Synthesis of functionalised mesoporous silica for
capture and transformation of carbon dioxide
Thèse
Maria Zakharova
Doctorat en chimie - Chimie
Philosophiae doctor
(
Ph. D.
)
Québec, Canada
Synthesis of functionalised mesoporous silica for
capture and transformation of carbon dioxide
Thèse
Maria Zakharova
Sous la direction de :
Frédéric-Georges Fontaine, directeur de recherche
Freddy Kleitz, codirecteur de recherche
iii
Résumé
De nos jours, plus de 80% de l'industrie chimique est basée sur des processus catalytiques hétérogènes nous fournissant ainsi de l'énergie, des aliments, des médicaments, la protection des cultures et de nouveaux produits. Bien que la catalyse soit un domaine stratégique de la chimie, le niveau de compréhension de la catalyse hétérogène est quant à lui assez limité, surtout lorsqu'il est comparé à celui de la catalyse homogène. Dans le travail présenté, nous essayons d'élargir la connaissance des systèmes catalytiques hétérogènes basés sur la silice mésoporeuse hybride fonctionnalisée et de les analyser dans différents processus de chimie verte, spécialement ceux en rapport avec la capture et la transformation du CO2.
Pour la capture du CO2, le concept bien connu des paires de Lewis frustrées a été appliqué à la silice mésoporeuse, avec pour résultat la première synthèse de paires d'acides et de bases de Lewis hétérogènes stables. Tout d'abord, la synthèse de la silice mésoporeuse Al-, Ti-, Zr-SBA-15 portant un caractère acide de Lewis très fort grâce à la réaction de silanols de surface avec des complexes métalliques homogènes est présentée. La capacité des matériaux à catalyser l'amidation directe d'amines pauvres en électrons et stériquement encombrées soutient la présence de centres métalliques acides de Lewis hautement actifs et de leur tolérance à l'eau. De plus, le développement de paires de Lewis frustrées solides à l’aide des silices mésoporeuses Al-, Ti- et Zr-SBA-15 est discuté. Une série de bases de Lewis classiques, tels que la diéthylènetriamine, les dérivés de la diphénylphosphine, la triéthylamine et le tétraméthylpipéridine sont greffées ou imprégnées sur la surface de Ti-,Al-, Zr-SBA-15 pour générer des paires acide-base de Lewis solides et stables à l’air. La préservation des deux propriétés acides et basiques est examinée après la formation des paires d’acide-base de Lewis solides. Une étude de leurs interactions avec le CO2 est effectuée à l’aide de la spectroscopie RMN à l’état solide et d’expériences d’adsorption de CO2, ce qui donne une nouvelle vision de leur applicabilité comme adsorbants solides du CO2. Une corrélation entre la force des couples acide-base de Lewis et l’affinité au CO2 est proposée sur la base du calcul de la chaleur isostérique d’adsorption.
Pour la transformation du CO2, l'étude de l'effet de confinement dans les nanopores de silice et son application dans la cycloaddition catalytique du dioxyde de carbone aux époxydes est présentée. La synthèse d’adsorbants mésoporeux hybrides de CO2 avec les silices MCM-41 et SBA-15 est réalisée et les critères d'un système catalytique efficace sont définis et optimisés. Cela a pour résultat un nouveau catalyseur hétérogène très efficace, capable d'effectuer la transformation du dioxyde de carbone
iv
à température ambiante et à pression atmosphérique sans pré-activation chimique des matières de départ.
v
Abstract
Nowadays over 80% of the chemical industry is based on heterogeneous catalytic processes supplying us with energy, aliments, medicines, crop protection, and new commodities. Even though catalysis remains a strategic field of chemistry, the level of understanding of heterogeneous catalysis is still quite limited, especially when compared to that of homogeneous catalysis. In the present work, we try to expand the knowledge of heterogeneous catalytic systems based on functionalized hybrid mesoporous silica and probe them in different green chemistry processes, especially in relation to the capture and transformation of CO2.
For CO2 capture, the well-known concept of frustrated Lewis pairs is translated on the surface of mesoporous silica, resulting in the synthesis of stable heterogenized Lewis acid-base pairs. Firstly, the synthesis of Al-, Ti-, Zr-SBA-15 mesoporous silica carrying very strong Lewis acidic character through the reaction of surface silanol groups with homogeneous metallic complexes is presented. The ability of these materials to catalyse the direct amidation of electron-poor and bulky amines supports the presence of highly active Lewis acidic metallic centers and their water-tolerance. Furthermore, the development of solid supported frustrated Lewis pairs (sFLPs) using Al-, Ti-, Zr-SBA-15 mesoporous silica is discussed. A series of conventional Lewis bases, such as diethylenetriamine, diphenylphosphine derivatives, triethylamine, and tetramethylpiperidine are grafted or impregnated on the surface of Ti-, Al-, Zr-SBA-15 to generate air-stable solid-supported Lewis acid-base pairs. The preservation of both Lewis acidic and basic properties after the solid Lewis acid-base pairs are formed is examined. Study of their interactions with CO2 is performed using solid state NMR spectroscopy and CO2 adsorption experiments, which provides a new insight in their applicability as solid CO2 adsorbents. A correlation between the solid supported Lewis acid-base pair strength and the affinity to CO2 is proposed based on the calculation of isosteric heat of adsorption.
For CO2 transformation, the study of confinement effect in silica nanopores and its application in catalytic cycloaddition of carbon dioxide to epoxides is presented. The synthesis of hybrid mesoporous adsorbents of CO2 on the base of MCM-41 and SBA-15 silica is performed and the criteria for an efficient catalytic system are defined and optimised, providing a novel and very efficient heterogeneous catalyst, capable of performing the transformation of carbon dioxide at room temperature under an atmospheric pressure without any chemical pre-activation of starting materials.
vi
Table of Contents
Résumé iii Abstract v Table of contents vi List of tables x List of schemes xiList of figures xii
List of abbreviations xvii
Acknowledgements xxi
Inserted research articles and author contributions xxiii
Chapter 1. Introduction 1
1.1 Origin of the project 1
1.2 Scope of thesis 3
1.3 Bibliography 5
Chapter 2. Literature review and chemical concepts of importance 6 2.1 The "status quo" for the carbon dioxide emissions and green house
effect 6
2.2 Carbon dioxide separation, capture and storage 8
2.3 Conventional chemical absorption 9
2.4 Physical absorbents 11
2.5 New materials for CO2 capture 11
2.5.1 Metal–organic frameworks 12
2.5.2 Microporous and mesoporous materials 14
2.6 Concept of green chemistry 16
2.7 The carbon dioxide molecule and its conversion into chemical
feedstock 17
2.8 Chemistry of frustrated Lewis pairs 22
2.9 Heterogenization of molecular FLPs 25
2.10 Concept of catalysis 26
2.11 Chemistry of nanoporous materials 28
2.12 Synthesis and functionalization of mesoporous materials 29
vii
2.14 Surface functionalisation of mesoporous silica 33
2.15 Bibliography 35
Chapter 3. Methodology 44
3.1 Experimental methods 44
3.1.1 Manipulations under inert atmosphere 44
3.1.2 Schlenk line 44
3.1.3 Glovebox 44
3.1.4 Manipulations with pressurized gas 45
3.2 Characterization techniques 46
3.2.1 Nuclear magnetic resonance spectroscopy 46
3.2.2 Solid-state nuclear magnetic resonance 46
3.2.3 Mass spectrometry 47
3.2.4 Attenuated total reflectance Fourier transform infrared
