HAL Id: hal-02392948
https://hal.archives-ouvertes.fr/hal-02392948
Submitted on 20 Aug 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Organosulfur Compounds Formed by Sulfur Ion
Bombardment of Astrophysical Ice Analogs:
Implications for Moons, Comets, and Kuiper Belt
Objects
Alexander Ruf, Alexis Bouquet, Philippe Boduch, Philippe Schmitt-Kopplin,
Vassilissa Vinogradoff, Fabrice Duvernay, Riccardo Giovanni Urso, Rosario
Brunetto, Louis Le Sergeant d’Hendecourt, Olivier Mousis, et al.
To cite this version:
Alexander Ruf, Alexis Bouquet, Philippe Boduch, Philippe Schmitt-Kopplin, Vassilissa Vinogradoff,
et al.. Organosulfur Compounds Formed by Sulfur Ion Bombardment of Astrophysical Ice Analogs:
Implications for Moons, Comets, and Kuiper Belt Objects. The Astrophysical journal letters, Bristol :
IOP Publishing, 2019, 885 (2), pp.L40. �10.3847/2041-8213/ab4e9f�. �hal-02392948�
Organosulfur Compounds Formed by Sulfur Ion Bombardment of Astrophysical Ice
Analogs: Implications for Moons, Comets, and Kuiper Belt Objects
Alexander Ruf
1, Alexis Bouquet
2, Philippe Boduch
3, Philippe Schmitt-Kopplin
4,5, Vassilissa Vinogradoff
1, Fabrice Duvernay
1,
Riccardo Giovanni Urso
6, Rosario Brunetto
6, Louis Le Sergeant d’Hendecourt
1, Olivier Mousis
2, and Grégoire Danger
11
Université Aix-Marseille, CNRS, Laboratoire de Physique des Interactions Ioniques et Moléculaires (PIIM), Marseille, France;gregoire.danger@univ-amu.fr
2Aix Marseille Université, CNRS, CNES, LAM, Marseille, France 3
Centre de Recherche sur les Ions, les Matériaux et la Photonique (CEA/CNRS/ENSICAEN/UCBN), CIMAP, CIRIL, GANIL, France4 Helmholtz Zentrum München, Analytical BioGeoChemistry, Neuherberg, Germany
5
Technische Universität München, Chair of Analytical Food Chemistry, Freising-Weihenstephan, Germany
6
Université d’Orsay, Institut d’Astrophysique Spatiale, CNRS, CNES, France
Abstract
Carbon, hydrogen, nitrogen, oxygen, and sulfur are the main elements involved in the solid-phase chemistry of
various astrophysical environments. Among these elements, sulfur chemistry is probably the least well understood.
We investigated whether sulfur ion bombardment within simple astrophysical ice analogs (originating from
H
2O:CH
3OH:NH
3, 2:1:1) could trigger the formation of complex organosulfur molecules. Over 1100 organosulfur
(CHNOS) molecular formulas (12% of all assigned signals) were detected in resulting refractory residues within a
broad mass range (from 100 to 900 amu, atomic mass unit). This finding indicates a diverse, rich and active sulfur
chemistry that could be relevant for Kuiper Belt objects (KBO) ices, triggered by high-energy ion implantation.
The putative presence of organosulfur compounds within KBO ices or on other icy bodies might influence our
view on the search of habitability and biosignatures.
Unified Astronomy Thesaurus concepts:
Comets (280)
;
Trans-Neptunian objects (1705)
;
Classical Kuiper belt
objects (250)
;
Astrochemistry (75)
;
Astrobiology (74)
;
Experimental techniques (2078)
;
Laboratory
astro-physics (2004)
1. Introduction
Sulfur is of high interest in the context of (astro)chemical
evolution and habitability. Several sulfur-bearing molecules
have been observed in various astronomical environments. In
the diffuse interstellar medium (ISM), various sulfur-bearing
molecules, such as CS (Penzias et al.
1971
), have been detected
and the sulfur abundance has been observed to be close to the
cosmic value (Sofia et al.
1994
; Neufeld et al.
2015
)
whereas in
dense molecular clouds, sulfur is found to be depleted (Prasad
& Huntress
1982
; Van Steenberg & Shull
1988
;
Jiménez-Escobar & Caro
2011
; Vastel et al.
2018
). A hypothesis
suggested that the sulfur is locked in the form of OCS in icy
grain mantles (Palumbo et al.
1997
). A recent study has
modeled that the “missing” sulfur is indeed trapped in the form
of organosulfur molecules on grains (Laas & Caselli
2019
). In
protoplanetary disks, the planets’ places of birth around young
stars, CS or H
2CS chemistry has been discussed (Semenov
et al.
2018
; Le Gal et al.
2019
). Nevertheless, the number of
sulfur molecules detected in disks is significantly lower than in
the diffuse ISM, indicating a high reactivity of sulfur in the gas
phase (Semenov et al.
2018
). In the solar system, comet 67P/
Churyumov–Gerasimenko has been extensively characterized
regarding its sulfur contents. C-, H-, and S-bearing molecules
were found to be the third most abundant species, next to CH
and CHO compounds (Altwegg et al.
2017
). Among all sulfur
compounds in comet 67P/Churyumov–Gerasimenko, even
fairly complex organosulfur compounds have been detected
(e.g., CH
3SH, methanethiol and C
2H
6S, ethanethiol, and/or
dimethyl sulfide; Calmonte et al.
2016
). On icy moons, as on
the surface of Europa, the production of sulfuric acid and other
sulfur compounds has been discussed (Carlson et al.
2002
).
The reservoir(s) and evolution of organosulfur molecules,
from the ISM toward their incorporation onto (proto)planetary
systems, is poorly constrained. Radiation, as a ubiquitous source
of energy in various astronomical environments, might be a
potential trigger of organosulfur chemistry in space. For
instance, energetic nuclei including sulfur are present in
planetary radiation belts, solar wind or galactic and anomalous
cosmic rays (GCR/ACR) (Mueller et al.
1991
; Takashima et al.
1997
; Von Steiger et al.
2000
; Mauk et al.
2004
; Paranicas et al.
2009
). On Europa, the formation of H
2SO
4has been supposed
via radiolysis from energetic charged particle bombardment
and is supported both by experiments (Strazzulla et al.
2007
)
and observations (Dalton et al.
2013
). Next to the presented
numerous observations of sulfur molecules in space, their
chemistry, especially those of organosulfur, is not well
under-stood yet (e.g., problem of sulfur depletion in dense clouds and
star-forming regions but no sulfur depletion in diffuse clouds in
the ISM; Calmonte et al.
2016
; Le Gal et al.
2019
).
Along with observations, laboratory experiments enable a
better understanding of the chemical evolution of extraterrestrial
ices (Herbst & Van Dishoeck
2009
; Danger et al.
2013
,
2016
; Van Dishoeck
2014
; Schlemmer et al.
2015
; Öberg
2016
; Fresneau et al.
2017
). Sulfur chemistry in laboratory ice
experiments has been mainly studied in two ways, (i) irradiation
by nonsulfurous particles of sulfur-bearing ices (e.g., via UV
photons (Chen et al.
2014
), electrons (Maity & Kaiser
2013
;
Mahjoub et al.
2017
; Poston et al.
2018
)), or protons (Moore
1984
; Moore et al.
2007
; Ferrante et al.
2008
; Strazzulla et al.
2009
; Garozzo et al.
2010
; Loeffler et al.
2011
)
and (ii) irradiation
by S projectiles of non-sulfur-bearing ices (Strazzulla et al.
2007
,
2009
; Ding et al.
2013
; Lv et al.
2013
; Boduch et al.
2016
). UV
photons can induce the formation of more complex sulfur-bearing
molecules (OCS or CS
2)
inside initial H
2S ices (Chen et al.
2014
).
Also, electrons can trigger the formation of complex S molecules
(small sulfur allotropes or even complex organosulfur compounds
within H
2S-bearing ices) (Mahjoub et al.
2017
). Protons enable
the formation of OCS out of initially bearing SO
2or H
2S ices as
well (Ferrante et al.
2008
; Garozzo et al.
2010
). The implantation
of S ions inside pure water ices leads to the production of sulfuric
acid (Strazzulla et al.
2007
; Ding et al.
2013
). Inside CO and CO
2ices, the bombardment of sulfur ions initiate the formation of SO
2(Lv et al.
2013
). Furthermore, molecular dynamics simulations
suggest the formation of complex organosulfur molecules via S
ion bombardment of H
2O:CO:NH
3:CH
3OH ices (Anders &
Urbassek
2018
,
2019
). However, the formation of complex
organosulfur molecules has never been experimentally
demon-strated up to now.
In this study, the role of sulfur implantation into
astrophysi-cally relevant, realistic ice analogs (de Marcellus et al.
2015
;
Meinert et al.
2016
)
was tested. The resulting refractory residue
was probed for the formation of organosulfur compounds via
infrared spectroscopy (IR) and ultra-high-resolving electrospray
ionization Fourier transform resonance cyclotron mass
spectro-metry (FT-ICR-MS). The sulfur-irradiated sample (S
7+ions) was
compared to a reference sample which was processed with
Argon (Ar
7+ions). FT-ICR-MS analyses enabled the first
detection of organosulfur compounds within H
2O:CH
3OH:NH
3ices (2:1:1), implanted with sulfur ions. Their detection and
chemical characterization is discussed in the context of
S
7+-irradiated ices of various astrophysical environments,
particularly focusing on Kuiper Belt objects (KBO).
2. Methods
2.1. Formation of Ion-irradiated Ice Residue and IR In Situ
Analysis
The ion beam used for irradiation was generated at the
ARIBE low-energy line of the Grand Accélérateur National
d’Ions Lourds (GANIL), Large Heavy Ion National
Accel-erator, facilities in Caen, France. For both Ar
7+and S
7+, the
ion beam was formed by ions at an energy of 105 keV, with a
flux of ≈1×10
11cm
−2s
−1. The current reaching the vacuum
chamber was controlled by a Faraday cup. The main vacuum
chamber contained three windows out of ZnSe that can be
cooled down to 9 K and could hold one sample each. Ice
samples were formed by preparing a gas mixture in a premixing
chamber and depositing this vapor using a mobile needle,
allowing target deposition onto a desired window. This device
effectively enabled the generation and irradiation of three
different deposited samples at similar vacuum conditions.
Details on beam generation (Lv et al.
2012
)
and the IGLIAS
device (Augé et al.
2018
)
have been described previously.
The IGLIAS setup also enabled in situ chemical analysis of
the sample by a Brüker V70 Fourier Transform Infrared
Spectrometer (FT-IR), under primary vacuum while it is
exposed to the beam. IR spectra were acquired before
deposition (as a reference background), during irradiation
(one spectrum after a new layer was deposited and before it was
exposed to the beam, Figure
5
)
and after warming up to room
temperature (one spectrum of the residue at 300 K,
Appendix
A
)
. A residue is defined here as the refractory
remaining material from irradiated ices at 300 K.
Ices were formed with an initial gas mixture of
H
2O:CH
3OH:NH
3(2:1:1). The quantity deposited corresponds
roughly to 0.5 μm which is thicker than the penetration depths of
105 keV S
7+or Ar
7+, calculated to be <0.3 μm (Ziegler et al.
2010
). Thus, implantation of projectile ions into the ice could be
ensured. The windows were kept at 10 K during deposition and
irradiation. To increase the yield of formed residue, we
deposited several layers of each sample. A new layer is
deposited when the underneath irradiated layer reaches a steady
state. The evolution during the irradiation is monitored by FT-IR
spectroscopy on both methanol and ammonia infrared
absorp-tion bands. This procedure was repeated. In total, 15 layers were
formed for the argon-irradiated sample (Ar
7+ions) and 10 layers
for the sulfur-irradiated sample (S
7+ions). The experiment was
repeated (technical duplicates) later generating two 15-layer
samples to test for reproducibility (for both Ar
7+-irradiated and
S
7+-irradiated). Both duplicates show reproducible results.
We performed SRIM simulations (SRIM, The Stopping and
Range of Ions in Matter software; Ziegler et al.
2010
)
to
calculate the effective volume of atom implantation. We
assume a sample density of 1 g/cm
−3. We find that sulfur ions
stop on average at a depth of 0.21±0.1 μm within the sample
(2σ interval). Within the implanted volume, we estimate the
signal-to-noise ratio (S/N) and S/C ratio to be 9E-4 at the end
of the irradiation experiments (after 20 minutes). Even
accounting for uncertainties on SRIM simulations and the
sample density probably being below 1 g/cm
−3, it is readily
apparent that the amount of implanted sulfur is small compared
to the elements it can react with.
