•M UfilWXSlTiXm
SHERBROOKE
Faculte de genie
Departement de genie chimique
DEVELOPPEMENT DE SURFACES BIOACTIVES POUR CONTROLER
LE COMPORTEMENT CELLULAIRE
UTILISATION DE PUCES DE POLYMERES ET DE BIOMOLECULES
DEVELOPMENT OF BIO ACTIVE SURFACES TO CONTROL CELL
BEHAVIOR
USE OF POLYMER AND BIOMOLECULE ARRAYS
These de doctorat es sciences appliquees
Specialite genie chimique
Emmanuelle MONCHAUX
1*1
Library and Archives Canada Published Heritage Branch 395 Wellington Street Ottawa ON K1A0N4 Canada Bibliotheque et Archives Canada Direction du Patrimoine de I'edition 395, rue Wellington Ottawa ON K1A0N4 CanadaYour file Votre reference ISBN: 978-0-494-37988-2 Our file Notre reference ISBN: 978-0-494-37988-2
NOTICE:
The author has granted a non-exclusive license allowing Library and Archives Canada to reproduce, publish, archive, preserve, conserve, communicate to the public by
telecommunication or on the Internet, loan, distribute and sell theses
worldwide, for commercial or non-commercial purposes, in microform, paper, electronic and/or any other formats.
AVIS:
L'auteur a accorde une licence non exclusive permettant a la Bibliotheque et Archives Canada de reproduire, publier, archiver,
sauvegarder, conserver, transmettre au public par telecommunication ou par Plntemet, prefer, distribuer et vendre des theses partout dans le monde, a des fins commerciales ou autres, sur support microforme, papier, electronique et/ou autres formats.
The author retains copyright ownership and moral rights in this thesis. Neither the thesis nor substantial extracts from it may be printed or otherwise reproduced without the author's permission.
L'auteur conserve la propriete du droit d'auteur et des droits moraux qui protege cette these. Ni la these ni des extraits substantiels de celle-ci ne doivent etre imprimes ou autrement reproduits sans son autorisation.
In compliance with the Canadian Privacy Act some supporting forms may have been removed from this thesis.
Conformement a la loi canadienne sur la protection de la vie privee, quelques formulaires secondaires ont ete enleves de cette these.
While these forms may be included in the document page count,
their removal does not represent any loss of content from the thesis.
Canada
Bien que ces formulaires
aient inclus dans la pagination, il n'y aura aucun contenu manquant.
A mes parents, Yves et Marie A mon frere et ma soeur, Geraud et Stephanie
Resume
La comprehension et le controle des interactions entre les cellules et les surfaces des materiaux sont essentiels pour la conception d'implants biocompatibles et fonctionnels. Ainsi, des surfaces bioactives prevenant toute reaction biologique non-specifique et fournissant des signaux dirigeant le comportement cellulaire sont developpees.
Les interactions entre les surfaces et les cellules endotheliales sont particulierement importantes pour des applications medicales telles que l'endothelisation des protheses vasculaires synthetiques et le developpement de tissus artificiels vascularises par genie tissulaire. L'objectif de ce travail de recherche est done de developper une surface bioactive interagissant specifiquement avec les cellules endotheliales par le developpement et l'utilisation de puces de polymeres et de surfaces bioactives.
Des puces de carboxy-methyl-dextran (CMD) sont developpees afin de determiner une surface anti-adherente resistant aux interactions non-specifiques. Les surfaces de CMD immobilisees selon differentes conditions sont caracterisees par spectroscopie des photoelectrons-X (XPS), microscopie a force atomique (AFM), exposees a un melange de proteines puis a des fibroblastes afin d'identifier les conditions d'immobilisation influencant leur structure et leur capacite a resister a 1'adsorption non-specifique de proteines et a 1'adhesion cellulaire. Une couche de CMD optimisee se revele aussi resistante a 1'adhesion cellulaire que le polyethylene glycol, polymere le plus utilise pour prevenir toute interaction non specifique.
Des puces de surfaces bioactives ciblant specifiquement les cellules endotheliales et comprenant les sequences peptidiques REDV, SVVYGLR et/ou le facteur de croissance VEGF sont fabriquees et exposees a des cellules endotheliales et des fibroblastes. Les molecules immobilisees n'induisent pas d'adhesion selective mais induisent la reorganisation du cytosquelette et des adhesions focales specifiquement chez les cellules endotheliales.
L'utilisation des puces de polymeres a permis le developpement d'une surface anti-adherente efficace pour la fabrication de surfaces bioactives et les puces de molecules bioactives rendent possible 1'etude de la reponse cellulaire face a des surfaces de composition variee.
Summary
Understanding and controlling interactions between cells and material surfaces are essential to design biocompatible and functional medical implants. To this end, bioactive surfaces preventing non-specific biological reactions and providing signals that guide cell behavior are designed.
Interactions between surfaces and endothelial cells are particularly important for biomedical applications such as endothelialization of vascular grafts and the development of vascularized artificial tissues by tissue engineering. The aim of this research project is then to develop a bioactive surface specifically interacting with endothelial cells by developing and using arrays of polymers and bioactive surfaces.
Arrays of carboxy-methyl-dextran (CMD) are made to determine a low-fouling surface preventing non-specific protein adsorption and cell adhesion. CMD layers are grafted with various conditions and are characterized by X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), exposed to proteins and then fibroblasts, to identify immobilization parameters that influence layers structure and ability to resist non-specific interactions. An optimized surface of CMD is obtained and is as resistant to cell adhesion as a layer of poly(ethylene glycol), the most used low-fouling polymer.
Arrays of bioactive surfaces specifically directed toward endothelial cells and made with peptide sequences REDV, SVVYGLR and/or vascular endothelial growth factor (VEGF) are synthesized and exposed to endothelial cells and fibroblasts. Immobilized biomolecules do not promote a selective endothelial adhesion but induce cytoskeleton and focal adhesion reorganization specifically for endothelial cells.
Use of polymer arrays allowed the development of a low-fouling surface efficient for the making of bioactive surfaces and biomolecule arrays allowed to study cell responses toward surfaces of various molecular composition.
Remerciements
Mes remerciements s'adressent tout d'abord a mon directeur de recherche, Patrick Vermette, qui m'a permis de venir finir mes etudes a Sherbrooke. Merci pour m'avoir confie ce sujet de recherche et soutenue financierement.
Je remercie les autres membres de l'equipe, etudiants gradues, post-docs et assistants de recherche, pour leurs conseils constructifs et la bonne ambiance de travail au laboratoire.
Je tiens a remercier tous les membres de mon jury de these qui me font l'honneur d'evaluer mon travail.
J'adresse egalement mes remerciements au personnel technique du departement de genie chimique et du centre de caracterisation des materiaux de 1'IMSI ainsi qu'au personnel administratif du departement de genie chimique pour leur bonne humeur et leurs coups de main toujours bienvenus.
Je remercie tous les etudiants gradues et post-docs de genie chimique qui m'ont permis de passer ces annees a Sherbrooke dans une ambiance multinationale enrichissante et des plus agreables.
Enfin, j'adresse mes remerciements les plus sinceres a ma mere, mon frere et ma soeur pour leur soutien durant ces annees d'etudes, surtout dans les moments de doute. Merci...