spectroscopy 49
3.2.5 Measurements of surface acidity 51
3.2.6 Low-temperature nitrogen physisorption 54
3.2.7 Calculation of isosteric heat of adsorption 58
3.2.8 Thermogravimetric analysis 60
3.2.9 X-Ray photoelectron spectroscopy 60
3.2.10 Electron microscopy 61
3.2.11 Transmission electron microscopy (TEM) 62
3.2.12 Scanning electron microscopy (SEM) 63
3.2.13 Energy dispersive X-ray spectrometry 64
3.2.14 Ultraviolet–visible spectroscopy 65
3.3 Bibliography 67
Chapter 4. Research article: Lewis acidity quantification and catalytic activity of
Ti, Zr and Al-supported mesoporous silica 69
4.1 Context of the research 69
4.2 Résumé 69
4.3 Abstract 70
4.4 Introduction 70
4.5 Results and Discussion 72
4.5.1 Materials characterization 72
viii 4.5.3 Catalytic amidation 90 4.6 Conclusions 93 4.7 Future perspectives 94 4.8 Experimental part 95 4.9 Bibliography 101
Chapter 5. Synthesis and CO2 Adsorption Properties of Solid Supported
Frustrated Lewis Acid-Base Pairs 107
5.1 Context of the research 107
5.2 State of art in supported FLPs (sFLPs) 107
5.3 Motivation of the project 109
5.4 Results and discussions. Synthesis 109
5.4.1 Synthesis 109
5.4.2 Materials characterization 113
5.4.3 Characterization of Lewis acidity 120
5.4.4 Isosteric Heat of CO2 Adsorption 121
5.4.5 Reaction of solid ambiphilic systems with carbon dioxide 128
5.5 Conclusion 132
5.6 Future perspectives 134
5.7 Experimental section 135
5.8 Bibliography 139
Chapter 6. Research article: Carbon Dioxide Oversolubility in Nanoconfined
Liquids for the Synthesis of Cyclic Carbonates 144
6.1 Context of the research 144
6.2. Résumé 145
6.3 Abstract 146
6.4 Introduction 147
6.5 Results and discussion 148
6.6 Conclusions 156
6.7 Future perspectives 157
6.8 Experimental procedures 159
6.9 Bibliography 163
Chapter 7. Closing remarks 167
ix
7.2 Future prospectives 169
7.3 Final words 170
7.4 Bibliography 171
x
List of tables
Table 2-1 Annual production and E-factors in the chemical industry 16 Table 2-2 Comparison of the homogeneous and heterogeneous catalysis 27
Table 3-1 Table of adsorption bands of selected functional groups 50
Table 3-2 SEM and TEM comparison chart 63
Table 4-1 Physicochemical properties derived from N2 sorption measurements at -196 °C and XPS analysis performed on the different Ti-, Al- and Zr-SBA-15 materials
75
Table 4-2 Hammett indicators used in the study, colors of the corresponding acidic and basic forms and their pKa values
86
Table 4-3 Results of titration of Ti-, Al-, Zr-SBA-15 materials with Hammett
indicators 87
Table 4-4 Acidity of Al-, Ti-, Zr-SBA-15 materials 90 Table 4-5 Catalytic amidation of aniline catalyzed by metalated SBA-15
materials 91
Table 5-1 Physicochemical properties derived from N2 sorption measurements
at -196 °C and TGA results 114
Table 5-2 Values of net isosteric heat of CO2 adsorption for the different
materials 127
Table 6-1 Physicochemical properties derived from N2 sorption measurements at 77.4 K, TGA and XPS analyses results performed on the SBA-15 and MCM-41 based material
150
Table 6-2 Reactions of synthesis of styrene carbonate performed without effect
of oversolubility 152
Table 6-3 Yields of styrene carbonate for different solid catalytic systems 154
Table 6-4 Physicochemical properties derived from N2 sorption measurements and TGA analysis performed on the SBA-15 and MCM-41 modified materials, and yield of styrene carbonate after 1 and 3 cycles
155
Table 6-5 Results of catalytic cycloaddition of carbon dioxide to styrene oxide
xi
List of schemes
Scheme 2-1 General reaction schemes for the chemical absorption-desorption of CO2 by A) primary or secondary and B) tertiary amine-containing solvents
10
Scheme 2-2 Examples of organic synthesis starting from CO2 19
Scheme 2-3 Synthesis of polycarbonates through alternating copolymerization of epoxides and CO2
21
Scheme 2-4 Representation of Frustrated Lewis pair reactivity for hydrogen splitting
23
Scheme 2-5 Reversible metal-free activation of CO2 by intramolecular FLP 23
Scheme 2-6 Formation of mesoporous materials by structure-directing agents: true liquid-crystal template mechanism (A) and cooperative liquid crystal template mechanism (B)
30
Scheme 5-1 Synthesis of 2-diphenylphosphinetromethoxysilane 112
Scheme 6-1 Synthesis of tripropylammonium salt III; B. Grafting of ammonium salt onto the surface of mesoporous silica 148 Scheme 6-2 Side reaction occurring during the synthesis of styrene oxide 153
xii
List of figures
Figure 1-1 Carbon dioxide utilization cycle 3
Figure 2-1 A. Global surface temperature chart from 1880 to 2010 base period; B. Decadal surface temperature anomalies relative to 1951–1980 base period
7
Figure 2-2 The process flow chart based on post-combustion CO2 capture, pre-combustion CO2 capture, oxyfuel process and the existing industrial process
9
Figure 2-3 Structure of Selexol© solvent 11
Figure 2-4 A portion of the crystal structure of Zn4O-based MOF (MOF-5) 13
Figure 2-5 Representation of the ambiphilic character of the CO2 molecule (A) and MOs diagram of the CO2 molecule
18
Figure 2-6 Existing pathways of CO2 convertion into valuable chemicals: without formal reduction, with complete reduction of CO2 to methane and saturated hydrocarbons, or combined reduction and bond formation to result in a large synthetic diversity
20
Figure 2-7 Selected examples of reported CO2-FLP adducts 24
Figure 2-8 Structures of mesoporous M41S materials: a) 4, b)
MCM-48, and c) MCM-50 29
Figure 2-9 Interactions between the inorganic species and the head group of the surfactant with consideration of the possible synthetic pathway in acidic, basic, or neutral media
31
Figure 3-1 Fischer-porter tube 45
Figure 3-2 27Al MAS NMR spectra of Al-4%-SBA-15 material 47 Figure 3-3 Schematic representation of mass spectrometric analysis, using
electron ionisation technique 48
Figure 3-4 A multiple reflection ATR system 49
Figure 3-5 Infrared spectra of a sample of Aerosil 300 after sample
pretreatment in vacuum at increasing temperatures 51 Figure 3-6 Titration technique for identification of surface acidity 53 Figure 3-7 Terminology used for the description of adsorption process 55
xiii
Figure 3-9 Schematic representation of adsorption process and
corresponding isotherm 57
Figure 3-10 Type H1 hysteresis loop typically observed for mesoporous
materials 58
Figure 3-11 Schematic representation of photoelectron generation 61 Figure 3-12 TEM image of functionalised SBA-15 material 62 Figure 3-13 SEM image of modified SBA-15 material under x1000 folds
magnification 64
Figure 4-1 N2 adsorption–desorption isotherms measured at 77.4 K for Al-
(A), Ti- (B), Zr-SBA-15 (C) 74
Figure 4-2 Low-angle X-ray diffractograms of Ti-SBA-15 samples with
different Ti loadings 76
Figure 4-3 Wide-angle X-ray powder diffractograms of Ti-SBA-15, Al-SBA-15 and Zr-SBA-Al-SBA-15 silica materials, as well as the respective crystalline metal oxides as references
78
Figure 4-4 SEM images of Ti-15%-SBA-15 material under x1000 (A) and x100 (B) folds magnification; SEM image of Zr-6%-SBA-15* under x1000 (C) fold magnification and EDX spectra of Zr-6%-SBA-15* containing zirconia crystalline region (D) and amorphous region (E)
79
Figure 4-5 FTIR spectra of A. Ti-SBA-15 materials; B. Al2O3 and Al-SBA15 materials; C. ZrO2 and Zr-SBA15 materials with different metal concentrations
81
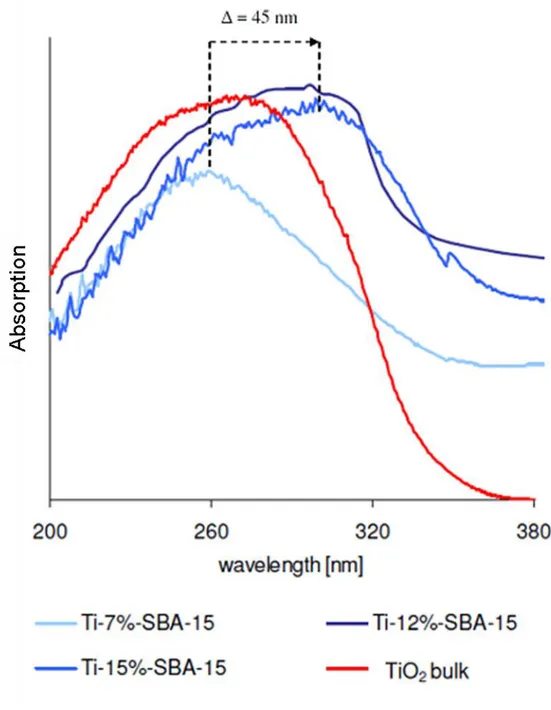
Figure 4-6 UV-vis DR spectra of Ti-SBA-15 samples with different Ti
concentrations 82
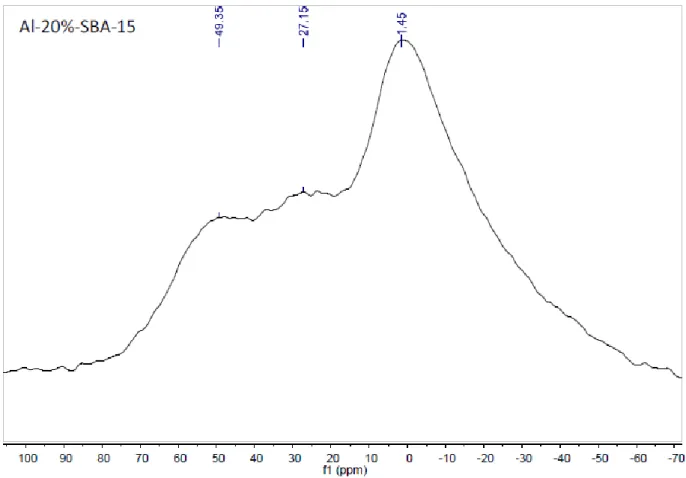
Figure 4-7 27Al MAS NMR spectrum of A. Al-3%-SBA-15 material; B.
