HAL Id: hal-02491567
https://hal.archives-ouvertes.fr/hal-02491567
Submitted on 8 Jul 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Photosensitive Resins: Towards Aromatic Amine-Free
Systems?
Mira Abdallah, Assi Al Mousawi, Malek Nechab, Akram Hijazi, Jean-Pierre
Fouassier, Frederic Dumur, Jacques Lalevee
To cite this version:
Mira Abdallah, Assi Al Mousawi, Malek Nechab, Akram Hijazi, Jean-Pierre Fouassier, et al.. Di-ethoxyacetate Salts as Co-initiators for Radical Photosensitive Resins: Towards Aromatic
Amine-Free Systems?. ChemPhotoChem, Wiley, 2019, Photopolymerisation, 3 (11), pp.1162–1170.
1
Diethoxyacetate salts as co-initiators for Radical Photosensitive Resins:
Towards Aromatic Amine-free Systems?
Mira Abdallah1,2,3, Assi Al Mousawi1,2,3, Malek Nechab4, Akram Hijazi3, Jean-Pierre Fouassier1,2, Frédéric Dumur*4, Jacques Lalevée*1,2
1
Université de Haute-Alsace, CNRS, IS2M UMR 7361, F-68100 Mulhouse, France
2
Université de Strasbourg, France
3
EDST, Université Libanaise, Campus Hariri, Hadath, Beyrouth, Liban.
4
Aix Marseille Univ, CNRS, ICR UMR 7273, F-13397 Marseille, France
Corresponding author: jacques.lalevee@uha.fr; frederic.dumur@univ-amu.fr
ABSTRACT: In this article, diethoxyacetate salts are investigated as new co-initiators for the
free radical polymerization (FRP) of (meth)acrylates upon visible light exposure using Light Emitting Diodes (LEDs) @405 nm and @477 nm. Interestingly, the impact of the counter cation on their initiating ability is clearly highlighted. When these co-initiators are combined with commercial photoinitiators such as 2-isopropylthioxanthone (ITX) or Camphorquinone (CQ), good to excellent free radical polymerization initiating abilities are found and high final reactive function conversions are obtained. In absence of these co-initiators, no or poor polymerization occurs, clearly highlighting the importance of these co-initiators for an efficient process. These systems can be interesting for the replacement of aromatic amine opening the way for amine-free Type II photoinitiating systems.
2
1. INTRODUCTION
A photopolymerization process occurs when a liquid monomer (or a soft film) is transformed into a solid material (or a solid film) upon light exposure. In fact, our daily life is surrounded by many products that are manufactured with the use of light with examples such as in 3D printing, dentistry, cosmetics, composites, adhesives, coatings, inks and many other fields... The polymerization processes induced by light are so efficient because of the usage of low temperature and low energy consumption [1-5]. They are also green technology because they occur at room temperature and there is no release of significant volatile organic compounds.[2-15] The usage of radical and cationic photopolymerization processes in industry rely on high intensity UV light sources (that can be harmful for the environment and human health). On the other hand, a small part of their emission spectra is used to generate initiating radicals or cations. From these previously mentioned drawbacks, the use of safer light sources based on near-UV or visible light is desired for mild irradiation conditions. A perfect example of safer sources is the Light Emitting Diodes (LEDs) [2,7-15]. These irradiation devices are characterized by several advantages: low energy consumption and operating costs, long lifetime, and it is safe to the environment. There is an important need to introduce new photoinitiating systems with absorption profiles matching the LED emission for faster polymerization under mild irradiation conditions.[13-15]. Recently, some of the substantial academic efforts on the development of LED sensitive photoinitiating systems has resulted in patent applications e.g. see [10-17].
The search of safer photoinitiating systems is today another important topic specially for applications in food packaging, medicine and so on. For example, in these areas, most of the aromatic amines used as co-initiators (such as EDB) are now considered as toxic.
3
In this work, a set of diethoxyacetate salts, varying by their counter cations, are tested as new co-initiators (see Scheme 1) to initiate free radical polymerization (FRP) process under near UV or visible light delivered by LEDs (e.g. 405 or/and 477 nm). The photoinitiating systems will be based on the co-initiator (1) that is characterized by good solubility in radical monomers. Recently, Wang and co-authors [18] have used sodium diethoxyacetate (CoI-3) in organo-photoredox catalyzed hydroformylation of styrenes. This group has proposed a free radical pathway mechanism and the formation of formyl radical equivalent, produced from 2,2-diethoxyacetic acid via a 1,2,3,5-tetrakis(carbazolyl)-4,6-dicyanobenzene (4CzIPN) visible light photocatalyzed oxidation−decarboxylation via a single electron transfer mechanism (SET). In this paper, we believe that the diethoxyacetyl radical formation can be reached upon visible light illumination with well-known photoinitiators (PIs) such as 2-isopropylthioxanthone (ITX) or camphorquinone (CQ) to initiate a polymerization process.
The comparison with reference PI systems will be provided to show the role of the co-initiator for the access to efficient systems. Indeed, the comparison of the new co-co-initiator with aromatic amine (such as ethyldimethylaminobenzoate - EDB) will also be studied to highlight their ability to be considered as alternatives to toxic amines.
