HAL Id: hal-01259433
https://hal.sorbonne-universite.fr/hal-01259433
Submitted on 20 Jan 2016
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution| 4.0 International License
Current challenges in the management of heart failure
Michel Komajda
To cite this version:
Michel Komajda.
Current challenges in the management of heart failure.
Circulation Journal,
Official Journal of the Japanese Circulation Society http://www.j-circ.or.jp
tify remaining challenges.
Chronic HF With Low EF: A Success Story
Evidence-based medicine in HF has been essentially devel-oped in patients with low EF.Pharmacotherapy (Table 1)
Two key randomized controlled trials using enalapril in approx-imately 2,800 patients, CONSENSUS and SOLVD in mild, moderate or severe symptomatic patients have demonstrated that the addition of an ACEI to diuretics and in most cases digoxin and/or spironolactone significantly reduced overall mortality by approximately 20%.1–3 In symptomatic patients
enrolled in SOLVD there was also a significant reduction of HF hospitalizations. Finally, the benefit of ACEIs on death or HF hospitalization was also demonstrated in 3 large trials enrolling patients with HF and/or left ventricular dysfunction after acute myocardial infarction (SAVE, TRACE, AIRE).4
These impressive results led to the recommendation in inter-national guidelines of using ACEIs as first-line therapy in chronic HF with low EF.5
The evidence supporting the use of β-blockers in the same
indication comes from the results of the large clinical trials published in the late 90 s or early 2000 s: the U.S. carvedilol studies showed a reduction in mortality with carvedilol.6 This
he management of heart failure (HF) has considerably changed during the past 30 years with the introduc-tion of both major classes of drugs and devices. Angio-tensin-converting enzyme inhibitors (ACEIs), β-adrenergic
blockers, angiotensin-receptor blockers (ARBs), mineralocor-ticoid-receptor antagonists (MRAs), “funny” channel (If)
block-ers and more recently, dual inhibitors blocking neprilysin and angiotensin receptors have been shown to improve mortality and morbidity in large randomized clinical trials including patients with mild to moderate chronic HF and reduced ejec-tion fracejec-tion (EF).1–17 Similarly, implantable cardiac
defibril-lators (ICDs) and cardiac resynchronization therapy (CRT) have shown benefit on the prevention of sudden cardiac death (SCD)18 in patients with mild to moderate HF with low
sys-tolic function, and on cardiovascular mortality and morbid-ity19–22 in both severe and in moderate HF with reduced EF.
There are, however, areas where there is a lack of evidence or a gap in knowledge despite numerous pharmacological or non-pharmacological attempts at treatment. This includes in particular acute HF and HF with preserved EF (HFpEF) where little or no progress has been made. In addition, lengthy and recurrent hospitalizations for HF remain a burden on health-care systems because of the costs incurred and the associated poor quality of life.