Al-4%-SBA-15 material; C. Al-20%-Al-4%-SBA-15 material 84
Figure 4-8 High resolution transmission electron microscopy images of Ti-SBA-15, where Ti 1% (A, B), Ti 7% (C, D), Ti 15% (E, F) and energy-dispersive X-ray spectroscopy data of Ti-SBA-15
85
Figure 4-9 FT-IR spectra for pyridine adsorbed on Ti-, Al-, Zr-SBA-15 materials before and after water addition (A) and schematic representations of the structure of grafted Lewis and Bronsted acidic centers (B)
89
xiv anilines (A-D), morpholine (E)
Figure 5-1 Development of solid ambiphilic systems: A. Metalation of SBA-15; B. Grafting of Lewis bases on the surface of the metalated material; C. Impregnation of Lewis bases in the pores of the metalated materials
111
Figure 5-2 Solid ambiphilic systems obtained after the grafting (A) and
impregnation (B) of Lewis bases. 113
Figure 5-3 EDX mapping analysis of solid ambiphilic solid 116 Figure 5-4 13C CP and 31P MAS NMR spectra and of PPh2-based ambiphilic
systems 117
Figure 5-5 13C MAS NMR spectra of DIETA-based ambiphilic systems 118 Figure 5-6 13C CP MAS NMR spectra of TMP-based ambiphilic systems 119 Figure 5-7 13C CP MAS NMR spectra of NEt3-based ambiphilic systems 120
Figure 5-8 31P MAS NMR spectra of tBu3P-type ambiphilic systems 121
Figure 5-9 FTIR spectra of initial Ti-SBA-15 and Zr-SBA-15 materials and pyridine adsorbed on Ti-PPh2-SBA-15 and Zr-PPh2-SBA-15 materials
122
Figure 5-10 A. Adsorption isotherms obtained at 273 K for reference materials; B. Net heat as a function of loading for reference
materials 124
Figure 5-11 A. CO2 Adsorption isotherms obtained at 273 K for PPh2-based materials; B. CO2 Adsorption isotherms obtained at 273 K for DIETA-based materials
125
Figure 5-12 A. CO2 Adsorption isotherms obtained at 273 K for TMP-based materials; B. CO2 Adsorption isotherms obtained at 273 K for NEt3-based materials
126
Figure 5-13 Simplified schematic representation of CO2-FLP adduct 129
Figure 5-14 31P MAS NMR spectra of reaction between Zr-PPh2-SBA-15 and 13CO
2
130
Figure 5-15 13C MAS NMR of reaction between Zr-DIETA-SBA-15 and 13CO2 131
Figure 5-16 31P MAS NMR of Al-SBA-15-TMDS- tBu3P before and after the reaction with CO2
132
Figure 5-17 13C MAS NMR of reaction between TMP- and NEt3 based solid ambiphilic systems and 13CO2
xv
Figure 5-18 Acetyl acetone and the thio-analogues stabilizing agents 135 Figure 5-19 Representation of P–C coupling route to polyphosphines 136
Figure 6-1 N2 physisorption isotherms and (NLDFT) pore size distribution of SBA-15 (A and C correspondingly) and MCM-41 (B and D, correspondingly)-based materials.
149
Figure 6-2 13C CP-MAS NMR spectra of MCM-41-TMDS-A (i) and
SBA-15-TMDS-A (ii). 151
Figure 6-3 FTIR spectra of MCM-41-A-TMDS (dark blue) and
SBA-15-A-TMDS (light blue) 151
Figure 7-1 Objectives achieved in presented thesis work, formulated in the
frame of CCU concept. 169
Annex
Figure 4-1 1H NMR spectra of 1-Phenyl-undecan-1-one 172 Figure 4-2 13C{1H} NMR spectra of 1-Phenyl-undecan-1-one 173 Figure 4-3 1H NMR spectra of 1-(4-Nitro-phenyl)-undecan-1-one 174 Figure 4-4 13C{1H} NMR spectra of 1-(4-Nitro-phenyl)-undecan-1-one 175 Figure 4-5 1H NMR spectra of 1-(4-Fluoro-phenyl)-undecan-1-one 176 Figure 4-6 13C{1H} NMR spectra of 1-(4-Fluoro-phenyl)-undecan-1-one 177 Figure 4-7 1H NMR of 1-(4-Methoxy-phenyl)-undecan-1-one 178 Figure 4-8 13C{1H} NMR of 1-p-Tolyl-undecan-1-one 179 Figure 4-9 1H NMR of 1-Morpholin-4-yl-undecan-1-one 180 Figure 4-10 13C{1H} NMR of 1-Morpholin-4-yl-undecan-1-one 181
Figure 5-1 Net heat as a function of loading for PPh2-based ambiphilic
systems 182
Figure 5-2 Net heat as a function of loading for DIETA-based ambiphilic
systems 182
Figure 5-3 Net heat as a function of loading for TMP-based ambiphilic
systems 183
Figure 5-4 Net heat as a function of loading for NEt3-based ambiphilic
systems 183
Figure 6-1 1H NMR spectrum of 1-tetrapropylammonium
xvi
Figure 6-2 13C NMR spectrum of 1-tetrapropylammonium
iodide-3-trimethoxysilyl propane (III) 185
Figure 6-3 29Si NMR spectrum of 1-tetrapropylammonium
iodide-3-trimethoxysilyl propane (III) 186
Figure 6-4 MASS spectrum of 1-tetrapropylammonium
iodide-3-trimethoxysilyl propane (III) 187
Figure 6-5 1H NMR spectra of iodohydrin (IV) 188
Figure 6-6 13C{1H} NMR spectra of iodohydrin (IV) 189
Figure 6-7 13C{1H} NMR spectra of iodohydrin (IV) 190
xvii
List of abbreviations
AA Acetyl acetone
AMP 2-amino-2-methyl-1-propanol
APCI Atmospheric Pressure Chemical Ionisation APTES Aminopropyl triethoxysilane
ATR FTIR Attenuated total reflection Fourier transform infrared spectroscopy
BE Binding energy
SBET Specific surface area (calculated by the Brunauer–Emmett–Teller method) BSE Backscattered electrons
BTB 1,3,5-Benzenetribenzoate CCS Carbon capture and storage
CCU Carbon dioxide capture and utilisation CP Cross-polarization
CSA Chemical shift anisotropy
CTAB Cetyltrimethylammonium bromide
DEA Diethanolamine
DIETA N1-(3-trimethoxysilylpropyl)diethylenetriamine
DTPMP Diethylenetriamine penta(methylene phosphonic acid) EDS Energy Dispersive X-ray Spectrometry
EDTA Ethylenediaminetetraacetic acid ESI Electrospray Ionisation
EQ Environmental quotient FLP Frustrated Lewis pairs
GC Gas chromatography
GHG Greenhouse gases
HR-TEM High resolution transmission electron microscopy ICDD International Center for Diffraction Data
KE Kinetic energy LNG Liquefied natural gas LC Liquid chromatography MDEA N-Methyldiethanolamine
MALDI Matrix assisted laser desorption ionisation MAS Magic-angle spinning
xviii
MEA Methyethylamine
MOF Metal–organic frameworks
MQ Multiple Quantum
NHCs N-Heterocyclic carbenes
NLDFT Non-local density functional theory NMR Nuclear Magnetic Resonance OCCs Organic cyclic carbonates P123 Pluronic P123
PDF-2 Powder Diffraction File 2
PMO Periodically-ordered mesoporous organosilica ppm 1 part per million
ppm yr-1 1 part per million per year PSD Pore size distribution
RF Radio frequency
Sarea Surface area
SBA-15 Santa-Barbara Amorphous Number 15 SDAs Structure-directing agents
SE Secondary electron
SEM Scanning electron microscope SiOH Silanol groups
sFLPs Supported Frustrated Lewis Pairs tBu
3P Tri-tert-butyl phosphine
TEM Transmission electron microscopy
TESPtBC (3-Triethoxysilylpropyl)- tert-butylcarbamate TGA Thermogravimetric analysis
TLCT True liquid-crystal templating TMDS Tetramethyldisilazane TMP Tetramethylpiperidine TMS Tetramethylsilane
TON Turnover number
TOF Turnover frequency
TPAI Tetrapropylammonium iodide TS-1 Titanium silicalite-1
UV-vis
DRS UV-visible diffuse reflectance spectra Vpore Pore volume
xix
vs Versus
XPS X-ray Photoelectron Spectroscopy XRD Low-angle X-ray diffraction
xx
There are but two roads that lead to an important goal and to the doing of
great things: strength and perseverance. Strength is the lot of but a few
privileged men; but austere perseverance, harsh and continuous, may be
employed by the smallest of us and rarely fails of its purpose, for its silent
power grows irresistibly greater with time...
