Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Combustion Institute/Canadian Section 2003 Spring Technical Meeting [Proceedings], pp. 13.1-13.5, 2003-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=6d5dda6b-d1cf-4667-a3d4-3f6786c90740 https://publications-cnrc.canada.ca/fra/voir/objet/?id=6d5dda6b-d1cf-4667-a3d4-3f6786c90740
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Study of fire and explosion in a compartment
Study of fire and explosion in a compartment
Liu, Z.; Kim, A.K.; Crampton, G.; Kanabus-Kaminska, M.
NRCC-46137
A version of this document is published in / Une version de ce document se trouve dans: The Combustion Institute, Canadian Section, 2003 Spring Technical Meeting, Vancouver, B.C.,
May 11-14, 2003, pp. 13.1-13.5
Figure 1 Photograph of test compartment
STUDY OF FIRE AND EXPLOSION IN A COMPARTMENT
Zhigang Liu, Andrew K. Kim, George Crampton and M. Kanabus-Kaminska Fire Risk Management Program
National Research Council of Canada, Ottawa, Canada, K1A 0R6, Telephone: 1-613-990-5075, Email: zhigang.liu@nrc-cnrc.gc.ca
Introduction
The deflagration-type explosion that occurs inside industrial facilities and military equipment presents a serious threat to lives and property [1, 2]. The phenomena of the deflagration-type explosion in a compartment are complex and there is a need to study the capability and limitation of the current extinguishing systems in suppressing this type of explosion.
The National Research Council of Canada (NRC) has recently built a test
compartment to study deflagration-type explosions and their suppression by different types of extinguishing systems. During full-scale experiments, the impact of fuel quantity and the length of the ignition delay period on the explosion intensity was investigated. Overpressure, temperature, gas composition and possible skin burn caused by the explosion were measured. Corresponding explosion damage to the equipment and the potential risk to personnel in the compartment were studied. In this paper, the
experimental facilities used for the study of the deflagration-type explosions in the
compartment are reported and potential threats generated by this type of the explosion are discussed.
Experimental Facilities
A test compartment with a cross-section area of 2.52 m2 and 1.5 m deep was built
in the NRC testing hall (see Figure 1). It was built with steel tubing as the frame and the compartment walls were covered with lexan, which allowed viewing of the interior of the compartment during explosion suppression testing. The test
compartment had an access door to the compartment with a dimension of 1.04 m x 1.04 m that was
located at the north side wall of the compartment. This door also acted as a pressure relief vent to protect the compartment integrity.
A deflagration-type explosion was generated with a fuel spray using a twin-fluid (gasoline and air) nozzle (Securiplex 90o, 5L/min) and a hot wire ignition source. They
were located at one corner of the compartment and 0.74 m high from the floor. The igniter was made of 0.91 m of 14 gauge nichrome wire looped around a 15.87 mm rod. It was located 0.47 m away from the fuel spray nozzle.
Gasoline was used as the testing fuel, which made the explosion very challenging for suppression due to its volatility. During the experiments, the fuel spray discharge was maintained for approximately 2 to 3 seconds (corresponding to 170 mL to 250 mL of gasoline), depending on the test conditions.
During the experiments, air was continuously supplied to the compartment through the twin fluid nozzle, and at the same time the hot wire igniter was maintained at a high temperature. This increased the possibility of fire re-ignition, resulting in a very
challenging scenario for explosion suppression.
One simulated skin indicator was placed inside the compartment and located near the gas sampling port. It was used to study possible skin burns during explosion
experiments. The skin indicator was made from 2 layers of polyethylene plastic sheet, each of 0.051 mm thickness. One 36 gauge thermocouple and one non-reversible OMEGALABEL temperature monitor (8MA) were placed between the plastic sheets. The temperature monitor had 6 display temperature indicators, ranging from 88 to 116oC.
The temperature indicator turned black as soon as the temperature reached the rated temperature.
