constructs comprising the genomic sequence of RRP41 including 1000 bp upstream of the translation start site and were previously described in ref.10. All other GFP fusion proteins were expressed from the UBIQUITIN 10 promoter. C-terminal fusion constructs contained the genomic sequence of the respective genes, including the 5′ UTR, but lacking the Stop codon. For N-terminal fusions, the genomic sequences without the 5′ UTR, but including the 3′ UTR were used. All sequences were amplified from genomic DNA, cloned into pENTR1a (Invitrogen) and transferred to pUBC-GFP and pUBN-GFP destination vectors75, respectively, using GatewayRrecombinases. Expression vectors were transferred to Agro-bacterium and used to transform rst1-3 (for RST1-GFP and GFP-RST1), cer7-2 for CER7-GFP, rrp45a for RRP45a-GFP and Col-0 plants for both GFP-RIPR and RIPR-GFP.
Co-immunopurification experiments. Plants were selected by testing crude flower extracts by western blots using homemade antibodies specific to GFP. For each IP, 200–500 mg of flower buds pooled from at least four individual plants were ground in liquid nitrogen or directly in 2 ml of ice-cold lysis buffer (50 mM Tris HCL pH 7.5, 25 or 50 mM NaCl, 1% Triton, protease inhibitors (Complete–EDTA, Roche). After removal of cell debris by centrifugation (two times 5 min, 16000×g, 4 °C) the cleared supernatants were incubated for 30 min with 50 µl of magnetic microbeads coupled to GFP antibodies (Miltenyi, catalogue number 130-091-125). Beads were loaded on magnetised MACS separation columns equilibrated with lysis buffer, and washedfive times with 300 µl of washing buffer (50 mM Tris HCl pH 7.5, 25 or 50 mM NaCl, 0.1% Triton). Samples were eluted in 50 µl of pre-warmed elution buffer (Milteny). Control IPs were carried out with GFP antibodies in Col-0 or in plants expressing RFP or GFP alone. Additional control IPs were performed with antibodies directed against myc or HA epitopes (Miltenyi, catalogue numbers 130-091-123 and 130-091-122).
Eluted proteins were digested with sequencing-grade trypsin (Promega) and analysed by nanoLC-MS/MS on a QExactive+ mass spectrometer coupled to an EASY-nanoLC-1000 (Thermo-Fisher Scientific, USA). The data were searched against the TAIR10 database with a decoy strategy. Peptides were identified with Mascot algorithm (version 2.5, Matrix Science, London, UK), and the data were imported into Proline 1.4 software [http://proline.profiproteomics.fr/]. The protein identification was validated using the following settings: Mascot pretty rank < = 1, FDR <= 1% for PSM scores, FDR < = 1% for protein set scores. The total number of MS/MS fragmentation spectra was used to quantify each protein from at least two independent biological replicates. If not specified otherwise, biological replicates consisted of plants of the same genotype grown at different dates and in different growth chambers.
For the statistical analysis of the co-immunoprecipitation data, we compared the data collected from multiple experiments for each bait against a set of 20 control IPs using R v3.5.1, R-studio v1.1.453. The size factor used to scale samples were calculated according to the DESeq2 normalisation method (i.e., median of ratios method)76. edgeR v3.14.0 and Stats v3.3.1 were used to perform a negative-binomial test and calculate the fold change and an adjusted p-value corrected by Benjamini–Hochberg for each identified protein. MDS plots were calculated with Stats v3.3.1. Annotation of proteins was retrieved using BiomartR v2.28.0, and volcano plots were drawn with ggplot2 v3.1.0. The RST1 data set comprised the data collected from 15 immunoprecipitation experiments performed in 6 biological replicates of each GFP-RST1 and RST1-GFP. Fourteen IPs from four biological replicates were performed with RRP45A. Six experiments from two biological replicates were used for each of RRP41-GFP and CER7-GFP, and the RIPR data set contained four IPs from two biological replicates of each RIPR-GFP and GFP-RIPR. Control IPs included four biological replicates of Col-0 incubated with GFP antibodies, six IPs from four biological replicates of GFP-expressing plants treated with GFP antibodies and ten IPs performed with anti-HA antibodies in three biological replicates RST1-GFP, three replicates GFP-RST1 and in 1 RFP sample. The mass spectrometry proteomics data have been deposited to the
ProteomeXchange Consortium [http://proteomecentral.proteomexchange.org] via the PRIDE partner repository77with the data set identifierPXD013435.