xxi
Acknowledgements
There is no better place and better moment, than here and now to acknowledge the people, who have been guiding and bravely following me throughout this amazing and incredibly difficult journey.
First and foremost, I would like to thank my supervisor, Prof. Frédéric-Georges Fontaine for this great opportunity to be a part of your group and to make this work. Now I understand your way of thinking and see its reflection in the personalities of your students. Real research requires many resources, making us strong, independent and scientists with a perspective. You showed me how it is important to stay positive even in the darkest periods of Ph.D. studies and avoid taking research personally. Now I just have to tell myself: ”I have not failed. I’ve just found 10,000 ways that won’t work". Thank you for the constant push of my professional and personal boundaries. From the first day I met you, back in 2009, when you accepted me as your summer student until now, there was not even a day, when I wouldn't appreciate the chance to be here and to work hard.
Further, I would like to say thank you to my co-supervisor Prof. Freddy Kleitz. Similarly, without you, I wouldn't be here, and this work wouldn't be done. I am very grateful for the countless scientific discussions and your valuable advises to help me find a solution to all my problems. Thank you both for giving me the chance to attend some great conferences within Canada, I absolutely loved each and every of them.
Additionally, I would like to express my gratitude to my second co-supervisor Prof. Faïçal Larachi for your help to get me here and this chance to perform some studies in your laboratory with one of you student Amin Sarvaramini.
I would like to thank the committee members Audrey Moores, Jesse Greener and Dominic Larivière for taking some of their precious time for reading my thesis and coming to my defense.
I would like to thank all the members of chemistry and chemical engineering department, especially Pierre Audet for his help with solid state NMR spectroscopy, Alan Adnot for XPS analysis and André Ferland for SEM analysis.
My experience would have been incomplete without my colleagues from Fontaine's and Kleitz's research teams. I would like to thank Cérine Karine Nahi, Nima Masoumifard, Yen Hoang, Mahesh Muraleedharan Nair and Meryem Bouchoucha, Marc-André Courtemanche, Marc-André Légaré and Ambreen Mushtaq. You are very inspirational and intelligent people, I wish you all the best and hope to stay connected for a long time. I cannot forget the second generation of Fontaine's team: Luis Mical Castro, Yimu
xxii
Hu, Étienne Rochette, Nicolas Bouchard, Hugo Boutin, Julien Légaré Lavergne, Théo Rongère, Thomas Bura and Arumugam Jayaraman. You are such awesome colleagues to work with, and I am convinced you will have a great future. Also, thanks to the students of Boukouvalas research team, you are great chemists.
I would like to thank my summer student Charles Boutin for your perseverance and infinite motivation. It was a great collaboration, and I hope I was able to show you some interesting research.
I would like to thank my former supervisors Dr. Igor Sivaev, Dr. Sergey Timofeev and Prof. Vladimir Bregadze for incredible experience during my bachelor and master studies. I wish you only the best and will never forget you.
Special thanks to my secondary school advisors Irina Kuznetsova and Valentina Folcovitch. I will never forget you because of your kindness and wisdom.
My sincere gratitude belongs to my friends. Nicolas, Karine, Emilie, Natasha, Nikunj, Niraj, Ramesh, Dimitry K., Dimitry O., Eugene, Mikhail, Leonid, Ekaterina, Zinaida, my new soul-mate Senja and many more, thank you for being a constant source of motivation, making me a better person and making me feel happy. Special thanks to Justin W. and Cérine Karine Nahi who spent his precious time reading my thesis and sharing with me their suggestions.
Finally, I would like to sincerely acknowledge my family. Thank you to my mother, Irina, for being very strong, positive, brave, real. Without you, I would have never make it. Thank you to my grandmothers, Galina and Raisa, I love you very much. Many deep thanks to the member of my new family: Hélène Dorval, André Thibault, Serge and Alain Dorval, Michael Acosta, Joceline C., Bianka Labbé, Julien Thibault, Thierry Dorval and Sandra Tremblay. You brought so much sense in my life; I love you. And the last word is addressed to Charles Thibault, without who anything would be possible. Thank you for inspiring me every single day, thank you for your kindness and intelligence, thank you for your infinite love and support. You are simply the best person in the world.
xxiii
Inserted research articles and author contributions
The following section lists the published work that is included in every chapter as well as the contribution of authors for every publication.
Chapter 4: V. M. Zakharova, F. Kleitz, F.-G. Fontaine, Lewis Acidity Quantification and
Catalytic Activity of Ti, Zr and Al-Supported Mesoporous Silica, Dalton Trans., 2017, 46, 3864-3876.
Author contributions: The experimental work and manuscript writing was done by MZ.
Editing was done by FGF and FK.
Chapter 6: V. M. Zakharova, F. Kleitz, F.-G. Fontaine, Carbon Dioxide Oversolubility in
Nanoconfined Liquids for the Synthesis of Cyclic Carbonates, ChemCatChem, 2017,
accepted. (DOI: 10.1002/cctc.201700247).
Author contributions: The experimental work and the writing was done by MZ. Editing
1
Chapter 1 - Introduction
1.1 Origin of the projectCarbon dioxide (CO2) is the final product of the combustion of organic matter with oxygen, notably generated by the respiration of animals. An estimate quantity of 750 Gt of CO2 is in the atmosphere and the “carbon-flow” between the atmosphere and the water basins are of about 90 Gt of carbon per year while the fixation by terrestrial plants and microorganisms, and the underground capture amounts to about 90 Gt per year.1 The anthropogenic emissions amount to about 7 Gt y-1 (including the indirect effects of deforestation, cattle industry, etc.). However, these emissions are not balanced, and, as a result, the concentration of CO2 in the atmosphere has kept steadily increasing for the last 200 years.The escalating level of atmospheric carbon dioxide is one of the most pressing environmental concerns of our age.