The objective of this review is to discuss the successes and the failures in the management of this condition and to
iden-T
Received March 12, 2015; accepted April 2, 2015; released online April 14, 2015
Department of Cardiology, Pitie-Salpetriere Hospital, Pierre & Marie Curie University (ICAN), Paris, France
Mailing address: Michel Komajda, MD, Pitie Salpetriere Hospital, Department of Cardiology, 47/83 Boulevard de l’Hôpital, 75013 Paris, France. E-mail: michel.komajda@psl.aphp.fr
ISSN-1346-9843 doi: 10.1253/circj.CJ-15-0368
All rights are reserved to the Japanese Circulation Society. For permissions, please e-mail: cj@j-circ.or.jp
Current Challenges in the Management of Heart Failure
Michel Komajda, MD
The management of chronic heart failure (HF) with low ejection fraction (EF) has changed considerably over the past 30 years: the introduction of angiotensin-converting enzyme inhibitors (ACEIs), β-blockers, angiotensin-receptor blockers, mineralocorticoid-receptor antagonists and recently, the If blocker, ivabradine, has led to a significant reduction in overall mortality and HF mortality. Recently, a trial testing a dual inhibitor blocking the angiotensin-II receptor and neprylisin, the enzyme responsible for B-type natriuretic peptide degradation, showed that this complex molecule improved clinical outcomes compared with the ACEI enalapril. However, challenges remain in the manage-ment of HF, with suboptimal implemanage-mentation of guideline-recommended therapies, a changing profile of patients who are older and have multiple comorbidities and a high rate of early rehospitalization for HF. Use of devices such as implantable cardiac defibrillators and cardiac resynchronization therapy are also associated with an improvement in outcomes in this condition. HF with preserved EF (HFpEF), a growing fraction of the HF population, remains a clinical dilemma: no pharmacological intervention has so far demonstrated any convincing benefit on outcome. Heterogeneity of the populations tested, role of comorbidities, difficulties in identifying patients with HFpEF, as well as a mismatch between the clinical phenotypes and the treatments tested, can explain the failure to find beneficial interventions. Overall, the management of HF after discharge remains fragmented and concerted action by all pro-fessionals concerned is needed. (Circ J 2015; 79: 948 – 953)
Key Words: Cardiac resynchronization therapy; Dual inhibitors; Heart failure; Implantable cardiac defibrillators
949 Management of HF
Ivabradine is a drug that inhibits the “funny” (If) channel in
the sino-atrial node and slows down heart rate in patients in sinus rhythm. The magnitude of heart rate reduction is directly dependent on the baseline heart rate and is more pronounced in patients with a markedly elevated heart rate.
This new drug was tested on top of ACEIs/ARBs, β-blockers
and in the majority of cases, a MRA in a large, controlled randomized trial including patients with mild to moderate HF, low EF and in sinus rhythm with heart rate ≥70 beats/min.16
There was a significant (18%) reduction in the primary com-posite endpoint of cardiovascular death or HF hospitalization. The reduction of cardiovascular death or all-cause death alone was not significant whereas HF hospitalizations were reduced by 26%. A significant improvement in quality of life was also observed, as well as a reverse remodeling effect.23,24 Ivabradine
is now recommended for symptomatic patients with low EF, in sinus rhythm ≥70 beats/min despite treatment with maxi-mally tolerated β-blocker (or when β-blockers are
contraindi-cated), ACEI/ARB and a MRA.
There are therefore 5 available classes of drug that favor-ably influence outcomes of patients with chronic HF with reduced EF and the current guidelines of the European Society of Cardiology recommend an additive strategy starting with ACEI (or ARB if not tolerated), β-blocker, MRA and finally
ivabradine for patients in sinus rhythm and with elevated heart rate.
Recently, a large randomized controlled trial evaluated a dual inhibitor (LCZ 696) blocking the ARB receptor and neprylisin, the enzyme that cleaves B-type natriuretic peptide (BNP) and makes it inactive, in a large population of mild to moderate chronic HF patients.17 In PARADIGM-HF, the
com-parator was enalapril 20 mg/day. The trial was prematurely interrupted because of benefit: there was a significant 20% reduction of the composite endpoint of cardiovascular mortal-ity or HF hospitalization, of cardiovascular mortalmortal-ity alone (20%) (which was the predefined trigger for recommending early termination), as well as all-cause death (16%) and of HF hospitalizations (21%). Overall, the safety was good; there were more episodes of hypotension but renal tolerability was program was followed by 3 randomized clinical trials, CIBIS
II (bisoprolol), MERIT HF (metoprolol succinate CR/XL) and COPERNICUS (carvedilol), which enrolled almost 9,000 patients and showed consistent results with a mortality reduc-tion of approximately one-third and a reducreduc-tion in HF hospi-talizations on top of background medication including ACEIs.7–9
These positive results were later confirmed in the elderly population (≥70 years) enrolled in SENIORS, where nebivolol reduced significantly the composite of death or HF hospital-ization but not mortality.10
These findings led to the recommendation of using β-blockers
on top of ACEIs in all patients with chronic HF and low EF. The evidence supporting the use of MRAs came early in severe HF. In RALES, spironolactone reduced by almost one-third death and HF hospitalizations in severely symptomatic (NYHA class III) patients.11 However, at that time only 11%
of the patients were treated with a β-blocker. More recently,
the EMPHASIS HF trial demonstrated that addition of eplere-none to mildly symptomatic patients treated with ACEIs or ARBs and β-blockers reduced significantly by 37%
cardiovas-cular mortality or HF hospitalizations and other clinical end-points.12 European Society of Cardiology (ESC) guidelines
therefore recommend considering this class of drug in all patients with HF and low EF and with persistent symptoms despite ACEIs (or ARBs if ACEIs are not tolerated) and β-blockers.