Four SFH 205F silicon planar PIN photodiodes were used in the experiments to monitor the times of fire ignition, extinguishment and re-ignition. They were installed on three side walls of the compartment, near the fire source.
Seven 36 gauge thermocouples were used in the experiments, including
thermocouples #1 and #2 near the gas sampling ports, thermocouples #3 and #4 near the fire source, thermocouples #5 placed in the simulated skin indicator, thermocouple #6 near the extinguisher, and thermocouple #7 near the compartment floor. One thermocouple tree with five 26 gauge thermocouples was set up in the middle of the testing
compartment. The thermocouples were placed in the tree at 100 mm intervals vertically from the bottom to the ceiling of the compartment.
One pressure sensor with measuring range from 0 to 6896 Pa was installed near the gas sampling port to monitor pressure changes in the compartment.
A CO2/CO analyzer, a O2 analyzer and a FTIR spectrometer were used to monitor
the production of fire gases during the experiments. Fire gases were drawn from the compartment through a copper sampling port with12 mm in diameter.
One standard and one digital video camera were set up outside the test
compartment to obtain visual records of fire ignition and development in the compartment during the experiment.
Experimental data from a wide array of instrumentation were collected by two data acquisition systems: a high speed system and a standard system. The high speed data
acquisition system was used to monitor times of fuel discharge and fire ignition, and the pressure rise in the compartment. All channels were scanned at 100 kHz (10 µs/channel). The standard data acquisition system was used to monitor the average compartment temperatures and fire gas compositions generated during the experiments. The standard data acquisition system collected data at 1 s intervals.
Experiment Results
In five explosion experiments, the fuel spray duration ranged from 1624 to 3000 ms, and approximately 135 to 250 ml of the gasoline was sprayed into the compartment. The ignition delay period in the experiments ranged from 119 to 1040 ms.
As observed in the experiments, once the fuel was ignited, the fire quickly spread and filled the whole compartment. The compartment door located near the fire source was pushed to fully open and flames were spreading out of the compartment as the explosion occurred. With the door fully open, the overpressure in the compartment was quickly released, and some fire gases vented out of the compartment and fresh air was brought into the compartment through the opening door.
Figures 2 to 5 show experimental results obtained in an experiment, including flame signals measured by optical devices, and changes in compartment overpressure and temperatures. The explosion occurred at 1124 ms into the experiment (124 ms after fuel discharge) and the fire duration lasted 2904 ms (see Figure 2). The maximum
overpressure of 1100 Pa was generated in the early stage of the explosion at 155 ms after the fuel discharge, and with the door being open, the overpressure in the compartment quickly declined (see Figure 3).
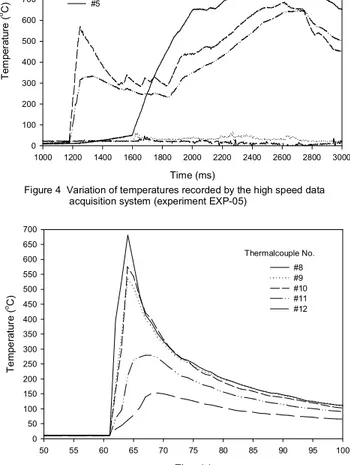
The temperatures measured near the fire source showed a quick increase as the fuel ignited, and the maximum compartment temperature reached was higher than 800oC (see Figure 4). The maximum temperature in the middle of the compartment, as measured at the thermocouple tree, was also higher than 650oC. It also showed that temperatures at
the upper portion of the compartment were much higher than temperatures at the lower portion of the compartment during the experiment (see Figure 5).
During the experiment, the minimum oxygen concentration in the compartment dropped to 10.65%, and the maximum CO concentration generated in the experiment was 0.55%, while the maximum CO2 concentration exceeded 4%, the maximum range of the
gas analyzer setting.