Epicuticular wax analysis. For each sample, three stem sections of 6 cm were immersed for 30 s in 10 ml of chloroform. Extracts were dried under N2gas,
dissolved in 150 µl of chloroform, transferred in an insert and again dried under N2
gas. Extracts were derivatized in a mix of BSTFA [N,O bis(trimethylsilyl) tri-fluoroacetamide) (> 99%, Sigma)]/pyridine (> 99.5%, Sigma) (50/50, V/V) (1 h at 80 °C with shaking at 300 rpm) before BSTFA-pyridine extracts were evaporated under N2gas. The samples were dissolved in chloroform containing a mix of nine
alkanes (C10–C12–C15–C18–C19–C22–C28–C32–C36) as internal standards. Derivatized silylated samples were analysed by GC-MS (436-GC, Bruker; column 30-m, 0.25-mm, 0.25 µm; HP-5-MS) with He carrier gas inlet pressure pro-grammed for constantflow of 1 ml/min and mass spectrometric detector (SCION TQ, Bruker; 70 eV, mass to charge ratio 50–800). GC was carried out with temperature-programmed injection at 50 °C over 2 min. The temperature was increased by 40 °C/min to 200 °C, held for 1 min at 200 °C, further increased by 3 °C/min to 320 °C and held for 15 min at 320 °C. Injector temperature was set to 230 °C with a split ratio of 3:1. Peaks in the chromatogram were identified based on of their mass spectra and retention indices. Mass spectra detected by GC-MS were compared with the spectra of known compounds stored in the National Institute Standard and Technology (NIST) and in the Golm Metabolome databases. Non-acosane, 15-nonacosanone and 1-octacosanol were identified with match values of 933, 865 and 952, respectively. Mass spectrometric detector peak areas were used for relative quantification with octacosane as internal standard.
RNA extraction and northern blots. RNA was extracted from the top 3 cm of inflorescence stems or from flowers with TRI-reagent (MRC) following the man-ufacturers instructions. After precipitation with 0.8 vol isopropanol for 1–3 h at −80 °C, RNAs were collected by centrifugation (30 min 16,000 × g, 4 °C), washed twice with 70% EtOH, dissolved in water and further purified by adding 1 vol of phenol:chloroform:isoamylalcohol (25:24:1). The aqueous phase was transferred in a fresh tube, RNAs were precipitated overnight with 2.5 vol of EtOH at−80 °C, collected by centrifugation, washed twice with 70% EtOH and dissolved in water to ~2.5 µg/µl. For high-molecular-weight northern blots, 20μg of the total RNA were separated in a 2% denaturing agarose gel containing 30 mM Tricine, 30 mM trie-thanolamine and 40 mM formaldehyde (4-6 h at 50 V). The RNA was blotted to Amersham Hybond-N+ membranes (GE Healthcare Life Sciences) and UV-cross-linked (254 nm). Membranes were stained with methylene blue and hybridised to 32P radiolabelled DNA probes (DecaLabel, ThermoFischer) in PerfectHybTM (Sigma) overnight at 65 °C. Sequences for primers used for amplification of probe templates are listed in Supplementary Table 1.
For low-molecular-weight and small RNA northern blots, 20 µg of the total RNA were separated in 5 and 17% polyacrylamide gels (19:1), respectively, containing 7 M urea in 100 mM Tris, 100 mM borate, 2 mM EDTA. RNA was transferred to Amersham Hybond-NX membranes (GE Healthcare Life Sciences) and either UV-cross-linked (LMW blots) or chemically cross-linked (small RNA blots) by incubation with 0.16 M l-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, Sigma) in 0.13 M 1-methylimidazole (Sigma), pH 8, for 1.5 h at 60 °C. Membranes were hybridised to radiolabelled DNA probes overnight at 45 °C. For loading controls, blots were stripped with boiling 0.1% SDS, and hybridised to a radiolabelled oligonucleotide specific to 7SL RNA, U6 snRNA or miR156. Oligonucleotide sequences are listed in Supplementary Table 1.