Scheme 1. Investigated Compounds. 2. EXPERIMENTAL PART
4
2.1. Synthesis of Co-initiators
Synthesis of liquid CoI-1: tributylammonium 2,2-diethoxyacetate
2,2-Diethoxyacetic acid (1 g, 6.75 mmol) and tributylamine (1.61 mL, 1 eq.) were suspended in ethanol (30 mL) and the solution was stirred at room temperature overnight. The solvent was removed under reduced pressure and used without any further purification. 1H NMR (CDCl3) δ:
0.93 (t, 9H, J = 7.3 Hz), 1.23 (t, 6H, J = 7.0 Hz), 1.29-1.38 (m, 6H), 1.57-1.65 (m, 6H), 2.92-2.94 (m, 6H), 3.61-3.71 (m, 4H), 4.80 (s, 1H); 13C NMR (CDCl3) δ: 13.7, 15.3, 20.3, 25.2, 51.2, 61.3,
99.8, 173.5; HRMS (ESI MS) m/z: theor: 147.0663 found: 147.0662 ([M]- detected); HRMS (ESI MS) m/z: theor: 186.2216 found: 186.2218 ([M]+ detected)
Synthesis of viscous CoI-2: tetrabutylammonium 2,2-diethoxyacetate
2,2-Diethoxyacetic acid (1 g, 6.75 mmol) and tetrabutylammonium hydrate 30-hydrate (5.40 g, 1 eq.) were suspended in ethanol (30 mL) and the solution was stirred at room temperature overnight. The solvent was removed under reduced pressure and used without any further purification. 1H NMR (CDCl3) δ: 0.96 (t, 12H, J = 8.0 Hz), 1.16-1.21 (m, 6H), 1.38-1.45 (m,
8H), 1.58-1.65 (m, 8H), 3.33-3.37 (m, 8H), 3.58-3.67 (m, 4H), 4.71 (s, 1H); 13C NMR (CDCl3)
δ:13.7, 15.4, 19.7, 24.2, 58.9, 61.0, 101.3, 172.0; HRMS (ESI MS) m/z: theor: 147.0663 found: 147.0660 ([M]- detected); HRMS (ESI MS) m/z: theor: 242.2842 found: 242.2840 ([M]+ detected)
Synthesis of solid CoI-3: sodium 2,2-diethoxyacetate
2,2-Diethoxyacetic acid (1 g, 6.75 mmol) in THF (20 mL) and aq. NaOH (270 mg in 20 mL water) were refluxed overnight. The solvents were removed under reduced pressure and the resulting solid was dried under vacuum. 1H NMR (DMSO-d6) δ: 1.08 (t, 6H, J = 7.1 Hz),
3.36-5
3.56 (m, 4H), 4.33 (s, 1H); 13C NMR (DMSO-d6) δ: 15.3, 60.4, 101.5, 169.8; HRMS (ESI MS)
m/z: theor: 147.0663 found: 147.0665 ([M]- detected).
2.2. Other chemical compounds
All the other chemical compounds were selected with the highest purity available and used as received, their structures are shown in Scheme 2. 2-Isopropylthioxanthone (ITX) was obtained from Lambson UK. Camphorquinone (CQ) was obtained from Tokyo Chemical Industry. Etyl-4-(dimethylamino)benzoate (EDB), bisphenol A-glycidyl methacrylate (BisGMA) and triethyleneglycol dimethacrylate (TEGDMA) were purchased from Sigma Aldrich. Trimethylolpropane triacrylate (TMPTA) was obtained from Allnex. TMPTA and BisGMA/TEGDMA were selected as benchmarked resins for radical polymerization.
6
2.3. Irradiation Sources
The following Light Emitting Diodes (LEDs) were used as irradiation sources: i) LED @405 nm with an incident light intensity at the sample surface: I0 = 110 mW.cm-2; ii) LED @477nm; I0 =
300 mW.cm-2.
2.4. Free Radical Photopolymerization (FRP)
The two-component photoinitiating systems (PISs) are mainly based on commercial photoinitiator/CoI (1%/x% w/w, with 0.1%<x<2%) or commercial photoinitiator/EDB (1%/1% w/w). The weight percent of the different chemical compounds in the photoinitiating system is calculated from the monomer content (w/w). The FRP of TMPTA was done in laminate; the photosensitive formulation (~25 m of thickness) is sandwiched between two polypropylene films to reduce the O2 inhibition. The 1.4 mm thick samples of (meth)acrylate were polymerized
under air into a rounded plastic mold of ~ 7 mm diameter and 1.4 mm of thickness. For thin samples, the evolution of the double bond content of acrylate functions was continuously followed by real time FTIR spectroscopy (JASCO FTIR 4100) at about 1630 cm−1. The evolution of the (meth)acrylate characteristic peak for the thick samples (1.4 mm) was followed in the near-infrared range at ∼6160 cm-1. The procedure used to monitor the photopolymerization profile has been described in details in [19,20,21].
2.5. UV-Visible absorption and Photolysis Experiments
The UV-Visible absorbance properties of the compounds were studied using JASCO V730 UV– visible spectrometer.
7
Molecular orbital calculations were carried out with the Gaussian 03 suite of programs [22,23]. The electronic absorption spectra for the different compounds were calculated with the time-dependent density functional theory at the MPW1PW91-FC/6-31G* level of theory on the relaxed geometries calculated at the UB3LYP/6-31G* level of theory. The triplet state energy levels were calculated at this level of theory.