ARBs are recommended as an alternative to ACEIs in patients who are intolerant to this class following the publica-tion of CHARM alternative, which demonstrated that in patients with low EF and intolerance for ACEIs, candesartan reduced cardiovascular mortality or HF hospitalization by 23%.13
Two additional trials, Val Heft (valsartan) and CHARM added, explored the potential benefit provided by a combina-tion of an ARB with an ACEI.14,15 There was a reduction in
HF hospitalizations, and in CHARM added there was a reduc-tion in cardiovascular death. However, because the magnitude of the benefit brought by this combination was smaller than that of the combination of an ACEI with a MRA, this class of drug is now primarily indicated as an alternative to ACEIs in the case of intolerance.
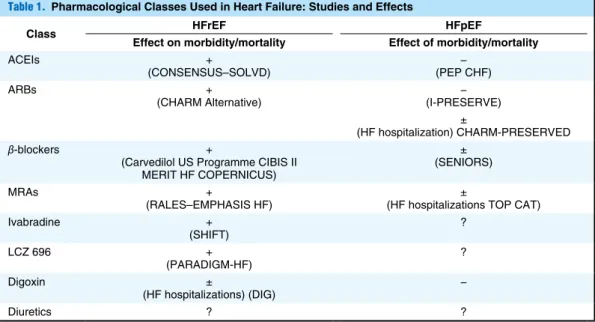
Table 1. Pharmacological Classes Used in Heart Failure: Studies and Effects
Class HFrEF HFpEF
Effect on morbidity/mortality Effect of morbidity/mortality
ACEIs +
(CONSENSUS–SOLVD) (PEP CHF)− ARBs +
(CHARM Alternative) (I-PRESERVE)− ±
(HF hospitalization) CHARM-PRESERVED
β-blockers +
(Carvedilol US Programme CIBIS II MERIT HF COPERNICUS)
± (SENIORS) MRAs +
(RALES–EMPHASIS HF) (HF hospitalizations TOP CAT)± Ivabradine + (SHIFT) ? LCZ 696 + (PARADIGM-HF) ? Digoxin ± (HF hospitalizations) (DIG) − Diuretics ? ?
ACEI, angiotensin-converting enzyme inhibitors; ARBs, angiotensin-receptor blockers; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MRAs, mineralocorticoid-receptor antagonists.
HF patients in 2 controlled trials: MADIT CRT and RAFT.21,22
In these trials the thresholds for EF and QRS duration at inclu-sion were different from those used in trials with more severe patients: ≤30% for EF in both trials, QRS duration ≥130 ms in MADIT CRT and ≥120 ms in RAFT. Moreover, the 2 strate-gies compared in each of these trials were optimal therapy plus ICD vs. optimal therapy vs. CRT plus ICD (CRT-D).
The conclusions of the 2 trials were similar and showed that CRT-D reduced the composite of all-cause death or HF hos-pitalization, although mortality alone was not reduced in MADIT CRT. There was also an improvement in symptoms and quality of life. These 2 trials also showed that QRS dura-tion and morphology (left bundle branch block (LBBB) vs. non-LBBB) influenced the outcome: the maximal benefit was observed patients with very wide QRS >150 ms and in those with a LBBB morphology and a QRS duration ≥130 ms. These thresholds have been used in the ESC guidelines for the use of CRT in patients with mild symptoms of HF.
