Publisher’s version / Version de l'éditeur:
Journal of Paint Technology, 39, 506, pp. 134-143, 1967-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Chemical kinetics of photo-oxidative degradation of dried trilinolein film
Yamasaki, R. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=04a1b7f3-f758-4100-9bb5-64b9e8ecb7e1 https://publications-cnrc.canada.ca/fra/voir/objet/?id=04a1b7f3-f758-4100-9bb5-64b9e8ecb7e1Chemical Minetics
Of
Photo-Oxidative Degradation
Of
Dried Trilinollein Film
By R . S. YAMASAKI
National Research Council of Canada%:
I n a colitinuirig cflort to\\.;1rd bcttcr ~~ntlcl-standing of tlic basic degradation processes t;~kirig ,place in cx- tcrior p a i n t coatings, frcc fil~lis of dl-ictl t i - ~ l ~ n o l c i n were sl~l),jectcd to co~itrollctl tlcgradation. Fil~lis \\'ere exposed to oxygen, ultraviolet radiatioii, aiid \\later vapor in a closctl systcn~ in various co~nbinations, and the c l ~ a ~ l g c s in c h c l ~ ~ i c a l conipositio~l of tlie fill11 a ~ i t l of the volatiles cvolvctl \\.ere analyzctl pcriodic;llly by illtl-arctl sl)cctro- pllotollletry.
T l i c f o ~ l i ~ a t i o n oC c : ~ r l ) o n ~ l c o t ~ ~ p o u ~ ~ t l s iu t l ~ c fill11 and tlie evolution of vol;ililc c;lrl)on c o ~ ~ ~ p o u ~ ~ t l s were a t - tril,utcd to internal hotly scissio~l.~ a ~ i t l c ~ l t l ellaill-scission ~.cactio~is, res])cctivcly. C:o~~~l,inctl, t l ~ c s c reactions give the ~ ~ r c ; ~ s u r c of t l ~ c t l c t c r i o l - ; ~ t i o ~ ~ untl non-fill11 f o r ~ i ~ a t i o n rc- a c t i o ~ ~ s o c c ~ i ~ r i ~ i g ill tllc 1il111 ill ~ I i c cai-ly stagcs o l e s - I"'" I re.
INTRODUCTION
A better ~~iiclerst;~ntling ol the clegradation proc-
esses t l i ; ~ ~ take place i l l p:tint filiii lias becollie csscntial
~ v i tli tlie clemancl for niore t l t ~ r a l ~ l e protccti~re coatings.
isVit11 respect co exterior llousc paint and ill particular
to its biilcler, linseed oil, Elin' 1i;ls s ~ ~ ~ n i i l ; t r i ~ c d the
work o n tlrietl linseed oil fill11 111) to 1949; ;tild Crecel-
ills ct (11 have since st~ltlietl tlie clleniical ell'ects ol' ~ l l t r a -
violet radiation ant1 oxygen on tltick and tliin drietl
liilseed oil filins by inir~recl sl,cctroscol)y.~~~ Millel-"
and cow an^ have uli.itten illore recent revie~vs. Tliese
sllow that when linseed oil is exl~oscd to oxygen i t
oxidizes to forin llyclroperosides at carbons ;ltlj;lcellt to the clouble carboil-to-carbon 1)oilcls. 1:rcc ratlicals are
formed and becoine carriers o l filiil re;~ctioi~s. Tllc 11)'-
tlroperoxicles nlay, in turrl, colilbine with tlie uni-eactecl esters to l'orin tlimers ;uld polyiners (~vitll l)e~~li;il)s
peroxicle or ether linkages) ;tilt1 solitlily, or they iiiny
initiate cliain reactions to split oil ;tntl poly11ie1.s. O n exposure to oxygen ant1 ~lltraviolet rat1i:ttion tlie 1)oly-
mers )nay f~lrtllcr cross-link ancl gr;~tlu;~lly for111 brittle
filins or may break tlowil to protluce, :lmoilg other sub-
st:~~iccs, cai bon tliositle :tild low i n o l e c u l ; ~ weight al-
tle1i)tles anel ;tcitls. T h u s ieactions peitaining to toi-
iii~~tioii, 11011-l'oiin;ttioii ~1ne1 cleteiioiation of film takc
placc siinultaneously.
T h e briel sununary oE the lilin reactions serves to s l l o ~ ~ tltitt r e i t ~ t i ~ l l s o c c ~ ~ r r i i l g i l l linsccd oil are com-
l'lex, itltlio~1g.11 only oxygen and ~tltraviolet radiation
1ia1.e bccn considerctl, and are far Lroin aclequately
~~nclcrstoocl. Tlte importaiit area of the elFects of inois-
cure has not been stucliecl. Furtllerinore, to~vards ob- taining a i1iol-e b;llanced picture ol' tllc relative effects
o l tlic various clegr;~cl;~ti\.e agents, the effects of ratlia-
tioil u~llosc sl>ct:tr;tl dist1,ibution is similai- to that of
the suit sliot~ltl be consitlerccl. B e c a ~ ~ s e ol' the complex-
ity oT tlie fill11 rc;1ctioi1s, st~ic1y 01' ;t syste111 simpler tliail
tliat o l linseccl oil, \\.l~icll is a inistu~.e, \ v o ~ ~ l t l be de-
sirable. 'IYlcsc coiisitlcr~ttioiis let1 to tlte present inves-
tigation oT tlic cllc~uic;~l kinctics of tlie plioto-oxidative
tlcg~.aclatioii o l r11.ictl fill11 ol' olie of tlte coInljonents o l
liiiseecl oil, trilinolcin.
EXPERIMENTAL
Selection of Film Material
As linseed oil is 21 coinplcx i i l i s t ~ ~ r e ol' seven com-
1>011ait Patty acids, ic was decided to s t ~ l d y a simpler
~.el)lacenient substance. T h e prini;try reactions iiivolvecl
i l l thc tli-ying and cleterioratioil ol' linseed oil are os-
itlative" and tlie site ol' this osidatioil is the clouble
bontl. I t follo~vs, tllerelore, that the reactivity of the
oil is dctcrininetl by the concentration o l these bonds. Linseecl oil consists inainly ol' triglycerides ol' three, two, ant1 ollc tlouble-bonclecl acids: liilolenic, liiloleic
aiitl olcic, resl)ecti\,ely. Tlle intlivicl~~al esters may be
pure or mixed. Thus, esters with (2 x 3
=
6) sixtlo~11,le 1)ontls sllould repi-eseilt the average reactivity
of tlie coinl~o~leilts in oil. T h e r e are t11rec possibilities
(incl~lcling one incorporatii~g a saturated fatty acid),
* Organic illatcrials Section, Di\ision of Building Research, Xlor~ircnl bLlt ollly Olle ester of tllrce f a t t y acicl raclicals Krl., Ottn\\.a 7, Ont., Canada.