Table des matieres
1. Mise en contexte 1 2. Effets des proprietes de surface des biomateriaux et de la bioactivation sur les
cellules endotheliales 10
2.1. Resume 10 2.2. Abstract 11 2.3. Introduction 12 2.4. The endothelial tissue and its matrix 13
2.4.1. Morphological and functional heterogeneity 13 2.4.2. Endothelial cells - matrix interactions 16
2.4.3 Formation of blood vessels 18 2.5. Interactions between endothelial cells and biomaterial surfaces 20
2.5.1. Surface properties and the mechanical environment 21
2.5.2. Pre-coatings made of matrix proteins 24
2.5.3. Grafting of peptides 26 2.5.4. Growth factors immobilization 29
2.6. Conclusions and perspectives 31
2.7. References 33
3. Developpement de puces d'un derive du dextran pour identifier les proprietes
physico-chimiques impliquees dans l'adsorption de proteines du serum 41
3.1. Resume 41 3.2. Abstract 42 3.3. Introduction 43 3.4. Experimental section 45 3.4.1. Materials 45 3.4.2 Fabrication of CMD arrays 46
3.4.3 Elemental composition of CMD spots by X-ray photoelectron
spectroscopy (XPS) 48 3.4.4 CMD spot layer structure by AFM force measurements 49
3.4.5 CMD spot homogeneity and CMD fouling from serum measured by
3.5. Results and discussion 50 3.5.1 Elemental composition of CMD spots by XPS 50
3.5.2 AFM colloidal probe interaction forces with HApp layers 53 3.5.3 AFM colloidal probe interaction forces with CMD spots grafted using
high EDC+NHS/COOH ratios 54 3.5.4 AFM colloidal probe interaction forces with CMD spots grafted using low
EDC+NHS/COOH ratios 1 56 3.5.5 CMD spot homogeneity and CMD fouling from serum measured by SPR
microscopy 58 3.6. Conclusions 62 3.7. Acknowledgements 63
3.8. References 63
4. Etude des mecanismes de resistance a 1'adhesion cellulaire par utilisation de
puces d'un derive du dextran 66
4.1. Resume 66 4.2. Abstract 67 4.3. Introduction 68 4.4. Materials and methods 69
4.4.1 Carboxy-methyl-dextran (CMD) synthesis 69 4.4.2 Fabrication of arrays of CMD graft layers 69 4.4.3 Testing arrays of CMD graft layers towards cell responses 71
4.5. Results 72 4.5.1 Initial cell interaction with CMD graft layers: short-term assays 73
4.5.2 CMD resistance following 3 days of cell confluence 77
4.6. Discussion 82 4.7. Conclusions 88 4.8. Acknowledgements 88
4.9. References 88
5. Puces bioactives immobilisees sur des surfaces anti-adherentes pour etudier
l'adhesion specifique des cellules endothelials 92
5.1. Resume 92 5.2. Abstract 93 5.3. Introduction 94 5.4. Materials and methods 95
5.4.1. Carboxy-methyl-dextran (CMD) layers 95 5.4.2. Fabrication of arrays of bioactive molecules 96 5.4.3. Testing bioactive arrays towards cell responses 97
5.4.4. Cell adhesion assay 97 5.4.5. Cell labelling 98
5.5. Results 98 5.5.1. RGD peptide is required to initiate cell adhesion 99
5.5.2. REDV, SVVYGLR and VEGF specifically affect endothelial cells
adhesion when co-immobilized with RGD 99 5.5.3. REDV, SVVYGLR and VEGF affect actin filaments organization and
focal adhesion assembly in endothelial cells 101
5.6. Discussion 101 5.7. Conclusions 105 5.8. Acknowledgements 106
5.9. References 106
Conclusions et perspectives 109 Annexe A: Synthese du carboxy-methyl-dextran (CMD) 114
Annexe B : Imagerie par microscopie a force atomique (AFM) des puces de CMD
hydrates 117 Annexe C : Marquages immunocytochimiques 119
Liste des figures
Figure 3.1: Guiding system to localize individual CMD spots for XPS and AFM analyses. 48
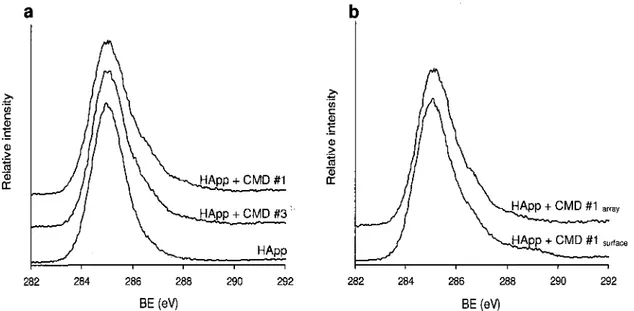
Figure 3.2: a) High-resolution XPS C Is spectra of a freshly deposited HApp layer and of CMD spots made under conditions no. 1 and no. 3. b) High-resolution XPS C Is spectra of CMD layers produced under condition no. 1 on a fully covered surface and on an
array. 51
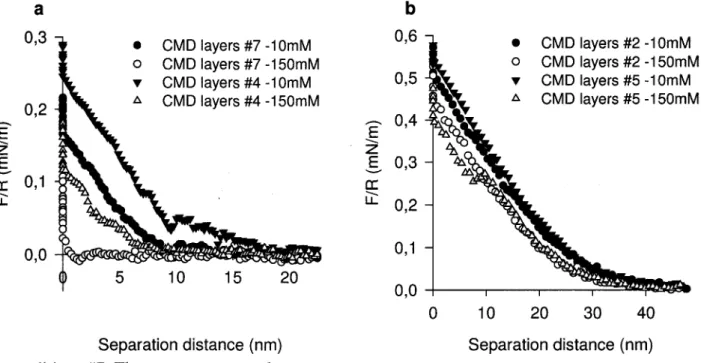
Figure 3.3: Representative colloidal probe force measurements between a silica colloidal
probe and HApp layers on a polymer array in two PBS solutions (pH 7.4). 53
Figure 3.4: Representative colloidal probe force measurements between a silica colloidal probe and spots of CMD grafted on HApp surface using high EDC+NHS/COOH ratios in two PBS solutions, (a) silica-CMD layers no. 1 and no. 6 and silica-CMD layers no. 3;
(b) silica-CMD layers no. 8. 55
Figure 3.5: Representative colloidal probe force measurements between a silica colloidal probe and spots of CMD grafted on HApp surface using low EDC+NHS/COOH ratios in two PBS solutions, (a) silica-CMD layers no. 4 and no. 7; (b) silica-CMD layers no. 2
and no. 5. 57
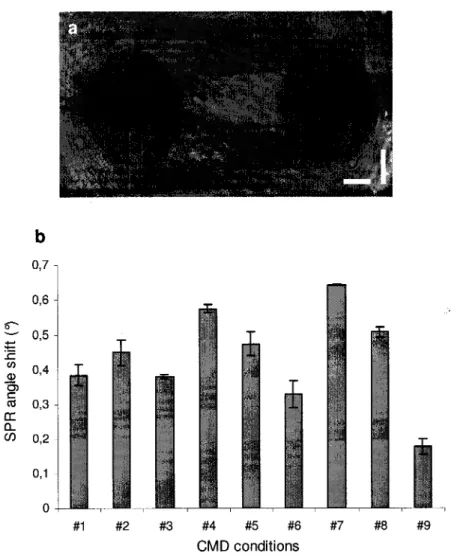
Figure 3.6: Protein adsorption on spots of CMD graft layers evaluated by SPR microscopy, a) Image of CMD layers produced in conditions no. 2 and no. 5 obtained at plasmon angle 0SPR (scale bars: 500pm). b) SPR angle shifts (°) resulting from FBS protein adsorption on CMD arrays. Condition no. 9 involves the use of 70kDa CMD, 25% carboxylation
degree, high EDC+NHS/COOH ratio, and a 2mg/ml CMD solution concentration. 59
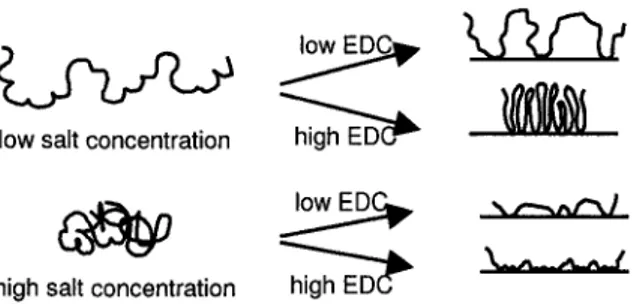
Figure 3.7: Schematic picture (not to scale) of immobilized CMD molecules. Final layer conformation can be controlled by electrolyte concentration and coupling agent ratio (EDC+NHS/COOH) during immobilization. CMD graft layers structures are
hypothesized based on the present XPS and AFM results. 63
Figure 4.1: Optical microscope images of fibroblasts seeded on CMD arrays following 4h (a,b) and 12h (c,d) cell seeding on CMD spots made using condition no. 8 (a,c) and
condition no. 3 (b,d). 73
Figure 4.2: Initial cell behavior on CMD arrays. Cells were fixed 4h following cell seeding. Cells on the HApp layer (a,b,c) and on a CMD spot (d,e,f: condition no.l and g,h,i:
Figure 4.3: Initial cell behavior on CMD arrays. Cells were fixed 12h following cell seeding. Cells on the HApp layer (a-c), on cell-resistant CMD spots (d-f, condition no. 9), and on
CMD layers made using condition no. 4 (g-i). 76
Figure 4.4: CMD spots surface coverage following 3-day exposition to confluent cells. Spot size was measured each day and compared to initial spot size. (*) Some spots were completely covered on Day 3, therefore the average initial size was not 1, resulting in
larger standard deviations. 77
Figure 4.5: CMD spots exposed to confluent fibroblasts for a period of 3 days. CMD layers