3. RESULTS AND DISCUSSION
3.1. Light Absorption Properties of the Investigated Compounds
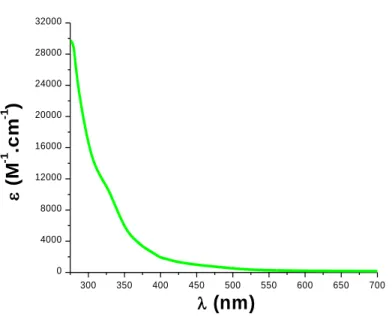
The UV-Visible absorption spectrum of the new proposed co-initiator (CoI-1) in acetonitrile (ACN) is reported in Figure 1. Remarkably, this compound has no intense absorption in the emission spectra of the visible Light Emitting Diodes (LEDs) used in this work (e.g., @405 and @477 nm), showing that this compound can only act as co-initiator and requires the presence of a photoinitiator or photosensitizer that will absorb the light. In addition, the absorption spectra of the two other proposed co-initiators (CoI-2 and CoI-3) are given in Figure S1 in SI. Some backgrounds experiments for CoI-1 alone in (meth)acrylate resins confirm the lack of ability of CoI-1 to initiate polymerization alone.
The optimized geometries as well as the frontier orbitals (Highest Occupied Molecular Orbital HOMO and Lowest Unoccupied Molecular Orbital LUMO) are shown in Figure 2. The HOMO is mainly centered on the carboxylate group suggesting its electron donating character (see the mechanism part below).
8
Figure 1. Absorption spectrum of CoI-1 in acetonitrile.
Figure 2. Contour plots of HOMOs and LUMOs for CoI-1 and CoI-2 structures optimized at the
B3LYP/6-31G* level of theory.
300 350 400 450 500 550 600 650 700 0 4000 8000 12000 16000 20000 24000 28000 32000 ( M -1 .c m -1 ) (nm)
HOMO
LUMO
Liquid CoI-1
Viscous CoI-2
9
3.2. Free radical photopolymerization of acrylates (TMPTA) 3.2.1. FRP of acrylates with ITX as photoinitiator
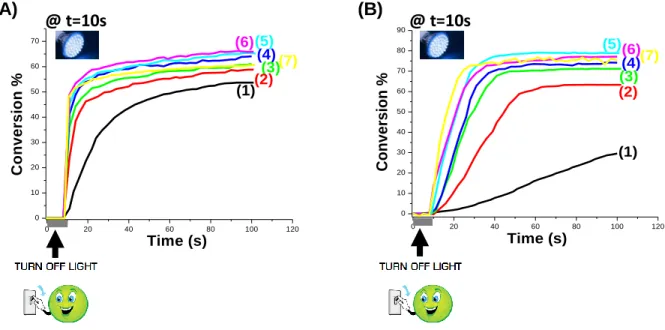
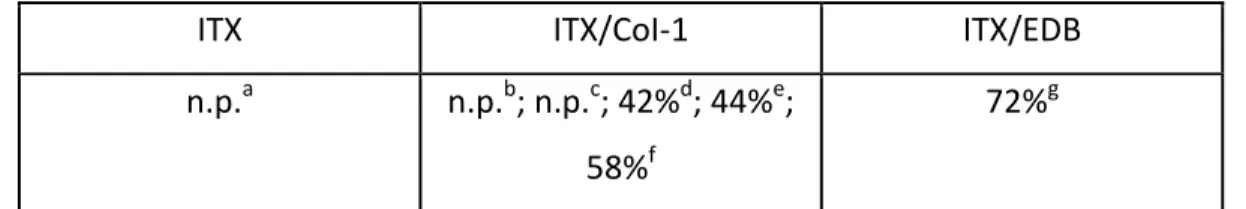
Typical photopolymerization profiles (acrylate function conversion vs. time) for ITX based photoinitiating systems are given in Figure 3 and the final acrylate function conversions (FCs) are summarized in Table 1. The FRP of TMPTA in thin films (25 m), in laminate and in the presence of the ITX/CoI-1 couple is quite efficient using the LED@405 nm (Figure 3A; curves 2-6) i.e. it is well obvious that ITX/CoI-1 systems have very high polymerization rates (Rp) and final conversions when irradiated with LED @405 nm. Clearly, the ITX/CoI-1 system has the ability to overcome the oxygen inhibition usually observed in FRP.
In comparison with ITX alone, the addition of the co-initiator leads to an increase of the performance. The final acrylate function conversion (FC) of TMPTA monomer previously reached was about 54% with one component photoinitiating system (ITX alone) (Figure 3A, curve 1), whereas higher FCs are reached when combined with the new co-initiator e.g. FC =66% with two-component photoinitiating system ITX/CoI-1 (1%/2% w/w) (Figure 3A, curve 6 and Table 1) under the same irradiation conditions, highlighting the crucial role of the co-initiator.
Interestingly, a higher final conversion is obtained for this new co-initiator than that achieved with the reference amine EDB (Figure 3A, curve 5 with 1% CoI-1 vs. curve 7 with 1% EDB, respectively; see also in Table 1), showing that this co-initiator can be an interesting substitute for amines.
The good performance of the ITX/CoI-1 two-component systems is also well observed for thick samples (1.4 mm) as shown in Figure 3B (curves 2-6) i.e. high polymerization rates (Rp)
10
were also clearly achieved. Again, a better performance is noted in presence of the proposed co-initiator e.g. FC increases up to 63% with ITX/CoI-1 (1%/0.1% w/w) compared to ITX alone, for which almost poor polymerization occurs (Figure 3B, curve 2 vs. curve 1, respectively - see also in Table 1). In comparison with EDB, the same performance was achieved (Figure 3B, curve 7 with 1% EDB vs. curve 5 with 1% CoI-1, respectively).
Remarkably, a tack free polymer is obtained for these CoI-1 samples and they also exhibit good bleaching properties (photos before and after polymerization are given in Figure S2).
