CRYO-EXTRACTION DES CIRES DES
PRODUITS CÉRÉALIERS
(SORGHO, BLÉ ET RIZ BRUN)
MÉMOIRE
Thi Can Tho PHAM
Maîtrise en Sciences et technologie des aliments
Maître ès Sciences (M.Sc.)
Québec, Canada
iii
Résumé court
La cire est un lipide naturel et possède beaucoup d‟applications intéressantes dans l‟industrie cosmétique et pharmaceutique. Nous visons à étudier l‟extraction des cires du sorgho, du blé et du riz brun par l‟azote liquide comme méthode „verte‟. Ces céréales ont été immergées dans l‟azote liquide de 1 à 3 cycles à différentes durées et différentes du temps entre les cycles. Nos résultats ont montré que l'extraction de grains de céréales par l'azote liquide est réalisable. En comparant avec la méthode utilisant n-hexane, la quantités de cires extraites par l'azote liquide était de 5, 7.5 et 9.3 fois plus faible mais le temps d‟extraction était de 2.3, 5.5 et 11.25 fois plus court pour le blé, riz et sorgho, respectivement. Selon l‟analyse par SEM, les cires superficielles des grains ont pu être extraites par l‟azote liquide. La composition des cires a été identifiée par MS et GC-FID.
v
Short Abstract
Wax is a natural lipid and has many interesting applications in cosmetics and pharmaceuticals. The purpose of this study is to use liquid nitrogen as a “green method” to extract waxes from rice, sorghum and wheat. These cereals were treated in liquid nitrogen in 1 to 3 cycles with different time intervals and different time between the cycles. The results showed that waxes could be extracted with liquid nitrogen. When compared to the traditional method (n-hexane), the extracted amounts of waxes with liquid nitrogen were 5, 7.5 and 9.3 times lower but the extraction time was 2.3, 5.5 and 11.25 times shorter for wheat, rice and sorghum, respectively. SEM microphotographs depicted that superficial waxes was extracted from grains by liquid nitrogen. GC-MS and GC-FID confirmed that extracted waxes had similar compositions in both extraction methods. This study points out a new friendly-environmental way to extract waxes from cereals.
vii
Résumé long
L‟extraction des cires végétales est fréquemment réalisée en utilisant des solvants tels que l‟hexane, le cyclohexane, le méthanol, etc. Ces solvants sont très efficaces pour dissoudre les lipides. Cependant, ils sont aussi inflammables, très volatils et toxiques. Récemment, des recherches ont montré que les fluides cryogéniques peuvent aussi servir à cette fin. Par exemple, l‟immersion cyclique des fruits entiers dans l‟azote liquide (bleuet, argousier, raisin vert) a permis de diminuer les cires cuticulaires jusqu‟à 50%. Le but de ce travail est donc d‟étudier l‟immersion dans l‟azote liquide comme méthode „verte‟ pour extraire la cire des grains.
Du sorgho, du blé et du riz brun ont été immergés dans l‟azote liquide en 1 à 3 cycles à différentes durées. L‟impact du temps de repos entre les cycles (1-5 minutes à température ambiante) sur le rendement d'extraction a aussi été analysé. La performance d‟extraction a été comparée à celle par le n-hexane avec la méthode reflux. La microscopie électronique à balayage (SEM) a permis de visualiser l'effet de l'immersion dans l'azote liquide et dans l‟hexane sur la surface des grains. La chromatographie en phase gazeuse couplée à un spectromètre de masse (GC-MS) et à un détecteur à ionisation de flamme (GC-FID) a été utilisée pour la détermination de la composition chimique des extraits obtenus par les deux méthodes.
Les résultats ont montré que le rendement d‟extraction le plus élevé était de 0.026% (g pds cire/100g pds grain) (riz-11minutes d'extraction), 0.032% (sorgho-4 minutes) et 0.025% (blé-13 minutes). En comparaison, le rendement des cires extraites par le n-hexane (70oC) a été de 0.196% (riz-60 minutes d'extraction), 0.298% (sorgho-45 minutes) et 0.126% (blé-30 minutes). Pour le blé et le riz, une augmentation significative du rendement a été observée pour des temps de repos entre 3 et 5 minutes entre les cycles par rapport à celui de 1 minute.
viii
Les résultats de GC-MS et GC-FID nous ont confirmé que de la cire a bien été extraire par l‟azote liquide et le n-hexane, dont la composition est similaire dans les deux cas.