We have calculated that the irradiated volume (from the
surface of the sample to a depth of 0.31 μm) receives a dose of
65 MGy (about 11 eV/16 amu).
The residues, resulting from irradiated ices, were kept under
argon atmosphere in a stainless steel vessel, to minimize
oxidation prior to analysis (de Marcellus et al.
2015
).
2.2. FT-ICR-MS Analysis
Electrospray ionization Fourier transform ion cyclotron
resonance mass spectrometry (FT-ICR-MS), ran in negative
ionization mode, was used for in-depth molecular
characteriza-tion of the organic matter formed within the residue, resulted
from irradiated ices. High resolution mass spectrometry has been
widely used for in-depth molecular characterization of
extra-terrestrial organic matter (Schmitt-Kopplin et al.
2010
; Ruf et al.
2017
,
2018
,
2019
; Danger et al.
2016
; Fresneau et al.
2017
).
FT-ICR-MS represents the highest performance in mass resolving
power and mass precision among all mass spectrometers.
The residue was dissolved in 50 μL methanol (extraction
solvent, LC-MS grade; Fluka). This step was repeated four
times. The complete residue got dissolved in methanol.
Afterwards, 20 μL of the dissolved residue was diluted in
200 μL methanol. The solution was removed with a
micro-syringe, ready for flow injection into the ESI source. A solvent
methanolic blank was measured in accordance to be able to
detect the indigenous soluble organic matter in each ice sample.
The experimental study was performed on a high-field
FT-ICR-MS from Bruker Daltonics with a 12-T magnet from
Magnex. A time domain transient with four MWords was
obtained and Fourier-transformed into a frequency domain
spectrum. The frequency domain was afterward converted to a
mass spectrum by the solariX control program of Bruker
Daltonics. The ion excitations were generated in broadband
mode (frequency sweep radial ion excitation) and 3000 scans
were accumulated for each mass spectrum in a mass range of
147 to 1000 amu (atomic mass unit). Ions were accumulated for
300 ms before ICR ion detection. The pressure in the
quadrupole/hexapole
and
ICR
vacuum
chamber
was
3×10
−6mbar and 6×10
−10mbar, respectively. For
CID-MS/MS, ions were accumulated for 3 s.
The electrospray ionization source (ESI, Apollo II; Bruker
Daltonics) was operated in negative ionization mode. The
methanolic solutions were injected directly into the ionization
source by means of a microliter pump at a flow rate of
120 μL h
−1. A source heating temperature of 200°C was
maintained and no nozzle-skimmer fragmentation was
per-formed in the ionization source. The instrument was previously
externally calibrated by using arginine negative cluster ions (5
mg L
−1arginine in methanol).
Mass spectra with m/z from 147 to 1000 amu were calibrated
externally and internally to preclude alignment errors.
Subse-quently, mass spectra were exported to peak lists at a
signal-to-noise ratio 3. Mass resolving power was 400,000 at m/z=
400 with a mass accuracy of <200 ppb, enabling the separate
detection of isobars differing by less than the mass of an
electron (Ruf et al.
2017
). Practically, this approach enables a
direct assignment of molecular compositions with C, H, N, O,
and S atoms (and isotopologues in natural abundance) for each
individual exact mass (m/z value).
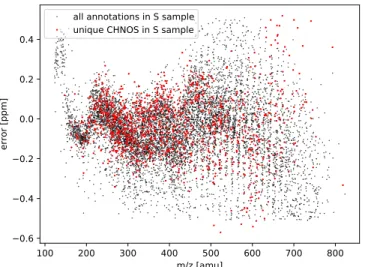
Molecular formulas were assigned from exact m/z values by
mass difference network analysis for each peak in batch mode
by an in-house software tool (Tziotis et al.
2011
)
and validated
via the senior-rule approach/cyclomatic number (Senior
1951
).
For formula assignment, 50.1% of all formulas have an error of
±0.1 ppm, 78.5% of all formulas have an error of ±0.2 ppm
and 100% of formula assignments within ±0.5 ppm (Figure
8
).
Further details on the assignment of molecular formulas from
FT-ICR-MS big data and their visualization in astrochemical
context are given in previous studies (Schmitt-Kopplin et al.
2010
; Ruf et al.
2018
; Bischoff et al.
2019
).
Data mining on organosulfur (CHNOS) compounds
repre-sent those m/z signals which were uniquely detected in the
S
7+-irradiated sample and absent in the Ar
7+-irradiated sample.
Double bond equivalent (DBE) was calculated according
DBE=C −(H/2) + (N/2) + 1.
3. Results and Discussion
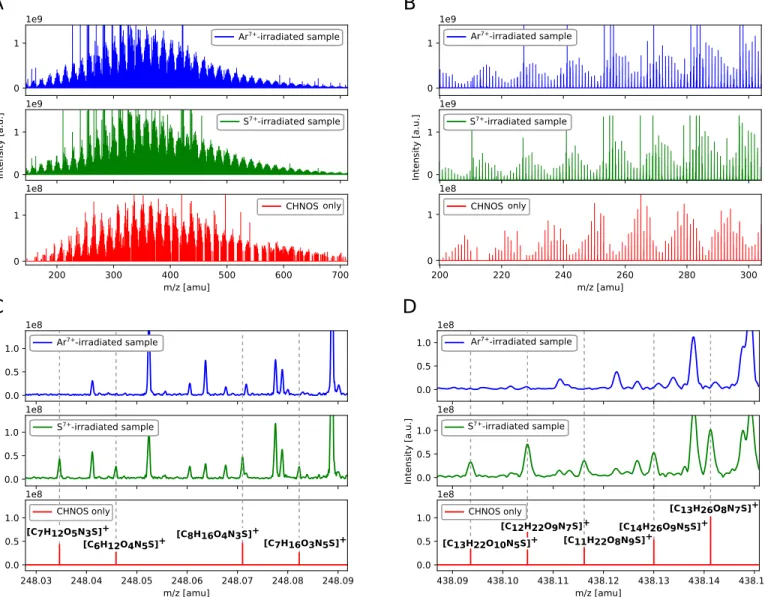
3.1. Detection of Organosulfur Compounds
IR spectra of S
7+- and Ar
7+-irradiated ices and residues show
the presence of organic compounds which have also been
previously observed in UV-irradiated ices (e.g., HNCO or
H
2CO; Figure
5
, Table
1
)
. Nevertheless, no significant difference
between both irradiation sources, S
7+and Ar
7+, could be
deciphered with infrared spectroscopy, and neither organosulfur
molecules could be identified in the S
7+-irradiated sample.
On a coarse level, mass spectra from FT-ICR-MS analysis
show similar features for Ar
7+-irradiated and S
7+-irradiated ices
as well (Figures
1
(
A) and (B)). Nevertheless, the fine structure
obtained with ultrahigh resolving power and ultrahigh mass
accuracy, FT-ICR-MS unambiguously revealed the detection of
organosulfur compounds in S
7+-irradiated ices (Figures
1
(C) and
(D)). m/z signals which correspond to organosulfur compounds
were absent in the Ar
7+-irradiated reference sample (Figures
1
(C)
and (D)). Within one nominal mass, up to five organosulfur
(CHNOS) signals are present as shown for two representative
examples (m/z=248 and m/z=438, Figures
1
(C) and (D)).
In general, the mass spectra of S
7+- and Ar
7+-irradiated
refractory residues show dense m/z signal patterns over a broad
mass range. From 30,000 experimental m/z signals, ranging
from 100 to 900 amu (atomic mass unit), 9,616 molecular
formulas, based on C, H, N, O, and S, could be unequivocally
calculated (5.3% CHO, 82.6% CHNO, and 12.1% CHNOS,
Figures
9
and
10
). This high molecular diversity indicates a rich
and active sulfur chemistry within these processed ices, triggered
by high-energy ion implantation. m/z signals appear as regular
patterns reflecting a pure chemosynthetic process as observed in
UV photon-irradiated ices (Danger et al.
2013
,
2016
; Fresneau
et al.
2017
)
or meteorites (Schmitt-Kopplin et al.
2010
; Ruf
et al.
2017
,
2019
; Figures
1
(A) and (B)). Regular patterns are
observed both for the global organic signature and for
organosulfur (CHNOS) compounds (Figures
1
(A) and (B)).
3.2. Characterization of Organosulfur Compounds
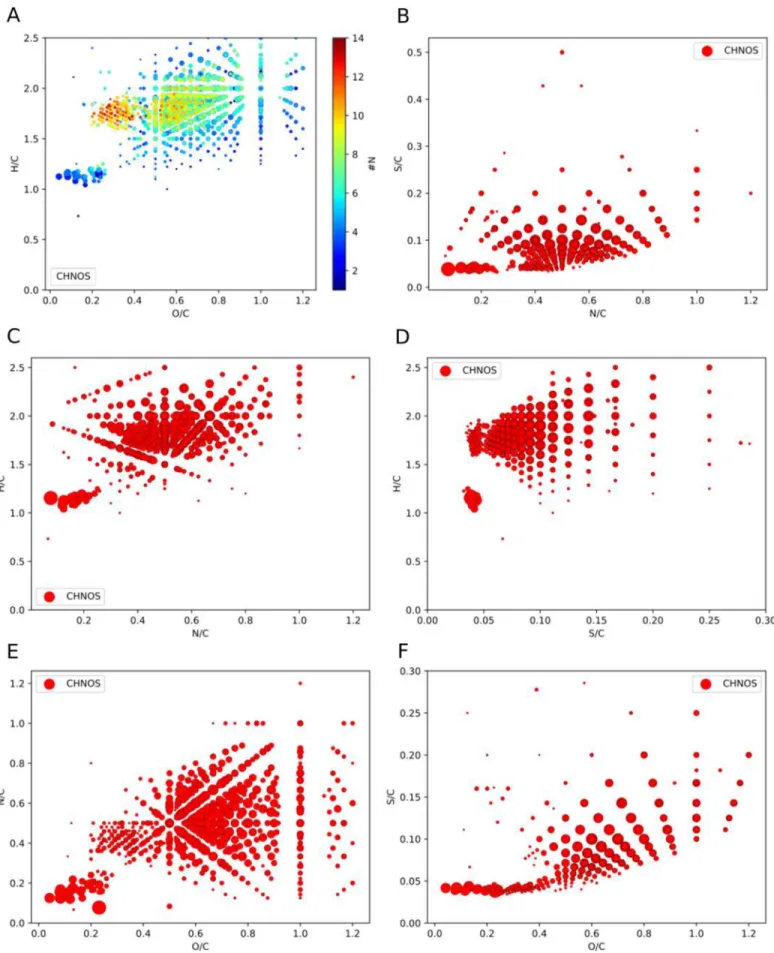
Atomic ratio plots, known as van Krevelen diagram (Van
Krevelen
1950
), were used to characterize in detail the detected
organosulfur (CHNOS) compounds. H/C against O/C
repre-sentation enables a first screening of complex mixtures with
respect to chemical families (Kim et al.
2003
; Wu et al.
2004
;
Danger et al.
2016
; Ruf et al.
2018
; Schmitt-Kopplin et al.
2019
). Organosulfur compounds present in S
7+-irradiated ices
show a low degree of unsaturation and a significant degree of
oxygenation (O/C
>0.5, H/C>1.5, Figure
2
). The degree of
oxygenation of organosulfur compounds is inversely related
with DBE (Figures
2
and
12
).
Basically, three groups of compounds can be extracted
(Figure
2
). Group 1 (Figure
2
, top right corner) basically
consists of small mass molecules up to 400 amu. This group of
compounds has high H/C and high O/C ratios with a small
number of DBE. Group 2 (Figure
2
, top left corner) bears
molecules with high masses (mostly 500–800 amu). Their DBE
is higher than those in group 1 (DBE
>8, Figure
12
). In
addition, this group of compounds is enriched in nitrogen
counts, bearing up to 14 N atoms per molecular formula
(Figure
11
). Group 3 (Figure
2
, bottom left corner) is
characterized by high numbers of DBE within a mass range
between 400 and 600 amu (Figures
2
and
12
).
