Publisher’s version / Version de l'éditeur:
Canadian Paint and Varnish, 36, 5, pp. 36-42, 1962-06-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Properties of paints in relation to their protection of steel
Harris, J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=4da05a90-a876-4611-990e-89f9cdaa5d63
https://publications-cnrc.canada.ca/fra/voir/objet/?id=4da05a90-a876-4611-990e-89f9cdaa5d63
W L L
TH1
N21r
2
no.
158
c .2
BLDG
NATIONAL RESEARCH COUNCIL
CANADA
DIVISION
OF
BUILDING RESEARCH
PROPERTIES OF PAINTS IN RELATION
TO THEIR PROTECTION OF STEEL
by
J. HARRIS
Reprinted from
Canadian Paint and Varnish
Vol. 36, No.
5,
M a y 1962, p. 36
-
Price 25 cents
Research Paper No. 158
of the
Division of Building Research
Ottawa
This publication is being distributed by the Division of Building
Research of the National Research Council. It should not be reproduced
in whole or in part, without permission of the original publisher. The
Division would be glad to be of assistance in obtaining such permission.
Publications of the Division of Building Research may be obtained
by mailing the appropriate remittance (a Bank, Express, or Post Office
Money Order or a cheque made payable at par in Ottawa, to the Re-
ceiver General of Canada, credit National Research Council), to the
National Research Council, Ottawa. Stamps are not acceptable.
A coupon system has been introduced to make payments for publi-
cations relatively simple. Coupons are available in denominations of 5,
25 and 50 ccnts, and may be obtained by making a remittance as indi-
cated above. These coupons may be used for the purchase of all National
Research Council publications including specifications of the Canadian
Government Specifications Board.
Properties of Paints in
Re
To Their Protection of Stee
The various relationships between water and p a i n t
@ This paper reports upon work that
has b-en done in a study of the be- haviour of paints on stecl and their ability to protect steel under a variety of environmental conditions. Environ- mental conditions are a complex com- bination of such factors as tempera- ture, wetncss, pollution, solar irradia- tion, cycling, and probably others. Of these factors, the prescnce of watcr in different forms is undoubtedly one of the I I I O S ~ important. It was therefore thought desirable to study the various relationships between water and paints. Questions that form the basis for this continuing program are:
1. What are the rates a n d maxi- n1un1 quantities of water absorbed by paints in free film form and when at- tached to steel?
2. What relation is therc between rates and quantities of water absorbed by free films and by the samc paints attached to stcel substrates?
3. What are the relationships be- tween water absorption and such ef- fects as blistering, rusting and loss of adhesion when the paints have bcen applied to steel and subjected to con- tinued wetness or immersion?
4. How d o these same paints ap- plied to steel behave in a nunlber of environments including simulated nat- ural conditions and laboratory test conditions?
5. What are the rates of drying o u t of coated steel panels that have ab-
sorbed water?
6. What is the effect of tempera- ture on water absorption and how does it influence blister fornlation?
Studies on these questions raise many more related ones but even those stated here have not yet been worked out exhaustively. From a large nunlber of observations and measure- ments an attempt has been made to obtain simple general explanations of the effect of water on the protec- tivc ability of paints on steel. The steel used in these experiments has been, for the most part, a clean, rather ideal surface. A p e a t d-a1 has yet to be learned about the application of the
BY J. HARRIS
Paint Laboratory, Building Materials Section, Division o f Building Re- search, National Research Council, Ottawa.
results obtained to thc less ideal sur- laces encountered in practice.
B r o c e d ~ ~ r e - Eighty-two experi- mental paints were prepared in order to vary con~position systenlatically with respect to pigment, pign~cnt vol- ume, type of vehiclc, varying levels of inhibiting pigments, and type of in- hibiting pigments. T h e paints were used in free film form and as coat- ings on standard clean stcel panels. Measurements were madc of amounts of water absorbed when immersed for varying periods. Coated panels were immcrsed in distilled water and the cficcts observed at frequent intervals. Similar coated panels wcre subjected to outdoor exposures at a nunlber of different sites at which pollution var- icd. A number of laboratory condi- tions wcre used for evaluating the cf- fects of cycling condensation.