made using condition nos. 5 (a-c), 2 (d-f), and 9 (g-i). 78
Figure 4.6: Fibroblast actin cytoskeleton (a,d,g,j), focal adhesion formation (b,e,h,k) and human fibronectin deposition and reorganization (c,f,i,l) after 3 days of confluence on CMD spots. Cells found on HApp surfaces (a,b), at the edge of CMD spots made using condition nos. 1, 3, and 9 (c-f), on CMD spot made using condition no. 6 (g,h,i,k), and on
CMD spot made using condition no. 5 (j,l). 79
Figure 4.7: Determination of an optimal cell-resistant CMD surface. CMD surfaces
immobilized using optimized conditions and various carboxylation degrees (referred to as CMD-5, -25 and -50) and PEG surfaces made under cloud point conditions (PEG-CP) were exposed to confluent fibroblasts for a period of 3 days. A: spot size evolution. B:
optical microscope images of spots over the 3 days. 81
Figure 5.1. Reaction scheme for the grafting of carboxy-methyl-dextran (CMD) to the HApp-modified surface and subsequent bioactive molecule (peptide or growth factor)
immobilization. 98
Figure 5.2. Microscopy images of phalloidin-actin labeling of (A) human umbilical vein endothelial cells (HUVECs) and (B) human foreskin fibroblasts adhering on RGD spots, 6 h after cell seeding, and of (C) confluent HUVECs cultured on a RGD spot for 5 days
with 10% serum. Scale bar is 250 jam. 99
Figure 5.3. (A) Human umbilical vein endothelial cells (HUVECs) and (B) human foreskin fibroblasts adhesion on bioactive spots 6 h after cell seeding. Significant difference
compared with RGD alone (R25) at *p< 0.05 or ** p< 0.01. 100
Figure 5.4. Spreading levels measured for human umbilical vein endothelial cells (HUVECs)
on bioactive spots 6 h after cell seeding. 100
Figure 5.5. Actin filaments labeled with phalloidin (A, C, E, G, I, K, N, O) and focal
adhesions labeled with anti-vinculin (B, D, F, H, J, L, N, P) in human foreskin fibroblasts and human umbilical vein endothelial cells (HUVECs) on R25 (A, B, E, F), R25V (C, D, G, H), RE1 (I and J), REIV (K and L), SV1 (M and N), and SV1V (O and P) 6 h after
cell seeding. Scale bars are 25um. 102
Figure A.2: Representation schematique d'un monomere carboxyle de la chaine de CMD. 115
Figure A.3: Spectre H'-RMN dans D2O du dextran non modifie. f: hydrogene anomerique. 116
Figure A.4: Spectre H'-RMN dans D2O du CMD obtenu avec 1M d'acide bromoacetique. f:
hydrogene anomerique, *: hydrogenes du groupe carboxymethyl. 116
Figure B.l: Image AFM de l|am2 de la surface HApp prise dans le PBS 150mM. 117
Figure B.2: Images AFM de lum2 prises dans le PBS 150mM des "spots" de CMD de 70kDa
immobilises selon les conditions #5 (a) et #9 (b). 118
Figure B.3: Images AFM de lum2 prises dans le PBS 150mM des "spots" de CMD de 500kDa
Liste des tableaux
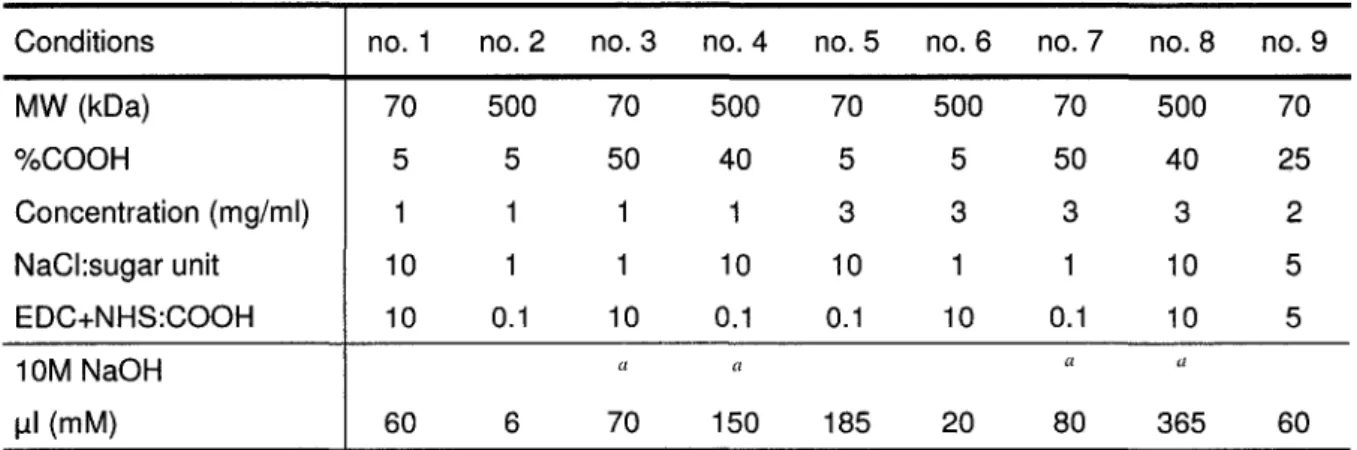
TABLE 3.1: CMD immobilization conditions used to produce spots of CMD graft layers. 47
TABLE 3.2: Elemental composition of CMD arrays and fully covered control CMD surfaces
on borosilicate glass determined by XPS analyses. 50
TABLE 4.1: CMD immobilization conditions used to produce arrays of CMD graft layers. 70
TABLE 4.2: Physico-chemical characterization of CMD layers and their resistance to protein
Liste des abreviations
AFM: atomic force microscopy Ang: angiopoietin
BSA: bovine serum albumin CMD: carboxymethyl dextran EC: vascular endothelial cell ECM: extracellular matrix FBS: foetal bovine serum
FEP: fluoroethylenepropylene (Teflon) FGF: fibroblast growth factor
Fn: fibronectin
HApp: R-heptylamine plasma polymer HFF: human foreskin fibroblast HGF: hepatocyte growth factor HS: heparan sulfate
HSPG: heparan sulfate proteoglycan
HUVEC: human umbilical vein endothelial cell ICAM: intercellular adhesion molecule
Ln: laminin
MMP: matrix metalloproteinase NMR: nuclear magnetic resonance NO: nitric oxide
OEG: oligo(ethylene glycol) PA: plasminogen activator PBS: phosphate-buffered saline PDGF: platelet-derived growth factor
PEG, PEO: poly (ethylene glycol), poly (ethylene oxide) PETP: polyethyleneterephthalate (Dacron)
PG: proteoglycan PGI2: prostacyclin
PTFE: polytetrafluoroethylene QCM: quartz crystal microbalance SMC: smooth muscle cell
SPR: surface plasmon resonance TCPS: tissue culture polystyrene TGF: transforming growth factor Tsp: thrombospondin
VCAM: vascular cell adhesion molecule VEGF: vascular endothelial growth factor VEGFR: VEGF receptor
Vn: vitronectin
Chapitre 1
Mise en contexte
Comprendre, optimiser et controler les interactions entre les materiaux et leur environnement biologique, pouvant contenir des proteines, des lipides et/ou des cellules, est essentiel pour le developpement de biomateriaux integres et fonctionnels une fois implantes dans l'organisme.1'2 Dans le domaine biomedical, les interactions materiaux - biomolecules
sont responsables du succes ou de l'echec de 1'implantation de protheses, destinees a remplacer ou ameliorer une fonction vitale perdue ou defaillante. Ces interactions dictent aussi le destin de biosenseurs, de membranes de dialyse, de systemes de liberation controlee de principes actifs ou bien encore, ces interactions sont essentielles pour la conception de structures polymeriques permettant le developpement controle d'un tissu fonctionnel par genie tissulaire.1'3
Surfaces bioactives
La plupart des biomateriaux implantes sont rapidement recouverts d'une couche de proteines, via des interactions de type van der Waals, hydrophobiques, electrostatiques et des liaisons hydrogenes. Des cellules, dont des monocytes, adherent a la couche de proteines pre-adsorbees; l'activation des monocytes mene a une reaction inflammatoire prolongee et a la formation d'une capsule fibreuse autour des implants, dont l'integration et la fonction sont alors alterees.4'5
Les surfaces des biomateriaux doivent done etre con§ues pour prevenir toute interaction non specifique menant a une reponse chaotique et non desiree. Elles doivent aussi pouvoir induire la reaction biologique desiree, interagissant de facon specifique avec leur environnement, pour une meilleure integration des implants. Dans cette optique, il est possible d'immobiliser une couche anti-adherente sur les surfaces des biomateriaux pour empecher toute interaction non specifique et ensuite, de greffer des molecules bioactives
pre-determinees, reconnues par les cellules environnantes et leur fournissant des signaux specifiques.