The performance of the system increased when increasing the co-initiator concentration. The ITX/CoI-1 (1%/2% w/w) combination leads both to the highest polymerization rate and final conversion (FC) (e.g. for thin samples, FC = 66% compared to 59% with 0.1% CoI-1, respectively; Figure 3A, curve 6 vs. curve 2, respectively; see also in Table 1, and for thick samples, FC = 77% compared to 63% with 0.1% CoI-1, respectively; Figure 3B, curve 6 vs. curve 2, respectively; see also in Table 1). In conclusion, a higher concentration of co-initiator is better to efficiently initiate a FRP process.
The ITX/CoI-1 systems show no efficiency when using LED @477 nm (Figure S3). This is related to the well-known lack of absorption for ITX @477 nm.
By testing the other co-initiators (CoI-2, CoI-3), we see that they are not able to initiate the FRP of acrylates in combination with ITX (under exposure to the LED@405 nm). This can be probably ascribed to their low initiating radical yield that is not able to overcome the oxygen inhibition.
11
Figure 3. (A): Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation
time) in laminate (thickness=25 m) upon exposure to LED@405 nm in the presence of one or two-component photoinitiating systems: (1) ITX: (1% w/w); (2): ITX/CoI-1: (1%/0.1% w/w); (3): ITX/CoI-1: (1%/0.3% w/w); (4): ITX/CoI-1: (1%/0.5% w/w); (5): ITX/CoI-1: (1%/1% w/w); (6): ITX/CoI-1: (1%/2% w/w); and (7): ITX/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s. (B): Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation time) under air (thickness=1.4 mm) upon exposure to LED@405 nm in the presence of one and two-component photoinitiating systems: (1) ITX: (1% w/w); (2): ITX/CoI-1: (1%/0.1% w/w); (3): ITX/CoI-1: (1%/0.3% w/w); (4): ITX/CoI-1: (1%/0.5% w/w); (5): ITX/CoI-1: (1%/1% w/w); (6): ITX/CoI-1: (1%/2% w/w); and (7): ITX/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s.
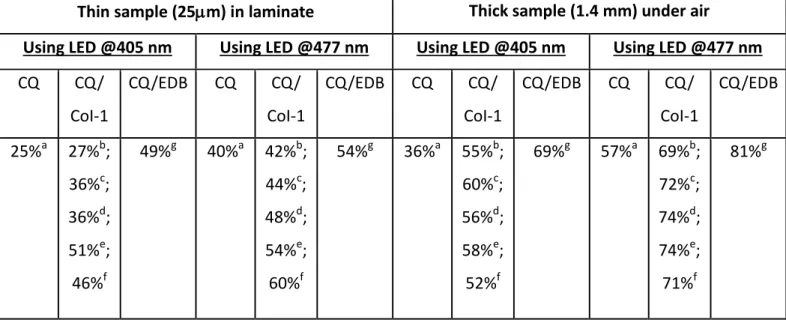
Table 1. Final Acrylate Function Conversion (FC) for TMPTA Using Different Photoinitiating
Systems after 100 s of irradiation with LED @405 nm.
Thin sample (25m) in laminate Thick sample (1.4 mm) under air
ITX ITX/CoI-1 ITX/EDB ITX ITX/CoI-1 ITX/EDB
54%a 59%b; 61%c; 64%d; 65%e; 66%f 61%g 30%a 63%b; 71%c; 74%d; 79%e; 77%f 75%g
a: ITX: 1% w/w; b: ITX/CoI-1: 1%/0.1% w/w; c: ITX/CoI-1: 1%/0.3% w/w; d: ITX/CoI-1: 1%/0.5% w/w; e: ITX/CoI-1: 1%/1% w/w; f: ITX/CoI-1: 1%/2% w/w; g: ITX/EDB: 1%/1% w/w
0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 (6)(5) (4) (3)(7) (2) (1) Time (s) C o n v e rs io n % 0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 80 90 (1) (2) (3) (7) (4) (5)(6) Time (s) C o n v e rs io n % (A) (B) @ t=10s @ t=10s
12
3.2.2. FRP of acrylates using CQ as photoinitiator
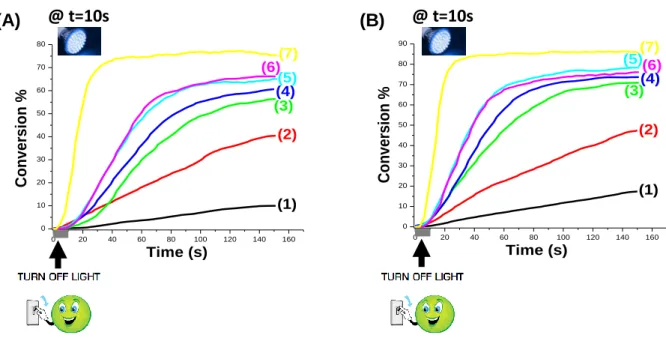
Typical photopolymerization profiles (acrylate function conversion vs. time) for CQ based systems are given in Figure 4, and the FCs are gathered in Table 2. The experimental results show that CQ/CoI-1 system is quite efficient using the two LEDs (@405, and @477 nm). For example, using LED@405 nm as a convenient mild irradiation source, the FRP of TMPTA in thin films (25 m, in laminate) in the presence of a two-component photoinitiating system based on CQ/CoI-1 combinations (1%/x% w/w) exhibits high efficiency in term of final acrylate function conversion (FC) (Figure 4A, Table 2). The efficiency is increased to reach FC = 51% with the two-component system CQ/CoI-1 (1%/1% w/w) after 100 s of irradiation compared to only 25% for CQ alone (Figure 4A, curve 5 vs. curve 1; respectively, Table 2), showing that the presence of the co-initiator is required for an efficient process.