EXPERIIMENTAL DETAILS
Paints
-
Paints were preparedmostly in groups of four ranging from flat paints with a high pigment volunle to glossy paints with a lower pigment volunic. The pigment base for the most part was iron oxide and ex- tenders. Varying proportions of zinc chromate or red lead wcre introduccd. A number of scts were based o n titanium oxide and a fcw individual paints used single pigments. Vehicles chosen were alkyd, raw linseed oil, mixtures of bodied and raw linseed oils, estcr gun1 modified alkyds, lin- seed oil alkyd mixtures, phenolic varnish, plasticized chlorinated rubber, and vinyl. These selections are some- what incidental. since the main con-
Paper was presented at the 1962
annual Coatings Conference, Coat- ings Section, Chemical Institute o f Canada.
sidcration was to vary properties. Propcrties were varied in a systematic way however, by forn~ulating paints iron1 known ingredients.
Paint Films - Wet paints were
drawn down on photographic paper held o n a suction plate. T h e s c were dried under standard conditions of 23 deg. C and 50 per cent relative humidity f o r 7 days, stripped after being loosened by wetting t h e papcr trom the back, and aged for an addi- tional 7 days. During this aging pe- r ~ o d , the large films were c u t into ap- propriate specinlens. Film thicknesses were of the order of 1 to 1.5 mils.
Painted Steel Specimens--'Q' pan-
els, 4 by 6 in. were coated by dipping to approxinlately 1.5 mils d r y thick- ness and allowed to dry a n d age iln- der standard conditions. T h e panels vvere used in this largc size for im- nlcrsion tests and cut t o smaller sizes foi \, ater absorption nleasurenlents.
The cdges wcre protected b y a bend of paint or wax.
Water Absorption-Frce films werc
cut to 1.42 by 1.42, in. for t h e meas- i~rement of weight of water absorbed and 0.3 by 2 in. for measurement of changc in dimension. T h e latter speci- mens were cut in the direction of drawdown a n d at right angles t o it. A separate spccimen was cut f o r each in~niersion period. They were nleas- urcd or weighed as requircd and im- mersed in distilled water f o r periods of 95, I , 2, 4, 8, 1 6 , 2 8 , 4 8 , 7 2 , 9 6 , 120, 144 a n d 168 hours, a t 22 deg. to 23 dcg. C. At the end of each im- mersion period, a specimcn f o r weight gain was removed, blotted off with tissue to remove surface water, and wcighed quickly. Specimens for di- mcnsional change werc removed at the sanlc time and while still wet, their lcngth measured by placing the specimcn o n a machinist's steel rule. A low power nlicroscope was used in reading length to 0.01 in. Weight and dimensional increases were calculated as perccntages of thc original. In some cases actual weight gain was used.
TABLE 1 -WATER ABSORPTION IN RELATION TO PIGMENT VOLUME
(Sums and averages of 17 sets)
- -
froni the larger panels to provide ex- posed surface area the same as for free films after the edges were beaded with paraffin wax. These were im- mersed for varying periods up to 168 hours to determine weight gain.
Measurements were also made on free film specinlens immersed at 0 to 1 deg. C and at 3 5 deg. C .
Pigment Volume Level 1 (high) 2 3 4 (low)
Immersion Tests-Panels with all
edges protected by paint were im- mersed in distilled water in 2-litre beakers at 22 to 23 deg. C, in some cases for 112 days. They were exarn- ined mainly for blistering, rusting, loss of adhesion and any other effects at frequent intervals at first and less frequently as time went on. Many of the tests were repeated a number o f times to obtain niore detailed results. The ASTM niethod of rating blisters was used and transcribed subsequently to a numerical rating. Adhesion was estiniated by a tape test and sometimes augmented by a knife test. When pan- els were finally removed, they were examined, stripped of paint and cor- rosion noted.
Outdoor Exposures - Panels sim-
ilar to those used for immersion were exposed at three N R C exposure sites of increasing corrosiveness, i.e., Ot- tawa, Montreal and Halifax. Some of these have now been exposed for over 2 years, others for only 6 months. At intervals these panels have been re- turned to the laboratory for examin- ation.