Les couches anti-adherentes peuvent etre utilisees seules lorsque aucune reaction n'est desiree a la surface du biomateriau. Cela peut concerner les membranes de dialyse, pour limiter leur obstruction, ou bien 1'extremite sensible des biosenseurs, afin d'augmenter le ratio signal/bruit.5 Un des principaux objectifs de la modification de surfaces de biomateriaux par
immobilisation d'une couche anti-adherente et le greffage subsequent de molecules bioactives choisies est de favoriser l'integration du biomateriau (c'est a dire une cicatrisation controlee) et ainsi sa fonctionnalite. Des electrodes implantees dans le systeme nerveux induisent la formation d'une cicatrice gliale qui gene la mesure d l'activite neuronale (a long terme).6 La
surface de l'electrode peut etre modifiee dans le but de limiter la proliferation des cellules gliales et la formation de cette cicatrice tout en favorisant l'adhesion des neurones. De meme, l'implantation de protheses vasculaires ou d'implants en contact avec le sang induit l'adhesion des plaquettes a leur surface et la formation d'un caillot, et a plus long terme, d'autres complications. II est done necessaire de modifier la surface de ces implants de maniere a empecher ces reactions et favoriser la formation d'une monocouche de cellules endotheliales aux proprietes thrombiques, c'est a dire dans leur etat differencie. Enfin, les surfaces anti-adherentes sur lesquelles ont ete greffees des biomolecules peuvent servir de modeles pour l'etude de l'interaction entre une molecule et un type cellulaire et de ses consequences sur le comportement cellulaire.
Couches anti-adherentes
Des couches anti-adherentes composees de chaines moleculaires sont immobilisees sur les surfaces des biomateriaux pour prevenir toute interaction non specifique menant a l'adsorption de proteines et a l'adhesion cellulaire non specifiques. Le caractere repulsif de ces couches peut etre explique par differents mecanismes.8 La resistance a l'adsorption proteique
peut etre expliquee par un phenomene de repulsion sterique lorsqu'une proteine interagit avec une couche dense et compressible.9'10 Elle peut egalement etre due a la presence d'une couche
d'eau a l'interface "surface anti-adherente/environnement", prevenant toute interaction avec la surface.1112 La stabilite de cette couche d'eau depend de l'orientation des fragments des
environnantes. Ainsi, la capacite des couches immobilisees a prevenir les interactions non specifiques depend de leur structure. Ces couches, le plus souvent constitutes de polymeres tels que le poly(ethylene glycol) (PEG) et les polysaccharides, sont generalement caracterisees par une forte densite de chaines, une faible densite de charge et presentent une interface hydratee.12"15
Techniques de fabrication. Les surfaces de biomateriaux a modifier peuvent etre
1 f\ 17
activees par oxydation, par silanisation (pour les surfaces contenant de la silice) ' ou bien par deposition de molecules organiques par plasma (incorporation de groupements amines ou carboxyles par exemple).15'18 Les groupements reactifs ainsi introduits a la surface permettent
l'immobilisation covalente des couches anti-adherentes.
Les couches les plus etudiees et utilisees sont composees de PEG (aussi appele oxyde de polyethylene, PEO), un polymere synthetique, hydrophile et neutre. L'efficacite des couches de PEG depend de la densite des chaines a l'interface.9'15 Les chaines de PEG portant
un groupement reactif tel qu'un aldehyde ou une amine primaire a une extremite, peuvent etre covalemment immobilisees sur une surface activee par Taction d'agents de couplage.1 Afin
d'augmenter la densite de chaines l'interface, le PEG peut etre immobilise sous les conditions de solvatation limite ou "cloud point": sous ces conditions, les repulsions inter-chaines sont reduites durant la reaction d'immobilisation, resultant en une plus grande densite de chaines a la surface.15 Une forte densite de groupements oligo(ethylene glycol) (OEG) peut aussi etre
1 -3 obtenue par la formation de couches auto-assemblees ("self-assembled monolayers", SAMs). Ces couches de structure et de chimie definies decoulent de l'assemblage organise de molecules d'alkyle-silane ou d'alcane-thiol exposees respectivement a une surface de silice ou a une surface metallique (or ou argent le plus souvent). Un bout de la chaine moleculaire se lie fortement a la surface alors que l'autre extremite de la chaine, qui peut etre constitute d'une variete de groupements fonctionnels, reste libre a la face externe de la couche. Les SAMs terminees par des groupements OEG previennent l'adsorption non specifique de proteines.
Les couches formees de polysaccharides recoivent un interet grandissant puisque la barriere protectrice presente a la surface des cellules, le glycocalyx, est composee de polysaccharides. Ces polymeres naturels, hautement hydrates, portent des groupements hydroxyles qui peuvent etre modifies a differents degres, soit par oxydation (formation de
groupements aldehydes), ' soit par addition de groupements reactifs (carboxyles, amines primaires).18 Les chaines de polysaccharides peuvent egalement etre thiolatees et adsorbees
sur une surface d'or.14 Ainsi, des surfaces de biomateriaux recouvertes de dextran, alginate,
hyaluronane ou de leurs derives montrent un certain degre de resistance, selon leur structure a la surface,11'18,21 et les couches de polysaccharides peuvent etre aussi repulsives que le
00 0%
PEG, ' tout en presentant plusieurs sites de liaison.
Immobilisation de biomolecules
Des biomolecules peuvent etre greffees sur une couche anti-adherente dans le but de transmettre un signal promouvant l'adhesion cellulaire, la proliferation, la migration, la differentiation ou bien la maintenance d'un phenotype differencie. Le signal est efficacement transmis aux cellules si les biomolecules sont presentes dans une conformation stable, reconnaissable par les recepteurs cellulaires, avec une densite et une orientation appropriees. II est aussi necessaire de souligner l'importance de l'absence d'interactions non specifiques pour une transmission du signal optimale. Le but ultime de l'immobilisation de biomolecules est la preservation de leur activite et de leur specificite. II peut s'agir de proteines, de peptides ou sequences-signal, ou bien de fragments de proteine, immobilises seuls ou en combinaison.
Modes d'immobilisation. La formation d'une liaison covalente entre la surface anti-adherente et les biomolecules est le mode de greffage le plus utilise. La liaison se fait entre un groupement fonctionnel expose a la surface de la couche anti-adherente et un groupement fonctionnel present dans un peptide (amine primaire, carboxyle ou thiol d'un acide amine terminal) ou bien expose a l'exterieur d'une proteine (amine de la lysine, thiol de la cysteine), via des agents de couplage.24'25 Si necessaire, la liaison peut etre faite via une molecule
d'espacement ou "spacer", tel que le PEG, pour eloigner la molecule de la surface et augmenter son exposition et sa liberte de conformation.26 La liaison covalente via l'utilisation
d'agents de couplage est une technique simple et polyvalente, cependant, ce mode d'immobilisation est non specifique et, dans le cas de proteines, offre peu de controle sur l'orientation et l'activite de la molecule immobilisee.
Aussi, il existe des techniques de greffage par l'intermediaire de complexes recepteur-ligand reposant sur la reconnaissance specifique des biomolecules, permettant l'attachement
de molecules dans leur conformation native et avec une orientation connue. Ainsi, des anticorps monoclonaux diriges contre la biomolecule d'interet peuvent etre immobilises sur une couche anti-adherente; un systeme plus polyvalent repose sur l'utilisation de l'avidine comme agent de liaison entre la surface et une biomolecule biotinylees.28 Enfin, des sequences
composees d'histidines peuvent etre inserees dans des sequences proteiques ("histidine-tags"), elles permettent l'attachement dans une orientation connue de ces molecules sur une couche presentant des atomes metalliques chelates.29
Enfin, l'incorporation de molecules bioactives dans une couche anti-adherente lors de la formation de celle-ci permet de controler leur densite a la surface.2 '30 Ainsi, un hydrogel de
PEG bioactif peut resulter de la polymerisation de molecules de PEG acrylees et de molecules de PEG liees a une sequence bioactive. Des molecules bioactives peuvent aussi etre incorporees dans une bicouche lipidique, composee de molecules amphiphiles (queue di-alkyle lipidique et tete hydrophile contenant la sequence bioactive ou bien un groupement OEG sur la couche exterieure).31,32 Ces structures, mimant les membranes cellulaires,
conferent stabilite et mobilite aux biomolecules immobilisees.