Carbon capture and storage (CCS) from large point sources is one option for reducing anthropogenic CO2 emissions.2 The capture of carbon dioxide from the flue gases of coal-, oil-, or gas-fired power plants and from industrial processes is a mature technology and is commercially available, but costly. Basic liquids, such as amines are used as chemical adsorbents. Alternatively, membranes have been introduced for CO2 separation. The latter are less space-demanding, while being more expensive than methylethylamine (MEA). The capture of CO2 is already implemented at the Mt level, mainly for the separation of liquefied natural gas (LNG) into CO2 and methane.2 Separated CO2 is further confined in natural sites such as aquifers, deep geological cavities, spent oil- or gas-fields, coal-mines and in the ocean. Liquid physical adsorbents, such as inorganic carbonates and alcohols are currently used by a series of pilot plants. High regeneration cost, the necessity of inhibitors to control corrosion and oxidative degradation of the adsorbent, and the short life-time of equipment due to the presence of highly basic amines lead to reduced efficiencies and increased costs for electricity production.2
It is estimated that the capture of CO2 alone will increase the energy requirements of a power plant by 25–40%.2
This is why alternative strategies based on carbon dioxide capture and utilisation (CCU) start to play a predominant role in CO2 mitigation scenarios.3 CCU can be defined as a process where the CO2 molecule ends up in a new molecule. An approach that integrates the efficient capture of CO2 with subsequent transformation may be the most practical solution for the decrease in emissions while providing the industry with important chemicals. After capture, the CO2
2
can be converted via chemical and electrochemical processes to other energy storage chemicals or inorganic minerals that may be used for building materials, as C1- chemical feedstock or as supercritical fluids. There is no single, universally applicable pathway for CO2 utilization. Depending on the industry, location, and other constraints, one or more technologies may fit better than others. On this basis, the exploration of CO2 capture and transformation systems becomes one of the most relevant subject of research in the last decade.
Chemistry of frustrated Lewis pairs (FLP) has been extensively investigated since 2006, when Stephan reported the first active FLP system.4 Similar reactivity to transition metal chemistry has been observed, and in some systems superior efficiency was also found. If we include the lower price and low toxicity of FLPs, this topic is one of the most fundamentally interesting class of compounds currently studied in academia. Their high reactivity towards CO2 at low temperature and gas pressure inspired the scientists to develop FLP-based adsorbents, but the high air- and moisture-sensitivity lowers the chance of commercial viability dramatically.4
One problem of very active FLP systems is their strong bonding of CO2 with formation of very stable adduct. Furthermore, the use of very active centers will demand a high energy input in order to strip CO2 upon the regeneration cycle. In the same time, unlike conventional soluble FLPs, adsorption of CO2 on the surface of solid FLP-type adsorbent is associated with a high entropic loss, making FLP with moderately strong Lewis acid and base incapable of effective capture. Unfortunately, theoretical prediction is currently difficult and only empiric comparisons of the adsorption efficiencies can help us to find the optimal acidity and basicity for supported FLPs. Therefore, the variation of Lewis acids and bases, followed by the synthesis of solid adsorbents carrying (1) both strong Lewis acid and Lewis base, (2) strong Lewis acid and weak Lewis base and (3) both weak Lewis acid and Lewis base must be performed.
In our work, we present a small-scale demonstration of CCU approach using mesoporous SBA-15 and MCM-41 silica. Carbon dioxide adsorption and subsequent catalytic transformation to the organic cyclic carbonates (OCCs), using heterogeneous materials, is discussed. Initially, we optimized the protocol for the synthesis of air- and moisture-stable Lewis acidic catalysts based on metalated SBA-15 silica. After a series of metalated materials was fully characterized, a novel surface modification protocol to introduce rigid and non-rigid air-stable Lewis acid-base pairs was developed. The obtained materials were shown to be capable of binding CO2 with low energy penalty. Finally, a simple surface functionalization of the parent SBA-15 and MCM-41 silicas with alkylammonium groups provides a very efficient catalyst for the synthesis of OCCs from abundant starting materials, such as organic epoxides and gaseous CO2. The
3
physical phenomena of CO2 oversolubility inside the mesopores is responsible for high cyclic carbonates yield under extremely smooth conditions, such as room temperature and 1 atm of CO2. Our work involves both CO2 capture and transformation, closing the cycle required for CCU technology. The importance of the presented solid systems is defined by the relatively straightforward and inexpensive synthesis, promising and cost-effective capture and transformation of CO2 processes and easy scalability. According to the principles of green chemistry, the concept of CO2 recycling after the burning of fossil fuels has an enormous potential to reduce the carbon footprint of many processes, while producing green chemicals and renewable fuel (Figure 1-1).
Figure 1-1. Carbon dioxide utilization cycle5
1.2 Scope of thesis
As a whole, the thesis is dedicated to better understanding of functionalized mesoporous silica materials in the context of CO2 capture and catalytic transformations. Before each chapter, a short introduction section will describe the most important advances on the topic covered in the chapter, in order to get the reader up to speed on the developments.
In Chapter 1, an overview of the chemistry that lead to the formulation of this thesis has been presented.
4
In Chapter 2 the context on which this work fits in the literature and the progress of this field in the last few years is presented. The fundamental concepts that are key to the proper appreciation of the work in this dissertation are also included.
Chapter 3 is a brief compilation of the methodology and techniques that made possible the development of the present work.
The first part of our project, presented in Chapter 4, demonstrates the exploration of the properties of mesoporous silica carrying very strong Lewis acidic character and the study of their catalytic applications. The corresponding results were recently accepted for publication in Dalton Transaction journal (for details, see Inserted research articles
and author contributions section).
The Chapter 5, is dedicated to the design and synthesis of "ambient" solid supported frustrated Lewis pairs (sFLPs). Promising reactivity toward carbon dioxide is explored by means of solid state NMR spectroscopy and the affinity of materials to CO2 is quantified by measuring the isosteric heat of carbon dioxide adsorption.
In the final part of the work, described in Chapter 6, we reconsider the physical phenomenon of gas oversolubility in nanoconfined liquids in the context of catalytic cycloaddition of carbon dioxide to epoxides. Effect of a high local concentration of CO2, the absence of pre-activation step and extremely mild reaction conditions are highlighted. The results of this project were recently published in ChemCatChem journal (for details, see Inserted research articles and author contributions section). For the sake of clarity, every chapter offers its own conclusion, perspective on future discoveries and applications, as well as the bibliography consulted for this dissertation. The thesis is ended by closing remarks including a general conclusion of the project.
5 1.3 Bibliography:
1. M. Aresta and A. Dibenedettob, Dalton Trans., 2007, 2975–2992.
2. D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058-6082.
3. S. Santos, presented at Methanol Technology and Policy Congress, Frankfurt, Germany, 2015.
4. a) G. C. Welch, R.R. San Juan, J.D. Masuda and D.W. Stephan, Science, 2006,
314, 1124-1126; b) C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D.
W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2009, 121, 6770–6773; c)
Angew. Chem., Int. Ed., 2009, 48, 6643-6646.
5. Carbon Dioxide Utilisation Network, information is available from: http://co2chem.co.uk/
6
Chapter 2 - Literature review and chemical concepts of
importance
2.1 The "status quo" for the carbon dioxide emissions and green house effect
There is clear evidence for fundamental shifts in the state and functioning of the earth system, obviously driven by human activities. The tendency of global energy use projecting a continuously increasing dependence on the utilization of fossil energy1, which in combination with the cattle breeding2 is considered to be the major contributor to the anthropogenic greenhouse gases (GHG) emission, ruining the climate of our planet.