The possibility of using CRT in patients with QRS <120 ms and evidence of mechanical dyssynchrony has been tested recently in Echo-CRT.25 The trial was prematurely interrupted
upon recommendation by the Data Safety Monitoring Board because of an excess in mortality and futility. There is there-fore no indication for CRT in patients with narrow QRS dura-tion.
One area of uncertainty is atrial fibrillation because the CRT trials listed above excluded this condition, with the exception of RAFT in which 13% of the patients had atrial fibrillation at inclusion. Although a subgroup analysis did not show a sig-nificant interaction between basal rhythm and CRT effect, it is based on a limited number of patients and therefore the evi-dence is weak. Another area of uncertainty is patients with a conventional indication of cardiac pacing who were excluded from clinical trials. Considering that conventional right ven-tricular pacing can lead to cardiac function deterioration and modifies the normal sequence of mechanical activation, ESC guidelines therefore recommend CRT in severely symptom-atic HF and low EF, irrespective of QRS duration, as well as in less symptomatic patients requiring conventional pacing but with a low class level of recommendation.
These multiple progresses made in both pharmacology and devices have undoubtedly improved the outcomes of patients with chronic HF with low EF. Only 20 years ago the annual mortality rate was approximately 16% in the placebo arm of SOLVD. In the SHIFT trial published in 2010, the rate had dropped to 9%. In addition, mortality rate related to HF as the cause of death has been halved in the time between those 2 trials. The difference is not observed only in populations enrolled in clinical trials, which are by definition highly selected. An analysis of mortality directly related to HF in 8 European better and cough was significantly less frequent than with
enalapril, and the frequency of angioedema, a well-known side effect of blockers of neprilysin, related to the increase in bradykinin plasma concentration, was only slightly higher than in the control group.
These impressive results should lead to a substantial revi-sion of the currently recommended strategy and to a replace-ment of ACEIs as first-line therapy by this new complex molecule in order to further reduce adverse outcomes in chronic HF with low EF.
There are, however, pending questions, including the mar-ket price and the definition of the population that should receive LCZ 696 (simple clinical criteria or restriction to patients with evidence of increased BNP/N-terminal pro-BNP, a criterion used for inclusion in the trial).
These important matters are currently subject to discussion with regulatory agencies.
Implantable Devices (Table 2)
Because a substantial number of deaths occur suddenly in HF and are presumed to be related to a fatal ventricular arrhyth-mia in most cases, prevention of SCD is a major goal, particu-larly in patients with mild to moderate symptoms in whom SCD is more prevalent than in those with more advanced HF. Neurohumoral modulators, particularly β-blockers and MRAs,
reduce significantly the risk of SCD, but they do not prevent it. Class I antiarrhythmic agents are contraindicated in HF with low EF and amiodarone failed to demonstrate any benefit on mortality in a large clinical trial comparing standard ther-apy, amiodarone and ICD.18 The trial (SCD-Heft) enrolled
2,521 patients with EF <35% in NYHA class II or III, without prior ventricular arrhythmia. The ICD strategy led to a 23% relative risk reduction in all-cause mortality. Primary preven-tion by ICD is therefore recommended for patients in class II or III with a good life expectancy >1 year after a sufficient period of optimization of medical therapy (≥3 months) in order to reduce the risk of SCD in patients with chronic HF of ischemic or nonischemic origin.
CRT was developed in the early 2000 s in patients with severely symptomatic HF despite optimal therapy, low EF <35% and wide QRS. COMPANION compared 3 strategies: conventional therapy, CRT and CRT plus ICD.19 CARE HF
compared CRT with conventional therapy alone.20 The 2 trials
demonstrated that CRT alone or combined with an ICD reduced all-cause mortality and HF hospitalizations signifi-cantly compared with conventional therapy (24% and 36%, respectively, for CRT and CRT-D in COMPANION, 36% for CRT in CARE HF). There was also a benefit on symptom improvement, quality of life and ventricular remodeling.