Objectifs du travail
Les interactions specifiques entre une surface et des cellules endotheliales sont recherchees pour de nombreuses applications biomedicales. Par exemple, le recouvrement de la surface luminale des protheses vasculaires de petit diametre par une couche de cellules endotheliales stable et complete permettrait d'exposer au sang une surface non thrombogene et ainsi de limiter leur occlusion.7'33 Egalement, l'interaction des cellules avec une surface
pourrait promouvoir 1'infiltration de cellules endotheliales dans une structure polymerique suivie de la formation de vaisseaux sanguins pour le developpement d'un tissu fonctionnel par genie tissulaire. Un tissu sain necessite un apport constant en oxygene et en nutriments pour croitre et survivre.34,35 Enfin, inciter la formation d'un reseau de capillaires sanguins autour
d'un implant favoriserait son integration dans l'organisme.4 Des surfaces favorisant
1'adhesion, la proliferation et/ou la migration plus ou moins controlees des cellules endotheliales ont ete developpees mais peu permettent 1'adhesion selective et une action
specifique sur le comportement de ces cellules dans des systemes de co-culture en 2D voire en 3D, pour le developpement de reseaux vasculaires fonctionnels.
L'objectif principal de ce travail est done de developper et de caracteriser une surface bioactive modele capable d'interagir specifiquement avec des cellules endotheliales. Tout d'abord, (i) le travail de recherche consiste a developper un revetement de surface inerte, resistant a 1'adsorption non specifique de proteines ainsi qu'a 1'adhesion cellulaire. Pour ce faire, des couches d'un polymere, immobilisees selon differentes conditions, sont caracterisees et exposees a un melange de proteines et a des cellules afin de determiner les parametres influencant leur structure et leur resistance et egalement, de trouver une surface inerte optimisee. Ensuite, (ii) des molecules bioactives specifiques pour les cellules endotheliales sont immobilisees sur ce revetement et la reponse de differents types cellulaires exposes a ces surfaces est etudiee.
Originalite
L'originalite de ce projet de recherche repose sur la methode experimental employee. Des puces de polymeres sont developpees et utilisees pour la caracterisation et l'optimisation des surfaces inertes tandis que des puces de molecules bioactives sont fabriquees pour l'etude du comportement cellulaire face a des surfaces de composition moleculaire variee. Les puces sont realisees a l'aide d'un robot habituellement utilise pour la fabrication de puces d'ADN ou de proteines. La possibilite de deposer sur une meme surface une multitude de solutions de compositions differentes permet non seulement de caracteriser simultanement les differentes surfaces creees, mais aussi d'etudier et de comparer l'effet d'un plus grand nombre de variables (facteurs influen§ant 1'immobilisation du polymere, composition des surfaces bioactives). Enfin, la polyvalence du robot permet de creer des surfaces avec une taille, un espacement, et un nombre d'echantillons varies et determines selon les besoins de l'experience donnee.
Structure de la these
Ainsi, apres avoir presente dans un premier chapitre le contexte dans lequel s'inscrit ce travail de recherche, une revue de litterature abordant les interactions entre les cellules endotheliales et leur environnement in vivo et exposant les modifications de surfaces experimentees pour favoriser 1'adhesion des cellules endotheliales est presentee dans le deuxieme chapitre. En effet, la connaissance et la comprehension des interactions entre les cellules et leur environnement dans l'organisme d'une part et au laboratoire d'autre part sont essentielles pour la conception de surfaces intelligentes permettant l'integration d'un biomateriau et guidant la reparation ou le developpement d'un tissu fonctionnel. Ce chapitre sera soumis a la revue Frontiers in Bioscience.
Dans le troisieme chapitre, la fabrication de puces de polymeres est detaillee, leurs proprietes physico-chimiques sont caracterisees par spectroscopie des photoelectrons-X (XPS) et par microscopie a force atomique (AFM). Les puces sont egalement exposees aux proteines de serum. L'analyse des resultats a permis de correler les conditions d'immobilisation des couches de polymere avec leur structure et leur capacite a repousser les proteines. Cette etude a fait l'objet d'un article publie dans le journal Langmuir.
Dans le quatrieme chapitre, les puces de polymeres sont exposees a des cellules afin de poursuivre la caracterisation des conditions plus ou moins resistantes. Le mecanisme d'invasion des cellules selon le degre de resistance et la structure de la couche est etudie et un revetement inerte optimal est determine grace a l'ensemble des resultats obtenus. Ce travail est decrit dans un article sous presse pour le periodique Journal of Biomedical Material Research- Part A.
Le cinquieme chapitre est dedie a 1'etude de puces bioactives synthetisees par immobilisation de molecules specifiques pour cellules endotheliales sur la couche inerte. L'effet de la composition moleculaire des spots bioactifs sur l'adhesion et la morphologie des cellules endotheliales et des fibroblastes est analyse. Ce travail a ete publie dans le journal Biomacromolecules.
References
(1 (2 (3 (4 (5 (6 (7 (8 (9(io:
(11 (12 (13 (14; (15 (i6; (17 (18 (19 (2o; (21Ratner, B. D.; Hoffman, A. S.; Schoen, F. J.; Lemons, J. E. Biomaterials science, an introduction to materials in medicine.; 2nd ed.; Elsevier: 2004.
Lutolf, M. P.; Hubbell, J. A. Nat. Biotechnol. 2005,23, 47-55.
Langer, R.; Vacanti, J. P. Science 1993,260, 920-926.
Ratner, B. D. J. Control Release 2002, 78, 211-218.
Wisniewski, N.; Reichert, M. Colloids Surf. B Biointerfaces 2000,18, 197-219.
Fawcett, J. W.; Asher, R. A. Brain Res Bull 1999,49, 377-391.
Merzkirch, C ; Davies, N.; Zilla, P. Anat Rec 2001,263, 379-387.
Morra, M. J. Biomater. Sci. Polym. £J2000,11, 547-569.
Jeon, S. I.; Lee, J. H.; Andrade, J. D.; DeGennes, P. G. Journal of Colloid and Interface Science 1991,142, 149-158.
Halperin, A. Langmuir 1999,15, 2525-2533.
Morra, M.; Cassinelli, C. Langmuir 1999,15, 4658-4663.
Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G. M.; Laibinis, P. E. J. Phys. Chem. B 1998,102, 426-436.
Prime, K.; Whitesides, G. Science 1991,252, 1164-1167.
Frazier, R. A.; Matthijs, G.; Davies, M. C.; Roberts, C. J.; Schacht, E.; Tendler, S. J. Biomaterials 2000,21, 957-966.
Kingshott, P.; Thissen, H.; Griesser, H. J. Biomaterials 2002, 23, 2043-2056.
Tasker Langmuir 1996,12, 6436-6442.
Martwiset, S.; Koh, A. E.; Chen, W. Langmuir 2006,22, 8192-8196.
McArthur, S. L.; McLean, K. M.; Kingshott, P.; St John, H. A. W.; Chatelier, R. C.; Griesser, H. J. Colloids and Surfaces B-Biointerfaces 2000, 17, 37-48.
Vermette, P.; Meagher, L. Colloids and Surfaces B-Biointerfaces 2003,28, 153-198.
senaratne, W.; Andruzzi, L.; Ober, C. K. Biomacromolecules 2005, 6, 2427-2448.
Osterberg, E.; Bergstrom, K.; Holmberg, K.; Schuman, T. P.; Riggs, J. A.; Burns, N. L.; Van Alstine, J. M.; Harris, J. M. J. Biomed. Mater. Res. 1995,29, 741-747.
Pallarola, D.; Domenianni, L.; Priano, G.; Battaglini, F. Electroanalysis 2007,19, 690-697.
Johnsson, B.; Lofas, S.; Lindquist, G. Anal. Biochem. 1991,198, 268-277.
Lahiri, J.; Isaacs, L.; Tien, J.; Whitesides, G. M. Anal Chem 1999, 71, 777-790.
Hern, D. L.; Hubbell, J. A. J. Biomed. Mater. Res. 1998,39, 266-276.
Calonder, C ; Matthew, H. W.; Van Tassel, P. R. J. Biomed. Mater. Res. A 2005, 75, 316-323.
Marie, R.; Beech, J. P.; Voros, J.; Tegenfeldt, J. O.; Hook, F. Langmuir 2006, 22, 10103-10108.
Kato, K.; Sato, H.; Iwata, H. Langmuir 2005,21, 7071-7075.
Murugesan, G.; Ruegsegger, M. A.; Kligman, F.; Marchant, R. E.; Kottke-Marchant, K. Cell Commun. Adhes. 2002, 9, 59-73.
Dillow, A. K.; Ochsenhirt, S. E.; McCarthy, J. B.; Fields, G. B.; Tirrell, M. Biomaterials 2001,22, 1493-1505.
Ochsenhirt, S. E.; Kokkoli, E.; McCarthy, J. B.; Tirrell, M. Biomaterials 2006,27, 3863-3874.
Dardik, A.; Liu, A.; Ballerman, B. J. J Vase Surg 1999,29, 157-167.
Bouhadir, K. H.; Mooney, D. G. J. Drug Target. 2001, 9, 397-406.