Carbon dioxide (CO2) and water (H2O) are the primarily component of anthropogenic GHG emissions. They are released during the anthropogenic activity, such as energy production through the combustion of fossil fuels: coal, oil, gas, the production of cement, deforestation process as well as a series of natural phenomena, such as the respiration of plants and animals, annual forest fires, volcano eruption, etc. An issue that deserves attention is the fact that only 30–35% of the chemical energy content associated with the carbon used in anthropogenic activities is converted into and used as various forms of energy (electric, mechanical, etc.), while the remaining 65–70% is lost in the form of heat.3 The CO2 and H2O accumulated in the Earth's atmosphere absorb thermal radiation from the surface of the earth and re-radiate this energy to the lower atmosphere, resulting in a net increase of earth’s temperature, a process called the greenhouse effect.4 Even though negative effects from the increase of GHG concentration have been partially mitigated by natural sinks such as oceans, trees and soil5, emissions have been increasing constantly since the industrial revolution and its concentration in the atmosphere has recently reached over 400 ppm for the first time in the history of mankind.6
Starting from the year of 1958 when the continuous observation on atmospheric CO2 concentration was initiated, its growth rate has accelerated from less than 1 part per million per year (ppm yr-1) prior to 1970 to the level beyond 2 ppm yr-1 in recent times.7 Unless taking effective and proper measures, the energy-related CO2 emission by 2050 is estimated to double the level of 32.3 billion metric tons in 2012.8
The increased amount of GHGs in the atmosphere has already led to an overall rise of temperature on a planetary scale ((A,B)).9
7
Figure 2-1. A. Global surface temperature chart from 1880 to 2010 base period; B. decadal surface temperature anomalies relative to 1951–1980 base period9
The capture and sequestration of carbon dioxide is a primary strategy, as it represents the first step of the CO2 utilisation cycle. Carbon capture and storage (CCS) schemes embody a group of technologies for the capture of CO2 from power plants, followed by compression, transport, and permanent storage. CCS in some sectors provides cost-effective emission reductions, but has significant shortcomings: it has high investment costs, the potential storage capacity has uncertainties, public resistance to CCS has been increasing, and it costs energy. In parallel to CCS, CCU can contribute to a green economy and has been suggested as a partial alternative to divert some carbon dioxide from the transport and storage route. Captured CO2 can be utilized by means of
8
free principal ways: conversion of CO2 back into fuel, utilization of CO2 as a C1 feedstock for chemicals, and use of CO2 as a technological fluid. Obviously, there are other crucial strategies, such as improving energy efficiency, switching to less carbon-intensive fuels such as natural gas and phasing in the use of renewable energy resources (e.g., solar energy, wind, and biomass).10
2.2 Carbon dioxide separation, capture and storage
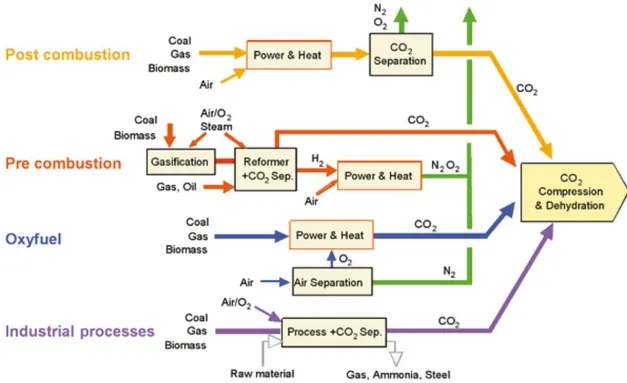
In order to efficiently store and transform CO2, it must be first separated from the other products of the combustion process, such as H2O, NOx and SOx. Three CO2 separation technologies are considered to hold promise. It includes the separation from the power plant flue streams, the separation from sour natural gas wells, and the separation from fuel gas (i.e., syngas). Each application requires different separation methods which impose distinct constraints for adsorbents. Nowadays, there are three major systems to capture CO2 from industrial power plants: postcombustion, precombustion and the oxyfuel process, schematically presented in Figure 2-2. Using postcombustion systems, CO2 is separated from flue gases that originates from the combustion of fossil fuels in the boiler. In a precombustion approach, the CO2 is separated from the fossil fuel before the actual combustion process. For the oxyfuel capture systems, the air used for the combustion process consists of nearly only molar equivalent of molecular oxygen, resulting in a flue gas that is very pure CO2.11
For the postcombustion capture from flue gas, a major obstacle is the low pressure of the flue gas (ca. 1 atm). Since the CO2 concentration is very low, the capture requires separation from a high volume stream of flue gas containing other components, predominantly N2 and H2O.12 Natural gas reserves (mainly CH4) are typically contaminated with over 40% CO2 and N2, and its use is acceptable only if this CO2 can be separated and sequestered at the source of production. This application requires an efficient separation of CO2 from the natural gas components at high pressures. CO2 separation from fuel gas (e.g., output from gasification, water-gas shift reactors) also occurs under high pressure conditions at high temperatures (250–450 °C). Therefore, each process has differences in properties making the capture technologies more challenging. The conventional technologies for large-scale capture are focused on the separation of CO2 from flue gases using amine absorbers (so-called scrubbers).12a
9
Figure 2-2. The process flow chart based on postcombustion CO2 capture (yellow), precombustion CO2 capture (red), oxyfuel process (blue) and the existing industrial
process (purple)13
However, the existing methods of capture are energy intensive and are not cost effective.14 Therefore, a serious need for research on innovative new materials presently exists.
2.3 Conventional chemical absorption
Wet-scrubbing-based CO2 capture technologies have been used industrially for over 50 years and are predominantly based on the chemistry of MEA.12a The typical scrubbing process involves the passage of an aqueous amine solution (typically 25–30 wt.%) down the top of an absorption tower, while a gaseous stream of flue gas containing CO2 is introduced at the bottom. A blower is required to pump the gas through the absorber. At a temperature of approximately 40 °C, the reaction of the amine with CO2 occurs through the formation of carbamates (Scheme 2-1).15
10 C O HN R R' O O C HO N R' R carbamate H2N R' R + low T H2O C O HO O H2N R' R HNR R' + A B C O N R R' O + R'' + H2O C O HO O NH R' R hydrogen carbonate R'' 2
Scheme 2-1. General reaction schemes for the chemical absorption-desorption of CO2 by A) primary or secondary and B) tertiary amine-containing solvents.
After the CO2-enrichment step, the solvent passes through inhibitors, such as diethylenetriamine penta(methylene phosphonic acid) (DTPMP) or ethylenediaminetetraacetic acid (EDTA) to control corrosion and oxidative degradation due to residual oxygen in the flue stream. The sensitivity of the solvents to chemical degradation from the by-products in the flue gas streams, such as SOx and NOx, also leads to increased costs for CO2 regeneration. Finally, the solution of amine enriched with CO2 passes from the absorber column to a stripping tower where the mixture is heated with steam to liberate the CO2. The regeneration of the chemical solvent is carried out at elevated temperatures (100–140 °C) and pressures not much higher than 1 atm. Importantly, the nature of amine defines the stability of the corresponding carbamate, which will consequently define the temperature of CO2 liberation upon desorption step. Secondary amines such as diethanolamine (DEA) possess a lower heat of reaction compared with primary amines: the lower stability of the carbamate formed upon CO2 absorption gives rise to a more economical regeneration. Tertiary amines such as N-methyldiethanolamine (MDEA) is commonly employed for natural gas treatment and exhibits lower solvent degradation rates in addition to a low energy penalty for the regeneration of the solvent. In practice, the addition of small amounts of primary and secondary amines enhances the CO2 absorption rates for tertiary amines. However sterically hindered tertiary amines, such as 2-amino-2-methyl-1-propanol (AMP) which contain bulky substituents are the most promising absorption solvents nowadays, comparing to conventional primary, secondary, and tertiary amines. The very low stability of the corresponding carbamates makes possible the reversible capture of CO2 with the lowest energy consumptions. On the basis of these results, steric hindrance and basicity of the amine can be identified as the major factors controlling the efficiency of CO2 capture reactions.15,16 Other alternatives include,
11
inorganic solvents such as aqueous potassium and sodium carbonate as well as aqueous ammonia solutions. Finally, CO2 capture from ambient air using chemical absorption of aqueous alkali hydroxide solutions has also been proposed.17
2.4 Physical absorbents
A promising alternative to chemical absorption is the use of physical solvents in which the solvent selectively binds CO2 at high partial pressures and low temperatures, accordingly to Henry law. For example, a mixture of dimethylethers of polyethylene glycol, known as Selexol, or methanol chilled to 40 °C, known as Rectisol (Figure 2-3), have been used industrially for 40 years for natural gas sweetening and the treatment of synthesis gas. Sweetening is referred to the processes of removal of H2S and CO2 from sour natural gas.