2111 the same. B e c a ~ ~ s e lie ~ ~ ~ i i n i x e t l ester c;~ii be ob-
t>~iiletl in 21 1 ~ 1 r e r state t h a n tlie ~ l l i s e t l one, glyceryl
trilinoleate, o r trilinolein, Ivas cllosen 21s the Lilm ma- terial.
For driers, octoates ol' leatl ; ~ n t l c o b ; ~ l t 1vei.e em-
ployetl because they are non-ositlizing ; ~ n t l c;111 be ob-
tained in pure 1'01-in. T h e y were atltletl i n minimum
; I I ~ ~ O U I ~ ~ S to ret111ce c o ~ ~ t a m i n a t i o n antl y c ~ prot111ce ;I
s;~tislactory tlrietl film.
Free films r ; ~ t h e r tlian s ~ ~ l ) p o r t e t l films were se-
lectetl l o r s t ~ ~ t l y in ortlcr to l>rovitle t l o ~ t b l e the surCace
lor reaction ~ 1 1 l c 1 s u b s e q ~ ~ e n t analysis, ant1 to C ~ ~ c i l i t ; ~ t c
inC~.aretl an;~lysis by obviating tlie tlilliculty of finding
21 s ~ t b s t r a ~ e both inert ant1 : ~ t l e q ~ ~ a t e l y tlxnsparent L O
1K ratliation.
Selection of Degradative Agents
01' tile ~\jeatlleririg agents to ~vhicll exterior 1);1ii1t
fihns are normally exl)osetl, osygen, ~11tra1.iolet r a d i ; ~ -
lion, a n d water vapor were co~~sitlerecl lor study. Gas-
eous tank osygeil (Y9.7%,) was usccl Cor tlic test at- mosphere.
T h e ultraviolet r:~tliation s h o ~ ~ l c l i n the first ill-
stance simulate that of the sun with respect to spectral tlistribution. T o isolate possible reactions promoted
by different ~ v a v e l e ~ l g t l ~ s , however, a p p r o l x i a t e mono-
chromatic radiations s h o ~ ~ l c l be used. Kacliations at
two wave lengths were eml)loyecl simult:~neously, o n e
a t 3130.4 to simulate short UV l'rom tlie sun, the o t h e r
a t 366OX to s i m ~ ~ l a t e long UV, wit11 intensities com-
p;~rable to those o f the noon-day sun. Specific:~lly, 21
I-Ianovia higli-l)ressure, mercury-quartz l a m p (-135 watts) i n conjunction with filter G527B a n d Ilcat-clc-
flectiilg vycor (i527I: was ~ ~ s e c l . Prior to the exposure
test the ratliation O L I ~ ~ ) L I L 01' tlie lanlp was i~ie:~sui-etl
by t h e Di~zision o l Al)l)liecl Pliysics o l the National Ke-
se;~rch C o ~ ~ i ~ c i l . ~ , i
I t is known that w;Iter contl.ibutes to tlie t l e g ~ . ; ~ t l ; ~ -
tion o l p a i n t films by c a ~ ~ s i n g swelliilg a n d leaching 01'
the solubles.fi Here the efEect oE the atmosphei-e in-
itially s a t ~ ~ r a t e c l ~ v i t h water vapor W A S s t ~ ~ t l i e d .
Apparatus
CONTROLL,ED-A-I'AIOSPI-IEKE I ~ s - T O illi~liinize pre-
m a t ~ ~ l - e cleterioration o l ti-iliilolein by osygen prior to
film prepai-ation, it was desirable to prepare s o l ~ ~ t i o n s in a n inert atmosphere. A conti-olletl-atmos1111ere box
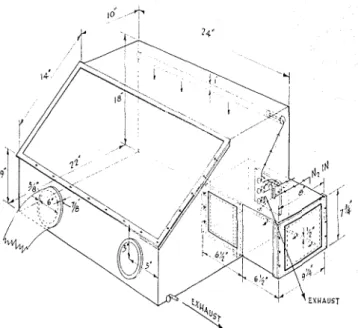
was clesigiiecl a n d c o n s t r ~ ~ c t e d of L ~ ~ c i t e , @ as is shoxvn
in F i g w e 1, i n which necessary m a n i p ~ ~ l a r i o n s coulcl be carriecl o u t in tlry nitrogen, free 01' oxygen.
I n operation the m a i n c11aml)er (127 litres) antl
an auxiliary transler c h a ~ n b e r (1 2 li tres) w e r e purged
~ r i t h certifiecl l ~ ~ l i - l ~ ~ ~ r i t y , dry nitrogen (0, m a s . 'LO
1~~111, H , O < I0 p p m ) u n t i l tlie osygen content i n the
effluent, as monitored ~ v i t h a Beckman Oxygen AII- alyzer, fell to O.OOvl. Objects, sollle oC which were flushecl o u t ulith nitrogen beEoreliand, were introduced
Lucite and N e o l ~ r e n c arc registered tradcrnarks of E. I. du Pont dc
S e ~ n o i ~ r s & Co., Inc.
PHOTO-OXIDATIVE DEGRADATION OF DRIED TRlLlNOLElN FILM
id -j-
Figure l-Controlled-;1tnlosl)11ere bos for 1)rel)aring clricr-
t~.ilinolein solution in ;rbsence ol' osygen
into tlie transfer cliambcr, 1)~lrged of osygen, then
transierretl i n t o tlie n ~ a i n c l ~ ~ ~ n i l ~ e r . Gas V ; I ~ I . C S were
closet1 to prevent s u b s e c l ~ ~ e n t suctio~l o l a i r illto the
110s antl s o l u t i o ~ l s 1vei.e p ~ ' e ~ ~ ; w c c l gr;~vinietrically. T h e
b o s was l.cl>urgetl in tlle i ~ ~ i t l t l l e o l ol,er;itions to re-
nlove oxygen that lii~tl t l i l l ~ ~ s c t l into the syscein (luring
r n a n i l ) ~ ~ l a t i o ~ l , m:~inly t l i r o ~ ~ g h t l ~ e rubber g l o ~ , e s . T h e
osygcn content i~icrc;~sctl tyl)ically l'ro~n 0.00 to 0.2'70
; ~ l t e r use.