The benefit of CRT was later evaluated in mild to moderate Table 2. Devices Used in Heart Failure
Type and name of study
HFrEF HFpEF
Effect on
morbidity/mortality morbidity/mortalityEffect on
CRT
Severe heart failure (COMPANION–CARE HF) + ? ICD
(SCD-Heft) + ? CRT+ICD
Mild/moderate heart failure (MADIT CRT–RAFT) + ?
951 Management of HF
patients with cognitive disorders, for whom the role of the family becomes crucial.
The use of serial biomarker measurements is another way to address this issue and a recent meta-analysis has shown that a natriuretic peptide-guided strategy reduced HF rehospitaliza-tions by approximately 25%.36 Of note, however, the
magni-tude of the benefit on mortality was observed only in younger patients (<75 years) and there was no benefit on all-cause death in older patients, who represent the majority of HF populations.
Telemedicine using structured telephone calls or remote monitoring of biomarkers is another tool to improve outcomes and reduce HF hospitalizations, although not all studies show benefit.37 One limitation to the development of telemedicine is
the fact that it is generally not reimbursed and therefore the medical time spent for this approach does not generate any fee.
HFpEF: A Clinical Dilemma
Unlike what is observed for HFrEF, all attempts to reduce the mortality and morbidity rates of HFpEF have failed (Table 1). One trial (PEP CHF) tested the ACE I, perindopril, in an elderly population of 850 patients and failed to demonstrate any benefit on the primary outcome of all-cause mortality or HF hospitalization.38 Two trials tested an ARB:
CHARM-PRESERVED evaluated candesartan in a group of 3,023 patients,39 and I-PRESERVE tested irbesartan in an elderly
population of 4,128 patients.40 In CHARM-PRESERVED,
there was a nonsignificant trend in risk reduction of the pri-mary endpoint of cardiovascular death or HF hospitalization, which was driven by a reduction in HF hospitalizations. In I-PRESERVE, there was no significant reduction in the com-posite of all-cause death or cardiovascular hospitalizations and no secondary endpoint showed any trend in favor of irbesar-tan. Recently, the TOPCAT trial testing spironolactone showed only a nonsignificant 11% relative risk reduction of the primary composite of cardiovascular death, HF hospital-ization or aborted cardiac arrest.41 HF hospitalization was the
only component of the primary endpoint that showed a sig-nificant reduction and the trial was criticized because of huge geographic variations in event rates.
Recently, the SUPPORT study evaluated the addition of an ARB, olmesartan, on top of existing therapy in a large popula-tion of patients with hypertension, stable HF and preserved EF.42 It concluded that the addition of the ARB was not
asso-ciated with improved outcomes, induced more episodes of renal impairment and that the combination of an ACEI, olmes-artan and a β-blocker was associated with more cardiovascular
events.
These results are all the more worrying because this subset of patients represents a growing part of the overall HF popula-tion.43 Several factors can explain the disappointing results
observed in the large, randomized clinical trials, as well as in the proof of concept studies. One explanation is that patients with HFpEF are usually older and have multiple comorbidi-ties, which makes them more complicated to manage.44,45
Another reason, possibly related to the greater noncardiac comorbidity burden, is that the relative proportion of noncar-diovascular death is greater in HFpEF than in HFrEF.46
Another explanation is that the diagnosis of HFpEF is chal-lenging because it requires the combination of signs and symptoms of HF, a “preserved” EF >50% and objective evi-dence of abnormal distensibility, left ventricular filling or relaxation.47 This third component may be difficult to
evi-countries shows that it has been constantly falling over the past 15 years.26
All-cause mortality remains, however, very high after a hospitalization for acute HF; in the Euro Observational Study on HF the annual mortality rate was 17% and the cause was cardiovascular in 60% of cases.27
Challenge of Hospitalization and Rehospitalization
The fact that life-saving medications do not always translate into a reduction in hospitalizations and, notably, early rehos-pitalizations, in the general population is a matter of concern. Although recent data from all US discharge summaries of HF hospitalizations show a decline over time,28 it remains themajor cause of referral to hospital, as shown by an analysis of hospitalizations in Sweden.29
The 4–6 weeks following discharge is known as a “vulner-able phase”, associated with high rates of mortality and rehos-pitalization for HF.30 The risk of dying in the first 30 days after