Water Absorbed, %
First
?h hour Max. Final
66.7 218.7 150.2
47.0 324.7 266.6
41.3 515.5 452.5
39.9 575.6 562.9
Simulated Tests - I t was decided
that the niost appropriate simulated tests would be those providing inter- mittent wetting. A humidity cabinet was adjusted to produce condensation at regular daily intervals followed by a drying-off period. Panels similar to those used for irnniersion tests were placed in the cabinet for continued long-term tests and examined at regu- lar intervals. T h c temperature during wetness was in the range 8 5 to 9 5 deg. F. Similar panels were also ex-
posed in a twin-arc weatherometer using a C.G.S.B. cycle. Av. time to max., hr 65.6 89.6 102.1 120.2 RESULTS
Water Absorption by Free Films-
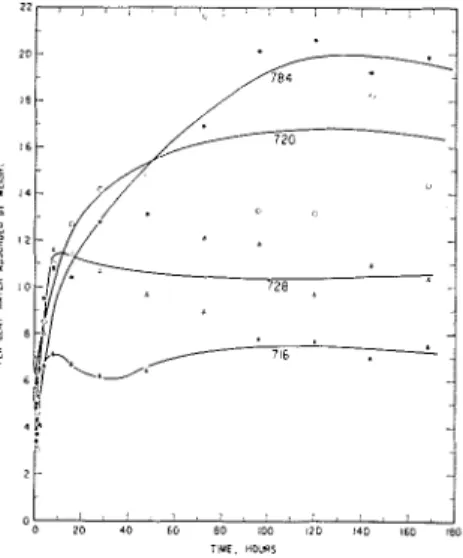
Water absorption measurements were made over a period of 168 hours. When plotted, the graphs approximate parabolic curves. For most of the paints studied a maximum quantity of absorbed water was attained within the 165 hours and a decrease followed. Di~iiensional changes in ternis of length and width increases followed changes in water absorbed. Paints pig- mented to a high pignient volume reached their maximum, or near max- imum, relatively quickly and the graphs decreased and flattened out. In some of the cases involving very high water absorption, the curves did not flatten out in 168 hours. Fig. 1
Ratios ?A hour Final Max. Max. 30.4 0.69 14.4 0.82 8 .0 0.89 6.9 0.98
FIGURE 1 , W a t e r absorbed b y f r e e films of a set o f iron o x i d e - a l k y d points o n im- mersion.
illustrates water absorption character- istics of a set of four paints and Fig. 2 shows the concurrent change in one of the dinlensions (width).
The percentages of niaximum water absorbed and the percentages of maxi- nium width increase for 17 sets each consisting of four paints ranging froni
'b
' 20 i C K 80 IW 110 I t 0 -0lk
T Y E . HdAI
FIGURE 2. Chonge i n width of free p o i n t films o f a sel of iron o x i d e - a l k y d points o n immersion.
high t o low pignient volunie have been summed up for each of four broad levels of pignient volume. Sim- ilarly the sum of weight gains in the first half hour a n d the final values were obtained. Average time to reach maxiniuni values and certain pertin- ent ratios were calculated. (These a r e given in Tables I and 11.) They show the following:
(I) T h a t water absorbed and di- mensional increase gain markedly with increase in vehicle.
(2) Water absorption and dimen- sional changes attain a maximum val- ue and then appear to decrease.
(3) Paints with higher pigment vol- umes attain niaximum values more quicldy and thereafter fall off to pro- portionately lower values than d o paints formulated to lower pigment volunies.
(4) Maximum diniensional increase precedes maximum water absorption since tinie to maximum dimensional changes is less than f o r maximum water absorption. This is shown ir, Fig. 3. where water absorption is plotted against width increase.
Water Absorption by Coated Steel
Specimens - Water absorption was
nieasured for coated steel panels pre- senting the same test area as the free films for short intervals up to a week
TABLE II
-
DIMENSIONAL CHANGE IN RELATION TO PIGMENT VOLUME(Sums and averages of 17 sets)
and at weekly intervals thereafter. Conlparisons were made between wa- ter absorbed by free films, and films attached to steel at stages of 1 hour, 16 hours and 168 hours, and at longer times. T h e 16-hour time was chosen because at that stage most of the painted panels were free of blisters; the 168-hour time represented the stage at which free film absorption had in most cases slowed down or stopped. (Table I11 shows observations
Pigment Volume Level 1 (high) 2 3 4 (low) 20 - 8 - , 16 - r I 4 - * - 4 i n - I l o -
=
,-
6 - I - CHAWGE IW I I D T K , %FIGURE 3. Relationship between water a b - sorption and width change.