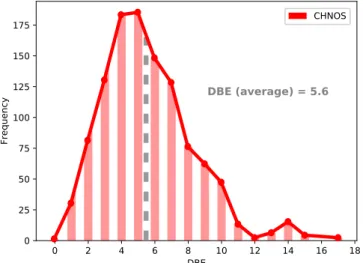
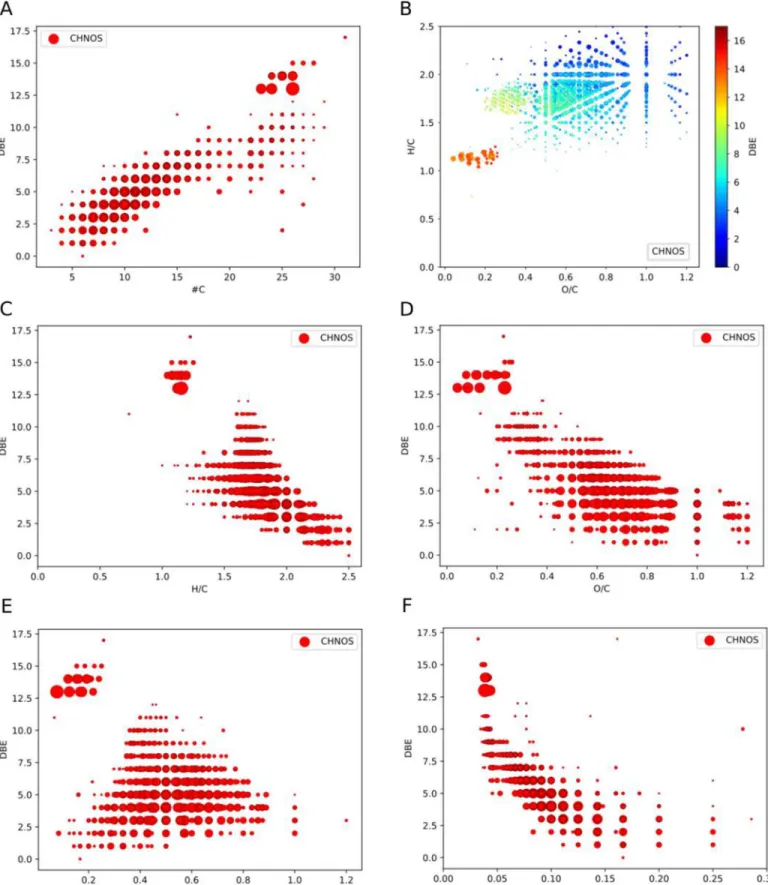
A large number of identified organosulfur compounds are
saturated molecules, having an averaged DBE of 5.6 (Figure
3
).
Nevertheless, DBEs of up to 17 are observed, especially for
high-mass molecules up to 900 amu (Figure
3
and
10
). Compounds
with high DBE have low H/C and low O/C ratios (Figure
12
).
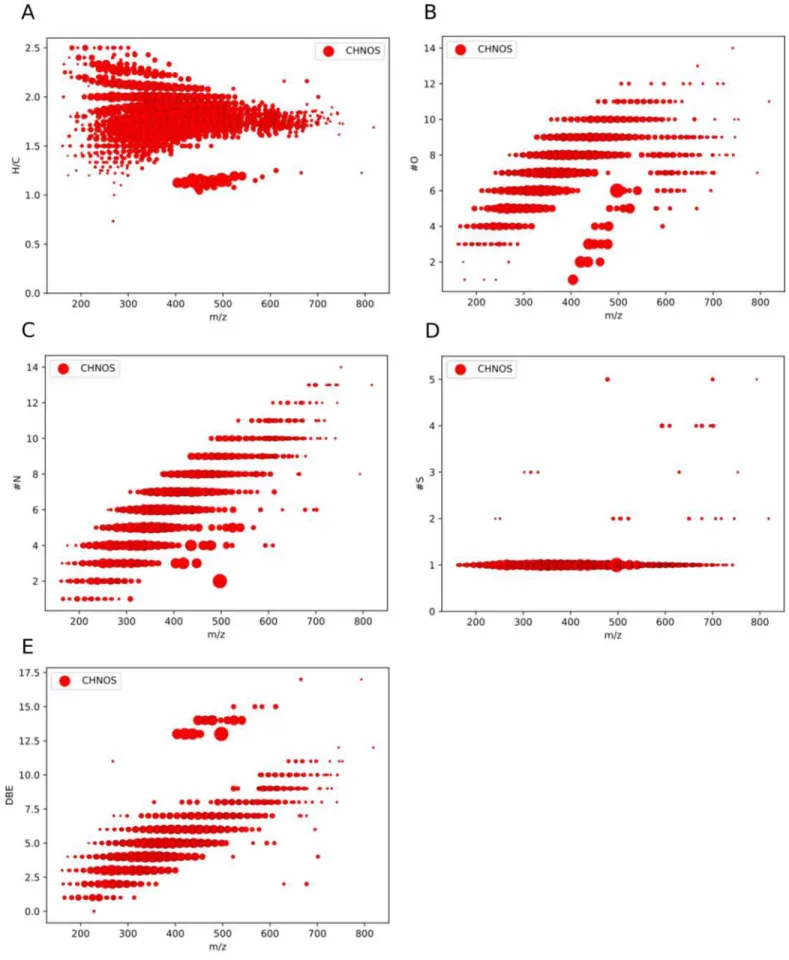
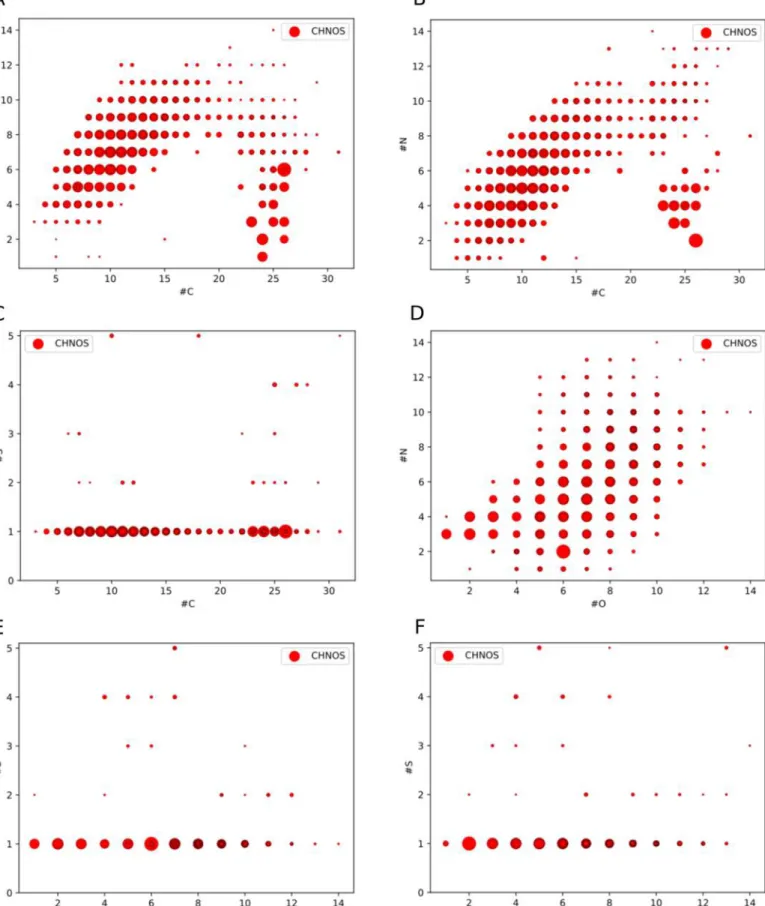
The number of atoms for every element varies for the
identified organosulfur compounds (Figures
4
and
13
). Almost
all organosulfur (CHNOS) compounds bear one sulfur atom
and only a few molecular formulas have up to five S atoms.
The aforementioned high degree of oxygenation is directly
related to the number of oxygen atoms. Most CHNOS formulas
possess eight O atoms. This might be related to the partially
oxidized carbon starting material (CH
3OH, O/C
=1). This
enables the formation of a complex organic molecules with a
oxygen-rich carbon backbone (Theulé et al.
2013
). These
findings are in agreement with results from UV-irradiated ices
(Danger et al.
2016
). Nitrogen counts are also widespread,
ranging mostly from 3 to 10 N atoms per CHNOS molecular
formula. The carbon backbone counts indicate two local
maxima in stability in C counts, for C
=9 and C=25.
Overall, the diversity in CHNOS formulas is related to a
diversity in atom counts therein. The observed large diversity
in atom counts indicates a rich and active sulfur chemistry.
On average, a stoichiometric formula of C
13H
23O
7N
6S is
calculated, having a DBE (average)
=5.6, O/C
(aver-age)=0.6 and H/C (average)=1.8.
As observed from UV-irradiated ices (Danger et al.
2016
),
Ar
7+-irradiated and S
7+-irradiated ices (Figure
9
), CHNO
represents the major chemical family. This implies that
pathways for CHNO formation in all cases are likely based
on radical chemistry (Theulé et al.
2013
; Butscher et al.
2017
).
Among the organosulfur (CHNOS) compounds observed in
S
7+-irradiated residues, almost all of them bear only one sulfur
atom (Figures
4
,
10
, and
13
)
and all organosulfur species bear
nitrogen atoms (only CHNOS and no CHOS compounds got
detected, Figure
9
)
. These findings indicate a selective
mechanism of organosulfur formation that is not well
under-stood yet. The irradiating agent, S
7+or Ar
7+, might act
chemically inert in a first step (similarly as UV photons), by
breaking bonds and triggering radical and ion formation
leading to precursor of complex CHNO compounds. Then
CHNO precursors might selectively react with sulfur
imple-mented in the water matrix forming CHNOS
1species. The low
number of S in organosulfur compounds can be explained by
low S/N and S/C ratios (9E-4) in the implantation zone (see
“SRIM simulations,” Methods Section
2.1
). Thus, the
like-lihood of implanting a sulfur atom into more complex organic
molecules is low. The test of this hypothesis is beyond this
manuscript and is part of ongoing work.
3.3. Astrophysical Implications
Several astrophysical environments reflect parallels with the
here presented experiments.
The icy surface of the Jovian satellite Europa is exposed to a
bombardment of energetic electrons and ions, including sulfur
(Paranicas et al.
2009
)
. This flux is abundant in the 100 keV
range which was used in the experiments presented here. A
calculation, based on flux (Paranicas et al.
2009
), shows that 20
to 30 minutes of exposure to the sulfur beam in the IGLIAS
chamber correspond to a few days on Europa’s surface in terms
of fluence. However, neither methanol nor ammonia have been
detected on Europa (Carlson et al.
2009
)
and the surface
temperature of 80–130 K (Spencer et al.
1999
)
is noticeably
higher than the temperature at which irradiation was performed
in our experiments. Nevertheless, the parallel with Enceladus
allows us to consider the putative presence of both ammonia
and methanol within Europa’s internal ocean (Hodyss et al.
2009
; Waite et al.
2017
); and micrometeoroid bombardment
represents a possible source of organic matter being transported
to its surface (Carlson et al.
2009
). However, as CO
2represents
the dominant form of carbon and the presence of NH
3is
uncertain, one should be cautious about direct implications of
the results discussed here to Europa.
Further from the Sun, trans-Neptunian objects (TNOs) and
KBOs present surface temperatures closer to our experimental
conditions (e.g., 44 K on Orcus: Barucci et al.
2008
). Ammonia
has been detected on several of these objects, e.g., on Charon
(Brown & Calvin
2000
; Grundy et al.
2016
), on Orcus (Barucci
et al.
2008
), or on Quaoar (Jewitt & Luu
2004
). Both ammonia
and methanol are present in comets (Bockelée-Morvan et al.
2004
; Altwegg et al.
2017
)
suggesting their presence in the
material that has formed TNOs/KBOs. These objects are
exposed to irradiation by solar wind including small quantities
of sulfur at about 32 keV/nucleus (derived from the solar wind
velocity; Von Steiger et al.
2000
), an energy comparable to the
one used in the experiments presented here. Using the S/O
ratios measured in the solar wind at 1 au (von Steiger et al.
2010
)
and extrapolating to the flux at 50 au, the fluence used in
our experiments is approximated to be reached in 2 Myr. This
relatively short time (on astronomical timescales) indicates that
icy surfaces of objects such as TNOs/KBOs, possibly rich in
ammonia and methanol, may have endured the same
transformation as our laboratory samples. Interestingly, 2 Myr
is shorter than the predicted time of destruction of ammonia
hydrates by solar wind (Cooper et al.