an index hospitalization is increased 6-fold compared with patients not hospitalized. Similarly, in the EVEREST trial, 25% of patients were rehospitalized during the same period of time after an index hospitalization for decompensated HF.31 In
an analysis of hospital discharge summaries in the USA, HF was first as the condition for recurrent hospitalizations.32 An
analysis in elderly patients hospitalized for HF in the USA suggests a bidirectional trend, with a reduction in post-dis-charge mortality but an increase in early rehospitalization rates.33 Because hospitalization represents approximately 70%
of the cost related to the management of HF, it is not surpris-ing that the cost of HF management should more than double between 2010 and 2030 in the USA.34
In addition, the specific management of acute HF has not changed over the past 20 years. A number of trials using very different drugs, such as new inotropes, arginine vasopressin antagonists, nesiritide, adenosine receptor A1 antagonists or endothelin-receptor antagonists, failed to demonstrate any improvement in short- or mid-term outcomes and the ESC guidelines still recommend that intravenous diuretics, oxygen, opiates if needed, nitrates, or “old” inotropes play a central role in the management of this condition.
Several explanations can be put forward to explain the explosion of rehospitalizations related to HF: (1) patients who previously died now survive at the expense of an increase in the rehospitalization rate; (2) a changing profile of HF patients who are older and have often multiple comorbidities that can make the management of their condition more complex because of contraindications or intolerances and puts them at higher risk of rehospitalization due to increased frailty; (3) subopti-mal titration of life-saving drugs, which is observed in all registries (a recent survey conducted by the ESC suggests that, at best, 25–30% of patients reach the target dose of β-blockers27);
and (4) a fragmented chain of command is frequently observed when patients leave the hospital, resulting in a gap in follow-up care after discharge and many patients do not see any healthcare professionals for a long period of time. In a national survey conducted in France, only 29% of the patients had consulted a cardiologist in the first 3 months after discharge from hospital.35
Solutions to this worrying situation include better patient education schemes so that every HF patient is aware of the objectives of their treatment, of the symptoms of decompensa-tion, of the most common side effects of drugs used to manage their condition and of dietary requirements. This approach has been shown to be effective, but is limited in very elderly
Acknowledgments
Michel Komajda has received honoraria exceeding 1,000,000 Yen from Servier and Amgen for consulting, and from Servier for lectures for an amount exceeding 500,000 Yen.
Disclosures
Name(s) of Grant(s): N/A.
References
1. The CONSENSUS Trial Study Group. Effects of enalapril on mor-tality in severe congestive heart failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429 – 1435.
2. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293 – 302.
3. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure: Collaborative Group on ACE Inhibitor Trial. JAMA 1995; 273: 1450 – 1456.
4. Flather MD, Yusuf F, Kober L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A systematic overview of date from individual patients: ACE-Inhibitor Myocardial Infarction Collabora-tive Group. Lancet 2000; 355: 1575 – 1581.
5. McMurray JJV, Adamopoulos S, Anker SF, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the diag-nosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology developed in collaboration with the Heart failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787 – 1847.
6. Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure: U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334: 1349 – 1355.
7. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A ran-domised trial. Lancet 1999; 353: 9 – 13.
8. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF): MERIT-HF Study Group. JAMA 2000; 283: 1295 – 1302.
9. Krum H, Roecker EB, Mohacsi P, Rouleau JL, Tendera M, Coats AJ, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: Results from the COPERNICUS Study. JAMA 2009; 289: 712 – 718.
10. Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26: 215 – 225.
11. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure: Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709 – 717.
12. Zannad F, McMurray JJ, Krum H, Van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11 – 21.
13. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alterna-tive trial. Lancet 2003; 362: 772 – 776.