Ces résultats montrent que la méthode d‟extraction par l'azote liquide est réalisable malgré son rendement plus faible que celle par l'n-hexane mais avec un temps d‟extraction plus court. L‟azote liquide n‟est pas toxique et c‟est un solvant « vert » qui peut être éliminé du produit très facilement par simple évaporation, sans laisser aucune trace problématique. L'utilisation de l'azote liquide comme solvant d'extraction s'avère une méthode prometteuse pour la séparation des composés cireux des grains. La qualité des céréales traitées par l‟azote liquide demeure inchangée donc elle pourra être considérée comme une étape secondaire dans le processus de polissage.
ix
Extended Abstract
Wax extraction from plants is conventionally carried out by using organic solvents such as
n-hexane, cyclohexane and methanol. Although, those solvents are very effective in
dissolving lipids, they are flammable, toxic and highly volatile. Recent studies show that cryogenic fluids could also be used for wax extraction. For instance, cyclic immersion of whole fruits (blueberry, seabuckthorn, grapes) in liquid nitrogen has significantly reduced cuticular waxes up to 50%. So, the main aim of this work is to use liquid nitrogen as a “green method” to extract waxes from cereals.
Several approaches were used to evaluate the extraction method by liquid nitrogen. Sorghum, wheat and brown rice were treated in liquid nitrogen in 1 to 3 cycles with different time intervals. The impact of rest time between cycles (1-5 minutes at room temperature) on the extraction yield was also reported by weighing waxes. The extraction efficiency of the method was compared to that of n-hexane which was referred as the control one. The surface of the grains treated by liquid nitrogen and n-hexane was visualized by the scanning electron microscopy (SEM). Finally, the gas chromatography coupled to mass spectrometry (GC-MS) and flame ionization detector (GC –FID) was used to determine the composition in the extracts resulted from the two methods.
Our results showed that the highest extraction efficiency was 0.026% (g cire/100g grain) (rice - 11 extraction minutes), 0.032% (Sorghum - 4 minutes) and 0.025% (wheat -13 minutes). Compared to the extraction by n-hexane method, its efficiency was 0.196% (rice – 60 extraction minutes), 0.298% (sorghum - 45 minutes) and 0.126% (wheat - 30 minutes). For wheat and rice, a significant increase in yield between 3 and 5 rest minutes with respect to the one of 1 minute was observed.
The results of GC-MS and GC –FID confirmed that the waxes were extracted out of the grains by both liquid nitrogen and n- hexane. The wax composition of the extract is similar in both cases.
x
These results conclude that the wax extraction method by liquid nitrogen is feasible despite it extracts waxes less than that of n-hexane, but the method benefits for the shorter extraction time. Liquid nitrogen is not toxic and is a "green" solvent which can be easily removed from the products by spontaneously simple evaporation leading no leftover residues. Therefore, using liquid nitrogen as the extraction solvent might be a promising way for separating waxes from waxy grains. The quality of grain processed by liquid nitrogen remains unchanged, thus it may be considered a sub-step in the milling process.