Water Absorbed, % Av. time Ratios
First
I
to m a . , '/Z hour Finalhr
1/2 hour Max. Final
I
MU. Max.for a number of sets of paints.) It is assumed that a comparison to be valid .for the early stages should be based on actual weight o f water absorbed for equal areas of paint since the transn~ission of water by paints with pigment volumes below the critical pigment volunie is probably by solu- tion and diffusion. T h e ultimate ab- sorption should, however, be on the basis of per cent of original weight or volunie, since there is more paint on the steel specimens and the ulti- niate capacity is greater.
There are two major classifications of paints with respect to water ab- sorption (see Table 111). There a r e
26.5 150.9 123.0
24.6 190.5 170.8
25.4 245.4 232.3
16.4 263.1 255.0
those paints that absorb more water at a faster rate when attached to steel and there are those that absorb lesser quantities of water at a reduced rate. Figs. 4 and 5 show comparisons of two sets with lower absorption for at- tached films. Weight gains are here expressed as percentages since we a r e concerned with ultimate trends. Both illustrations show tendencies for curves for paints on steel t o flatten at lower water absorption, sometimes much lower levels. Fig. 6 shows a com- parison of a set of four paints that absorb larger amounts of water at a greater rate when attached to steel than they d o in free film form.
Paints in which water absorption is suppressed when they are combined with steel have been found to show superior blister resistance. Superior blister resistance is also found for paints formulated a t o r near the critical pignient volunle, presumably because the water is acconimodated in voids. Paints with low free film water ab- sorption appear to be more critical to the effect of increased water absorp- tion when combined with steel. (See
Table IV: Alkyd - T i 0 2 - Z n C r
forniulation).
T I Y E , HOURS
FIGURE 4 . Comparisons of water absarptions of free films and coated steel panels.
43.6 77.1 90.0 124.7
TIYF, HOURI
FIGURE 5. Comporison of water absorptions of free film and coated steel panels.
17.5 0.82
12.9 0.90
10.3 0.95
6.2 0.97
NATURAL WEATHERING
Results from the Ottawa test site only will be considered. This is one of the more severe environments from the point of view of wetness and tem- peratures, but is relatively low in pol- lution. T h e factor of high sulphur dioxide concentrations is purposely avoided at present since its influence has not yet been adequately studied. About 8 0 experimental paints have been exposed, many f o r over two years. These were examined frequent- ly. At n o time has wet blistering been observed o n any panel during the ex- posure even after periods of wetness.
A few paints did show a mild rust-
are porous because they are formu- lated at about the critical pigment vol- ume o r those that are extremely sensi- tive to blistering and are not inhibited. Rusting on the porous paints appears in a few days as stains or spots (it was observed at 7 days) and does not thereafter develop to a more severe condition. An alkyd-iron oxide paint of about 4 5 % pigment volumc con- centration (PVC) which is very per- meable has been used in another project and has lasted a t the Ottawa exposure site f o r nine years without development of rust blisters but has shown rust stain in the intervening period. Presuniably porous paints have the ability under mild conditions to seal themselves with corrosion prod- ucts.
SIXULATEIB TESTS
A number of accelerated simulated ssrvice tests were used. Only the onc using the huniidity cabinet, adjusted to produce a daily condensation cycle, will be reported at this time. There are indications that accelerated weather- ing using a C.G.S.B. cycle produces
L2
8 f i >O r t 8 r c a,
" .
FIGURE 6. Comparison of vrofer obsorptions of free films ond coated steel ponels.
results siniilar to those observed at the Ottawa outdoor site.
The same paints exposed at the Ot-
tawa outdoor site were subjected to the cycling condensation treatment. The niean temperature during wetness is considerably higher than that out- doors, and the treatment recurs regu- larly each day. This brings about ac- celeration of effects. T h e paints a f - fected arc the same ones that showed changes when exposed to natural weathering at Ottawa a n d some addi- tional paints that will likely develop corrosion effects on the outdoor pan- els with increased exposure time. This is probable since they a r e similar in conlposition to others that have al- ready shown rusting effects.