The level of overpressure generated in the experiments was mainly determined by the length of the ignition delay period, instead of the total discharged fuel quantity, as the fuel quantity involved in the explosion increased with the ignition delay period. In another experiment, approximately 170 ml of gasoline was discharged into the compartment during 2080 ms of the discharge period. The fuel ignition was significantly delayed until 1040 ms after the start of the fuel discharge. With the occurrence of the explosion, a large
fire ball was generated in the compartment. The overpressure (2300 Pa) generated in the explosion was two times higher than those generated in other experiments with the ignition delay times of approximately 125 ms. However, the length of fire duration, and the production of fire gas in the compartment were still determined by the total discharged fuel quantity. With an increase in the total discharged fuel quantity, the fire duration and CO and CO2 concentrations in the compartment increased, and O2 concentration
decreased.
In the five explosion experiments, the maximum overpressures generated ranged from 1050 to 2300 Pa, and the maximum temperatures from 820 to 1020oC. The
minimum oxygen concentrations in the experiments ranged from 8.96 to 13.37%, depending on the amount of fuel discharged into the compartment. The maximum CO2
concentrations generated in the experiments exceeded 4.0%, the maximum setting of the gas analyzer. The maximum CO concentrations ranged from 0.55% to higher than 1.0%, the maximum range of the CO gas analyzer setting.
These experimental results indicate that although only a small fuel quantity was involved, the deflagration-type explosion generated in the compartment was very severe, creating high temperatures and overpressure, as well as low oxygen concentration and high CO and CO2 levels in the compartment. In all five experiments, the simulated skin
indicator placed inside the compartment was damaged, and its plastic sheet melted. With this type of explosion, the equipment and personnel in the compartment would have little chance of survival, when no protection was provided.
Conclusion
The deflagration-type explosion was studied in a test compartment by monitoring overpressure, temperatures and fire gas compositions. The intensity of the explosion was mainly determined by the ignition delay period, but the fire duration and fire gas
compositions generated in the explosion were determined by the total discharged fuel quantity. The deflagration-type explosion presented a serious threat to lives and property in the compartment, even though a small fuel quantity was involved.
Acknowledgements
This study was conducted under the Halon Alternatives Performance Evaluation Program, a joint research project between the Department of National Defence and the National Research Council of Canada. The authors would like to thank Dr. Joseph Su, Mr. Cameron McCartney, and Mr. Michael Ryan for their contributions in constructing the test facility and participating in the fire experiments.
References
1. Lunn, G., “Venting Gas and Dust Explosions – A Review,” An ICHEME Industrial Fellowship Report, The Institution of Chemical Engineers, England, 1985.
2. McCormick, S., Clauson, M. and Cross, H., “US Army Ground Vehicle Crew Compartment Halon Replacement Programs,” Halon Options Technical Working Conference, May 2000, pp.229-236, Albuquerque, New Mexico, USA
Time (ms) 1000 1200 1400 1600 1800 2000 2200 2400 2600 2800 3000 F lam e Si gnal 0 10 20 30 40 50 60 top side upper side lower side middle Time (ms) 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 P res su re (P a) 0 100 200 300 400 500 600 700 800 900 1000 1100 1200
Figure 2 Variation of flame signals with time (experiment EXP-05)
Sensor position
Figure 3 Variation of pressure with time (experiment EXP-05)
Time (ms) 1000 1200 1400 1600 1800 2000 2200 2400 2600 2800 3000 T em per at ur e ( oC) 0 100 200 300 400 500 600 700 800 900 1000 #1 #2 #3 #4 #5
Figure 4 Variation of temperatures recorded by the high speed data acquisition system (experiment EXP-05)
Thermalcouple No. Time (s) 50 55 60 65 70 75 80 85 90 95 100 T e m pe rat ur e ( oC) 0 50 100 150 200 250 300 350 400 450 500 550 600 650 700 #8 #9 #10 #11 #12
Figure 5 Variation of temperatures recorded by the regular data acquisition system (experiment EXP-05)