xi
Table of contents
Résumé court ... iii
Short Abstract... v
Résumé long ... vii
Extended Abstract ... ix
Table of contents ... xi
List of tables ... xiii
List of figures ... xv
Remerciements ... xvii
CHAPTER 1. INTRODUCTION ... 1
CHAPTER 2. LITERATURE REVIEW ... 5
2.1. Rice (Oryza sativa L.) ... 5
2.1.1. Production of rice ... 6
2.1.2. Structure and composition of rice grain ... 6
2.2. Wheat (Triticum aestivum) ... 9
2.2.1. Wheat production ... 10
2.2.2. Structure and composition of wheat ... 10
2.3. Sorghum (Sorghum bicolor L.) ... 11
2.3.1 Sorghum production ... 12
2.3.2 Structure and composition of sorghum. ... 13
2.4. Rice, wheat and sorghum waxes and their applications ... 15
2.4.1. Wax generalities: types and composition ... 15
2.4.2. Cereal wax applications ... 18
2.5. Extraction methods of lipid ... 19
2.6. Extraction methods of cereal waxes ... 21
2.7. Liquid nitrogen and its potentials for wax extraction ... 22
2.7.1. Properties of liquid nitrogen ... 22
2.7.2. Potential of wax extraction by liquid nitrogen ... 23
CHAPTER 3. HYPOTHESIS AND OBJECTIVES ... 25
3.1. Hypothesis ... 25
3.2. Objectives ... 25
3.2.1 General objective ... 25
3.2.2 Specific objectives ... 25
CHAPTER 4. MATERIALS AND METHODS ... 27
4.1. Materials ... 27
4.2. Methodology ... 27
4.2.1. Determination of moisture content ... 29
4.2.2. Wax extraction by liquid nitrogen... 29
4.2.3. Wax extraction by n-hexane ... 31
4.2.4. Calculation of wax yield ... 32
4.2.5. Examination of wax by scanning electron microscope (SEM) ... 32
4.2.6. Analysis of wax composition ... 33
4.3. Statistical analysis ... 34
CHAPTER 5. RESULTS AND DISCUSSION ... 35
xii
5.2. Extraction of cereal waxes with liquid nitrogen ... 38
5.3. Impact of number of immersion cycles and rest time with liquid nitrogen ... 41
5.3.1. Sorghum... 41
5.3.2 Rice ... 41
5.3.3 Wheat ... 47
5.4. Comparison between two methods of wax extraction ... 50
5.5. Color of sorghum, rice and wheat waxes ... 50
5.6. Scanning electron microscopy (SEM) ... 52
5.7. Composition of waxes - Gas chromatography ... 56
CHAPTER 6. CONCLUSION ... 69
References ... 71
ANNEX 1: ANOVA table and mean table of the comparison the means of percent rice wax extracted by n-hexane. ... 79
ANNEX 2: ANOVA table and mean table of the comparison the means of percent sorghum wax extracted by n-hexane. ... 80
ANNEX 3: ANOVA table and mean table of the comparison the means of percent wheat wax extracted by n-hexane. ... 81
ANNEX 4: ANOVA table and mean table of the comparison the means of percent sorghum wax extracted by liquid nitrogen with 1 minute rest ... 82
ANNEX 5: ANOVA table and mean table of the comparison the means of percent sorghum wax extracted by liquid nitrogen with 1, 3, 5 minutes rest ... 83
ANNEX 6: ANOVA table and mean table of the comparison the means of percent sorghum wax extracted by liquid nitrogen with 1 minutes rest ... 84
ANNEX 7: ANOVA table and mean table of the comparison the means of percent rice wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 85
ANNEX 8: ANOVA table and mean table of the comparison the means of percent rice wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 86
ANNEX 9: ANOVA table and mean table of the comparison the means of percent wheat wax extracted by liquid nitrogen with 1 minute of rest ... 87