An iron oxide-zinc chroniate-lin- seed oil series appears t o be anoma- lous since blistering occurs very read- ily in laboratory tests but not out- doors. T h e explanation may depend on the difference of temperature level since laboratory tests h a v e all been carried out at temperatures higher than the niean outdoor temperatures during times when panels are wet.
ANALYSIS O F RESULTS
Wafer Absorption-The frequency
of eitccts shown by immersed panels
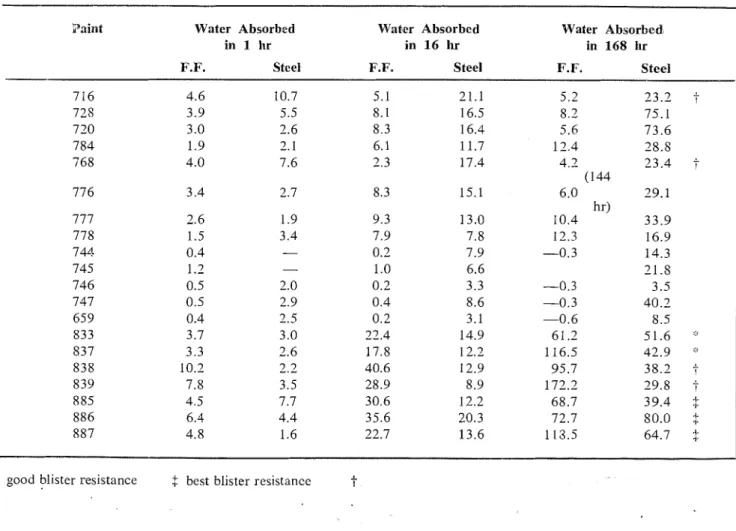
TABLE Ill -COMPARISON OF WATER ABSORBED BY FREE FILMS AND FILMS
ATTACHED TO STEEL
(Expressed in nigln for 4 sq in.)
h i n t Water Absorbed Waber Absorbed Water Absorbed
in 1 hr in 16 hr in 168 hr
F.F. Steel F.F. Steel F.F. Steel
was studied in relation to the levels of water absorption by free films with- out regard to any other classifications (Table V). T h e distribution is ran- dom. F r o m thcse obscrvations, the quantity of water absorbed by free paint films does not appear to be a reliable guide to the behavior of paints with respect to blistering, rust- ing, o r loss of adhesion of clean paint- cd stecl panels subjected to continuous immersion. High, low, and intermcdi- ate levels of water absorption can procluce poor blister resistance, rust- ing tendcncies, and proneness to loss o f adhesion but they can also go in hand with good blister and rust re- sistance and good adhesion retention. A second conlpilation was made ar- ranging frequency of effects on im- nlcrsed painted panels according to pigment v o l u n ~ c (Tablc VI). F o r the paints used, those formulated near the critical P V C were
no re
often en- dowcd with good resistance t o blister- ing, but poor protection against rust- ing and poor adhesion retention. There arc, howevcr, still a considerable number of paints possessing internicdi- ate and low pigment v o l u n ~ e t h a t show similar properties.Other relationships were notcd for a number of compositional factors and water absorption and effects by painted steel panels subjected to in]- mersion (Table IV) which may be summarized as follows:
(1) Oil paints have high water ab- sorption and possess good properties whcn fornli~lated with red lead at a n appropriate level; poor properties when form~llated with zinc chromate. (1) Alkyd paints have intermediate and low water absorption. T h e y tend toward loss of adhesion on immersion exccpt when the fornlulation includes red lead. Red lead is very effective in reducing rusting as is zinc chromate in adequate quantities.
(3) The phenolic (100%) varnish produced paints of relatively low water absorption. Paints based on a chlorinated rubber vehicle h a d low in- termediate water absorption. Both showed good blister resistance but poor rust resistance and adhesion re- tcntion on immersion tests.
Composition is a more important guide in preparing protective paints for steel than the knowledge of the water absorption characteristics of frce films.