?'he l ) o s was construc.~ctl oC 0.25-in. (0.64 cm)
L,ucite sec-tions pi~ss-sc;~lctl w i ~ l i c ~ l ~ l o r o f o r ~ n a s solvent.
I'orts ;111(1 tllc 1'roilt u ~ i ~ i t l o ~ v \\,ere 111;1tk O C 0,37-i1i.
(0.94 cni) Ld~lc:ite; the l a t l c ~ . \\.;IS r c ~ i i o ~ ~ ; ~ l ) l c ; ~ ~ i t l scz~letl
\\,it11 a 0.030-in. (O.OS(i ( x i ) r ~ ~ l ) l ) c r gasket ;III(I \\ling nuts. K e o p r c n e ' ' glovcs we1.c usctl, antl glovcs xntl
1)ortwvcre sealet1 witlt 0 . 5 - i l l . (O.(i-l cni) 0-ri~igs m t l
0.50-i1i. (I
."
tcm) r ~ ~ l > b c r L L I l)ings, rcs1)ecti vcly. 1-101-low gas inlct cylintlers wit11 0.015-i1i. (0.0:28 cm) t l i a ~ n -
eter holes spacetl 2 in. (5.1 ~111) ;~ l ) a r t were ~rsetl to
facilitate nitrogen gas t I i s t ~ . i l , ~ ~ t i o n . r l ' l ~ c m a i n cliaml~er
reclui~.etl 5.5 vol~~~::es 01' ~ ~ i t r o g c n at 3.6 litres/min to
lower tlie oxygen contcnt from 20.7 to 0.01
yl.
P l ~ ~ n i b -ing \\?as oS 0.25-in. (0.b.l c.111) col)l)cr lul>ing ant1 \t;lcutllil-l)ressure ~.alves.
E ~ I ~ ~ ~ U I < E - ~ X T ; I < , ~ I < I I I ) C~:~.r.-t'or L I I ~ [)resent ki~ictic s t ~ ~ d y non-tlestructi\~c, rcl;~tively i.;~l)itl riietliotl o l
an;~lysis I\T;LS i.cq~~irecl ~ v i t l i a r n i n i l i i ~ ~ m o l systelil tlis-
~11i.b;incc. T h e inf~xl-ctl rrictl~otl I \ ~ ; I S [IlcreEoi~e chosen
Lor fill11 a n d gas analyses.
A n u m b e r of exposure-infrarctl cells 21s illustratetl
in F i g l r ~ c 2 were tlcsignctl ; ~ i l c l k~l)ricatecl. 'The filril is
exl)oseetl in a n apl)rol)riate a t l n o s l ~ l ~ e r e to i.adiation
at position 1. A t s ~ ~ i t a b l c i~itervals tlic cell is removctl
ancl ~ ~ l a c e t l in the infi.:~rctl s p e c ~ r o l > h o t o r n e ~ e r ant1 ulit11 the aitl o l orclinate scale e s l ~ a n s i o n (20X) tlie spec- t r u m oE the gas is detcrrninetl lor analysis. T h e film is
F i g u r e 2-Exposure-inlrared cell i n whirl1 b o t h the e x p o s u r e of t h e f i l m a n d in sitti iilfl.ared analysis 01 the film ancl its
volatile p r o d u c t s m a y b e carried o u t
then movetl to position 2 and the film and the gas are
scanned a t 1X. T o obtain tlle sl)ectrum ol tlle film
only, the gas s p e c t r ~ ~ i n is next 1.1111 at 1X with film at
position 1 ant1 s~~btlactecl lrom the combined spec-
trum.
For exposirre in a dr) atlnosl)he~e, sodium chlo-
ride windows weie us11~11ly used and sealed wit11 Glyp-
tal.@ For a humid atmosphere, water-insoluble Irtran"
2 wintlows were employetl. T o maintain the oliginal
tliy atmos1)lleie the linger was k e l ~ t tooletl ~ v l t l ~ liquid
ni tiogen.
Spectia froin 10,000 to 650 cm-1 Iveie taken on a
Perkin-Elmel htotlel 221 in11 a1 etl sl~ecti opl~otometcl
with sot1111in cllloritle prisin optics.
Study of Degradation
Experiments were designed so tll;~t the effect of
each degr;~dati\.e agent could be studied. ;\II ampoule
containing 5 g of trilinolein (97C/(: +) 'k sealetl in ni-
trogen was ol)ened in t l ~ e controlletl-atmospl~el-e box
in the a1,sence o l oxygen antl a solution containing
0.030% lead and 0.025%) cobdt as octoates was pre-
pared gravimetrically. Drier solutions ~vel-e l~reviously
de-oxygenated by bubbling nitrogen through tlle.111.
About 1-ml portions of tile s o l ~ ~ t i o n \\ere t l ~ e i l dis-
pensed in gas-tigl~t, nitrogen-fillet1 vaccine bottles and
stored a t -20 C to minimize detecioratioil.
Prior to initial film preparation trilinolein solu-
tions were aged at room temper;lti~i-e lor about '18 ltrs.
to allow driers to reach full e f f e c t i ~ ~ e n e s s . ~ A l ~ o u t 0.1-
nll portions of the solution were removed with a syringe antl free filnls 25p tl~ick were prepared by
tlrauing the solution o n a tin panel wit11 a 12p doctor-
I>l;~cle. ?'he coati~ig was alloxvet1 to dry o ~ ~ e i n i g h t kor
17 h r at 2" 1 1, relatile humidity ok 5 3 1 l'/o, and
~ ~ n d e r noimal intloor illumination. It was subsequent-
1) looseiletl by mercury amalgamation of the substrate.
l;ilms wele cut to suitable s i ~ e and placed on rec-
tangular (2.0 x 1.5 cm) pyrex rod frames wit11 han- tlles. 1:ilins ielatively free o l imperlections were selected
lo1 stt~dy to m i n i ~ n i / e IIlemature ruptule. T h e frame
wit11 thc lilrn was then mounted i n the cell with the
aid 01 a flexible Teflon@ holder (FQu1.e 2)
.