Chapitre 2
Effets des proprietes de surface des biomateriaux et
de la bioactivation sur les cellules endothelials
2.1. Resume
Les interactions entre les cellules endotheliales vasculaires (ECs) et les materiaux sont centrales pour des applications biomedicales telles que l'endothelisation de pro theses vasculaires ou la vascularisation de substituts tissulaires. Afin d'ameliorer les resultats des implants, les surfaces des biomateriaux sont con§ues pour promouvoir 1'adhesion des ECs et diriger leur comportement. In vivo, les ECs recouvrent tous les vaisseaux sanguins ; leur morphologie, leur fonction et la matrice associee sont localement adaptees et specifiques au micro-environnement. Pour induire 1'adhesion et la croissance des ECs, des traitements modifiant les proprietes physico-chimiques et mecaniques des surfaces des materiaux ont ete developpes. Les materiaux peuvent aussi etre recouverts de molecules bioactives telles que des proteines de la matrice, des peptides et/ou des facteurs de croissance afin d'etudier et controler le comportement des ECs. Le but de cette revue est de donner un apergu des connaissances actuelles au sujet des ECs et de leur environnement solide in vivo et de leurs reponses face aux surfaces synthetiques in vitro.
Effects of biomaterials surface properties and
bioactivation on endothelial cells
Emmanuelle Monchaux, Patrick Vermette
2.2. Abstract
Interactions between vascular endothelial cells (ECs) and materials are central to biomedical applications such as vascular graft endothelialization or vascularization of an engineered tissue substitute. To improve implant success, biomaterial surfaces are designed to promote EC adhesion and direct their response. In vivo, ECs line all blood vessels; their morphology, function and associated matrix are locally adapted to and specific for the microenvironment. To enhance EC adhesion and growth, surface treatments have been developed that modify materials surface physico-chemical and mechanical properties. Materials may also be coated with bioactive molecules such as proteins from the matrix, peptides and/or growth factors to study and control EC behavior. The aim of this review is to give an overview of current knowledge about EC and their solid environment in vivo and their responses to synthetic surfaces in vitro.
2.3. Introduction
Adhesion of endothelial cells (ECs) on biomaterial surfaces and subsequent controlled behavior are of increasing importance in the biomedical field with implication in the endothelialization of vascular grafts or in the formation of a vascular network in engineered tissue substitutes. Prosthetic vascular grafts used to replace small-diameter blood vessels (diameter < 6mm) are characterized by a reduced patency and occlusion: thrombosis and intimal hyperplasia are the main reasons for the high failure.1 The lack of a complete
endothelium covering blood contacting devices is a major contributing factor to both phenomena. One approach to prevent thrombosis and to improve the hemocompatibility of synthetic vascular grafts is to create a functional, quiescent monolayer of ECs on the graft surface prior to implantation. Another solution is to develop implants that will enhance endothelialization upon implantation. ECs in-growth and formation of a functional, mature vascular network remain a challenge in tissue engineering research; this network is required for the construction or regeneration of hybrid tissues. ' Similar to normal tissues, engineered tissues need blood supply to grow and to remain viable. In addition, implant biocompatibility could be improved by promoting a normal wound healing response including peri-implant vascularization and reduced encapsulation.4
Biomaterial science and tissue engineering rely heavily on cell-material interactions: surfaces may induce cell adhesion, determine cell fate and promote the regulated development of functional structures.5 In vivo, cells are anchored to their extracellular matrix (ECM) and
cell-ECM interactions modulate cells survival and responses.6 Biomaterial surfaces should
thus be designed to mimic cells biological environment and the knowledge of the interactions of cells with their natural environment in an organism is essential to develop implants that will be integrated into the host organism.
The objectives of this review paper are to give an overview of ECs and their natural environment in vivo and to present surface modifications and their effect on EC responses in vitro. In the first part of this paper, normal endothelial tissue characteristics and functions are presented, and interactions with its ECM in vivo are described with a particular interest in the dynamic process of blood vessel formation through angiogenesis. The second part deals with EC interactions with synthetic surfaces. A multitude of surface chemical modifications and
bioactive coatings have been developed to promote EC adhesion onto biomaterials. Substrate properties and immobilization mode influence proteins binding to their receptors and consequently, cell response. Combined immobilization of various signaling molecules and its effects on cell responses are also discussed.
2.4. The endothelial tissue and its matrix
Vascular endothelial cells (ECs) are a specialized type of epithelial cells lining the inner surface of blood vessels of the entire circulatory system, from the heart, arteries and veins to smallest capillaries. They do not form a passive barrier between circulating blood and surrounding tissues. In fact, the endothelium provides a non-thrombogenic surface, communicates with the surrounding microenvironment and releases biochemical regulators. ECs hence form a heterogeneous tissue, they exhibit a great diversity in morphology and functions among the vascular tree depending on vessel type, tissue irrigated and activation state. Interactions between ECs and the ECM more particularly are crucial for the maintenance of ECs integrity and functions and for the controlled formation and regeneration of blood vessels.
2.4.1. Morphological and functional heterogeneity
'Morphological diversity
Walls of large vessels like arteries and veins consist of three layers: an inner intima made up of a layer of ECs attached to their basement membrane, an intermediate media mainly composed of smooth muscle cells (SMCs) and elastic fibers and an outer adventitia made of collagenous ECM with fibroblasts, blood vessels and nerves. Arteries are muscular, elastic blood vessels with thick walls that possess elastic laminae surrounding the intima and the media, and that pulsate. Veins have thin walls, they do not pulsate but possess valves.7 In
vivo, vascular ECs experience fluid shear stress, the tangential component of hemodynamic stresses. In large straight arteries of uniform geometry, the mean wall shear stress is between 10 and 20 dynes/cm2 while in regions of non-uniform geometries (branches and arches)
aligned in the direction of blood flow in straight segments of arteries but not at branch points; in veins, ECs are shorter and flat and are not aligned in the direction of blood flow.7
Arterioles and venules are intermediate vessels between capillaries and arteries and capillaries and veins, respectively. Pre-capillary arterioles are completely surrounded by one or two layers of SMCs and post-capillary venules are surrounded by pericytes embedded in their basement membrane.8
Capillary microvessels represent the most abundant vessels in an organism and consist of ECs surrounded by a basement membrane and occasional pericytes, allowing direct physical contact between endothelial and tissue cells. ECs in capillaries are flattened, elongated, they adapt to their microenvironment and acquire specialized characteristics to accommodate local physiological requirements.7'9,10 Continuous endothelium consists of ECs
tightly connected to each other via tight junctions and surrounded by a complete basement membrane. It is found in capillaries of the brain, heart, skin and lung, as well as in arteries and veins. Further specialization of the continuous endothelium is observed in brain, blood-retina and blood-testis barriers with acquisition of complex tight junctions and highly regulated transcellular transports. Fenestrated continuous capillaries are characterized by the presence of small openings called fenestrae, and are found in capillaries with an increased fluid exchange between blood and tissues: diaphragmed fenestrated capillaries are found in endocrine and exocrine glands, gastric and intestinal mucosa, and renal tubules whereas non diaphragmed fenestrated are present in renal glomerulus. Finally, discontinuous endothelium, characterized by the presence of many large fenestrations with no diaphragm and gaps, presents a poorly formed basement membrane (discontinuous or absent) and is found in more restricted regions such as capillaries of the liver, spleen and bone marrow.
'Functional diversity
Transport function. Capillaries form the main site of exchange of nutrients between blood and tissues. They use several specific transport mechanisms to meet the metabolic needs of the surrounding tissue cells. Fluids and small solutes move passively across the barrier via the paracellular pathway, regulated by intercellular tight junctions, whereas macromolecules use transcellular transports, controlled by the presence of specific membrane receptors or vesicular carriers such as caveolae and vesiculo-vacuolar organelles.9'11 Spatial heterogeneity
of permeability depends on differences in junctional properties and presence or absence of fenestrae and gaps.
Vasomotricity. Transport may be regulated by blood perfusion which is locally controlled by vasomotricity of pre-capillary arterioles. Generally speaking, ECs finely control blood flow in response to metabolic demand (oxygen tension and glucose concentration, for instance), cytokines and shear stress, by acting on SMCs in vessel walls of arterioles and large vessels. This regulation is short and local, through the production and catabolism of vasoactive molecules by ECs, either vasodilators such as nitric oxide (NO), prostacyclin (PGI2) or vasoconstrictors such as endothelins.11
Host defense and inflammation. The endothelium actively participates in the inflammatory response following tissue infection or irritation, mainly at post-capillary venule sites where cell-cell junctions are looser. Activated ECs (i) secrete vasoactive molecules to locally increase permeability, (ii) express receptors for immune cells adhesion such as vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM) and (iii) secrete cytokines for the recruitment of leucocytes and induction of angiogenesis. EC activation allows adhesion and transmigration of leucocytes to inflammation sites and neovascularization of the injured tissue.