Interestingly, the CQ/CoI-1 (1%/x% w/w) systems also efficiently initiate the FRP of TMPTA thick samples (1.4 mm), (Figure 4B, Table 2). The maximum acrylate function conversion (FC) of TMPTA was about 36% with CQ alone compared to 58% with 1% CoI-1 (Figure 4B, curve 1 vs. curve 5 and Table 2) under the same irradiation conditions, showing the huge effect of the co-initiator on the associated initiating ability.
In the same context, good polymerization profiles for the FRP of TMPTA thin and thick samples are also obtained when using LED@477 nm (Figures 4C and 4D). Clearly, CQ/CoI-1 PISs have the ability to overcome the oxygen inhibition usually observed in FRP, as shown in Figure 4D. With LED@477 nm, the addition of the co-initiator leads to an increase of the performance for FRP of thin samples and a FC up to 60% is obtained instead of only 40% for CQ alone (Figure 4C, curve 6 vs. curve 1, respectively; see also in Table 2). The same holds true
13
for FRP thick samples (FC = 74% with 1% CoI-1 compared to 57% with CQ alone, curve 5 vs. curve 1 in Figure 4D).
When replacing CoI-1 by EDB, the same performance was achieved for FRP of thin samples when using both LED@405 nm and LED@477 nm (for LED@405 nm: Figure 4A, curve 7 with 1% EDB vs. curve 5 for 1% CoI-1; for LED@477 nm: Figure 4C, curve 7 with 1% EDB vs. curve 5 for 1% CoI-1). However, higher polymerization rates were observed for FRP of thick samples in the presence of CQ/EDB system compared to CQ/CoI-1 system (Figure 4B, curve 7 vs. curve 5 and Figure 4D, curve 7 vs. curve 5).
In addition, some photos of TMPTA thick films (1.4 mm) in the presence of different two-component photoinitiating systems under air before and after polymerization are shown in Figure S4 in SI. Note that the color of the obtained thick and tack-free polymer remains light colored.
In fact, the FRP of TMPTA in thin and thick samples, and in the presence of the CQ/CoI-2 (or CoI-3) does not show any efficiency. The structure/reactivity/efficiency relationships for the co-initiators will be discussed below.
14
Figure 4. (A) Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation
time) in laminate (thickness=25 m) upon exposure to LED@405 nm in the presence of one or two-component photoinitiating systems: (1) CQ: (1% w/w); (2): CQ/CoI-1: (1%/0.1% w/w); (3): CQ/CoI-1: (1%/0.3% w/w); (4): CQ/CoI-1: (1%/0.5% w/w); (5): CQ/CoI-1: (1%/1% w/w); (6): CQ/CoI-1: (1%/2% w/w); and (7): CQ/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s. (B): Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation time) under air (thickness=1.4 mm) upon exposure to LED@405 nm in the presence of one or two-component photoinitiating systems: (1) CQ: (1% w/w); (2): CQ/CoI-1: (1%/0.1% w/w); (3): CQ/CoI-1: (1%/0.3% w/w); (4): CQ/CoI-1: (1%/0.5% w/w); (5): CQ/CoI-1: (1%/1% w/w); (6): CQ/CoI-1: (1%/2% w/w); and (7): CQ/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s. (C): Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation time) in laminate (thickness=25 m) upon exposure to LED@477 nm in the presence of one or two-component photoinitiating systems: (1) CQ: (1% w/w); (2): CQ/CoI-1: (1%/0.1% w/w); (3): CQ/CoI-1: (1%/0.3% w/w); (4): CQ/CoI-1: (1%/0.5% w/w); (5): CQ/CoI-1: (1%/1% w/w); (6):
0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 80 (5) (6) (7) (4) (3) (2) (1) Time (s) C o n v e rs io n % 0 20 40 60 80 100 120 0 10 20 30 40 50 60 (7) (6) (5) (4) (3) (2) (1) Time (s) C o n v e rs io n % 0 20 40 60 80 100 120 0 10 20 30 40 50 60 (1)(2) (3)(4) (6) (5) (7) Time (s) C o n v e rs io n % 0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 80 90 (6) (7) (5) (3) (4) (2) (1) Time (s) C o n v e rs io n % (D) (A) (B) (C)
15
CQ/CoI-1: (1%/2% w/w); and (7): CQ/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s. (D): Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation time) under air (thickness=1.4 mm) upon exposure to LED@477 nm in the presence of one or two-component photoinitiating systems: (1) CQ: (1% w/w); (2): CQ/CoI-1: (1%/0.1% w/w); (3): CQ/CoI-1: (1%/0.3% w/w); (4): CQ/CoI-1: (1%/0.5% w/w); (5): CQ/CoI-1: (1%/1% w/w); (6): CQ/CoI-1: (1%/2% w/w); and (7): CQ/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s.
Table 2. Final Acrylate Function Conversion (FC) for TMPTA Using Different Photoinitiating
Systems after 100 s of irradiation.