Cells containing onc film each were evacuated ancl
fillet1 wit11 the appropiiate ;~tmospl~ere at slightly less
t l ~ ~ n atmosphciic pressure: i.e., 6'3 cm o l mercury. At-
~ n o s l ~ l ~ e r e s usetl uieie clry oxygen, \\uter-vapor sat~~ratecl
(25 C) ox)gen, ; ~ n d clly nitrogen. I h y o s ) g e n was ob-
tained by passing tank oxygen througl~ 21 dl y ice-ace-
tone t1~1p. SIoisture-laden oxygen w;~s generated by
bubbling tlte gas via liltel- stick tllrougl~ water that hat1
~)ieviously l)een oxygen;~tetl. C ~ I tifictL l l i g l l - l ) ~ ~ ~ i t y tlry
ni t i ogen was usetl xvitl~ou t I L I ~ tllel- p ~ ~ r i f i c 21 t ion.
l;ilms Miel e s~tbjectctl to test cxl)osIlre ant1 ~lllrarecl
spectra o l the films ;11ld ;~tnlosl)llclcs were taken at suit-
al-ble intervals. Non-iii21diated filins were air-thcrnlos-
tatetl a t 2 5 f 1 C untlcl noimal indoor i l l ~ ~ m i n a t i o n .
T h e ir~atliated lilms n c i c n~aintainetl a t 2 7 t 1 C
( l f i g l ~ e ~ d u e to 1 a d i ; ~ t i o ~ ~ ltcating)
.
E x p o s ~ ~ i es weie us-ually run in clul)lic;~~c. I:~lins weie lollolvecl L Ito 120 ~
ant1 1200 111 s. lo1 ladi;~tioil-eul)osctl ant1 i~nexposed-
lilms, iesl~ec ti1 ell. T l ~ e lo1 mer pel iod was slloi t be-
cause o l Glm r ~ ~ ~ ) t i ~ r c . As tlle I>rii~~ilry volatile I I ~ O ~ U C L S
may subsecl~~ently undergo I ~ ~ r t h e r cl~emical changes,
auxiliary check runs were made. Finally, calibration
curx.es were determined lor each volatile ~ ~ r o d u c t antl
the analytical results were interpreted i n terms o l
cl~enlical changes in tlie film.
Analysis of Volatile Products
T l ~ e volatile ~)rotlucts observed, identified ancl
s t ~ ~ d i e d kinetic;~lly l\rcre formic acid vapor, carbon cli-
oxitle, carbon lnonoxide a n d water vapor. They were
.l.cllul~ is n rcgistcrcd t r n d r m n r k of E. 1. d u Pant d c Ncmours & C o . ,
Inc. I
DR. R . S. YAMASAKI rcccivcd his l3.S~. and M.Sc. D e ~ c e s in chemistry h o ~ n the University of hlxnitol,a, a n d his 1'h.D. D e g ~ e e i n physical clle~nistry from the 1Jniversity of \irisconsin i n 1958. Sincc 1959 he has bccn employed by thc Na- tional Research Council of Canada, Divi- sion of Building Research, a n d is current- ly cngagccl in tllc study of chc~nistry a n d physics of degradation of paint fillns.
T E S T - E X P O S U R E P E R I O D OF F I L M S , H O U R S
PHOTO-OXIDATIVE DEGRADATION OF DRIED TRlLlNOLElN FILM
Figure 3-Kinetics of evolution of formic acid from trilinolein films exposed to
different depadative agents
+-f- m u g 3 0 I 0 w Y M 0 z U -
-
u > ;a 4Figure 4-Kinetics of evolution of carbon I dioxide trom trilinolein films exposed to
=
-
I I I I I ! I I LEGEND - 0 o x y , r ~ , ,rill l l " -.
O.)Lrll 0 N i l r o k r n , l > d u \._
-*--.-
L m - 0 O X " & . t i I , $ * "I -.
O Y " L L " 0.
N i t r o k e n ,"d u \ h n r o p e nA Wa8.r V,pour l H % ~ h Con< ) ~ > d $ l v
./
A O x y & e n l n d W , l r r V%pour 0 I c ~ h Can, I,
- / - / /
,
/ /* 1 1 m y3 0 0 I 0 P: U - I U-
m r..,rup.nI A Oxypen W ~ t r r V n p u u r ( I l t h h Coltr I l l t r l ilu
-
u A 0 x ) p . n ti>d W l l e r V r p o i l r I H t c h Conr I >-
2 2 0 - 2 - 3 I 3 U-
n u > -4. A -A/ A.----.
1 1 2 5 10 20 50 IW 2W 5W 1WOdifferem degradative agents n
u > 2 510 - w t u 2 - , + 4 2 - 0 > I 1 2 5 1D 20 5 0 1W 200 5W low T E S T - E X P O S U R E P E R I O D OF F I L M S H O U R S I I I I I I I I L B - - 0 OxyLe,, ,"d .i
,
O l y i ~ r , 0.
N I I T Y ~ E I I ~ I I I I I I I h u r o & c "-
A or,,.cc>, w,!,, \ .,,",,r (IIL~I, culli I ,ICI Ilrw
.