* O O
n
Figure 2-3. Structure of Selexol© solvent
The advantage in this case is the significantly lower energy penalty for the solvent regeneration step, as the stripping process can be driven by heat or a pressure reduction (i.e., “flash distillation”). Ionic liquids (ILs) constitute another class of physical solvents which are also known to be selective for CO2 absorption.18 ILs are typically formed by combination of large organic cations and smaller inorganic anions and are typically viscous liquids near room temperature. In addition to their near zero vapor pressures, they are non-flammable, environmentally benign, highly viscous and can exhibit exceptional thermal stability.19 The introduction of functional groups such as amines into ILs, have allowed higher rates of adsorption to be achieved at pressures relevant to flue streams (ca. 1 bar).19
2.5 New materials for CO2 capture
Besides the significant progress in the field of liquid carbon dioxide adsorbents, their high energy-demand and the difficulty in the regeneration process remains the biggest challenge and require additional development. Solid adsorbents used in the form of packed adsorbent beds can be regarded as an energy efficient alternative. In this case, however, some practical difficulties in the heat exchange efficiency are to be expected. Whereas CO2 molecules dissolve into the bulk of the liquid in absorption processes, the
12
CO2 adsorption on solids involves the interaction between gas molecules and the surface of a material by either physisorption (through van der Waals molecular interactions) or chemisorption (through covalent bonds formation). After the solid is loaded with CO2, it can be regenerated in multiple stages using either pressure, vacuum, or temperature swing adsorption cycles to remove and concentrate the CO2.20 A variety of solid physical adsorbents have been considered for CO2 capture including microporous and mesoporous materials (carbon-based sorbents such as activated carbon and carbon molecular sieves, zeolites, metal-organic frameworks and functionalized mesoporous materials) and metal oxides.20,b Metal oxides (such as CaO and MgO) are promising capture materials given their ability to retain high adsorption capacities at temperatures above 300 °C.21 On the downside, adsorbent degradation has been observed after several cycles. Improved oxide materials containing lithium, such as Li2ZrO3 and Li4SiO4 have recently attracted the attention for their high CO2 adsorption capacities.22 The “Dry Carbonate Process” is currently in the experimental stages of development and is nearing implementation in postcombustion coal- and natural-gas pilot plants.23 Here, flue gas mixes with a solid dry powdered carbonate sorbent (e.g., Na2CO3 or K2CO3) in a bed in the presence of water to form the corresponding hydrogen carbonate salt (NaHCO3 or KHCO3). The regenerative decarbonation reaction can be achieved at a relatively low temperature of 120 °C. In addition, a diverse range of promising materials exist for CO2 capture. Significant research efforts have been directed towards overcoming the energy intensive adsorbent regeneration step and chemical degradation issues. The most promising examples of new materials include microporous and mesoporous adsorbents, and metal-organic frameworks.24 A brief review of these materials is required to put our work in context.
2.5.1 Metal–organic frameworks
The past 20 years have seen remarkable progress in the design, synthesis, and characterization of metal–organic frameworks (MOFs) owing to their large structural and chemical diversity and their potential applications in gas storage, ion exchange, molecular separation, and heterogeneous catalysis.25 These microporous crystalline solids are composed of organic bridging ligands coordinated to metal-based nodes to form a three-dimensional extended network with uniform pore diameters typically in the range 3 to 20 Å (Figure 2-4).26
13
Figure 2-4. A portion of the crystal structure of Zn4O-based MOF (MOF-5)27
The nodes generally consist of one or more metal ions (e.g., Al3+, Cr3+, Cu2+, Zn2+, Ti4+, Zr4+ etc.) to which the organic bridging ligands coordinate through a specific anionic or neutral functional group (e.g., carboxylate, pyridyl, etc). Their unique properties, including robustness, high thermal and chemical stabilities, unprecedented internal surface areas (up to 5000 m2 g-1), high void volumes (55–90%), and low densities (from 0.21 to 1.00 g cm-3) are the reasons for the intense efforts towards finding industrial applications for MOFs in gas storage, separation, and catalysis.28 The regular and monodisperse nature of the crystalline array of micropores is a key feature that distinguishes these systems from other porous materials (e.g., polymers, mesoporous silicas, carbons, etc.). In addition, the ability to tune systematically the pore dimensions and surface functionality within metal–organic frameworks is a feature that was previously not found in zeolite materials.29 The high surface area-to-weight ratio of MOFs is such that they have the highest capacity for CO2 capture at moderate pressures compared to other adsorbents. While zeolites capture more CO2 at the pressure below 10 bar, their maximum capacities are typically limited to one third to those of MOFs at the pressure above 10 bars.12,a The capacities of metal–organic frameworks up to high pressures scale correlates with the surface area: mesoporous silica and activated carbon have a surface area of 400–1000 m2 g-1, zeolites of up to 1500 m2 g-1, and MOFs of 1500–4500 m2 g-1.30 The highest gravimetric adsorption of CO2 has been reported in frameworks with high surface areas and pore diameters of greater than 15 Å. The framework [Zn4O(btb)2] (MOF-177, btb=1,3,5-benzenetribenzoate) with a surface area of 4500 m2 g-1 exhibits the highest capacity for CO2, taking up 33.5 mmol g-1 at 32 bar.30 By comparison, the benchmark material
14
zeolite 13X adsorbs 7.4 mmol g-1 at 32 bar.30 The mechanism for CO2 capture and separation from other gases by MOFs can often be determined predominantly by an interplay of factors including the molecular sieving effect, the kinetic effect and the thermodynamic equilibrium effect, which include the electrostatic interactions with both the ligand and on coordinatively unsaturated metallic sites31.
Zeolitic imidazolate frameworks (ZIFs) constitute a subclass of metal–organic frameworks that can adopt zeolite structure types based on the replacement of: 1) tetrahedral Si4+ and Al3+ ions with tetrahedral transition metal ions such as Zn2+ or Co2+ and 2) bridging O2- ions with bridging imidazolate-based ligands. Functionalization of the imidazolate and benzimidazolate linkers was also shown to permit fine-tuning of the interactions between the pore walls and guest molecules, thereby varying the selectivity of adsorption. Importantly, in a great contrast to many metal–organic frameworks, ZIFs exhibit high thermal stabilities and chemical stability in refluxing aqueous and organic media, which are required for practical separations processes.27 Besides the significant progress made, the stability of a framework toward long-term exposure to water vapor remains critical issue limiting its suitability for CO2 capture from the exhaust gas.32 Nevertheless, MOFs and ZIFs are certainly among the most promising adsorbent of CO2 developed so far.
2.5.2 Microporous and mesoporous materials
Zeolites are natural microporous alumosilicates and are the earliest and most widely reported physical adsorbents for CO2 capture.27 They constitute the primary adsorption materials for commercial hydrogen production (involving H2/CO2 separation) using pressure swing adsorption, with the most popular of these materials being based on zeolite 13X.33 Zeolites are typically employed at elevated pressures (above 2 bar), however their adsorption capacity is greatly dependent on the presence of moisture in the gas, thereby necessitating very high regeneration temperatures (mostly over 300 °C).34 Therefore, the recovery costs can be a significant disadvantage. Besides the zeolites, an advantage of mesoporous solid materials, particularly silica based ones, is the ability to tune their chemical properties by impregnating or tethering active groups such as amines onto their internal surfaces. This strategy has been exploited and provided very promising results for low pressure CO2 capture applications, such as those relevant in flue streams and for capture from ambient air. In last decade numerous amine-modified silica materials have been prepared and tested.35 The post-synthesis surface modification with primary amines facilitates the adsorption of CO2 through the formation of carbamate species, reminiscent of the amine–CO2 chemistry
15
in conventional liquid phase scrubbing. The stripping of CO2 can be achieved at lower temperatures than those required for the regeneration of amine solvents (typically >100 °C), decreasing the energy consumption of the capture process.36 Polyethylenamine impregnated inside the pores of periodic MCM-41 mesoporous silica (Mobil Catalytic Material Number 41) has also been shown to lead to a significant enhancement (about 24-fold) in the CO2 absorption capacity of the solid support using a pressure swing adsorption approach.37 Polymers, such as poly-methylmethacrylate modified with a series of amines exhibit increased CO2 absorption capacities.38 However, the lack of stability of the materials and leaching problems over repeated cycles are the principal issues of all the materials with non-tethered functionalities. To overcome these limitations, the covalent attachment of the functional groups on the surface with a solid adsorbent can be performed. In a multiple reports alkylamines have been covalently linked to the surface of mesoporous supports in an attempt to increase their stability. For example, aziridine polymerization at the surface of mesoporous silica was used to generate a hyperbranched materials which was shown to exhibit reversible CO2 binding (with a capacity of 2 mmol CO2/g adsorbent) and multi-cycle stability under simulated flue gas conditions using a temperature swing adsorption approach.35 Surface of SBA-15 (Santa-Barbara Amorphous Number SBA-15) silica was modified by grafting of monoamino, diamino, and triamino ethoxysilanes and the influence of the amine type and the presence of moisture on CO2 adsorption performance have also been investigated.39 Capacities of 0.52, 0.87, and 1.10 mmol CO2/g adsorbent, respectively, were obtained. In the presence of a moist CO2 stream, the capacity decreased slightly for the primary amine, but increased by ca. 3 and 10% for the secondary and tertiary amine grafted materials, respectively. It was demonstrated that CO2 adsorption performance was strictly dependent on the surface density of the amine groups.34b The synthesis of functionalized mesoporous materials through the grafting procedure requires an excess of amines, and will be described in more details in a following section. Furthermore, the amount which is ultimately grafted to the surface may vary significantly over repeated syntheses in hardly-controlled manner and may not result in an optimal amount of grafted amine for the particular CO2 capture process. Additionally, the efficiency of solid adsorbents depends on both the rate of adsorption and the adsorption capacity, which must be optimal for specific adsorption process. These considerations highlight the importance of in-depth investigation of the influence and nature of grafting reagent added on the actual amount of amine that is covalently attached to the surface and its adsorption properties.