Thin sample (25m) in laminate Thick sample (1.4 mm) under air
Using LED @405 nm Using LED @477 nm Using LED @405 nm Using LED @477 nm
CQ CQ/ CoI-1 CQ/EDB CQ CQ/ CoI-1 CQ/EDB CQ CQ/ CoI-1 CQ/EDB CQ CQ/ CoI-1 CQ/EDB 25%a 27%b; 36%c; 36%d; 51%e; 46%f 49%g 40%a 42%b; 44%c; 48%d; 54%e; 60%f 54%g 36%a 55%b; 60%c; 56%d; 58%e; 52%f 69%g 57%a 69%b; 72%c; 74%d; 74%e; 71%f 81%g
a: CQ: 1% w/w; b: CQ/CoI-1: 1%/0.1% w/w; c: CQ/CoI-1: 1%/0.3% w/w; d: CQ/CoI-1: 1%/0.5% w/w; e: CQ/CoI-1: 1%/1% w/w; f: CQ/CoI-1: 1%/2% w/w; g: CQ/EDB: 1%/1% w/w
3.3. Free radical photopolymerization of methacrylates (BisGMA/TEGDMA blend) 3.3.1. FRP of methacrylates using ITX as photoinitiator
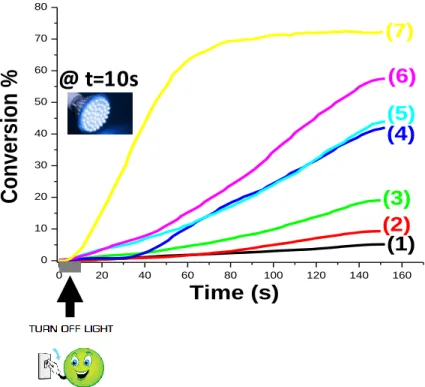
Interestingly, the ITX/CoI-1 (1%/0.5% w/w, 1%/1% w/w, and 1%/2% w/w) system efficiently initiates the FRP of a methacrylate blend (BisGMA/TEGDMA 70%/30% w/w) (under air; 1.4 mm thick sample; irradiation with LED@405 nm) (Figure 5; Table 3). Typical polymerization profiles (methacrylate function conversion vs. time) are given in Figure 5 and the FCs are gathered in Table 3.
16
As expected, for ITX alone, no polymerization was observed using LED@405 nm under air (Figure 5, curve 1) showing that the presence of the co-initiator is required. The ITX/EDB (1%/1% w/w) couple exhibits a better efficiency over the ITX/CoI-1 (1%/1% w/w) system. Indeed, the ITX/EDB couple shows both higher polymerization rate (Rp) and final conversion of methacrylate functions when using LED@405 nm (e.g. FC = 44% with ITX/CoI-1 vs. 72% with ITX/EDB; Figure 5 curve 5 vs. curve 7).
Remarkably, a tack-free polymer is obtained for these samples and exhibits low yellowing properties (photos before and after polymerization are given in Figure S5).
In addition, no polymerization occurs when using ITX/CoI-2 (or CoI-3) two-component system. This will be discussed below in the structure/reactivity/efficiency part.
Figure 5. Polymerization profiles (methacrylate function conversion vs. irradiation time) for a
BisGMA-TEGDMA blend under air (thickness = 1.4 mm) upon exposure to LED@405 nm in the presence of one or two-component photoinitiating systems: (1) ITX: (1% w/w); (2): ITX/CoI-1:
0 20 40 60 80 100 120 140 160 0 10 20 30 40 50 60 70 80
(1)
(2)
(3)
(4)
(5)
(6)
(7)
Time (s)
Conversio
n %
@ t=10s
17
(1%/0.1% w/w); (3): ITX/CoI-1: (1%/0.3% w/w); (4): ITX/CoI-1: (1%/0.5% w/w); (5): ITX/CoI-1: (1%/1% w/w); (6): ITX/CoI-1: (1%/2% w/w); and (7): ITX/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s.
Table 3. Final Methacrylate Function Conversion (FC) for a BisGMA/TEGDMA (70%/30% w/w)
blend after 150 s of Irradiation with LED@405 nm Using Different Photoinitiating Systems (under Air; thickness= 1.4 mm).
ITX ITX/CoI-1 ITX/EDB
n.p.a n.p.b; n.p.c; 42%d; 44%e; 58%f
72%g
n.p.: no polymerization; a: ITX: 1% w/w; b: ITX/CoI-1: 1%/0.1% w/w; c: ITX/CoI-1: 1%/0.3% w/w; d: ITX/CoI-1: 1%/0.5% w/w; e: ITX/CoI-1: 1%/1% w/w; f: ITX/CoI-1: 1%/2% w/w; g: ITX/EDB: 1%/1% w/w
3.3.2. FRP of methacrylates using CQ as photoinitiator
The FRP of BisGMA/TEGDMA in thick films (1.4 mm, under air) and in the presence of the CQ/CoI-1 (1%/x% w/w) couple is very efficient using different LEDs (e.g. LED@405 nm and @477 nm) (Figure 6A and 6B, respectively). Typical polymerization profiles (methacrylate function conversion vs. time) are depicted in Figure 6 and the FCs are summarized in Table 4. In these irradiation conditions, CQ alone does not lead to an efficient process, highlighting the crucial role of the co-initiator. The CQ/CoI-2 and CQ/CoI-3 systems were tested and no polymerization was observed. The structure/reactivity/efficiency relationships will also be discussed below.
It is well obvious that the CQ/EDB system exhibits higher polymerization rate (Rp) than the other system (CQ/CoI-1) when irradiated with both LED@405 nm or LED@477 nm (Figure 6A, curve 7 vs. curves 2-6 with LED@405 nm and Figure 6B curve 7 vs. curves 2-6 with LED@477 nm; see also in Table 4).
18
Some photos of BisGMA-TEGDMA thick films (1.4 mm) upon irradiation with the LEDs @405 and @477 nm for 150s in the presence of different two-component photoinitiating systems under air before and after polymerization are shown in Figure S6. The color of the obtained thick and tack-free polymers remains light colored, no strong yellowing is observed in presence of Co-I.