Vascular hemostasis. The endothelium lining arteries, veins and all blood vessels provides a non-thrombogenic, anti-coagulant surface by the secretion and/or surface expression of several regulatory factors that maintain blood in a fluid state. An intact EC monolayer is covered by a layer of glycocalyx containing anti-coagulant heparan sulfate proteoglycans (HSPGs) and anti-thrombic thrombomodulin. ECs also secrete vasodilators that prevent platelet adhesion. When a vascular lesion occurs, platelets adhere to exposed vessel walls, ECs and surrounding cells secrete pro-coagulant molecules leading to the formation of a fibrin clot and finally EC produce fibrinolytic effectors to limit clot formation. The nature of factors involved in vascular hemostasis depend on location in the vascular tree.''
2.4.2. Endothelial cells - matrix interactions
mCell-ECM interactions
ECs interactions with their underlying ECM are essential for maintenance of cell integrity and functional activity, and for the formation of functional mature blood vessels. The ECM provides mechanical support and biochemical cues for cell adhesion, migration, proliferation and differentiation via interactions with cell membrane receptors and through growth factor sequestering. Matrix proteins and more particularly adhesive ones such as fibronectin (Fn), laminins (Ln) and vitronectin (Vn) possess many binding domains capable of interacting with other ECM proteins as well as with cell surface receptors. Defined amino acid sequences present within ECM molecules specifically bind cell surface receptors to trigger various intracellular pathways. Cell-ECM adhesions are mediated primarily by integrin receptors, heterodimeric transmembrane proteins composed of a and P subunits that connect the ECM molecules to the cell cytoskeleton. When bound to their specific ligand, integrins cluster, form focal adhesion structures, mediate cell anchorage to the underlying matrix, and can also initiate signaling cascades transduced to the nucleus.12 These events may affect many
aspects of the cell responses such as proliferation, differentiation, migration and survival. A single cell binding motif can be found within several proteins, such as the most investigated Arg-Gly-Asp (RGD) sequence present in Fn, Vn and Ln among others. A protein is able to bind several receptors through various sequences which exposition depends on protein self-assembly into fibers or a network, its association with other ECM molecules or its proteolytic degradation. Moreover, cell membrane receptors frequently associate with other receptors such as integrins or growth factor receptors, allowing integration of diverse signaling pathways. Hence, signals transduced to cell nucleus depend on the set of membrane receptors expressed by cells, as well as on the composition, the structure and the spatial organization of the underlying ECM which are characteristic of a tissue at a given time.6
'Vascular basement membrane
Basement membranes are specialized types of ECM, highly cross-linked and organized in a sheet-like structure that separate the epithelium from the connective tissue. They function as selective filters, maintain mature tissue function and define spatial organization during
tissue development and reconstruction following tissue injury, by regulation of cell growth, differentiation, and migration.13 The upper layers, called basal lamina, are secreted by
epithelial cells and consist of a network of Ln and a network of collagen type IV interconnected via nidogen/entactin. The lower layer of the basement membrane is secreted by cells from the underlying connective tissue and contains fibrils of collagen type I and type in andFn.13'14
The Ln network assembly is necessary for the basal lamina formation and plays an essential role in cell adhesion and signaling.14 The laminins are a family of heterotrimeric
molecules composed of a-, (3- and y-chains. Ln oc-chains possess many receptor binding sites and they are expressed in a tissue-specific and developmentally regulated manner, conferring heterogeneity among basement membranes.13'15 ECs express only 2 Ln oc-chains: Ln oc4 which
is a component of Ln-8 (oc4plyl) and Ln-9 (cc4p2yl) and oc5 which is a component of Ln-10 (cc5piyl), Ln-11 (a5p2yl) and Ln-15 (Ct5p2y3). Ln a4 is the predominant a chain found in vascular basement membranes and is expressed by all types of ECs, both during development in the embryo and in the adult, while Ln cc5 is detectable in basement membranes of quiescent mature vessels, primarily in capillaries and some venules after birth and is not associated with a fenestrated endothelium.16'17 Ln-10 is believed to be involved in vessel maturation and
stability.16'18 ECs bind to the Ln network via integrin receptors including integrins a3(3l and
cc6pi for both Ln cc4 and oc5 chains and avp3 and avp5 for Ln a5 chain via exposed RGD sites. Ln a5 also binds the oc-dystroglycan and the Lutheran blood group transmembrane glycoproteins.16'19
Collagen type IV network provides the scaffold mechanical resistance. In addition, network forming collagen type VIII, closely associated with human vascular basement membranes and therefore used as a marker for blood vessels, maintains vascular basement membranes in an open porous structure.16 Ln and collagen IV networks are principally linked
by the nidogen/entactin-2 isoform.16
Proteoglycans (PGs), proteins with glycosaminoglycan side chains, associated with basement membranes play a structural role in maintaining tissue architecture via interactions with matrix proteins, help in selective filtration, sequester soluble growth factors via their heparan sulfate (HS) side chains and thus help in regulating cell differentiation.13,14 In addition
with basement membranes, leprecan and collagen type XV chondroitin sulfate PGs are detected in vascular basement membranes.14"16
Thrombospondins (Tsp) belong to a group of proteins called matricellular, which interact with cell receptors and matrix components and are defined as regulators of cell function; they are expressed at high levels during development and in response to injury.20
Tsp-1 and -2 are strong inhibitors of angiogenesis, they are both involved in pathological conditions and Tsp-2 is associated with developing vessels.16,20 von Willebrand factor, a
thrombogenic molecule released by EC that favors platelet adhesion, is primarily found in veins basement membranes. Finally, as ECs in capillaries may be in close contact to surrounding tissue cells, they interact with composite basement membranes containing other isoforms including Ln a-chains produced by surrounding cells.16
2.4.3 Formation of blood vessels
'Context and initiation
Blood vessels in the embryo develop through vasculogenesis i.e., through in situ differentiation of mesodermal precursor cells, called angioblasts, into ECs that assemble into a primary capillary plexus. The primitive network is then grown, remodeled and stabilized by the process of angiogenesis into a complex, mature, and functional network.21 In the adult,
ECs interact with a laminin-rich ECM that maintains mature vessel in a stable quiescent state. During regulated physiological processes such as endometrium vascularization or wound repair, ECs undergo rapid proliferation to form new vessels following matrix remodeling via sprouting angiogenesis. Activated ECs degrade the underlying basement membrane, migrate and proliferate in the perivascular matrix, form tubular structures that become mature and functional.22 Under these conditions, angiogenesis is transitory and highly regulated, spatially
and temporally. However, many diseases such as arthritis, diabetes and tumor growth are driven by a persistent unregulated angiogenesis. Thus, control of the angiogenic process is essential and relies especially on regulated cell-matrix interactions. Gene knock-out experiments in mice and in vitro/in vivo experiments allowed to define a model mechanism of sprouting angiogenesis.
Local hypoxia, hypoglycemia, shear stress or inflammation induce the release of pro-angiogenic vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)-2 that in turn attract ECs. ' " Activated ECs locally increase vascular permeability thereby allowing extravasation of plasma proteins that lay down a provisional matrix rich in fibrin and fibronectin. They also secrete proteinases such as plasminogen activators (PA) and matrix metalloproteinases (MMP) that degrade ECM proteins and liberate growth factors sequestered within the ECM. Angiopoietin (Ang)-2, antagonist of the Tie2 receptor, destabilizes existing vessels probably by loosening ECs adhesion with local basement membrane and periendothelial cells that surround and support blood vessels.
'Proliferation and migration
Local ECM remodeling not only creates a free path for ECs to migrate towards the angiogenic stimulus, but also induces the participation of a new set of cell-matrix interactions, eliciting new signals. The release of matrix-bound growth factors such as FGF-2 and heparin-binding forms of VEGF induces angiogenic signals promoting EC proliferation and migration and modulates cell integrin expression. " Matrix protein cleavage or conformational changes following remodeling expose cryptic sites within matrix proteins that alter their function promoting EC proliferation and migration.25 Collagen type I and type IV usually interact with
ocl|3l and a2(3l integrins whereas cleaved molecules expose cryptic sites interacting with ocv|33.15 Proteolytic degradation also induce production of soluble fragments such as
endostatin, from collagen type XVIII, that can exert anti-angiogenic effects by inhibiting EC
9S
proliferation and migration and thus permit control of the angiogenic process. Finally, ECs previously exposed to a laminin-rich stabilizing ECM, then interact with a new set of ECM molecules from the fibronectin-rich provisional matrix, enhancing proliferation. Formation of new blood vessels in embryos and in adult organisms relies upon different endothelial integrins and ECM ligands: in the embryo, a successful vascular development depends on Fn and its major receptor oc5[3l, but not on av(33 and avP5, which are up-regulated in adult and pathological angiogenesis. ' ' Non-proliferative cells located at migrating tip are exposed to the interstitial matrix rich in collagen type I and type in fibers.24 Concurrently, ECs
'Stabilization and maturation
Vessel mature or regress depending on their use in the network, which is modeled by blood flow generated forces. Hemodynamic forces induce modifications of cell and cell-ECM adhesions and are believed to up-regulate growth factors such as platelet-derived growth factor (PDGF).8'22'27 ECs recruit PDGF receptor-oc-expressing periendothelial cells and
contact between ECs and mural cells triggers the activation of transforming growth factor (TGF)-P that inhibits ECs proliferation and migration, induces SMCs differentiation, stimulates basement membrane production and deposition and alters integrin profiles.8'18'22'23,27 Ang-1, a Tie2 ligand, stabilizes EC-EC interactions and adhesions of mural
cells with ECs. ' Mural cells adhesion and deposition of a complete stable basement membrane provide stability against rupture or regression in absence of VEGF, except for a fenestrated endothelium. EC interaction with periendothelial cells is essential for deposition of a complete stable basement membrane.