2004
; Jewitt &
Luu
2004
). Thus, NH
3might still be stable enough to be
further processed by sulfur ion bombardment to putatively form
organosulfur compounds. This time is short enough that the
building blocks of these bodies (e.g., small grains, ice) may
interact with sulfur ions from solar wind before their accretion
(assuming a similar composition of the solar wind at that time).
Table 1
Spectral Features and their Attribution for the Ices Post-irradiation (Figure5) Position (cm−1) Assignment Species Source
2340 νas(CO2) CO2 (Gerakines et al.1994)
2275 ν(NCO)/ ? HOCN/CO2 (Schutte et al.1993; Signorell et al.2006)
2169 ν(NCO) OCN− (Hudson et al.2001; Raunier et al.2003)
2140 CO (Gerakines et al.1996)
2089 ν(CN)and/or ν(13CO) HCN and/or CO (Maki & Blaine1964
)
1750-1700 ν(C=O) H2CO and other aldehydes (Bisschop et al.2007)/(Bossa et al.2009)
1723 ν(C=O) H2CO (Schutte et al.1993)
1689 ν(C=O) HCOOH/NH2COOH? (Bisschop et al.2007)/(Bossa et al.2009)
1585 ν(CO-O) HCOO− (Schutte et al.1999)and ref. therein
1494 δ(CH) H2CO (Schutte et al.1993)
1478 NH+4 (Schutte et al.1999)and ref. therein
1461 NH+4 (Schutte et al.1999)and ref. therein
1387 δ(CH) HCOO−/HCOOH (Schutte et al.1999)and ref. therein
1353 ν(CO-O) HCOO− (Schutte et al.1999)and ref. therein
1304 CH4 (Kerkhof et al.1999)
660 CO2 (Colthup1950)
This is likely applicable to comets as well. The D/H ratio of
comet 67P, for example, indicates a formation at a large
distance from the Sun (Altwegg et al.
2015
). So, ion
implantation would have occurred at low temperatures. It
should be noted that due to their formation beyond the H
2S
snowline, the aforementioned objects (TNOs/KBOs) are likely
to include H
2S, another putative source of sulfur chemistry.
The presence of H
2S is supported by comparisons between
laboratory experiments and IR spectra of KBOs and Trojan
asteroids (Poston et al.
2018
).
It is critical to note that thermal processing might be a factor
driving the formation of complex organosulfur compounds. The
surfaces of a KBO or TNO is unlikely to have undergone such
processes. However, some of these objects could later get closer
to the Sun and lose the more volatile components of their
external layers, as has been suggested for Ceres (De Sanctis et al.
2015
), undergoing a more modest thermal processing than in our
experiments. On the other hand, we may also consider thermal
processing of the interior of these objects, combined with the
possibility that most of the ice, not only on the surface, has been
implanted with sulfur before the object accreted. Accretional
heat and radiogenic heating could have provided thermal
processing to relatively high temperatures, especially in the
larger objects such as Pluto, Charon, or Triton. In the case of
Triton tidal heating may also have been a contributor (Ross &
Schubert
1990
). Contemporary cryovolcanic activity (Jewitt &
Luu
2004
; Cook et al.
2007
; Desch et al.
2009
)
could then bring
possible organosulfur molecules to the surface. Future missions
to KBO/TNOs, comets, or even Neptune (if observations of
Triton are included) could uncover these compounds.
4. Conclusions
The formation of complex organosulfur molecules by sulfur
ion bombardment within simple, realistic ice analogs was
tested. H
2O, CH
3OH, and NH
3ices (2:1:1) were irradiated with
S
7+ions at 105 keV at GANIL, Large Heavy Ion National
Accelerator facilities in Caen, France. Sulfur-irradiated samples
Figure 1.FT-ICR-MS revealed the detection of organosulfur compounds. (A) Mass spectra show a large number of m/z signals over broad mass range, from 150 to 700 amu (atomic mass unit), for both the Ar7+- and S7+-irradiated ice sample. Over 1100 unique organosulfur signals (CHNOS) are distributed over that mass range
as well (Figure10, Table3). The “CHNOS only” spectrum has been reconstructed from experimental data (only CHNOS-corresponding m/z values are plotted). Organosulfur signals were absent in the Ar reference spectrum. (B) A zoomed-in representation shows repetition of signal patterns. (C, D) Detailed nominal mass spectra of m/z=248 and m/z=438 highlight the need for ultra-high-mass resolution and ultra-high-mass accuracy to enable the unambiguous differentiation of CHNOS molecular formulas in the S7+-irradiated sample from non-sulfur-bearing ones in the Ar7+-irradiated sample.
(S
7+ions) were compared to a reference sample which was
processed similarly with argon (Ar
7+ions). Residues formed
from ice processing were then analyzed by
ultra-high-resolution mass spectrometry (FT-ICR-MS).
We unambiguously detected organosulfur compounds within
sulfur-bombarded ices. Over 1100 organosulfur (CHNOS)
molecular formulas (12% of all assigned signals) were
observed within a broad mass range, from 100 to 900 amu
(Figure
10
, Table
3
). On average, a stoechiometric formula of
C
13H
23O
7N
6S is calculated, having an DBE (average)=5.6,
O/C (average)=0.6 and H/C (average)=1.8.
There are multiple instances in the outer solar system in which
water ice with methanol and ammonia could be undergoing or
have undergone sulfur implantation. These include icy moons,
KBOs/TNOs/comets, and their original building blocks. The
experiments presented here indicate that with later thermal
processing, these objects could feature a large diversity of
complex organosulfur molecules such as the ones detected in the
present work. These compounds could have participated in
prebiotic chemistry and could be accessible to detection by
future space missions performing in situ measurements.
This work has been funded by CNRS (Programme National
de Planétologie, P.N.P, INSU), Programme de Physique et
Chimie du Milieu Interstellaire (PCMI, INSU), and the Centre
National d’Etudes Spatiales (CNES, exobiology program). It
was also supported by the French Agence Nationale de la
Recherche (VAHIIA grant ANR-12-JS08-0001 and RAHIA
SSOM grant ANR-16-CE29-0015) and from the Excellence
Initiative of Aix-Marseille University—A
*MIDEX, a French
“Investissements d’Avenir programme.”
Appendix A
IR Spectra
Supplementary figures and tables regarding the IR analysis.
In the following, details on IR analysis are given. Spectra of
both ices at 10 K (Figure
5
, Table
1
, Figure
6
)
and of residues
at 300 K (Figure
7
, Table
2
)
are shown.
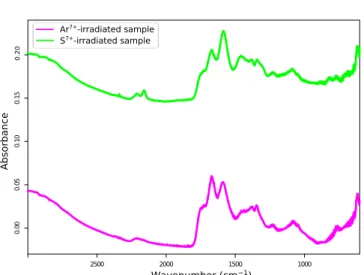
IR spectrum of ices at 10 K (before and after irradiation).
A
comparison between the spectra of the samples at 10 K after
irradiation (Figure
5
)
shows no significant difference in the
features present between the argon-irradiated sample (Ar
7+ions) and the sulfur-irradiated sample (S
7+ions). In addition to
the expected features of remaining water, methanol and
ammonia, the spectra show carbon monoxide and dioxide,
formaldehyde, and ammonium ions. Some other features are
attributable to nitrogenated molecules or ions such as HCN or
OCN
−. These products are consistent with previous
experi-ments on these type of ices.
IR spectrum of the residues at 300 K (after irradiation).
A
comparison between the spectra of the samples at 300 K
(refractory residues) is given in Figure
7
. Both spectra show
similar IR features, assigned to formiate or methanoate ions and
to chemical functions such as amides, carboxylic acids, imines,
and alcanes, consistent with previous studies (Table
2
). Of note,
in the sulfur-irradiated sample (S
7+ions), the presence of CO
2and OCN
−features, more pronounced than in the
argon-irradiated sample (argon-argon-irradiated sample; Ar
7+ions), indicates
Figure 2.van Krevelen diagram (H/C against O/C) of organosulfur (CHNOS) compounds reveals information on different chemical families. Chemical compositions can be grouped according to the degree of unsaturation and relative oxygen amounts. The plotted CHNOS data correspond to m/z signals which are uniquely present in the S7+-irradiated ice but not in the Ar7+
-irradiated ice sample. The bubble size scales with mass spectrometric intensity.
Figure 3.Frequency distribution of organosulfur (CHNOS) compounds as a function of DBE, double bond equivalent. The detected organosulfur compounds have an averaged DBE of 5.6, representing a significant amount of saturated molecules.
Figure 4.Frequency distribution of the number of molecular formulas as a function of the corresponding number of C, H, N, O, and S atoms in a CHNOS molecular formula. The observed large diversity in atom counts indicates a rich and active sulfur chemistry.
a trapping of these molecules in the residue. We note that the
sulfur-irradiated sample does not appear to display the features
of sulfuric acid seen in previous experiments involving sulfur in
water ice (Moore et al.
2007
; Strazzulla et al.
2007
). We attribute
this to a much lower sulfur amount present in our experiments
(
each layer was subjected to a fluence of ≈1×10
14cm
−2, to be
compared to 3×10
16cm
−2in the experiments of Strazzulla
et al.
2007
), along with the presence of NH
3that makes sulfuric
acid features at 1100 cm
−1difficult to distinguish. Other
possible explanations include the lower temperature of our
experiments (although the spectras during heating of the sample,
not shown here, do not appear to present sulfuric acid features)
or that in the presence of methanol and ammonia sulfur could be
mostly incorporated into other products than sulfuric acid.
Appendix B
FT-ICR-MS Data Analysis
Supplementary figures regarding the FT-ICR-MS analysis.
In
the following, details on data mining of experimental FT-ICR-MS
data are given. First, error analysis on formula assignment is
reported (Figure
8
). Then, distribution of organosulfur (CHNOS)
compounds in contrast to CHO and CHNO compounds highlights
Figure 5.IR spectra of ices (H2O:CH3OH:NH3ices, 2:1:1), taken at 10 K, one
before irradiation (black), one after irradiation by argon (Ar7+ions, magenta),
and one after sulfur (S7+ions, green), before warming up. Note that in the
pre-irradiation case, only one layer has been deposited, while argon-irradiated and sulfur-irradiated represent 15 and 10 layers, respectively, which explains the difference in absorbance in water, methanol, and ammonia features.
Figure 6.Spectra of the first ice layer at 10 K, irradiated with S7+. From top to
bottom: no irradiation, 3.3, 6.5, 16.3, 32.5, and 65.0 MGy. The features of CO2
at 2340 cm−1, of CO at 2140 cm−1, CH
4at 1304 cm−1, and OCN−at 2169
cm−1are evolving with irradiation.
Figure 7.IR spectra of the residue, taken at 300 K, after irradiation by argon (Ar7+ions, magenta) and sulfur (S7+ions, green). The residue resulted from
irradiated H2O:CH3OH:NH3ices (2:1:1).
Table 2
Spectral Features and Their Attribution for the Residue at 300 K (Figure7)
Position (cm−1) Assignment Species Source Difference between Spectras?
2338 ν(CO) CO2 (Gerakines et al.1996) More pronounced in S-irr
2214 Alkynes? (Colthup1950)
2160 ν(NCO) OCN− (Raunier et al.2003) More pronounced in S-irr
1726 Carboxylic acids/aldehydes/imines (Colthup1950)
1669 ν(C=O) Amides/carbamic acid (Muñoz Caro & Schutte2003) 1589 ν(COO−) HCOO− (Schutte et al.1999)
1457 ν(CH) Alkanes (Colthup1950), (Fresneau et al.2017) 1376 ν(CH) HCOO− (Schutte et al.1999)
1341 ν(COO−) HCOO− (Schutte et al.1999)
1300 ?
1228 ?
1084 Ethylene glycol (Hudson et al.2005) 1050 Ethylene glycol (Hudson et al.2005) 887 Ethylene glycol (Hudson et al.2005)
820 ?