Permeability to Water Vapor-The oil-base and alkyd-base paints exam- ined all possess permeability v a l ~ ~ e s of
TABLE V
-
INCIDENCE OF EFFECTS AT VARYING WATER ABSORPTION LEVELS(Numbers denote frequency)
the same order of magnitude. (Table
VII). F o r pigmentation levels around
the critical pigment volun~e, perme- ability becomes high, as would be ex- pected. With lower PVC levels there is a systematic variation of perme- ability with PVC. F o r alkyd paints there is a gradual increase; for oil paints a gradual decrease with de- creasing PVC. T h e values, however, remain small. Since permeability val- ues vary relatively little and show a relationship to w a t e r absorption (which is related to PVC), perme- ability measurements are of n o value
Maximum Water Absorbed % wt 0 - 2 2 - 4 4 - 6 6 - 8 8
-
10 10 - 12 12-
14 14-
16 16 - 18 18-
20 20-
22 22-
24 24-
26 26-
30 30-
40 40-
50 50 - 60 60-
70 70-
80 80 - 90 90 - 100 100 - 150in predicting the protective value of paints o n steel except when they are found to be high which indicates fornlulation at critical pigment vol- ume. These are known to resist blister- ing in many cases.
Relationship Between Laboratory Tests and Natural Weathering-Con-
tinuous immersion of painted steel panels at 23 deg. C. even for short pzriods produced a high incidence of wet blistering. Of 60 tests, only seven did not produce water blisters even after prolonged periods of immersion (four months in some cases). Twenty-
nine of the 60 tests, however, produc- ed blisters in less than three days. Clearly this behavior does not have any relationship to mild atmospheric weathering where water blisters a r e seldom observed even after periods of wetness. This discrepancy arises be- cause atmospheric weathering includes intermittent wetting which does not permit the absorption of sufficient water to produce blisters. Temperature may have an effect. With 17 different paints measured a t 0 t o 1 deg. C, 23 deg. C and 35 deg. C it was ob- served that the water absorbed in-
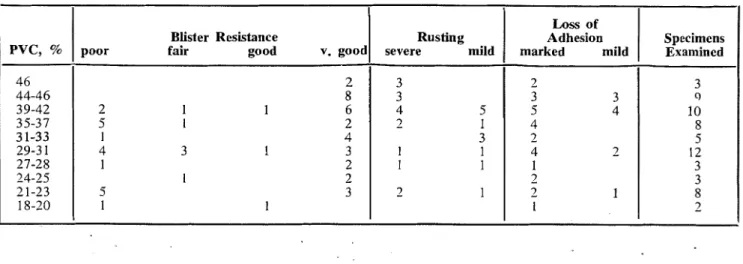
TABLE VI
-
INCIDENCE OF EFFECTS AT VARYING PIGMENT VOLUMEBlister Resistance
poor fair good v. good
6 6 2 4 3 1 4 1 1 4 1 1 1 3 3 1 1 1 2 1 1 1 1 1 3 3 1 ! 1 1 2 1 Rusting severe mild 5 2 1 1 1 1 3 1 1 1 1 1 1 3 1 1 1 2 1 Loss of Adhesion marked mild 5
5
1 3 4 2 2 2 1 7 1 1 2 Specinlens Examin& 6 6 6 4 7 6 2 2 8 1 5 1 1 0 1 3 3 1 1 1 1 3 I PVC, % 46 44-46 39-42 3 5-3 7 3 1-33 29-3 1 27-28 24-25 21-23 18-20 Blister Resistancepoor fair good v. good
2 8 2 1 1 6 5 1 2 1 4 4 3 1 3 1 2 1 2 5 3 1 1 Rusting severe mild 3 3 4 5 2 1 3 1 1 I 1 2 1 Loss of Adhesion marked mild 2 3 3 5 4 4 2 4 2 1 3 2 1 1 Specimens Examined 3 10 8 5 12 3 3 8 2
TABLE VII
-
PERMEABILITY OF EXPERIMENTAL PAINTS, PERMS/MILcreased markedly. F o r red lead in lin- seed oil the figures for maximum water absorption are 11.8, 32.5 and 72.4 per cent respectively. It is not known with certainty how this affects blistering. A few preliminary tests have shown a reduction in rate with lower temperature.