Further vessel specialization is realized by EC interaction with repelling cues for arterio-venous determination and guided vessel branching, heterotypic interaction with periendothelial cells such as astrocytes involved in the blood-brain barrier formation, interaction with organ-specific growth factors such as endocrine gland (EG)-VEGF for endocrine gland capillaries specialization, and other non determined processes.7"10
2.5. Interactions between endothelial cells and biomaterial surfaces
Endothelial cell adhesion on biomaterial surfaces is required to provide a non-thrombogenic surface to vascular prosthesis, to subsequently induce formation of blood vessels around implanted biomedical devices improving their biocompatibility or to promote vascularization of growing (hybrid) tissues for regenerative medicine. Cell-material interactions must then incite EC adhesion, but also allow cells to maintain their differentiated functional state and, in some cases, guide spouting i.e., regulated cell proliferation and migration.
The ability of a material to support cell adhesion depends on its surface properties. Treatments to modify a surface chemistry or topography have been used to induce protein adsorption and subsequent cell adhesion. Surface attachment of bioactive molecules such as
ECM proteins, adhesive peptide sequences and/or growth factors have been used to promote EC adhesion onto materials and to control cell processes such as proliferation, migration, differentiation and survival.
2.5.1. Surface properties and the mechanical environment
'Physico-chemical properties
Cell attachment to synthetic surfaces relies heavily on the presence of adsorbed proteins from the media containing serum, from blood plasma or from cellular secretion of matrix proteins. The physico-chemical properties of the surface (hydrophilicity, chemical composition, charge) determine the composition of the adsorbed protein layer as well as the amount and conformation of adsorbed proteins. Protein adsorption results from a combination of interactions with material surface including hydrophobic interactions, electrostatic forces, hydrogen bonding and van der Waals forces.28
It is generally observed that ECs adhere and spread on moderately hydrophilic surfaces such as tissue culture polystyrene (TCPS) and glass, while EC adhesion is reduced or even absent on hydrophobic surfaces such as polytetrafluoroethylene (PTFE), polyethyleneterephthalate (PETP, Dacron) and fluoroethylenepropylene (FEP, Teflon).29"31
Differences in surface hydrophilicity result in quantitative and qualitative variations in the composition of the adsorbed protein layer. Proteins preferentially bind to different surfaces dependent on their nature, and adsorption onto a surface induces protein conformation changes, more or less important, depending on the surface properties. Hydrophobic surfaces exert strong attraction with hydrophobic parts of the protein (inside); proteins strongly and usually irreversibly adhere to these surfaces and may undergo denaturation i.e., disruption of native conformational state, altering exposition of cell binding sites naturally exposed on the outside of the protein. When materials are exposed to blood plasma or serum, albumin strongly binds to hydrophobic surfaces while conformationally active adhesive proteins such as Fn and Vn preferentially adsorb on hydrophilic surfaces.33,34 As adsorption onto
hydrophilic surfaces is reversible, proteins can be displaced.29
Many polymeric biomaterials used for clinical applications (vascular grafts) are hydrophobic (PTFE, PETP, FEP, polyurethane). A solution to promote cell adhesion is to increase surface hydrophilicity by chemical treatments (UV exposition, alkaline hydrolysis) or
plasma treatment (ammonia or oxygen plasma treatment), all resulting in the addition of polar groups and charges.35"37 TCPS and Primaria, routinely used for cell culture, have been made
hydrophilic and cell adhesive by plasma treatment. Since EC adhesion to hydrophobic polymeric surfaces has been enhanced by plasma modification using either nitrogen- or oxygen-containing monomers: modified surfaces are more hydrophilic, adsorb more adhesive proteins such as Fn and support cell adhesion, spreading and growth.38"41 Moreover, EC
adhering on plasma treated surfaces show improved anti-coagulant and fibrinolytic activities and better resist to shear stress induced detachment.38,40
Surface exposition of various chemical groups and charges affect initial cell adhesion. In presence of serum, initial cell attachment on nitrogen rich surfaces such as Primaria is a result of adsorption of Vn and Fn, while cell adhesion on oxygen-rich surfaces such as TCPS is mediated by adsorbed Vn only, as Fn adsorption on these surfaces is sub-optimal for cell adhesion.39'40,42'43 Oxygen-rich surfaces present negatively charged groups while nitrogen rich
surfaces rather expose positively charged groups (at physiological pH). Fn is an acidic protein overall negatively charged that more abundantly adsorbs on positively charged surfaces. Introduction of electrical charges on a surface can enhance protein adsorption via electro-attractive forces. Addition of a polyelectrolyte film on a poorly adhesive surface, either as a monolayer or a multilayer film resulting from alternate adsorption of polycations and polyanions, enhances cell attachment.44'45 In case of weak polyelectrolyte gels, EC adhesion
increases with charge density while cells highly adhere and proliferate on strong polyeletrolytes, they also expose higher amount of anti-platelet HSPGs and are more resistant to shear stress than confluent ECs attached on glass or TCPS.44'46,47
'The mechanical environment
Cells sense and respond to underlying substrate stiffness, to topography and to fluid flow. External mechanical forces can be sensed by cytoskeleton linked receptors or the cytoskeleton structure itself. The mechanical coupling of the cytoskeleton with cell-ECM and cell-cell adhesions allow transduction of mechanical signals throughout the cell. Upon adhesion, cells form adhesive contacts with surfaces and pull on the substrate. Increased surface stiffness is associated with increased cell contractility.49 On stiff surfaces, cells have
actin stress fibers, resulting in cell spreading and a higher adhesion strength than on soft substrates.45,49'50 Substrate stiffness and cell contractility appear to regulate EC proliferation
and differentiation: ECs form tube-like structures on soft malleable substrates while they proliferate on rigid surfaces.45'51'52
ECs are also sensitive to surface topography.53 When attached to a surface presenting
micro- or even nano-features, they emit filopodia and lamellipodia, sensory organs of cells, and undergo morphological changes.54 On grooved surfaces, ECs elongate, they align parallel
to the channel direction; cell orientation persists until near confluence is reached and increases with channel depth.55"57 Cell actin stress fibers and focal adhesions align parallel to channel
direction and focal contacts are preferentially located at feature edges.5 Their localization
CO
correlates with preferential Fn fibrils formation at edges of grooves, pillars or wells. However, enhancement of cell elongation and orientation associated with actin stress fibers alignment has also been observed on wave features, in absence of sharp edges.59 Grooved and
waved topographies induce polarization of cell movement: cell orientation and directed movement in response to substrate topography may be referred to as "contact guidance".53
Random topography or increased surface roughness have been shown to enhance EC adhesion but also Fn and Vn adsorption.60'61 Hence, surface roughness and topography may affect cell
morphology directly by directing focal adhesions and stress fibers assembly, but they may also influence cell behavior indirectly by altering surface protein adsorption.
The shear stress applied to the luminal surface of cells can be sensed by the cell membrane and the associated receptors and this stress can be transmitted throughout the cell to cell-matrix and cell-cell adhesions. As a result, shear stress induces EC alignment and an increase of stress fibers and remodels cell-matrix adhesions to increase adhesion strength. In confluent ECs, shear stress increases the size of focal adhesions and activates integrins such as ccvp3 and oc5pl.48 In non confluent ECs, shear stress promotes cell spreading, then
lamellipodial extension in the flow direction, cell-cell adhesion dissociation and enhances directed cell migration. The existing focal adhesions under the main cell body increase in size while new transient focal adhesions assemble under the lamellipodia and align with the flow direction. ' Shear stress preconditioning may be used to orient ECs or to increase cell adhesion strength to the substrate.63