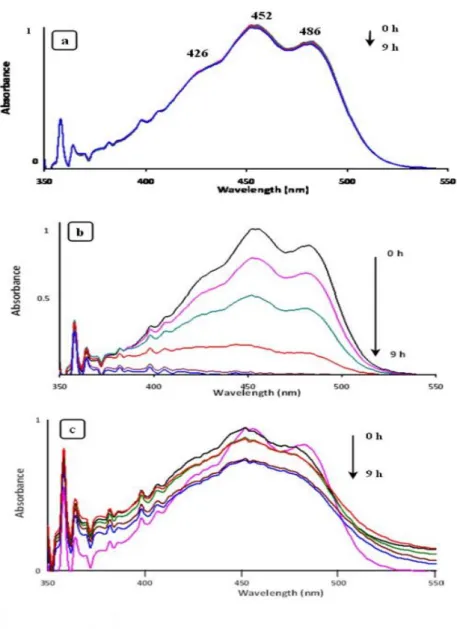
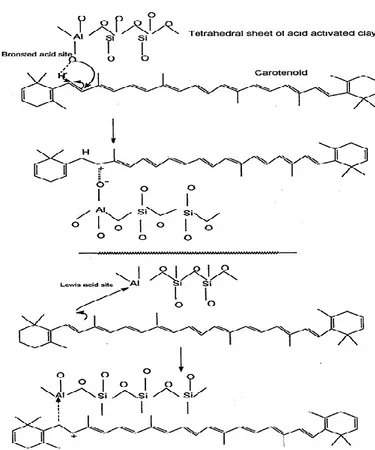
Documents relatifs
In acidic media, the shape of the adsorption isotherm leads to the following suggestions: firstly, for surfactant concentration below the CMC a surfactant monolayer is adsorbed on
By plotting the pre-exponential factors as a function of probe wavelength (yielding decay associated amplitude spectra), concerted changes in different parts of
Keyswords : Risk premiums · Distortion · Heavy-tailed distribution · Tail index · Extreme quantiles · Bias reduction..
In this paper, we propose a method to prove that a given class of models enjoys the minimality prop- erty, based on two main ingredients: the finite intersection property (fip) and
L’introduction de matériaux à propriétés pouzzolaniques comme le métakaolin, en remplacement d’un pourcentage du ciment Portland dans les mortiers et bétons peut être
In oblique shocks, that is in a 0^ range between ~ 50° - 60°, particles can get injected by the same mecha- nism that works at quasi-parallel shocks: we have pointed out that
Traditional budget targets, used for performance evaluation, focus on financial metrics. Such short-term targets nurture short-term thinking inside
Table 4: Relative impact (in %) of dominant experimental and theoretical uncertainties in the event yields for the top-quark background processes in the three signal regions (SR ggF