767 HCOO−NH4+? (Muñoz Caro & Schutte2003)
Table 3
List of Detected Organosulfur (CHNOS) Molecular Formulas
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
147.05974 0.119 C5H12N2OS- 5.05E+06 2.4 0.2 161.00264 −0.016 C4H6N2O3S- 8.10E+06 1.5 0.75 163.0183 −0.077 C4H8N2O3S- 9.97E+06 2 0.75 164.01356 −0.143 C3H7N3O3S- 7.28E+06 2.33 1 166.01796 −0.039 C4H9NO4S- 1.94E+07 2.25 1 172.0186 0.125 C5H7N3O2S- 3.70E+06 1.4 0.4 173.00264 −0.014 C5H6N2O3S- 4.23E+06 1.2 0.6 175.01831 −0.129 C5H8N2O3S- 1.36E+07 1.6 0.6 175.06592 −0.083 C5H12N4OS- 6.07E+06 2.4 0.2 176.99757 −0.099 C4H6N2O4S- 1.27E+07 1.5 1 178.01797 −0.093 C5H9NO4S- 7.47E+06 1.8 0.8 178.0292 −0.076 C4H9N3O3S- 1.37E+07 2.25 0.75 179.01321 −0.042 C4H8N2O4S- 2.31E+07 2 1 180.03361 −0.036 C5H11NO4S- 1.19E+07 2.2 0.8 181.02887 −0.097 C4H10N2O4S- 2.72E+07 2.5 1 188.01354 −0.019 C5H7N3O3S- 1.07E+07 1.4 0.6 189.03394 −0.013 C6H10N2O3S- 1.16E+07 1.67 0.5 189.9928 0.008 C4H5N3O4S- 6.09E+06 1.25 1 190.02921 −0.124 C5H9N3O3S- 1.47E+07 1.8 0.6 191.01322 −0.092 C5H8N2O4S- 3.06E+07 1.6 0.8 192.00848 −0.148 C4H7N3O4S- 1.64E+07 1.75 1 192.03364 −0.19 C6H11NO4S- 7.62E+06 1.83 0.67 192.04484 −0.018 C5H11N3O3S- 1.20E+07 2.2 0.6 193.02887 −0.091 C5H10N2O4S- 3.72E+07 2 0.8 193.0401 −0.075 C4H10N4O3S- 9.04E+06 2.5 0.75 193.06529 −0.272 C6H14N2O3S- 4.64E+06 2.33 0.5 194.01289 −0.111 C5H9NO5S- 1.62E+07 1.8 1 194.02412 −0.095 C4H9N3O4S- 5.39E+07 2.25 1 195.04452 −0.09 C5H12N2O4S- 3.78E+07 2.4 0.8 196.02854 −0.11 C5H11NO5S- 3.29E+07 2.2 1 201.03395 −0.062 C7H10N2O3S- 4.86E+06 1.43 0.43 202.0292 −0.067 C6H9N3O3S- 1.47E+07 1.5 0.5 203.04961 −0.111 C7H12N2O3S- 6.61E+06 1.71 0.43 204.00843 0.105 C5H7N3O4S- 1.67E+07 1.4 0.8 205.02886 −0.037 C6H10N2O4S- 2.34E+07 1.67 0.67 205.04011 −0.119 C5H10N4O3S- 9.80E+06 2 0.6 206.0241 0.007 C5H9N3O4S- 2.96E+07 1.8 0.8 206.06049 −0.017 C6H13N3O3S- 8.91E+06 2.17 0.5 207.01937 −0.094 C4H8N4O4S- 2.44E+07 2 1 207.0445 0.012 C6H12N2O4S- 2.88E+07 2 0.67 207.05574 −0.022 C5H12N4O3S- 1.43E+07 2.4 0.6 208.03976 −0.041 C5H11N3O4S- 4.63E+07 2.2 0.8 209.02378 −0.06 C5H10N2O5S- 5.78E+07 2 1 209.03499 0.05 C4H10N4O4S- 3.81E+07 2.5 1 209.06013 0.108 C6H14N2O4S- 1.80E+07 2.33 0.67 210.00776 0.112 C5H9NO6S- 1.64E+07 1.8 1.2 210.04417 −0.007 C6H13NO5S- 1.79E+07 2.17 0.83 211.03941 0.036 C5H12N2O5S- 3.94E+07 2.4 1 212.02343 0.017 C5H11NO6S- 2.41E+07 2.2 1.2 216.04484 −0.016 C7H11N3O3S- 1.26E+07 1.57 0.43 216.11761 −0.016 C9H19N3OS- 4.44E+06 2.11 0.11 217.00368 0.094 C5H6N4O4S- 4.80E+06 1.2 0.8 217.02889 −0.173 C7H10N2O4S- 9.44E+06 1.43 0.57 217.04011 −0.113 C6H10N4O3S- 9.67E+06 1.67 0.5 218.02409 0.053 C6H9N3O4S- 2.30E+07 1.5 0.67 218.06049 −0.016 C7H13N3O3S- 1.57E+07 1.86 0.43 219.00813 −0.057 C6H8N2O5S- 9.04E+06 1.33 0.83 219.01934 0.048 C5H8N4O4S- 1.42E+07 1.6 0.8 219.04449 0.057 C7H12N2O4S- 1.94E+07 1.71 0.57 219.05572 0.071 C6H12N4O3S- 1.04E+07 2 0.5 220.00337 −0.016 C5H7N3O5S- 1.80E+07 1.4 1 220.02853 −0.052 C7H11NO5S- 7.25E+06 1.57 0.71 220.03975 0.007 C6H11N3O4S- 4.60E+07 1.83 0.67 221.02377 −0.011 C6H10N2O5S- 3.28E+07 1.67 0.83
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
221.03499 0.048 C5H10N4O4S- 5.46E+07 2 0.8 221.06011 0.192 C7H14N2O4S- 1.78E+07 2 0.57 222.01899 0.119 C5H9N3O5S- 4.42E+07 1.8 1 222.04418 −0.052 C7H13NO5S- 1.10E+07 1.86 0.71 222.05539 0.052 C6H13N3O4S- 4.34E+07 2.17 0.67 223.00299 0.191 C5H8N2O6S- 1.79E+07 1.6 1.2 223.03942 −0.011 C6H12N2O5S- 4.68E+07 2 0.83 223.05063 0.092 C5H12N4O4S- 3.68E+07 2.4 0.8 224.02344 −0.029 C6H11NO6S- 1.44E+07 1.83 1 224.03464 0.118 C5H11N3O5S- 6.53E+07 2.2 1 224.05978 0.172 C7H15NO5S- 1.06E+07 2.14 0.71 225.01865 0.144 C5H10N2O6S- 6.43E+07 2 1.2 225.05505 0.078 C6H14N2O5S- 5.44E+07 2.33 0.83 226.03907 0.06 C6H13NO6S- 2.77E+07 2.17 1 227.03433 0.011 C5H12N2O6S- 1.80E+07 2.4 1.2 228.05475 −0.072 C6H15NO6S- 9.55E+06 2.5 1 229.04007 0.068 C7H10N4O3S- 9.27E+06 1.43 0.43 230.02405 0.224 C7H9N3O4S- 1.00E+07 1.29 0.57 230.06052 −0.146 C8H13N3O3S- 1.03E+07 1.63 0.38 231.01933 0.089 C6H8N4O4S- 1.11E+07 1.33 0.67 231.03058 0.015 C5H8N6O3S- 2.75E+07 1.6 0.6 231.04448 0.097 C8H12N2O4S- 7.78E+06 1.5 0.5 231.05569 0.197 C7H12N4O3S- 1.60E+07 1.71 0.43 232.03974 0.05 C7H11N3O4S- 3.33E+07 1.57 0.57 233.02376 0.032 C7H10N2O5S- 1.59E+07 1.43 0.71 233.03498 0.088 C6H10N4O4S- 2.44E+07 1.67 0.67 233.06016 −0.032 C8H14N2O4S- 1.42E+07 1.75 0.5 233.07136 0.109 C7H14N4O3S- 1.19E+07 2 0.43 234.01899 0.113 C6H9N3O5S- 3.38E+07 1.5 0.83 234.03026 −0.045 C5H9N5O4S- 8.38E+06 1.8 0.8 234.05539 0.049 C7H13N3O4S- 5.57E+07 1.86 0.57 235.00307 −0.16 C6H8N2O6S- 7.38E+06 1.33 1 235.01425 0.066 C5H8N4O5S- 1.46E+07 1.6 1 235.03941 0.032 C7H12N2O5S- 3.82E+07 1.71 0.71 235.05064 0.045 C6H12N4O4S- 7.32E+07 2 0.67 235.07582 −0.074 C8H16N2O4S- 8.59E+06 2 0.5 235.087 0.151 C7H16N4O3S- 4.47E+06 2.29 0.43 235.99828 0.006 C5H7N3O6S- 7.28E+06 1.4 1.2 236.02347 −0.155 C7H11NO6S- 7.20E+06 1.57 0.86 236.03466 0.028 C6H11N3O5S- 8.58E+07 1.83 0.83 236.04588 0.083 C5H11N5O4S- 2.02E+07 2.2 0.8 236.07106 −0.036 C7H15N3O4S- 3.54E+07 2.14 0.57 237.01868 0.011 C6H10N2O6S- 3.75E+07 1.67 1 237.02988 0.15 C5H10N4O5S- 7.56E+07 2 1 237.05504 0.116 C7H14N2O5S- 5.48E+07 2 0.71 237.06629 0.044 C6H14N4O4S- 6.48E+07 2.33 0.67 238.01389 0.174 C5H9N3O6S- 3.65E+07 1.8 1.2 238.03904 0.183 C7H13NO6S- 1.27E+07 1.86 0.86 238.0503 0.069 C6H13N3O5S- 9.16E+07 2.17 0.83 239.03432 0.052 C6H12N2O6S- 7.70E+07 2 1 239.04555 0.065 C5H12N4O5S- 4.86E+07 2.4 1 239.07071 0.031 C7H16N2O5S- 5.86E+07 2.29 0.71 240.01836 −0.048 C6H11NO7S- 1.70E+07 1.83 1.17 240.02956 0.09 C5H11N3O6S- 3.77E+07 2.2 1.2 240.04489 −0.223 C9H11N3O3S- 5.39E+06 1.22 0.33 240.0547 0.14 C7H15NO6S- 1.42E+07 2.14 0.86 240.06597 −0.015 C6H15N3O5S- 2.76E+07 2.5 0.83 241.02234 −0.056 C8H10N4OS2- 4.76E+06 1.25 0.13 241.04008 0.023 C8H10N4O3S- 6.84E+06 1.25 0.38 241.04995 0.135 C6H14N2O6S- 2.96E+07 2.33 1 242.03399 0.035 C6H13NO7S- 1.03E+07 2.17 1.17 242.06045 0.151 C9H13N3O3S- 5.57E+06 1.44 0.33 243.05571 0.105 C8H12N4O3S- 1.13E+07 1.5 0.38 244.03974 0.047 C8H11N3O4S- 1.48E+07 1.38 0.5
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