O."LC,, ,"<I S" , , ? r V , , I " > # ~ (ll,~l! < , ! I , , 1>
;20 -
5
z, Figure 5-Kinetics of evolution of carbon
I
z, monoxide from trilinolein films exposed
U-
C1 to dilferent degradative agents
u > 2 0 >lo - u u 2 - + 5 0 > I 2 5 10 20 50 1W 200 5W 10W T E S T - E X P O S U R E P E R I O D OF F I L M S . H O U R S
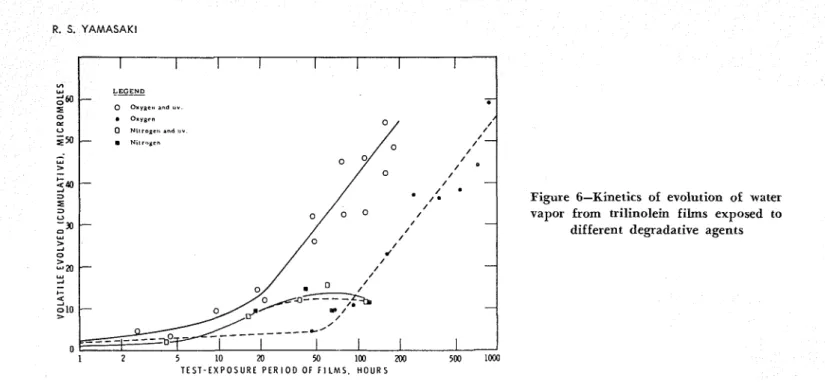
Figure 6-Kinetics of evolution of water vapor from trilinolein films exposed to
different degradative agents
T E S T - E X P O S U R E P E R I O D OF F I L M S . H O U R S
quantitatively analyzed by their bands at 1770 and 1122, 2300, 2250, and 1550 cm-l, respectively.
Calibration curves for formic acid vapor were de- termined using 98% formic acicl. Those of carbon di- oxide and carbon monoxide were obtained by employ- ing gas mixture G-7 (Phillips Petroleum CO., Special
Products Div., Bartlesville, Okla.)
.
Air of known rela-tive humidity was mixed with dry nitrogen to draw u p the water vapor curve.
T h e kinetic results for formic acicl vapor, carbon dioxide, carbon monoxide, and water vapor are shown in Figz1re.r 3, 4, 5, and 6, respectively. T h e results for formic acid vapor are uncorrected for possible subse- quent loss of the vapor in the test condition. For ex- ample, in an auxiliary run in oxygen atmosphere un-
der U V radiation, formic acid vapor concentration de- creased as a first order reaction with specific reaction rate constant of 0.011 hr-1. T h e other volatiles were not affected.
Film Analysis
As an illustration of changes observed, the infrared spectrum for the trilinolein oii and working spectra for the dried film and its corresponding atmospheres (be-
fore and a f ~ e r exposure to radiation and dry oxygen
for 85 hrs.) are given in Figzire 7. T h e bands at 967
cm-I and 910 cm-5 sshow the Dresence of isolated trans-
trans and cis-cis double bonds, respectively, in the orig- inal trilinolein oil. For the dried film subjected to ex-
W A V E N U M B E R , C M - '
A. T r i l i n o l e i n Oil. B. Cell a n d Atmosphere Before Exposure. C. Cell and Atmosphere After Exposure o f F i l m for 85 hr.
D. D r i e d i r i l i n o l e i n F i l m , Cell a n d Atmosphere Before Exposure. E. D r i e d i r i l i n o l e i n Film, Cell and Atmosphere After Exposure o f F i l m for 85 h r .
Figure 7-Infrared spectra of trilinolein oil; dried film and its environmental atmosphere before and after exposure to UV
PHOTO-OXIDATIVE DEGRADATION OF DRIED TRlLlNOLElN FILM
Table 1-Composition* of Tack-Free Trilinolein Film Dried for 13 Hourst''
Dried Film
Wt. or Number Original Oil Functional Groups lo-" Mole (Theory)
1. PeroxideX ... 22 0
2 . Hydroxyl ... 28 0
3. Alpha glycolic group ... 10
4. Unsaturated double bonds ... 39 84 0
...
5. Carbonyl (ketonic, aldehydic) 4-8 0
' For 14x10-0 mole equivalent in monomeric trilinolein. By analysis
of reduced fatty acids.
t Dried at 21°C and 61% relative humidity. Drier content 0.025 per cent Co and 0.03C/o Pb.
*' Analysis on original oxidized film.
posure the main changes occurred with bands at 3400, 1740 and 980 cm-l. These consisted of changes in band intensity, band width and band peak frequency.
T o make the intensity results of the bands amen- able to comparison among films of different thickness and to allow for thickness changes of the film in the course of exposure, each absorbance value was cor- rected with reference to that of the corresponding metllylene group at 1460 cm-1 (a function of thickness). This is possible because the methylene group is rela- tively stable to oxidation and much of the population of the film is composed of this group; even if some of it is lost during degradation, absorption will primarily depend upon film thickness.lO T h e baseline methodl1 was employed to determine absorbance of the bands.
T o aid in the interpretation of the spectra chem- ical analytical results for tack-free trilinolein film 3lP in thickness and dried for 13 hrs. were used and are given in Table 1.12
3400 em-1 band-Increase in band intensity and apparent half-band width and the apparent frequency shift of the absorption maximum at 3400 cm-I were
observed. Corresponding kinetics are illustrated in Fig- ures 8 ancl 9. Shifts were found to be characteristic of the exposure.
T h e increase in absorption at the initial stages of exposure is probably causecl by tlie formation of more
hydroperoxide (3450 cm-I) , carboxylic acids and al-
cohols (Table l ) . Broadening and shift of the band peak probably resulted from the increase in concentra- tion and hence association o i the monomers with poly- mers by hydrogen bonding.13 Thus formation of for- mic and other carboxylic acids brought about increase in band intensity, and as the acid concentration in- creased the monomer (3500 cm-l) reverted to dimer
(3150 cm-l) , causing broadening and shift in band
peak. T h e corresponding general increase in carbonyl band absorption at 1200 to 1300 cm-I tends to confirm this. Formation of monollydric ancl even dihydric al- cohols in any significant amount would also result in monomer hydrogen bonding, with consequent shiit in absorption bands as for acids. Here the band peaks are slightly higher, 3635 cm-I for monomer and 3500 cm-1
for polymer.l3~14~lThe mixture of acid and alcohol
hydrogen bondings could then account lor the ob- served peak at 3225 cm-1. For exposures in humid at- mosphere some of the water vapor was sorbed by the
film so that its infrared band at about 3350 cm-1 16
interfered with the normal analysis.