Microscopy. Plants were grown on MS agar plates supplemented with 0.5% sucrose. Roots from 10-day-old seedlings were excised, placed with water under a coverslip and examined with a ZEISS LSM 780 confocal microscope. The line expressing tRFP-PAB2 was a kind gift of C. Bousquet-Antonelli.
small RNA libraries and analysis. RNA was prepared as described above from flower buds of 6-week-old plants. For each genotype, three biological replicates of Col-0 (wild-type), cer7-3, rst1-2, rst1-3, ripr(insT), ripr(insC) and two of cer7-4 were grown at different dates in different growth chambers. Libraries were prepared from 1 µg of the total RNA using the NEB Next Multiplex Small RNA Library Prep Set for Illumina (NEB #E7300S and #E7580S) following the manufacturer’s instructions. After ligation of primers, cDNA synthesis and PCR amplification, pooled libraries were loaded on a NOVEX 6% polyacrylamide gel with 100 mM Tris, 100 mM borate, 2 mM EDTA. Five fractions corresponding to 130–180 bp products were excised and eluted overnight in water. After EtOH precipitation, size and concentration of the fractions were checked with an Agilent 2100 Bioanalyzer (Agilent Technologies). The fraction of 140–150 bp containing the 21–22 nt small RNAs of interest was sequenced on a HiSeq 4000 sequencer (single-end mode 1 × 50 bp).
Sequence reads were trimmed from 3′-adapters and low-quality bases (q < 30) using cutadapt v1.1878. Reads were aligned without mismatches to the Arabidopsis TAIR10 genome using ShortStack v3.8.579in unique mode (−u). Counts of 21 nt and 22 nt reads were extracted and annotated against TAIR10. Differential expression analysis was performed with DEseq2. The data obtained from the two alleles of cer7, rst1 and ripr were analysed together. Downstream analysis and data visualisation were done with R. Only loci with log2FC > 1 and an adjusted p-value of < 0.01 were considered. PhasiRNA were identified with the help of ShortStack’s phasing score (score >= 5). Potential miRNA target mRNAs were predicted using the psRNATarget webservice at [http://plantgrn.noble.org/psRNATarget]80. Statistical analysis. For the statistical analysis of proteomic and sequencing data, we used negative-binomial models based on the edgeR and DEseq2 packages, respectively, which calculate the fold change and adjusted p-values with a two-sided Wald test.
Gel and blot images. Uncropped blots, gels and stem images are provided in Supplementary Fig. 5 and at [https://doi.org/10.6084/m9.figshare.c.4483406]. Reporting summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The small RNA-seq and mass spectrometry proteomics raw data that support the findings of this study have been deposited to the NCBI Gene Expression Omnibus (GEO) database, accession codeGSE129736, and to the ProteomeXchange Consortium via the PRIDE75partner repository with the data set identifierPXD013435, respectively. Full
resolution versions of all images, the wax analysis data, the processed small RNA-seq data and interactive volcano blots are available atfigshare.
Received: 26 April 2019 Accepted: 5 August 2019
References
1. Vanacova, S. & Stefl, R. The exosome and RNA quality control in the nucleus. EMBO Rep. 8, 651–657 (2007).
2. Schaeffer, D., Clark, A., Klauer, A. A., Tsanova, B. & van Hoof, A. Functions of the cytoplasmic exosome. Adv. Exp. Med. Biol. 702, 79–90 (2011). 3. Lange, H. & Gagliardi, D. Plant exosomes and cofactors. Enzymes 31, 31–52
(2012).
4. Łabno, A., Tomecki, R., Dziembowski, A. & Cytoplasmic, R. N. A. decay pathways—enzymes and mechanisms. Biochim. Biophys. Acta 1863, 3125–3147 (2016).
5. Zinder, J. C. & Lima, C. D. Targeting RNA for processing or destruction by the eukaryotic RNA exosome and its cofactors. Genes Dev. 31, 88–100 (2017). 6. Tomecki, R. et al. The human core exosome interacts with differentially localized
processive RNases: hDIS3 and hDIS3L. EMBO J. 29, 2342–2357 (2010). 7. Lykke-Andersen, S., Tomecki, R., Jensen, T. H. & Dziembowski, A. The
eukaryotic RNA exosome: same scaffold but variable catalytic subunits. RNA Biol. 8, 61–66 (2011).
8. Wasmuth, E. V. & Lima, C. D. Exo- and endoribonucleolytic activities of yeast cytoplasmic and nuclear RNA exosomes are dependent on the noncatalytic core and central channel. Mol. Cell 48, 133–144 (2012).
9. Dziembowski, A., Lorentzen, E., Conti, E. & Séraphin, B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat. Struct. Mol. Biol. 14, 15–22 (2007).
10. Sikorska, N., Zuber, H., Gobert, A., Lange, H. & Gagliardi, D. RNA degradation by the plant RNA exosome involves both phosphorolytic and hydrolytic activities. Nat. Commun. 8, 2162 (2017).
11. Staals, R. H. J. et al. Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J. 29, 2358–2367 (2010).