16 2.6 Concept of green chemistry
In the 1990s, the concept of “green chemistry” was initiated both in the US and Europe, and has since been adopted worldwide.40 Green chemistry is based on the design of chemical products and processes that generate and use as less as possible hazardous substances. The green chemistry message is simple: "Seek prevention, not cure". Proper application of the principles of green chemistry decreases both environmental risks and operating costs.42,a
Several terms were formulated in order to define the criteria of the green chemistry. First method for quantifying a reaction's efficiency is by examining the reactant conversion, the product selectivity, and the product yield over time. The reactant conversion is the fraction of reactant molecules that have transformed to the product molecules (regardless of which product it is). The selectivity is the fraction (or percentage) of the converted reactant that has turned into desired product. The yield of desired product is simply a multiplication of conversion and selectivity. High conversions in short time is typically associated with smaller and safer reactors. Similarly, high selectivity means less waste, and simpler and cheaper separation units. Thus, conversion, selectivity, and yield are all measures of the reaction efficiency and the latter one determines to what extent reaction is green. Additionally, there are specific markers for measuring the "greenness or eco-friendliness" of processes and products. One such measure is the E-factor, introduced by Sheldon in 1994.41 A reaction's E-factor is the quotient unit weight of waste per unit weight of product (waste is referred as everything formed in the reaction except the desired product). The waste can be any gas, water, common inorganic salts, heavy metal salts, and other by- and side-products. Table 2-1 compares the production tonnage and E-factors of various industrial sectors. Notably, the petrochemicals and the bulk chemicals sectors are the least polluting.
17
The concept of atom economy, introduced by Trost in 1991, is similar to that of the E-factor.43 It considers how many and which atoms of the reactants are incorporated into the products. With these two concepts, one can quantitatively evaluate chemical reactions. Finally Sheldon put forward the concept of the environmental quotient (EQ).41a By multiplying the E-factor by Q, where Q is an arbitrarily assigned hazard quotient, this measure takes both the amount and the nature of the waste into account.
2.7 The carbon dioxide molecule and its conversion into chemical feedstock
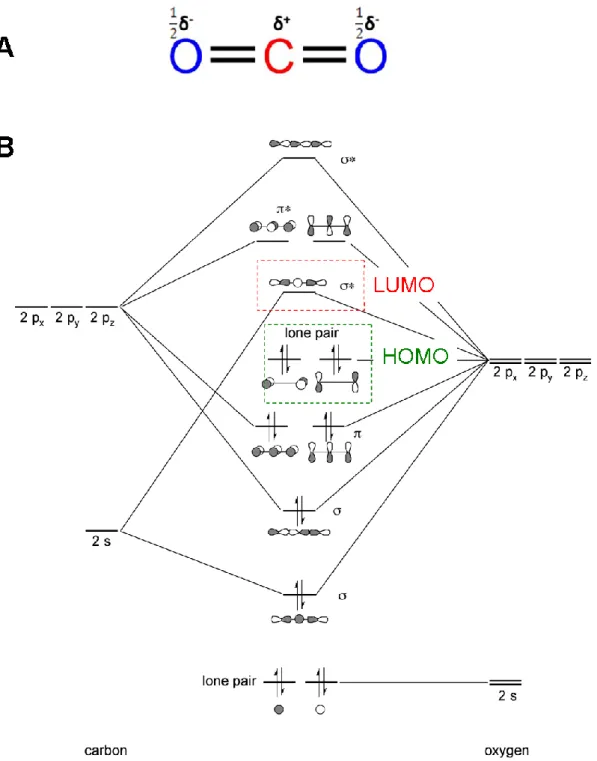
Carbon dioxide belongs to the D∞h point group and is linear in its ground state. Consequently, although it contains two polar C–O bonds, the molecule itself is non-polar. CO2 presents two different reaction sites, the carbon atom is an electrophile carrying +δ positive charge and the two oxygen atoms are nucleophiles carrying -1/2δ each: giving it an ambiphilic nature44 (Figure 2-5, A).
Due to the electron deficiency of the carbonyl carbon, CO2 has a strong affinity toward nucleophiles and electron-donating reagents. Because of this, CO2 is sometimes referred as an “anhydrous carbonic acid”,45 which rapidly reacts with basic compounds (Scheme 2-1). On the other hand, reactions of low-valent metal complexes (mainly Ni(0) and Pd(0)) with CO2 and unsaturated compounds lead to the formation of five-membered metallalactones.46 Hence, reactions involving CO2 can be categorized into two patterns: (1) formation of a carboxyl group through nucleophilic attack and (2) generation of a five-membered ring through oxidative cycloaddition.
When the carbon atom of CO2 is bonded to a third atom, the population of the LUMO-orbitals causes the distortion of the molecule from linearity, the O-C-O angle becomes close to 133°, the C–O bond length increases and the molecular energy of the molecule varies.
The molecular orbital diagram of CO2 is depicted in Figure 2-5 (B). The highest occupied molecular orbital (HOMO) is located preferentially on the oxygen atoms making them susceptible to electrophilic attacks (by Lewis acids), while the lowest unoccupied molecular orbital (LUMO) being energetically higher and thereby closer to the carbon atom, makes it reactive toward nucleophilic attacks (by Lewis bases). Such reactivity explains the ambiphilic nature of the CO2 molecule.
18
Figure 2-5. Representation of the ambiphilic character of the CO2 molecule (A) and MOs diagram of the CO2 molecule
In contrast, while one component of the lowest unoccupied molecular orbital LUMO remains largely unaffected, the out of plane component (corresponding to the anti-bonding orbital localized on the carbon atom) is drastically stabilized, to the point where it starts mixing with other bonding orbitals. Translating this observation in terms of reactivity reveals that introduction of electron density in this LUMO orbital will lead to bending of the CO2 molecule, drastically changing its fundamental properties.
19
Scheme 2-2. Examples of organic synthesis starting from CO245
Current industrial processes that use the most carbon dioxide are the synthesis of urea and polycarbonates (Scheme 2-2, highlighted in blue).47 About 70 Mt of CO2 per year are converted into urea, 30 Mt CO2 per year into inorganic carbonates and pigments and 6 Mt CO2 per year are used as an additive to carbon monoxide in the synthesis of methanol. Other chemicals, such as salicylic acid (20 kt CO2 per year) and propylene carbonate (a few kt per year), have a minor share of the market. In addition, 18 Mt of CO2 per year are used48 as a technological fluid. Comparing these numbers to the anthropogenic emissions of 26 gigatonnes (Gt) per year, we find that the combined