1740 em-I ba~zd-The apparent half-band width of this carbonyl band increased on film exposure. T h e kinetics are given in Figure 10. T h e 1740 cm-1 band is initially produced mostly by the carboxyl group of the ester as for the liquid in Figure 7. As the film dries and undergoes deterioration the band peak shifts to 1725 cm-I and broadens owing to the formation of
carboxylic acids (17 15-1690 cm-l)
,
ketones (1730-1600 cm-I) (Table I ) , and aldehydes (1740-1680 an-1) (Table 1 ) . Here again, for exposures in humid at- mosphere the infrared band at 1625 cm-l for water
.
o l y g c l , 0 N l l r o y e l l and u \ ;@,
-.
m N'L'DR." I O O x y ~ c n . W a l c r V ~ p v u r I H l c h Co8,r I r n d u u U O y y ~ c o and W n t c r V a p o u r l l l l f h Conc I I =;7W -.--.-
Figure 8-Increase in apparent half-band g A/+-
"-
width of 3400 an-' band for trilinolein
g
films exposed to different degradative 600 -a
. /
...
/ A agents LA. -I I 500 - - - --;/- -- - - . - - - m_ _ _ _
_ _ - - ----
--- m t - - - M O L 1 1 2 5 10 20 50 100 Dl 500 10W T E S T - E X P O S U R E P E R I O D OF F I L M S , H O U R SR. 5. YAMASAKI 3500 - 1
I
I I I A I U *- U zu Figure 9-Apparent frequency shift of
= 3400 cm-l band peak for trilinolein films
Z3m - L
-P:
L
0 o x y p r n a n d u r exposed to different degradative agents
.
0 x y p . n 0 N1,rog.n a n d u L N i t r o p e n A O x y ~ e n , W a t e r Vaprrllr I H t c h C v n r I ~ n d u v A 0 x y p . n a n d W l l r r V ~ P U Y I I I l l ~ h C o n c 1 32W-
-. I 1 2 5 10 20 50 IW no 5 W 1000 T E S T - E X P O S U R E P E R I O D OF F I L M S H O U R S 110 L B - 0 0.yp.n ""4 u r A -.
o x y g l " 0 N l l r o p r n a n d u,.
N l l r ~ f r n A' - - A 0 x y p . n . W a t e r V l p o u r l t l ~ ~ h C I I X 2nd u v /A A - r A O x y g e n and W , > l c i V l p o u r I S I l i h C u n r I U = 90-Figure 10-Increase in apparent half-band Z
width of carbonyl band a t 1740 cm-' for trilinolein films exposed to different
degradative agents - 56 1 2 5 10 20 50 1 W 2W 5 W 1WO T E S T - E X P O S U R E P E R I O D OF F I L M S . H O U R S 0 150 ur A A O x y p c n a n d W r t c r V a p n u r ( l l l l i h C o n < I - - 2 1, \ 1 \--&-:--a A ' \
\.
\ 1 2 5 10 20 50 I00 2w, 5W laxl.
I
Figure 11-Decrease in intensity of trans
bonding band a t 980 cm-l for trilinolein films exposed to different degradative
agents T E S T - E X P O S U R E P E R I O D OF F I L M S . H O U R S
..
'.
L n-
'.
'.
.
0 Oxygen and u v -.
.
.
O x y p c n-
-
.
0 N1trog.n a n d v v-- -
.
.
.
.
N l t r o i i " "-
.
Z \ A o x y l e n , waver v n p o v r (HI@ conr I a n dsorbed by the film interfered with the analysis, al- though to a lesser extent.
980 cm-1 band-The band decreased i n intensity
and in some cases disappeared o n film exposure. I t may be attributed to a mixture of original isolated tram-tmns (967 cm-1) a n d conjugated trans-tmns (988 cm-1) bonds formed on hydroperoxidation (Fig-
ure 7) ; these were gradually destroyed (Table 1 ) . T h e
kinetics are illustrated in Figure 11.
INTERPRETATION O F RESULTS
I n this study interest is centered o n the cleteriora- tions reactions. Nevertheless, as other reactions are tak- ing place simultaneously i n the film and some <lie even intimately connected with the degradation p~ocesses, the over-all reactions will be studied and interpreted to provide insight into the degradation reactions. Reac- tions will be classified as film formation, non-film formation and deterioration reactions, ancl probable mechanisms employing free radicals will be suggested. For convenience, reaction equations are given first. Thus:
2ROOII (metal d ~ i e r )
--
RO.+
RO,.+
H,O (2) (Hydroperoxide)13. Propaption"
R.
+
0, + KO,.RO,.
+
R H + ROOH+
R C. Polymcrization and cross-linking'~17KO,.
+
R. + ROORRO,.
+
RO,. + ROOR +02RO.
+
R. + ROR (7)ROOH
+
R H + ROR+
H,O (S)tRrOOI-I
+
R'H -+ R'OR'+
H 2 0 (9)R'O,.
+
R'C = CIS - C H = C R ' +RrO,R'CH,CHCH = CRf (10) D. Non-film for~nation or cleavagelo
ROOH + CH, (CH,)
,
CH C H - CHO (2-octenal)+
CH, (CH,),
CHO (n-hexanal)1
= S (11)+
CH, (CH,) C H = CH-
CH = CH - CHO (2, 4-dccadienal) CHx(CHz)r-CH = CH-CH -CH = CH(CH3);-COOCH? ir&;' I
glyc- CHn(CH?)l-CH = CH-CHz-CH = CH(CH2);-COOCH eryl I tri- C H ~ ( C H X ) I - C H z CH-CH--CH = CH(CHz)7-COOCHz lino- lrarr -... radi- cal+=
free radical t R' = polymer radicalPHOTO-OXIDATIVE DEGRADATION OF DRIED TRlLlNOLElN FILM
hv
S
+
O2 + HCOOH+
CO,+RCOOH+
H,O(formic acid) (acid) (12)
RUCH(OOH) R'+R"COR"'
+
H z O + R C O .+
Rot. (13) E. D e p o l y m e r i ~ a t i o n ~ ~ ~ ~ ~KtCH,OOR + RfCH,O.
+
RO.RrCH,O.
+
O z + R'.+
HCOOH ( l j )hv
RfCH,COR + R'COCOR
h e
R'COCOR + R'CO.
+
RCOR'CO. -t R'.
+
CO (18)R'CO.
+
0, + RO.+
CO, (19)R'CI-I (OOH) R --+ R'CHO
+
R O H(aldehyde) (alcohol) (20)
R'CI-IICO.
+
0, -t HCOOH+
CO,+
H 2 0+
R. (21)When trilinolein film that has been dried in air for 17 hrs. is placed in a n inert oxygen-free atmosphere, principally water vapor is evolved. Rate of evolution falls to zero rather rapidly, however, indicating initia-
tion (equation (2) ) and mostly polymerization ancl
cross-linking reactions (equations (8) , (9) )
.