14. Maggioni AP, Anand I, Gottlieb SO, Latini R, Tognoni G, Cohn JN. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol 2002; 40: 1414 – 1421.
15. McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-Added trial. Lancet 2003; 362: 767 – 771.
16. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I. Dubost-Brama A. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010; 376: 875 – 885. 17. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP,
Riskala AR, et al; for the PARADIGM-HF Investigators and Com-dence: usually a central place is given to the echo Doppler e/e’
ratio or to surrogate markers, including left atrial enlargement, left ventricular hypertrophy or raised natriuretic peptide lev-els. There is therefore concern that some patients enrolled in clinical trials of HFpEF did not have HF but simply had left ventricular hypertrophy and noncardiac reasons for dyspnea such as chronic pulmonary disease or obesity. However, the rate of cardiovascular mortality or HF hospitalizations is undoubtedly higher in the HEFpEF trials than in trials of hypertension with or without left ventricular hypetrophy.48
Another cause of failure is the heterogeneity of patients enrolled in large clinical trials with regard to age or natriuretic plasma levels, suggesting that these patients are at different stages of their disease. Factors related to the trials also play a role: difficulties in recruiting patients have resulted in pro-longed recruitment periods of time with associated drop out/ drop in issues.
Finally, drug factors may play a role. The understanding of the pathophysiology of HFpEF remains suboptimal and a uniform approach to its management may not work, because several phenotypes of this condition have been described.49,50
The recent failure of the RELAX trial to demonstrate any benefit of sildenafil, a phosphodiesterase 5 inhibitor, may be attributable to the fact that the patients did not have pulmonary hypertension unlike what was required in small previous trials or to the fact that the pathophysiological defect is not an increased degradation of cGMP but rather an insufficient pro-duction of cGMP.51 Future trials testing direct soluble
guanyl-ate cyclase stimulators should provide an answer to this question. Recently, the PARAMOUNT trial tested the dual inhibitor LCZ 696 in a group of 266 patients and showed that compared with valsartan there was a favorable change in NT-proBNP at 12 weeks.52 Based on these preliminary results and on the
positive results of PARADIGM-HF, a large outcome trial in patients with HF and low EF, PARAGON, has been initiated to evaluate this new molecule in a large population of HFpEF patients.
Several other proof of concept studies are currently evaluat-ing other strategies includevaluat-ing ivabradine, exercise trainevaluat-ing or a direct soluble guanylate cyclase stimulator.
In summary, HFpEF remains a challenging condition with imperfectly understood pathophysiology, difficult diagnosis and heterogeneous phenotype, all factors that make the man-agement of this condition difficult and which explain why no progress has been made in improving patients’ outcomes.53
Conclusions
The management of HF has considerably changed during the past 30 years, with the wide dissemination of neurohormonal therapies and, to a lesser extent of implantable devices includ-ing ICDs and CRT.
Progress made has exclusively concerned chronic HF with low EF and it translates into an overall reduction in mortality and hospitalizations because of HF.
However, the burden of hospitalizations and particularly of rehospitalization remains.
No progress has been made in HFpEF or in acute HF. Because of the cost of the management of HF, and the high prevalence of this condition in the elderly, we need to develop new concepts and novel therapies, to improve the implementa-tion of life-saving medicaimplementa-tions and to better coordinate the action of the professionals involved in the management of this condition, including cardiologists, general practitioners, nurses and dieteticians.
953 Management of HF
Eurlings LW, Erntell H, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hos-pitalization: An individual patient meta-analysis. Eur Heart J 2014; 35: 1559 – 1567.
37. Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective?: A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail 2011; 13: 1028 – 1040.
38. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27: 2338 – 2345.
39. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Pre-served trial. Lancet 2003; 362: 777 – 781.
40. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456 – 2467.
41. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al; for the TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383 – 1392.
42. Sakata Y, Shiba N, Takahashi J, Miyata S, Nochioka K, Miura M, et al; on Behalf of the SUPPORT Trial Investigators. Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: The supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. Eur Heart J 2015 January 30, doi: 10.1093/eurheartj/ehu504.
43. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with pre-served ejection fraction. N Engl J Med 2006; 355: 251 – 259. 44. Chan MM, Lam CS. How do patients with heart failure with
pre-served ejection fraction die? Eur J Heart Fail 2013; 15: 604 – 613. 45. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade
M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF registry. J Am Coll Car-diol 2007; 50: 768 – 777.
46. Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejec-tion fracejec-tion: Results from the irbesartan in heart failure with pre-served ejection fraction study (I-PRESERVE) trial. Circulation 2010; 121: 1393 – 1405.
47. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiogra-phy associations of the european society of cardiology. Eur Heart J 2007; 28: 2539 – 2550.
48. Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJ. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-PRESERVED, and I-PRESERVE? J Am Coll Cardiol 2012; 60: 2349 – 2356. 49. Shah SJ. Matchmaking for the optimization of heart failure with
preserved ejection fraction clinical trials: No laughing matter. J Am Coll Cardiol 2013; 62: 1339 – 1342.
50. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hyperten-sion in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124: 164 – 174.
51. Redfield M, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capac-ity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial [RELAX Trial]. JAMA 2013; 309: 1268 – 1277.
52. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 2012; 380: 1387 – 1395. 53. Komajda M, Lam CS. Heart failure with preserved ejection fraction:
A clinical dilemma. Eur Heart J 2014; 35: 1022 – 1032. mittees. Angiotensin-neprilysin inhibition versus enalapril in heart
failure. N Engl J Med 2014; 371: 993 – 1004.
18. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for con-gestive heart failure. N Engl J Med 2005; 352: 225 – 237.
19. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140 – 2150.
20. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539 – 1549.
21. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart failure events. N Engl J Med 2009; 361: 1329 – 1338.
22. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010; 363: 2385 – 2395.
23. Ekman I, Chassany O, Komajda M, Böhm M, Borer JS, Ford I, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure results from the SHIFT study. Eur Heart J 2011; 32: 2395 – 2404.
24. Tardif JC, O’Meara E, Komajda M, Böhm JS, Ford I, Tavazzi L, et al; SHIFT Investigators. Effects of selective heart rate reduction with ivabradine on left ventricular remodeling and function: Results from the SHIFT echocardiography substudy. Eur Heart J 2011; 32: 2507 – 2515.
25. Ruschitzka F, Abraham W, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac resynchronization therapy in heart failure with a nar-row QRS complex. N Engl J Med 2013; 369: 1395 – 1405. 26. Laribi S, Aouba S, Nikolaou M; GREAT Network. Trends in death
attributed to heart failure over the past two decades in Europe. Eur J Heart Fail 2012; 14: 234 – 239.
27. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EUEObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF-Pilot). Eur J Heart Fail 2010; 12: 1076 – 1084.
28. Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999– 2011. Circulation 2014; 130: 966 – 975.
29. Stewart S, Ekman I, Ekman T, Odén A, Rosengren A. Population impact of heart failure and the most common forms of cancer: A study of 1 162 309 hospital cases in Sweden (1988 to 2004). Circ Cardiovasc Qual Outcomes 2010; 3: 573 – 580.
30. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al; Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators. Influence of non-fatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116: 1482 – 1487.
31. O’Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: Results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J 2010; 159: 841 – 849.
32. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360: 1418 – 1428.
33. Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Diver-gent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs Health care system 2002 to 2006. J Am Coll Cardiol 2010; 56: 362 – 368.
34. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular dis-ease in the United States: A policy statement from the American Heart Association. Circulation 2011; 123: 933 – 944.
35. Cohen Solal A, Leurs I, Assyag P, Beauvais F, Clerson P, Contre C, et al. French National College of Cardiologists. Optimization of heart FailUre medical Treatment after hospital discharge according to left ventricUlaR Ejection fraction: The FUTURE survey. Arch Cardio-vasc Dis 2012; 105: 355 – 365.