245.02379 −0.092 C8H10N2O5S- 6.39E+06 1.25 0.63 245.03498 0.084 C7H10N4O4S- 2.14E+07 1.43 0.57 245.07132 0.267 C8H14N4O3S- 1.18E+07 1.75 0.38 246.01901 0.026 C7H9N3O5S- 1.02E+07 1.29 0.71 246.05538 0.087 C8H13N3O4S- 3.46E+07 1.63 0.5 247.01423 0.144 C6H8N4O5S- 8.15E+06 1.33 0.83 247.03941 0.03 C8H12N2O5S- 1.65E+07 1.5 0.63 247.05067 −0.079 C7H12N4O4S- 3.83E+07 1.71 0.57 247.08698 0.225 C8H16N4O3S- 9.11E+06 2 0.38 248.03465 0.067 C7H11N3O5S- 4.23E+07 1.57 0.71 248.04587 0.119 C6H11N5O4S- 2.65E+07 1.83 0.67 248.07102 0.127 C8H15N3O4S- 4.65E+07 1.88 0.5 248.08227 0.058 C7H15N5O3S- 2.60E+07 2.14 0.43 249.01869 −0.03 C7H10N2O6S- 1.19E+07 1.43 0.86 249.02989 0.102 C6H10N4O5S- 3.71E+07 1.67 0.83 249.05506 0.03 C8H14N2O5S- 2.88E+07 1.75 0.63 249.06627 0.122 C7H14N4O4S- 7.23E+07 2 0.57 250.01393 0.006 C6H9N3O6S- 2.58E+07 1.5 1 250.02516 0.018 C5H9N5O5S- 1.22E+07 1.8 1 250.05032 −0.014 C7H13N3O5S- 1.06E+08 1.86 0.71 250.06153 0.078 C6H13N5O4S- 3.95E+07 2.17 0.67 250.99791 0.149 C6H8N2O7S- 6.73E+06 1.33 1.17 251.00918 0.002 C5H8N4O6S- 9.77E+06 1.6 1.2 251.01655 0.094 C7H12N2O4S2- 6.54E+06 1.71 0.57 251.03433 0.01 C7H12N2O6S- 4.57E+07 1.71 0.86 251.04555 0.062 C6H12N4O5S- 1.12E+08 2 0.83 251.0707 0.07 C8H16N2O5S- 3.09E+07 2 0.63 251.08195 0.002 C7H16N4O4S- 5.37E+07 2.29 0.57 252.0184 −0.204 C7H11NO7S- 8.13E+06 1.57 1 252.02958 0.006 C6H11N3O6S- 6.87E+07 1.83 1 252.04078 0.137 C5H11N5O5S- 3.73E+07 2.2 1 252.05479 −0.224 C8H15NO6S- 8.80E+06 1.88 0.75 252.06595 0.065 C7H15N3O5S- 8.64E+07 2.14 0.71 253.0136 −0.01 C6H10N2O7S- 3.40E+07 1.67 1.17 253.02479 0.16 C5H10N4O6S- 4.40E+07 2 1.2 253.04997 0.049 C7H14N2O6S- 5.25E+07 2 0.86 253.06121 0.022 C6H14N4O5S- 8.22E+07 2.33 0.83 253.08636 0.03 C8H18N2O5S- 1.43E+07 2.25 0.63 254.03396 0.152 C7H13NO7S- 1.26E+07 1.86 1 254.04523 0.006 C6H13N3O6S- 5.19E+07 2.17 1 255.02925 −0.01 C6H12N2O7S- 3.03E+07 2 1.17 255.04046 0.08 C5H12N4O6S- 2.33E+07 2.4 1.2 255.05579 −0.214 C9H12N4O3S- 8.57E+06 1.33 0.33 256.03976 −0.033 C9H11N3O4S- 6.68E+06 1.22 0.44 256.04966 −0.045 C7H15NO7S- 1.71E+07 2.14 1 256.06087 0.045 C6H15N3O6S- 1.39E+07 2.5 1 257.03503 −0.115 C8H10N4O4S- 8.53E+06 1.25 0.5 257.04494 −0.165 C6H14N2O7S- 1.06E+07 2.33 1.17 258.05538 0.083 C9H13N3O4S- 1.65E+07 1.44 0.44 258.06665 −0.06 C8H13N5O3S- 1.41E+07 1.63 0.38 259.05066 −0.037 C8H12N4O4S- 3.38E+07 1.5 0.5 260.03465 0.063 C8H11N3O5S- 2.06E+07 1.38 0.63 260.04588 0.075 C7H11N5O4S- 2.14E+07 1.57 0.57 260.07107 −0.071 C9H15N3O4S- 2.71E+07 1.67 0.44 260.08229 −0.021 C8H15N5O3S- 1.53E+07 1.88 0.38 261.02994 −0.094 C7H10N4O5S- 2.29E+07 1.43 0.71 261.04117 −0.082 C6H10N6O4S- 5.57E+06 1.67 0.67 261.05505 0.067 C9H14N2O5S- 1.45E+07 1.56 0.56 261.06632 −0.075 C8H14N4O4S- 4.95E+07 1.75 0.5 262.01391 0.082 C7H9N3O6S- 1.12E+07 1.29 0.86 262.05034 −0.09 C8H13N3O5S- 5.19E+07 1.63 0.63 262.06155 −0.002 C7H13N5O4S- 4.75E+07 1.86 0.57 262.08675 −0.185 C9H17N3O4S- 2.69E+07 1.89 0.44 263.04555 0.059 C7H12N4O5S- 7.66E+07 1.71 0.71
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
263.07069 0.105 C9H16N2O5S- 1.92E+07 1.78 0.56 263.08197 −0.074 C8H16N4O4S- 4.88E+07 2 0.5 264.0296 −0.07 C7H11N3O6S- 4.80E+07 1.57 0.86 264.04081 0.017 C6H11N5O5S- 3.89E+07 1.83 0.83 264.06599 −0.089 C8H15N3O5S- 9.44E+07 1.88 0.63 264.07717 0.112 C7H15N5O4S- 3.95E+07 2.14 0.57 265.02484 −0.036 C6H10N4O6S- 3.96E+07 1.67 1 265.05 −0.066 C8H14N2O6S- 4.66E+07 1.75 0.75 265.06122 −0.017 C7H14N4O5S- 1.29E+08 2 0.71 265.08641 −0.16 C9H18N2O5S- 1.68E+07 2 0.56 265.09761 −0.036 C8H18N4O4S- 1.35E+07 2.25 0.5 266.00884 0.024 C6H9N3O7S- 1.83E+07 1.5 1.17 266.02012 −0.152 C5H9N5O6S- 8.59E+06 1.8 1.2 266.03398 0.07 C8H13NO7S- 1.00E+07 1.63 0.88 266.04522 0.043 C7H13N3O6S- 1.22E+08 1.86 0.86 266.05648 −0.058 C6H13N5O5S- 7.34E+07 2.17 0.83 266.08162 −0.013 C8H17N3O5S- 5.40E+07 2.13 0.63 267.02927 −0.084 C7H12N2O7S- 4.16E+07 1.71 1 267.04049 −0.036 C6H12N4O6S- 9.23E+07 2 1 267.05171 0.013 C5H12N6O5S- 1.23E+07 2.4 1 267.06564 −0.028 C8H16N2O6S- 4.85E+07 2 0.75 267.07686 0.021 C7H16N4O5S- 1.00E+08 2.29 0.71 268.0245 −0.013 C6H11N3O7S- 4.58E+07 1.83 1.17 268.03573 −0.002 C5H11N5O6S- 2.24E+07 2.2 1.2 268.04385 −0.285 C15H11NO2S- 7.57E+06 0.73 0.13 268.04974 −0.341 C8H15NO7S- 1.24E+07 1.88 0.88 268.06088 0.006 C7H15N3O6S- 7.93E+07 2.14 0.86 268.07208 0.129 C6H15N5O5S- 1.87E+07 2.5 0.83 269.04493 −0.121 C7H14N2O7S- 3.69E+07 2 1 269.05613 0.002 C6H14N4O6S- 4.46E+07 2.33 1 269.08132 −0.139 C8H18N2O6S- 3.03E+07 2.25 0.75 270.01906 −0.161 C9H9N3O5S- 5.82E+06 1 0.56 270.02891 0.013 C7H13NO8S- 1.10E+07 1.86 1.14 270.03024 0.035 C8H9N5O4S- 5.93E+06 1.13 0.5 270.04015 −0.013 C6H13N3O7S- 2.59E+07 2.17 1.17 270.06662 0.054 C9H13N5O3S- 7.01E+06 1.44 0.33 270.07653 0.006 C7H17N3O6S- 1.76E+07 2.43 0.86 271.05068 −0.109 C9H12N4O4S- 1.57E+07 1.33 0.44 271.06058 −0.12 C7H16N2O7S- 1.31E+07 2.29 1 272.03472 −0.197 C9H11N3O5S- 7.77E+06 1.22 0.56 272.04589 0.035 C8H11N5O4S- 1.33E+07 1.38 0.5 272.05584 −0.16 C6H15N3O7S- 8.60E+06 2.5 1.17 272.08233 −0.167 C9H15N5O3S- 1.48E+07 1.67 0.33 273.0663 0.002 C9H14N4O4S- 3.59E+07 1.56 0.44 274.05033 −0.049 C9H13N3O5S- 2.47E+07 1.44 0.56 274.06157 −0.075 C8H13N5O4S- 3.09E+07 1.63 0.5 275.03433 0.009 C9H12N2O6S- 9.40E+06 1.33 0.67 275.04561 −0.162 C8H12N4O5S- 4.04E+07 1.5 0.63 275.08197 −0.071 C9H16N4O4S- 4.35E+07 1.78 0.44 276.0296 −0.067 C8H11N3O6S- 1.83E+07 1.38 0.75 276.04083 −0.056 C7H11N5O5S- 2.41E+07 1.57 0.71 276.06596 0.024 C9H15N3O5S- 4.87E+07 1.67 0.56 276.07723 −0.11 C8H15N5O4S- 5.54E+07 1.88 0.5 277.02486 −0.106 C7H10N4O6S- 1.66E+07 1.43 0.86 277.05 −0.063 C9H14N2O6S- 1.79E+07 1.56 0.67 277.06125 −0.125 C8H14N4O5S- 9.00E+07 1.75 0.63 277.09767 −0.251 C9H18N4O4S- 2.75E+07 2 0.44 278.00881 0.131 C7H9N3O7S- 7.55E+06 1.29 1 278.04525 −0.067 C8H13N3O6S- 6.21E+07 1.63 0.75 278.05648 −0.056 C7H13N5O5S- 7.89E+07 1.86 0.71 278.08164 −0.085 C9H17N3O5S- 6.45E+07 1.89 0.56 278.09289 −0.146 C8H17N5O4S- 4.21E+07 2.13 0.5 279.02925 −0.009 C8H12N2O7S- 1.32E+07 1.5 0.88 279.0405 −0.07 C7H12N4O6S- 8.68E+07 1.71 0.86
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
279.05172 −0.023 C6H12N6O5S- 1.48E+07 2 0.83 279.06565 −0.063 C9H16N2O6S- 3.49E+07 1.78 0.67 279.0769 −0.124 C8H16N4O5S- 1.04E+08 2 0.63 280.02449 0.023 C7H11N3O7S- 3.98E+07 1.57 1 280.03577 −0.145 C6H11N5O6S- 3.09E+07 1.83 1 280.06087 0.041 C8H15N3O6S- 1.27E+08 1.88 0.75 280.07211 0.016 C7H15N5O5S- 8.59E+07 2.14 0.71 280.0973 −0.12 C9H19N3O5S- 3.21E+07 2.11 0.56 281.01977 −0.087 C6H10N4O7S- 1.99E+07 1.67 1.17 281.04486 0.133 C8H14N2O7S- 4.11E+07 1.75 0.88 281.05616 −0.105 C7H14N4O6S- 1.20E+08 2 0.86 281.06739 −0.094 C6H14N6O5S- 3.28E+07 2.33 0.83 281.08126 0.08 C9H18N2O6S- 3.23E+07 2 0.67 281.09252 −0.016 C8H18N4O5S- 6.69E+07 2.25 0.63 282.04016 −0.048 C7H13N3O7S- 6.44E+07 1.86 1 282.05141 −0.108 C6H13N5O6S- 4.88E+07 2.17 1 282.07652 0.041 C8H17N3O6S- 7.61E+07 2.13 0.75 283.02412 0.15 C7H12N2O8S- 2.22E+07 1.71 1.14 283.0354 −0.016 C6H12N4O7S- 4.95E+07 2 1.17 283.06055 −0.009 C8H16N2O7S- 3.41E+07 2 0.88 283.07176 0.072 C7H16N4O6S- 5.92E+07 2.29 0.86 283.09694 −0.026 C9H20N2O6S- 9.89E+06 2.22 0.67 284.03462 0.164 C10H11N3O5S- 5.53E+06 1.1 0.5 284.04458 −0.058 C8H15NO8S- 8.69E+06 1.88 1 284.05583 −0.118 C7H15N3O7S- 3.61E+07 2.14 1 284.06702 0.033 C6H15N5O6S- 1.90E+07 2.5 1 284.09215 0.111 C8H19N3O6S- 1.23E+07 2.38 0.75 285.0398 0.044 C7H14N2O8S- 1.52E+07 2 1.14 285.05105 −0.016 C6H14N4O7S- 2.52E+07 2.33 1.17 285.06635 −0.174 C10H14N4O4S- 1.63E+07 1.4 0.4 285.07625 −0.184 C8H18N2O7S- 1.27E+07 2.25 0.88 286.05036 −0.152 C10H13N3O5S- 9.31E+06 1.3 0.5 287.04562 −0.19 C9H12N4O5S- 1.91E+07 1.33 0.56 287.09314 0.152 C9H16N6O3S- 1.50E+07 1.78 