Smallamounts of formic acid a n d carbon dioxide are also produced, probably from residue cleavage (equations ( I ] ) , (12)) and depolymerization reactions (equa-
tions (15) , (19), (21) ) . I n the film further formation
or hydroperoxide (equation (4) ) , hydrosyl (equation
(20)) ancl carboxyl (equations (12) ), (15) , (2 1) ) prod-
ucts is terminatecl. Trans tlouble-bondings ale very
slowly lost (equation (10) )
.
I n short, o n exclusioil ofgaseous oxygen the reactiolls in the film, principally free radical formation and probably polymeriration and cross-linking, proceeded until the oxygen was exhausted; then tliey terminatecl.
On exposure to UV radiation, tliough still in an inert atmosphere, the film evolvecl greater quantities of formic acid vapor and carbon dioxide a n d a small amount of carbon monoxide. These volatiles were probably produced by photolytic decomposition of the carbonyl compo~incls, ketones and aldehydes (equa-
tions (12) , (16) , (17) , (18) , (21) ) , by radiations at
3130 A and to a lesser extent at 3660
A.
T h t i s chain-scission increased. I n the film there was a slight in- crease i n hydroperoxide a n d / o r hydroxyl products, but none for carbonyl. Trans double-bonclings were de- stroyecl at a greater rate. T h u s , radiation i n the ab- sence of oxygen increased deterioration, presumably by clepolymerization and cleavage.
O n exposure of tlle film to oxygen (in the absence of test UV racliation) the production of water vapor increased, signifying increasecl cross-linking reactions
(equations ( 8 ) , (9) )
.
Formic acid and carbon oxideswere evolvecl a t a greater rate ancl quantity, indicating acceleration of chain-scission reactions. I n the film
Inore llydroperoxitles a n d / o r hyclroxyls a n d / o r car- boxylic acicls were generated. T h e 3400 cin-l band peak shifted to a final position at 3225 cm-1, signifying in- creased concentration of carboxylic acicls and alcol~ols, to lorm hydrogen bonded polymerr. Carbonyl concen-
tration increased. Traws bonclings were more rapidly
destroyed. This, coupled with the fact that cis boncls
were absent, led to the conclusion that all double
boncls were lost, so that further film for~nation through
unsaturated hydroperoxicle terminatetl. T h u s it is clear that environmental oxygen is required for ful tller poly- merization and cross-linking, cleavage, ant1 increaseel depolymerization of the film after initial drying ancl solitlification of trilinolein.
T h e presence of UV radiation in addition to dry oxygen encouraged the formation of the protlucts. As trilinolein oil, through drying, assumed a more viscous cross-linked structure, further polymerization reaction, which involves linking of two large molecules, is re- tarded because of lower mobility of the molecules in
favor of cleavage of the molec~~les. Tllus, more carbon
oxides (equations (18) , (19) ) ~tlei-e produced, presum-
ably through the absorption of UV radiation by t l ~ e carbonyls. Increasecl forination of water vapor could
signify greater depolymerization (equation (21) ) and
cross-linking (equations (8) , (9) ) reactions. Increase
in changes in the 3400 cnl-I band probably reflects the
production of more carboxylic acids (equations (1 1) ,
(12)
,
(21) ).
Tlle destruction of tra7zs bonc1ing.s waseven more rapid (equation (10) ) . T h u s UV ratliation probably accelerated the cleavage, clepoly~nerization ancl cross-linking reactions. Conlparison of the present
results with those of Crecelius et nl wit11 thin linseetl
oil film (2OP) "llows that carbon tlioxide, carbon
~nonoxide and Eorinic acid were evolved in botli cases
althougl~ at clifl'erent relative rates, but that no volatile
carbonyls o r formaldehyde were o b s e r ~ ~ e d Ilere. I n the
film the concentration of tlle carbonyl groups and the groups associated wit11 the 3400 cm-1 band as for lin- seed oil film increased rather than decreased during the exposure period probably because of the absence of short 2600A radiation ancl milder intensity of radia- tion.
T h e presence of water vapor in high concentration in oxygen decreasetl tlle apparent rate oE evolution of formic acicl antl carbon oxicles as well as the formation of non-volatiles i n the film. T h e 3-100 cm-1 band peak shifted only slightly to 3375 cm-1. Tlle water sorbed by the film produced a strong band at 3355 cm-I that dominated and maskecl the other adjacent bands. T h e film became soft and tacky, presumably through sorp- tion of water vapor. I n the process of oxidative poly- merization and deterioration of the film, carbonyl and hydroxyl groups are formed. These are polar sub- stances a n d sorb water vapor from the surroundings. As a result, the film imbibes water and undergoes swelling. I n turn, the polar substances dissolve i n wa-
ter so that the film becomes tacky and weakens me- chanically, eventually rupturing. T h u s the presence of a high concentration of water vapor retarded the film reactions ancl mechanically weakened the film so that it ruptured. T h e results agree with Miller's interpreta- tion4 that water does not enter clleinically into degracl- ation reaction but leaches o u t solubles a n d contributes to mecllanical stlesses in the film.
Exposure of the film to UV radiation i n the pres- ence of highly llumidifiecl oxygen encouraged the rate of evolution of foimic acid and carbon oxides and the formation of non-volatile alcohols, carboxylic acids and other carbonyl compounds. T h e 3400 cm-l band peak slliftecl more, to 3330 cm-l. T h e rate of formation of the products was generally comparable to or slightly lower than that with UV antl dry oxygen. Film became soft a n d tacky ancl ruptured after about 100 hrs. of exposure to radiation. T h u s , more cleavage and depoly-
merization reactions probably took place, with possibly more cross-linking than without radiation.
CONCLUSIONS
Trilinolein film, dried for 17 hrs. and subsequent- ly exposed to oxygen, ultraviolet radiation, ancl water vapor, underwent chemical changes i n the early stages
that resulted in forination of l~ydroperoxides, hydrox-
yls, carboxylic acitls, ketones and probably aldehydes,
antl destruction of trans-trans double-bondings. Vola-
tiles ~t~el-e evolved in the form of formic acicl, carbon
tlioxide, carbon monoxide ancl water vapor. These protlucts probably lormecl as a result of reactions in- volviilg formation, non-formation and deterioration of polymer film.