Figure 6. (A): Polymerization profiles (methacrylate function conversion vs. irradiation time) for
a BisGMA-TEGDMA blend under air (thickness = 1.4 mm) upon exposure to LED@405 nm in the presence of the one or two-component photoinitiating systems: (1) CQ: (1% w/w); (2): CQ/CoI-1: (1%/0.1% w/w); (3): CQ/CoI-CQ/CoI-1: (1%/0.3% w/w); (4): CQ/CoI-CQ/CoI-1: (1%/0.5% w/w); (5): CQ/CoI-CQ/CoI-1: (1%/1% w/w); (6): CQ/CoI-1: (1%/2% w/w) and (7): CQ/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s. (B): Polymerization profiles (methacrylate function conversion vs. irradiation time) for a BisGMA-TEGDMA blend under air (thickness=1.4 mm) upon exposure to
LED@477 nm in the presence of the one or two-component photoinitiating systems: (1) CQ:
(1% w/w); (2): CQ/CoI-1: (1%/0.1% w/w); (3): CQ/CoI-1: (1%/0.3% w/w); (4): CQ/CoI-1: (1%/0.5% w/w); (5): CQ/CoI-1: (1%/1% w/w); (6): CQ/CoI-1: (1%/2% w/w) and (7): CQ/EDB: (1%/1% w/w); respectively. The irradiation starts for t = 10 s.
0 20 40 60 80 100 120 140 160 0 10 20 30 40 50 60 70 80 90 (7) (6) (5) (4) (3) (2) (1) Time (s) Conversion % 0 20 40 60 80 100 120 140 160 0 10 20 30 40 50 60 70 80 (1) (2) (3) (4) (7) (6) (5) Time (s) C o n v e rs io n % (A) @ t=10s (B) @ t=10s
19
Table 4. Final Methacrylate Function Conversion (FC) for a BisGMA/TEGDMA blend after 150s
of Irradiation with LEDs @405 nm and @477 nm Using Different Photoinitiating Systems (under Air; thickness= 1.4 mm).
One and two-component photoinitiating systems
(LED@405 nm)
One and two-component photoinitiating systems
(LED@477 nm)
CQ CQ/CoI-1 CQ/EDB CQ CQ/CoI-1 CQ/EDB
n.p.a 41%b; 56%c; 61%d; 65%e; 66%f 75%g n.p.a 47%b; 71%c; 74%d; 78%e; 76%f 86%g
n.p.: no polymerization; a: CQ: 1% w/w; b: 1: 1%/0.1% w/w; c: 1: 1%/0.3% w/w; d: CQ/CoI-1: 1%/0.5% w/w; e: CQ/CoI-CQ/CoI-1: 1%/1% w/w; f: CQ/CoI-CQ/CoI-1: 1%/2% w/w; g: CQ/EDB: 1%/1% w/w
3.4. Proposed initiation mechanism
The formation of the initiating radicals in the presence of diethoxyacetate salt combined with ITX or CQ can occur according to a single electron transfer mechanism (SET). In fact, the PI/CoI-1 interaction probably corresponds to the processes r1-r3 (Scheme 3). The RCOO● and the subsequent radical generated after decarboxylation (R● in r3) can be proposed as the initiating species for the free radical polymerization. To fully support the proposed mechanism, a peak ascribed to CO2 [24] appears at a wavenumber of 2337 cm-1 in the course of the
photopolymerization process in presence of CoI-1 (Figure 7) highlighting the formation of the radical (R●) after a decarboxylation process.
20
Figure 7. IR spectra recorded before and after polymerization when using ITX/CoI-1 (1%/2%
w/w) system upon exposure to LED@405 nm.
PI → *PI (h) (r1)
*
PI + RCOO- → PI●- + RCOO● → radical initiation via SET (r2) RCOO● → R● + CO2 (r3)
Scheme 3. Proposed free radical pathway mechanism
3.5. Structure/Reactivity/Efficiency Relationship
The most important parameter that can explain the better initiating ability of CoI-1 is its best solubility compared to CoI-3 which is not soluble in radical monomers which level off the performance of CoI-3. On the other hand, when taking into account the two co-initiators CoI-1 and CoI-2, which are both soluble in the investigated monomers, it is noted that CoI-1 is able to
2300 2305 2310 2315 2320 2325 2330 2335 2340 2345 2350 0.14 0.16 0.18 0.20 0.22 0.24 0.26
2337 cm
-1(2)
(1)
O
.D
(cm
-1)
(1)Before polymerization (2)After polymerization21
initiate the polymerization reaction while CoI-2 is not able to do so, this can be probably ascribed to a steric hindrance for CoI-2 (i.e. the countercation in CoI-2 is bigger than the one in CoI-1). Therefore, a higher yield of electron transfer can be expected with CoI-1 than with CoI-2 leading to a higher initiating radical yield.
4. Conclusion
In this article, new diethoxyacetate salts are investigated as co-initiators (CoI-1, CoI-2, CoI-3). The synthesis is reported as well as their initiating ability in combination with commercial Type II photoinitiator. The comparison with amine (such as EDB) has shown that one new co-initiator (CoI-1) can be an interesting alternative for amine-free Type II photoinitiating systems. Remarkably, CoI-1 is able to initiate the FRP of (meth)acrylate functions among the proposed series of co-initiators: for its good solubility in radical monomers, less steric hindrance, and high initiating radical yield.
Acknowledgments: The authors would like to thank “The Association of Specialization and
Scientific Guidance” (Beirut, Lebanon) for funding and supporting this scientific work.