0.33 288.02963 −0.168 C9H11N3O6S- 9.35E+06 1.22 0.67 288.04083 −0.054 C8H11N5O5S- 1.54E+07 1.38 0.63 288.06594 0.092 C10H15N3O5S- 2.24E+07 1.5 0.5 288.07722 −0.071 C9H15N5O4S- 4.31E+07 1.67 0.44 289.0612 0.054 C9H14N4O5S- 5.63E+07 1.56 0.56 289.07249 −0.144 C8H14N6O4S- 3.09E+07 1.75 0.5 290.04524 −0.029 C9H13N3O6S- 3.13E+07 1.44 0.67 290.05648 −0.053 C8H13N5O5S- 5.23E+07 1.63 0.63 290.08161 0.022 C10H17N3O5S- 3.86E+07 1.7 0.5 290.09287 −0.071 C9H17N5O4S- 5.15E+07 1.89 0.44 291.04049 −0.033 C8H12N4O6S- 4.39E+07 1.5 0.75 291.05176 −0.16 C7H12N6O5S- 1.91E+07 1.71 0.71 291.07685 0.053 C9H16N4O5S- 9.15E+07 1.78 0.56 291.08808 0.064 C8H16N6O4S- 3.79E+07 2 0.5 292.02451 −0.046 C8H11N3O7S- 1.60E+07 1.38 0.88 292.03573 −0.002 C7H11N5O6S- 2.71E+07 1.57 0.86 292.0609 −0.063 C9H15N3O6S- 6.86E+07 1.67 0.67 292.0721 0.05 C8H15N5O5S- 1.06E+08 1.88 0.63 292.09728 −0.046 C10H19N3O5S- 3.48E+07 1.9 0.5 292.10848 0.067 C9H19N5O4S- 2.78E+07 2.11 0.44 293.04488 0.06 C9H14N2O7S- 1.75E+07 1.56 0.78 293.05614 −0.032 C8H14N4O6S- 1.13E+08 1.75 0.75 293.06741 −0.159 C7H14N6O5S- 4.95E+07 2 0.71 293.08128 0.009 C10H18N2O6S- 1.88E+07 1.8 0.6 293.0925 0.053 C9H18N4O5S- 7.88E+07 2 0.56 294.04017 −0.08 C8H13N3O7S- 5.26E+07 1.63 0.88 294.05141 −0.104 C7H13N5O6S- 9.19E+07 1.86 0.86 294.07654 −0.029 C9H17N3O6S- 9.49E+07 1.89 0.67 294.0878 −0.121 C8H17N5O5S- 8.88E+07 2.13 0.63 295.03541 −0.049 C7H12N4O7S- 5.34E+07 1.71 1
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
295.0466 0.097 C6H12N6O6S- 1.65E+07 2 1 295.06057 −0.076 C9H16N2O7S- 3.25E+07 1.78 0.78 295.07182 −0.134 C8H16N4O6S- 1.27E+08 2 0.75 295.08304 −0.09 C7H16N6O5S- 2.89E+07 2.29 0.71 296.01947 −0.198 C7H11N3O8S- 2.22E+07 1.57 1.14 296.03069 −0.154 C6H11N5O7S- 1.65E+07 1.83 1.17 296.05578 0.056 C8H15N3O7S- 8.21E+07 1.88 0.88 296.06705 −0.069 C7H15N5O6S- 8.25E+07 2.14 0.86 296.09221 −0.096 C9H19N3O6S- 4.99E+07 2.11 0.67 297.03985 −0.126 C8H14N2O8S- 2.70E+07 1.75 1 297.05108 −0.116 C7H14N4O7S- 7.23E+07 2 1 297.06228 −0.005 C6H14N6O6S- 2.99E+07 2.33 1 297.06637 −0.234 C11H14N4O4S- 7.63E+06 1.27 0.36 297.0762 −0.008 C9H18N2O7S- 3.31E+07 2 0.78 297.08746 −0.099 C8H18N4O6S- 6.92E+07 2.25 0.75 298.03506 0.005 C7H13N3O8S- 3.55E+07 1.86 1.14 298.0463 −0.018 C6H13N5O7S- 2.85E+07 2.17 1.17 298.06158 −0.102 C10H13N5O4S- 1.15E+07 1.3 0.4 298.07148 −0.112 C8H17N3O7S- 4.39E+07 2.13 0.88 298.08267 0.032 C7H17N5O6S- 2.30E+07 2.43 0.86 299.04556 0.018 C10H12N4O5S- 1.02E+07 1.2 0.5 299.05546 0.008 C8H16N2O8S- 1.90E+07 2 1 299.06672 −0.082 C7H16N4O7S- 2.95E+07 2.29 1 300.05075 −0.128 C7H15N3O8S- 1.86E+07 2.14 1.14 300.06196 −0.052 C6H15N5O7S- 1.90E+07 2.5 1.17 300.07724 −0.135 C10H15N5O4S- 2.35E+07 1.5 0.4 301.06123 −0.048 C10H14N4O5S- 2.60E+07 1.4 0.5 301.99439 0.227 C6H13N3O5S3- 6.19E+06 2.17 0.83 302.05649 −0.084 C9H13N5O5S- 2.90E+07 1.44 0.56 303.04049 −0.031 C9H12N4O6S- 1.75E+07 1.33 0.67 303.07689 −0.081 C10H16N4O5S- 5.15E+07 1.6 0.5 303.08813 −0.104 C9H16N6O4S- 3.71E+07 1.78 0.44 304.06087 0.038 C10H15N3O6S- 2.81E+07 1.5 0.6 304.07212 −0.018 C9H15N5O5S- 6.70E+07 1.67 0.56 305.05615 −0.064 C9H14N4O6S- 6.05E+07 1.56 0.67 305.06743 −0.218 C8H14N6O5S- 3.99E+07 1.75 0.63 306.04014 0.021 C9H13N3O7S- 2.40E+07 1.44 0.78 306.05141 −0.1 C8H13N5O6S- 5.35E+07 1.63 0.75 306.07656 −0.093 C10H17N3O6S- 5.61E+07 1.7 0.6 306.08776 0.015 C9H17N5O5S- 1.04E+08 1.89 0.56 307.03543 −0.112 C8H12N4O7S- 3.51E+07 1.5 0.88 307.07183 −0.161 C9H16N4O6S- 1.23E+08 1.78 0.67 307.08306 −0.151 C8H16N6O5S- 7.49E+07 2 0.63 308.03065 −0.018 C7H11N5O7S- 1.40E+07 1.57 1 308.05584 −0.141 C9H15N3O7S- 6.47E+07 1.67 0.78 308.06703 −0.002 C8H15N5O6S- 1.18E+08 1.88 0.75 308.07826 0.008 C7H15N7O5S- 1.83E+07 2.14 0.71 308.0922 −0.06 C10H19N3O6S- 6.57E+07 1.9 0.6 308.10345 −0.115 C9H19N5O5S- 7.63E+07 2.11 0.56 308.11735 −0.054 C12H23NO6S- 3.28E+07 1.92 0.5 309.05109 −0.144 C8H14N4O7S- 9.45E+07 1.75 0.88 309.06234 −0.199 C7H14N6O6S- 5.53E+07 2 0.86 309.07621 −0.04 C10H18N2O7S- 2.34E+07 1.8 0.7 309.08743 0.002 C9H18N4O6S- 1.10E+08 2 0.67 310.03507 −0.027 C8H13N3O8S- 3.35E+07 1.63 1 310.04634 −0.147 C7H13N5O7S- 6.10E+07 1.86 1 310.07147 −0.076 C9H17N3O7S- 9.80E+07 1.89 0.78 310.08271 −0.098 C8H17N5O6S- 1.01E+08 2.13 0.75 311.03038 −0.223 C7H12N4O8S- 3.37E+07 1.71 1.14 311.05549 −0.088 C9H16N2O8S- 2.32E+07 1.78 0.89 311.06667 0.082 C8H16N4O7S- 1.02E+08 2 0.88 311.07791 0.059 C7H16N6O6S- 4.21E+07 2.29 0.86 312.05076 −0.155 C8H15N3O8S- 5.75E+07 1.88 1 312.06197 −0.082 C7H15N5O7S- 6.13E+07 2.14 1
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
312.08712 −0.075 C9H19N3O7S- 4.34E+07 2.11 0.78 313.04602 −0.19 C7H14N4O8S- 6.14E+07 2 1.14 313.05728 −0.276 C6H14N6O7S- 2.34E+07 2.33 1.17 313.08232 0.081 C8H18N4O7S- 3.38E+07 2.25 0.88 313.11877 −0.126 C9H22N4O6S- 1.89E+07 2.44 0.67 314.06638 −0.059 C8H17N3O8S- 2.46E+07 2.13 1 314.07763 −0.113 C7H17N5O7S- 2.71E+07 2.43 1 315.07684 0.081 C11H16N4O5S- 3.39E+07 1.45 0.45 315.08816 −0.195 C10H16N6O4S- 3.48E+07 1.6 0.4 316.01005 0.185 C7H15N3O5S3- 1.15E+07 2.14 0.71 316.07213 −0.049 C10H15N5O5S- 4.92E+07 1.5 0.5 317.05612 0.033 C10H14N4O6S- 3.38E+07 1.4 0.6 317.09252 −0.014 C11H18N4O5S- 5.29E+07 1.64 0.45 317.10377 −0.068 C10H18N6O4S- 4.13E+07 1.8 0.4 318.05139 −0.033 C9H13N5O6S- 3.21E+07 1.44 0.67 318.07657 −0.121 C11H17N3O6S- 3.14E+07 1.55 0.55 319.03543 −0.108 C9H12N4O7S- 1.65E+07 1.33 0.78 319.04666 −0.099 C8H12N6O6S- 1.68E+07 1.5 0.75 319.0718 −0.061 C10H16N4O6S- 8.22E+07 1.6 0.6 319.08307 −0.177 C9H16N6O5S- 7.62E+07 1.78 0.56 319.10815 0.049 C11H20N4O5S- 5.19E+07 1.82 0.45 320.05589 −0.292 C10H15N3O7S- 3.72E+07 1.5 0.7 320.06708 −0.158 C9H15N5O6S- 9.79E+07 1.67 0.67 320.10343 −0.048 C10H19N5O5S- 9.50E+07 1.9 0.5 321.05102 0.079 C9H14N4O7S- 6.06E+07 1.56 0.78 321.06225 0.089 C8H14N6O6S- 5.50E+07 1.75 0.75 321.0875 −0.216 C10H18N4O6S- 1.27E+08 1.8 0.6 321.09873 −0.207 C9H18N6O5S- 8.74E+07 2 0.56 322.03502 0.129 C9H13N3O8S- 1.81E+07 1.44 0.89 322.04628 0.045 C8H13N5O7S- 4.81E+07 1.63 0.88 322.07153 −0.259 C10H17N3O7S- 7.34E+07 1.7 0.7 322.08274 −0.188 C9H17N5O6S- 1.57E+08 1.89 0.67 322.11903 0.107 C10H21N5O5S- 4.61E+07 2.1 0.5 323.06677 −0.231 C9H16N4O7S- 1.31E+08 1.78 0.78 323.07797 −0.128 C8H16N6O6S- 1.03E+08 2 0.75 323.10307 0.032 C10H20N4O6S- 9.03E+07 2 0.6 324.05078 −0.211 C9H15N3O8S- 5.12E+07 1.67 0.89 324.06202 −0.233 C8H15N5O7S- 1.19E+08 1.88 0.88 324.07322 −0.131 C7H15N7O6S- 2.50E+07 2.14 0.86 324.08708 0.051 C10H19N3O7S- 7.70E+07 1.9 0.7 324.09833 −0.002 C9H19N5O6S- 1.10E+08 2.11 0.67 325.046 −0.122 C8H14N4O8S- 6.26E+07 1.75 1 325.05727 −0.235 C7H14N6O7S- 4.39E+07 2 1 325.07117 −0.177 C10H18N2O8S- 2.38E+07 1.8 0.8 325.08239 −0.137 C9H18N4O7S- 1.15E+08 2 0.78 325.09358 −0.005 C8H18N6O6S- 5.14E+07 2.25 0.75 326.02996 0.051 C8H13N3O9S- 2.19E+07 1.63 1.13 326.0412 0.029 C7H13N5O8S- 3.18E+07 1.86 1.14 326.0664 −0.118 C9H17N3O8S- 6.34E+07 1.89 0.89 326.07768 −0.262 C8H17N5O7S- 9.97E+07 2.13 0.88 326.10276 −0.041 C10H21N3O7S- 3.75E+07 2.1 0.7 327.05038 −0.008 C9H16N2O9S- 1.57E+07 1.78 1 327.06159 0.063 C8H16N4O8S- 6.52E+07 2 1 327.07279 0.164 C7H16N6O7S- 4.94E+07 2.29 1 328.04562 0.02 C8H15N3O9S- 3.13E+07 1.88 1.13 328.0569 −0.123 C7H15N5O8S- 5.14E+07 2.14 1.14 328.0722 −0.261 C11H15N5O5S- 2.85E+07 1.36 0.45 329.0562 −0.211 C11H14N4O6S- 1.71E+07 1.27 0.55 329.06741 −0.141 C10H14N6O5S- 2.23E+07 1.4 0.5 329.07723 0.093 C8H18N4O8S- 2.85E+07 2.25 1 331.02098 0.083 C7H16N4O5S3- 8.73E+06 2.29 0.71 331.07176 0.062 C11H16N4O6S- 4.11E+07 1.45 0.55 331.08301 0.011 C10H16N6O5S- 5.63E+07 1.6 0.5 332.06702 0.029 C10H15N5O6S- 5.01E+07 1.5 0.6