A cycling condensation test offers better correlation since it can be ad- justed to avoid blistering artifacts and accelerate corrosion effects. Cycling conditions prevent the cumulative ab- sorption of water because drying o u t periods are interspersed between wet- ness periods. It has been observed from a large nuniber of measurements that paints o n steel panels lose water in a niuch shorter time than it took to absorb it. Although intermittent wet- ting may produce cuniulative rusting and rust blisters may result, such ac- tion does not add u p to water blister development. A n immersion labora- tory test must be considered related only to an immersion service condi- tion. PVC 46+ 44-46 39-42 35-37 31-33 29-3 1 27-28 24-25 21-23 18-20 CONCLUSION
Water absorption studies have been carried o u t on a large number of ex- perimental paints varied in composi- tion to cover a presumed wide range of properties. Permeability measure- ments were made on the same paints. T h e behavior of the same paints was observed when applied to steel and subjected to immersion in distilled water, natural weathering and a cycl- ing condensation test. The relation- ships between water absorption, ef- fects o n imniersed steel panels, and service tests were examined.
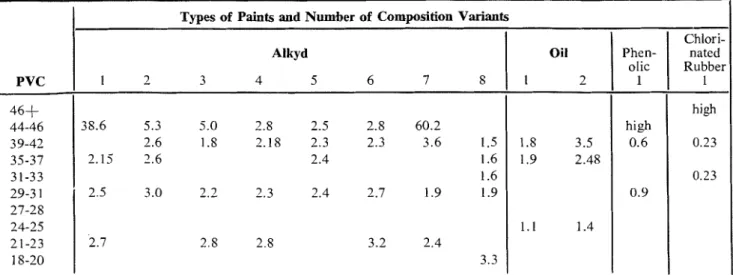
Types of Paints and Number of Composition Variants
There appears to be no simple rela- tionship between absolute quantities of water absorbed by free filni or painted steel panels and the effects noted o n inimersion of the same paints applied to steel. It was noted, however, that sonie paints absorb water at greater rates than they would in free film forni and this generally leads to blister- ing unless the paints are pigmented in the region of the critical pigment vol- ume. Other paints have reduced water absorption rates and quantities when attached to steel rather than as free films. This behavior typifies a nunibzr o f paints studied which showed im- proved blister resistance or complete freedom froni blistering for the ini- niersion periods used in the experi- ments.
There is some relation between water absorption and coniposition but this is not always clear cut. T h e vehicle initially determines the level of water absorption but this may be modified by pigment volunie and type of pig- ment. Generally, oil paints have high absorption; alkyds low or intermedi- ate. The phenolic varnish-base paints used had low water absorption and ch!orinated rubber-base paints intzr- niediate values.
Pigment volunie concentration at about the critical point favors blister resistance. Many of the paints of such pignientation show susceptibility to corrosion. Permeabilities are related to type of vehicle and its proportion. Except when permeability is high at critical pigment volunie, it does not provide any guide in judging the pro- tective value of a paint to steel. A s in
Alkyd 1 2 3 4 5 6 7 8 38.6 5.3 5.0 2.8 2.5 2.8 60.2 2.6 1.8 2.18 2.3 2.3 3.6 1.5 2.15 2.6 2.4 1.6 1.6 2.5 3.0 2.2 2.3 2.4 2.7 1.9 1.9 2.7 2.8 2.8 3.2 2.4 3.3
the case of water absorption there is a random variation with respect to blis- tering, rusting, and adhesion.
Blistering behavior of coated steel panels subjected to continuous im- niersion cannot be used to predict dur- ability in an atmospheric environment. In such an environment water blister- ing seldoni occurs whereas it is fre- quent in the case of inimersion. Rust effects on iinniersion show possible correlation with outdoor effects. Cy- cling condensation tests should b: used and further developed t o evaluate the protective capabilities of paints o n steel.
It was hoped that water absorption mzasurement of paint films would pro- vide a parameter for judging the pro- tective capabilities of paints o n steel. It appears. however, that other para- nieters or properties must b e sought, particularly for paint combinations with steel, and better basic infornia-
tion and procedures developed for simulated service tests.
ACKNOWLEDGEMENTS Chlori- nated Rubber 1 high 0.23 0.23 Oil 1 2 1.8 3.5 1.9 2.48 1.1 1.4
The writer wishes to express his appreciation for the help of R. C. Seeley, B. F. Stafford and J. J. Wood of the D B R Paint Research Labora-
Phen- olic 1 high 0.6 0.9
tory staff in carrying o u t many
preparations and measurements, and also to C. St. Jacques for his assistance in performing permeability measure- ments. This paper is a contribution from the Division of Building Re- search, National Research Council, and is published with the approval of the Director of the Division.