Supporting Information Available: Figure S1: The absorption spectra of the co-initiators
(CoI-2 and CoI-3) in acetonitrile; Figure S(CoI-2, S4-S6: Color before and after photopolymerization for the different polymers; Figure S3: Polymerization profiles of TMPTA (acrylate function conversion vs. irradiation time) in laminate (thickness=25 m) and under air (thickness=1.4 mm) upon exposure to LED@477 nm in the presence of the ITX/CoI (1%/x% w/w) couples.
22
REFERENCES
[1] J.P. Fouassier, Photoinitiator, Photopolymerization and Photocuring: Fundamentals and Applications; Gardner Publications: New York, 1995.
2 . . ouassier, . Lalev e, Photoinitiators for Polymer Synthesis, Scope, Reactivity, and Efficiency; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2012.
[3] K. A. Dietliker, Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd.: London, 2002.
[4] S. Davidson, Exploring the Science, Technology and Application of UV and EB Curing; Sita Technology Ltd.: London, 1999.
[5] J. V. Crivello, K. Dietliker, G. Bradley, Photoinitiators for Free Radical Cationic & Anionic Photopolymerisation; John Wiley & Sons: Chichester, U.K., 1999.
[6] W. A. Green, Industrial Photoinitiators: A Technical Guide, CRC Press, 2010.
[7] M. U. Kahveci, A. G. Yilmaz, Y. Yagci, in Photochemistry and Photophysics of Polymer Materials, ed. N. S. Allen, John Wiley & Sons, Inc., 2010, 421–478.
[8] B. Strehmel, T. Brömme, C. Schmitz, K. Reiner, S. Ernst, D. Keil, in Dyes and Chromophores in Polymer Science, Ed. J. Lalevée and J. P. Fouassier, John Wiley & Sons, Inc., 2015, 213–249.
[9] Y. Yagci, S. Jockusch, N.J. Turro, Photoinitiated Polymerization: Advances, Challenges, and Opportunities, Macromolecules, 2010, 43 (15), 6245-6260, 10.1021/ma1007545.
[10] J. Lalevée, J.P. Fouassier, in Dye Photosensitized Polymerization Reactions: Novel Perspectives, RSC Photochemistry Reports, Ed. A. Albini and E. Fasani, Photochemistry, London, UK, 2015, 215–232.
[11] C. I. Vallo, S. V. Asmussen, In Photocured Materials; A. Tiwari, A. Polykarpov, Eds.; RSC Smart Materials Series 13; The Royal Society of Chemistry: Cambridge, 2015, 321–346.
[12] M. Abdallah, H. Le, A. Hijazi, M. Schmitt, B. Graff, F. Dumur, T-T. Bui, F. Goubard, J.P. Fouassier, J. Lalevée, Acridone derivatives as high performance visible light photoinitiators for cationic and radical photosensitive resins for 3D printing technology and for low migration photopolymer property, Polymer, 2018, 159, 47-58.
[13] J. Crivello, in Dyes and Chromophores in Polymer Science, Ed. J. Lalevée and J.P. Fouassier, John Wiley & Sons, Inc., 2015, 45–79.
23
[14] M. Sangermano, N. Razza, J. V. Crivello, Cationic UV-Curing and Applications, Macromol. Mater. Eng., 2014, 299, 775–793.
[15] R. Bongiovanni, M. Sangermano, in Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2014, 1–20.
[16] J. Loccufier, Polymerisable photoinitiators for LED curable compositions, Patent No. US 8759412B2, 2014.
[17] P. G. Odell, E. Toma, Radiation curable inks, Patent No. US 7838570B2, 2010.
[18] H. Huang, C. Yu, Yueteng. Zhang, Yongqiang. Zhang, P. S. Mariano, W. Wang, Chemo- and Regioselective Organo-Photoredox Catalyzed Hydroformylation of Styrenes via a Radical Pathway, Journal of the American Chemical Society, 2017, 139, 9799-9802, 10.1021/jacs.7b05082.
1 . ietlin, S. Schweizer, . iao, . hang, . orlet-Savary, B. Graff, . . ouassier, . Lalev e, Photopolymerization upon LEDs: New Photoinitiating Systems and Strategies. Polym. hem. 2015, 6, 38 5−3 12, 10.103 / 5 Y00258 .
20 . Lalev e, N. Blanchard, M.A. Tehfe, F. Morlet-Savary, J.P. Fouassier, Green Bulb Light Source Induced Epoxy Cationic Polymerization under Air Using Tris(2,2′-bipyridine)ruthenium(II) and Silyl Radicals. acromolecules, 2010, 43, 101 1−101 5.
21 . Lalev e, N. Blanchard, M.A. Tehfe, M. Peter, F. Morlet- Savary, D. Gigmes, J.P. Fouassier, Efficient Dual Radical/Cationic Photoinitiator under Visible Light: A New Concept. olym. hem. 2011, 2, 1 86−1 1.
[22] J.B. Foresman, A. Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian Inc.: Pittsburgh, PA, 1996.
[23] M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. W. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, P. Salvador, J. J. Dannenberg, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen,
24
M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, J.A. Pople, Gaussian 03, Revision B-2; Gaussian Inc.: Pittsburgh, PA, 2003.
[24] A. Pyo, Y. H. Kim, K. Park, G. C. Kim, H. C. Choi, S. Lee, Mechanistic study of palladium-catalyzed decarboxylative coupling of phenylpropiolic acid and aryl iodide, Applied Organometallic Chemistry, 2012, 26, 650-654.
25
TOC:
CoI-1 CoI-2 CoI-3
Diethoxyacetate salts as co-initiators for Radical Photosensitive Resins:
0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 (6)(5) (4) (3)(7) (2) (1) Time (s) C o n v e rs io n % @ t=10s