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
332.07822 0.128 C9H15N7O5S- 2.60E+07 1.67 0.56 332.10344 −0.077 C11H19N5O5S- 8.17E+07 1.73 0.45 333.05107 −0.074 C10H14N4O7S- 2.69E+07 1.4 0.7 333.06231 −0.095 C9H14N6O6S- 3.87E+07 1.56 0.67 333.0874 0.092 C11H18N4O6S- 7.46E+07 1.64 0.55 334.04628 0.043 C9H13N5O7S- 2.74E+07 1.44 0.78 334.0827 −0.061 C10H17N5O6S- 1.21E+08 1.7 0.6 335.06671 −0.043 C10H16N4O7S- 8.51E+07 1.6 0.7 335.07791 0.055 C9H16N6O6S- 9.80E+07 1.78 0.67 335.10307 0.031 C11H20N4O6S- 9.44E+07 1.82 0.55 335.11437 −0.169 C10H20N6O5S- 7.49E+07 2 0.5 336.05074 −0.085 C10H15N3O8S- 2.79E+07 1.5 0.8 336.06199 −0.135 C9H15N5O7S- 9.30E+07 1.67 0.78 336.0871 −0.01 C11H19N3O7S- 5.68E+07 1.73 0.64 336.09833 −0.001 C10H19N5O6S- 1.43E+08 1.9 0.6 337.04596 0.001 C9H14N4O8S- 4.21E+07 1.56 0.89 337.05722 −0.079 C8H14N6O7S- 4.47E+07 1.75 0.88 337.08242 −0.221 C10H18N4O7S- 1.29E+08 1.8 0.7 337.09363 −0.153 C9H18N6O6S- 1.27E+08 2 0.67 338.06635 0.034 C10H17N3O8S- 4.99E+07 1.7 0.8 338.07759 0.013 C9H17N5O7S- 1.40E+08 1.89 0.78 338.08884 −0.037 C8H17N7O6S- 5.17E+07 2.13 0.75 338.10275 −0.01 C11H21N3O7S- 4.98E+07 1.91 0.64 338.11402 −0.12 C10H21N5O6S- 8.91E+07 2.1 0.6 339.06166 −0.146 C9H16N4O8S- 8.54E+07 1.78 0.89 339.07291 −0.196 C8H16N6O7S- 9.22E+07 2 0.88 339.098 −0.013 C10H20N4O7S- 8.89E+07 2 0.7 339.10921 0.055 C9H20N6O6S- 5.23E+07 2.22 0.67 340.05689 −0.09 C8H15N5O8S- 7.39E+07 1.88 1 340.06808 0.037 C7H15N7O7S- 2.06E+07 2.14 1 340.08201 0.004 C10H19N3O8S- 5.99E+07 1.9 0.8 340.09325 −0.016 C9H19N5O7S- 9.13E+07 2.11 0.78 341.04084 0.104 C8H14N4O9S- 3.60E+07 1.75 1.13 341.05208 0.084 C7H14N6O8S- 2.18E+07 2 1.14 341.07726 0.001 C9H18N4O8S- 7.30E+07 2 0.89 341.08852 −0.078 C8H18N6O7S- 5.96E+07 2.25 0.88 342.06135 −0.215 C9H17N3O9S- 3.39E+07 1.89 1 342.07257 −0.177 C8H17N5O8S- 6.76E+07 2.13 1 342.08379 −0.139 C7H17N7O7S- 2.90E+07 2.43 1 342.08779 −0.075 C12H17N5O5S- 3.01E+07 1.42 0.42 343.0566 −0.217 C8H16N4O9S- 4.66E+07 2 1.13 343.06784 −0.238 C7H16N6O8S- 3.21E+07 2.29 1.14 344.06711 −0.234 C11H15N5O6S- 3.11E+07 1.36 0.55 344.07698 −0.155 C9H19N3O9S- 2.23E+07 2.11 1 345.08739 0.117 C12H18N4O6S- 3.84E+07 1.5 0.5 345.09865 0.039 C11H18N6O5S- 6.37E+07 1.64 0.45 346.08273 −0.146 C11H17N5O6S- 7.26E+07 1.55 0.55 346.09396 −0.137 C10H17N7O5S- 4.46E+07 1.7 0.5 347.06671 −0.042 C11H16N4O7S- 3.83E+07 1.45 0.64 347.07795 −0.062 C10H16N6O6S- 6.55E+07 1.6 0.6 347.10304 0.117 C12H20N4O6S- 5.80E+07 1.67 0.5 348.06197 −0.073 C10H15N5O7S- 4.58E+07 1.5 0.7 348.09833 −0.001 C11H19N5O6S- 1.11E+08 1.73 0.55 348.10961 −0.136 C10H19N7O5S- 6.18E+07 1.9 0.5 349.08237 −0.07 C11H18N4O7S- 8.40E+07 1.64 0.64 349.0936 −0.062 C10H18N6O6S- 1.22E+08 1.8 0.6 350.0776 −0.016 C10H17N5O7S- 1.13E+08 1.7 0.7 350.08885 −0.064 C9H17N7O6S- 7.01E+07 1.89 0.67 350.11399 −0.03 C11H21N5O6S- 1.07E+08 1.91 0.55 351.06168 −0.198 C10H16N4O8S- 6.32E+07 1.6 0.8 351.0729 −0.161 C9H16N6O7S- 8.97E+07 1.78 0.78 351.09799 0.016 C11H20N4O7S- 1.00E+08 1.82 0.64 351.10921 0.053 C10H20N6O6S- 1.08E+08 2 0.6 352.05686 −0.001 C9H15N5O8S- 5.96E+07 1.67 0.89
Table 3 (Continued)
m/z (exp) Error (ppm) Molecular Ion Formula Intensity/Arbitrary Units H/C O/C
352.08197 0.118 C11H19N3O8S- 4.53E+07 1.73 0.73 352.09323 0.041 C10H19N5O7S- 1.43E+08 1.9 0.7 352.10452 −0.121 C9H19N7O6S- 6.70E+07 2.11 0.67 353.04087 0.016 C9H14N4O9S- 2.11E+07 1.56 1 353.05208 0.081 C8H14N6O8S- 2.66E+07 1.75 1 353.07729 −0.084 C10H18N4O8S- 9.30E+07 1.8 0.8 353.08856 −0.188 C9H18N6O7S- 1.24E+08 2 0.78 354.06134 −0.179 C10H17N3O9S- 3.24E+07 1.7 0.9 354.07257 −0.171 C9H17N5O8S- 1.04E+08 1.89 0.89 354.08381 −0.191 C8H17N7O7S- 5.49E+07 2.13 0.88 354.09767 −0.024 C11H21N3O8S- 4.15E+07 1.91 0.73 354.10889 0.013 C10H21N5O7S- 8.20E+07 2.1 0.7 355.05656 −0.097 C9H16N4O9S- 5.41E+07 1.78 1 355.06782 −0.173 C8H16N6O8S- 6.95E+07 2 1 355.08305 −0.103 C12H16N6O5S- 1.73E+07 1.33 0.42 355.09289 0.058 C10H20N4O8S- 6.75E+07 2 0.8 355.10412 0.066 C9H20N6O7S- 6.33E+07 2.22 0.78 356.05183 −0.156 C8H15N5O9S- 3.79E+07 1.88 1.13 356.07696 −0.094 C10H19N3O9S- 3.80E+07 1.9 0.9 356.08818 −0.058 C9H19N5O8S- 7.78E+07 2.11 0.89 356.09938 0.035 C8H19N7O7S- 2.94E+07 2.38 0.88 357.07219 −0.041 C9H18N4O9S- 5.05E+07 2 1 357.08342 −0.032 C8H18N6O8S- 5.23E+07 2.25 1 357.09863 0.094 C12H18N6O5S- 3.54E+07 1.5 0.42 358.05623 −0.108 C9H17N3O10S- 2.03E+07 1.89 1.11 358.06747 −0.127 C8H17N5O9S- 3.91E+07 2.13 1.13 359.07799 −0.171 C11H16N6O6S- 3.93E+07 1.45 0.55 359.08782 0.015 C9H20N4O9S- 2.46E+07 2.22 1 360.06194 0.012 C11H15N5O7S- 2.06E+07 1.36 0.64 360.09833 −0.001 C12H19N5O6S- 6.78E+07 1.58 0.5 360.10961 −0.132 C11H19N7O5S- 5.56E+07 1.73 0.45 361.09355 0.079 C11H18N6O6S- 8.19E+07 1.64 0.55 362.07767 −0.209 C11H17N5O7S- 6.41E+07 1.55 0.64 362.08891 −0.228 C10H17N7O6S- 5.73E+07 1.7 0.6 363.07284 0.01 C10H16N6O7S- 5.50E+07 1.6 0.7 363.09798 0.043 C12H20N4O7S- 6.49E+07 1.67 0.58 363.10926 −0.087 C11H20N6O6S- 1.18E+08 1.82 0.55 364.05692 −0.166 C10H15N5O8S- 3.06E+07 1.5 0.8 364.09326 −0.043 C11H19N5O7S- 1.19E+08 1.73 0.64 364.1045 −0.062 C10H19N7O6S- 8.94E+07 1.9 0.6 365.07723 0.084 C11H18N4O8S- 6.31E+07 1.64 0.73 365.08846 0.092 C10H18N6O7S- 1.21E+08 1.8 0.7 365.11359 0.152 C12H22N4O7S- 7.21E+07 1.83 0.58 365.12488 −0.004 C11H22N6O6S- 8.97E+07 2 0.55 366.07253 −0.056 C10H17N5O8S- 8.86E+07 1.7 0.8 366.08378 −0.102 C9H17N7O7S- 6.65E+07 1.89 0.78 366.09764 0.059 C12H21N3O8S- 3.56E+07 1.75 0.67 366.10893 −0.097 C11H21N5O7S- 1.29E+08 1.91 0.64 366.12015 −0.061 C10H21N7O6S- 6.62E+07 2.1 0.6 367.06781 −0.14 C9H16N6O8S- 6.73E+07 1.78 0.89 367.09291 0.001 C11H20N4O8S- 8.60E+07 1.82 0.73 367.10414 0.01 C10H20N6O7S- 1.19E+08 2 0.7 368.0518 −0.069 C9H15N5O9S- 3.32E+07 1.67 1 368.07691 0.045 C11H19N3O9S- 2.97E+07 1.73 0.82 368.08814 0.053 C10H19N5O8S- 1.13E+08 1.9 0.8 368.09937 0.061 C9H19N7O7S- 7.25E+07 2.11 0.78 369.07215 0.069 C10H18N4O9S- 5.42E+07 1.8 0.9 369.08339 0.05 C9H18N6O8S- 9.72E+07 2 0.89 370.05616 0.085 C10H17N3O10S- 1.33E+07 1.7 1 370.06739 0.093 C9H17N5O9S- 5.89E+07 1.89 1 370.07862 0.101 C8H17N7O8S- 3.75E+07 2.13 1 370.1038 0.026 C10H21N5O8S- 6.47E+07 2.1 0.8 371.05139 0.136 C9H16N4O10S- 2.14E+07 1.78 1.11 371.06264 0.09 C8H16N6O9S- 3.08E+07 2 1.13