Ti\Titl~ respect to degradation reactions occurring in the film, the inciease i n apparent half-band ~ v i d t h
oi tlle carbonyl band gi1.e~ a measure of the non-vola-
tile ketones, carboxylic acicls and probably altlel~ycles forinecl when the molecules undergo internal body scissions a n d break off relatively large molecules. Evolu- tion of the volatile carbon compounds is piobably mostly from the chain ends ancl as such gives tlle meas- ure of the end cllain-scission reactions. T h e evolution o l water vapor may reflect both beneficial ancl detri- mental film reactions. T h e first two, combined, then give the measure of the deterioration reactions oc- curring in the film in the early stages of exposure. Compaiison of the rates of formation of the above products ainong different exposure conditions provides an assessment of the degrading quality of the exposure agents ancl may be listed as follows i n descending order;
(a) Oxygen and UV,
(b) Oxygen, water vapor (high conc.) a n d UV, (c) Oxygen,
(d) Oxygen and water vapor (high conc.) ,
(e) Nitrogen and UV, (f) Nitrogen.
Oxygen is requirecl in the deterioration o l the film. Ultraviolet radiation promoted the clegraclatioll of the film by cleavage and depolymerization, pre- sumably through absorption of the radiation by the carbonyl compounds. Water vapor slightly clecreased the degradation of the film as lar as chain scission is concenlecl. I t made the film swell, however, and be- come tacky and lose its mechanical strength, and caused rupture.
SUMMARY
I n order to obtain better understanding of the degradation process taking place in exterior house
~ a i n t s , a pure constituent of linseed oil, trilinolein,
was selected for study. Free films of trilinolein were prepared and subjected to colltrolled degradation in specially designed exposure-inCrarec1 cells. Films were exposed to oxygen, UV radiation and water vapor in various combinations in such a way that the eil'ect of each Cactor could be studied. T h e changes i n chemical
c o m ~ o s i t i o n of the film and of the volatiles evolvetl
were periodically analyzed by incrared sl~ectrophotom- etry. T h e results were interpreted in terms oT reactions involving film Cormation, non-film Cormation, and de- terioration of the polymer film. Finally, conclusions were drawn as to the type of deterioration reaction taking place, the relative effectiveness oC the degracla- tive factors and the mode of influence of each factor.
ACKNOWLEDGMENTS
T h e author wishes to express his appreciation to J. J. Wood for preparing the films.
T h i s paper is a contribution from the Divisioll oC Building Research, National Research Council, Can-
ada, and is p~~blishecl with the approval oC the Director
of the Division.
+
PHOTO-OXIDATIVE DEGRADATION OF DRIED TRlLlNOLElN FILM
References
(1) Elm, '1. C., 111rl. Eng. C h e ~ ~ a . , 41, 319 (1919)
.
(2) C r c c c l i ~ ~ s , S. B., Kagarisc, R . E. antl Alexander, A. L., ibid., f'7, 1643 (1955).
(3) C ~ e c c l i ~ ~ s , S. B. antl A l c s a n d c ~ , A. I., N R L R c p o ~ t P B 121330, Naval R c ~ c a ~ c h L a b o ~ ~ l t o ~ y , IVashington, D. C. (1956).
(1) hI11lc1, C. D , J . A I I L . 0 1 1 Che177iils' loc., 36, 596 (1959)
.
(5) Cowan, J. C., O b c i n l DLCIST, 34, No. 449, 561 (1962). (6) Bcdford, K. E., N R C R e p o r t APHSSP-823, National Rc-
search C o ~ ~ n c i l of Canada, Ottawa (1959).
(7) I'cttit, E., A s t r o / ~ h g s . J., 91, 159 (1910) .
(8) B r o ~ v n c , F. L., Forest Procl. J., 9, 417 (1959).
(9) Bragdon, C. R., Editor, "Film Formation, Film Properties a n d Film Deterioration," Intcrscicncc Publishers, Inc., Ncw I'ork, 1958, p. 35.
(10) ibid., p. 124.
(11) Cl~icago Societl for P a ~ n t Technology, O f i c ~ n l Drcrsr, 33,
S o . 131, hIa1c11, Par1 2 (1961).
(12) Chipault, J. K. hIcMcans, E., OfJicinl Drcrar, 116, No. 351, 548 (1954)
.
(13) P i l ~ ~ c l ~ t c l , G C. a n d McClcllan, '1. L , " T h e Hydrogcn
Bond," I V IT. F ~ c e l n a n a n d Company, S a n F~ancisco, Calif. 1060.
(11) B l a ~ ~ h j , R . F. ' u ~ d P ~ r u ~ \ n i t / , J. M., J . Che17l. P h g ~ . , 39,
1500 (1963).
(15) Liddcl, I!. a n d Bccker, E. D., S p e c t ~ o c h i ~ ~ a i c n Actci, 10, 70 (1957)
.
(16) T l ~ i e l , M. 'i'., Bcckcr, E. I). and P i n ~ c n t c l , G. C., J .
Cf'le111. Phys., 117, 486 (1957) .
(17) Payne, H. I:., " O ~ g a n i c C o a t i n g Tcclunology. Vol. I. oil^,
Ilr\un\, 'iTallui\hcs and Polyrrucu\," J o h n \tlilcy and Sons, I n c , hTcw Yolk, 1951.
(18) h l a ) o , F. I< , 111r1. 1:11g. C ~ ~ I I I . , 52, 614 ( 1 9 6 0
.
(1'4) Bading\, T3. T , J . A I I I . Oil Che~~rists' Soc., 36, 648 (1959). (20) I:inkclslc.in, A . ; ~ n d Koycs, Jr., 'iV. A,, Discussio~~s, Fnrnday
Soc., KO. 14, 76 (1953)
.
(21) Gi~illet, J. E. a11c1 Nor~iali, I<. G. IV., N a t u ~ e , 173, 625 (1954)
.
Reprinted from the March 1967 issue of JOURNAL OF PAINT TECHNOLOGY Volume 39; Number 506; Pages 134-143
Copyright 1967 by the Federation of Societies for Paint Technology, Philadelphia, Pennsylvania, U. S. A.