ANNEX 10: ANOVA table and mean table of the comparison the means of percent wheat wax extracted by liquid nitrogen with 3 minutes of rest ... 88
ANNEX 11: ANOVA table and mean table of the comparison the means of percent wheat wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 89
xiii
List of tables
Table 2.1. World production of rice (Source FAO, 2012) ... 6
Table 2.2. Composition of brown, milled and parboiled rice (source: Adair, 1972) ... 8
Table 2.3. White rice and by-products from paddy milling process (Source: Kahlon, 2009) 8 Table 2.4. The production of wheat (Source FAO, 2012) ... 10
Table 2.5. The production of sorghum (Source FAO, 2012) ... 13
Table 2.6. Composition of the whole kernel and its part for sorghum (moisture-free basis) (Wall et Charles, 1970) ... 14
Table 2. 7. The most common substance classes of cuticular waxes (Luka et al., 2009) ... 16
Table 2.8. Properties of nitrogen (Source Afsset, 2008) ... 23
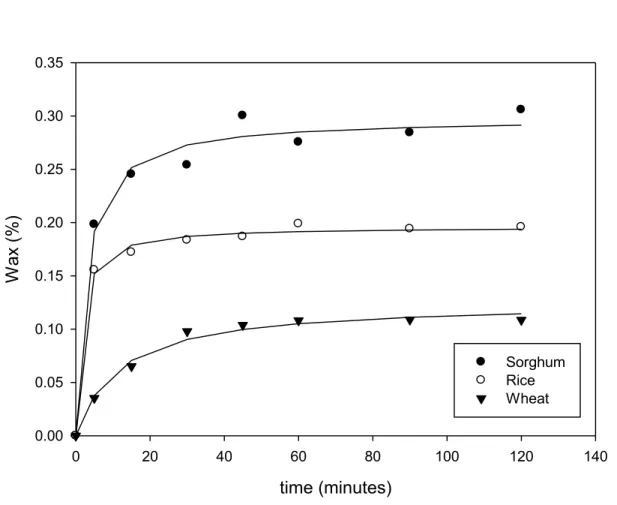
Table 5.1. Fitted parameters for kinetics constants Wmax and b from Eqn. (5.1) – hexane extraction (r2 is the coefficient of determination) ... 36
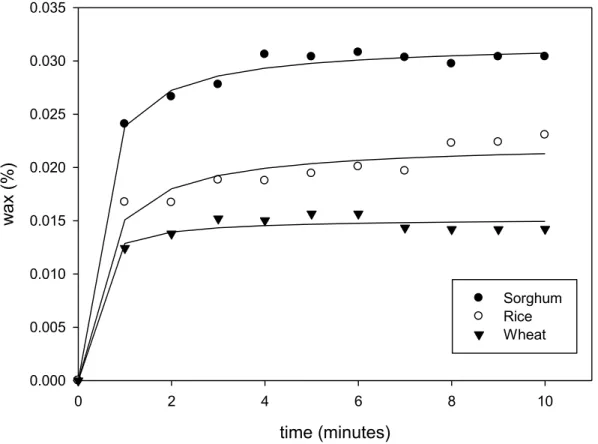
Table 5.2. Fitted parameters for kinetics constants Wmax and b from Eqn. (5.1) – liquid nitrogen extraction (r2 is the coefficient of determination) ... 40
Table 5.3. Comparison of liquid nitrogen and n-hexane extraction methods ... 50
Table 5.4. Classes of compounds present in the rice wax extracted with their retention time. Superscripts (1) to (7) indicates ratios that could not be calculated since amount obtained with n-hexane was zero. ... 64
Table 5.5. Classes of compounds present in the sorghum wax extracted with their retention time. Superscripts (1) to (7) indicates ratios that could not be calculated since amount obtained with n-hexane was zero. ... 65
Table 5.6. Classes compounds present in the wheat wax extracted with their retention time. Superscripts (1) to (7) indicates ratios that could not be calculated since amount obtained with n-hexane was zero. ... 66
Table A-1. Anova of the percentage of rice wax extracted by n-hexane ... 79
Table A-2. Rice wax extraction by n-hexane ... 79
Table A-3. Anova of the percentage of sorghum wax extracted by n-hexane ... 80
Table A-4. Percent of sorghum wax extraction by n-hexane ... 80
Table A-5. Anova of percentage of wheat wax extracted by n-hexane ... 81
Table A-6 . Percent of wheat wax extraction by n-hexane ... 81
Table A-7. Anova of percentage of sorghum wax extracted by liquid nitrogen with 1 minute of rest ... 82
Table A-8. Percent of sorghum wax extracted by liquid nitrogen with 1minute of rest ... 82
Table A-9. Anova of percentage of sorghum wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 83
Table A-10. Percent of sorghum wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 83
Table A-11. Anova of percentage of rice wax extracted by liquid nitrogen with 1 minute of rest ... 84
Table A-12. Percent of rice wax extracted by liquid nitrogen with 1 minute of rest ... 84
Table A-13. Anova of percentage of rice wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 85
Table A-14. Percent of rice wax extracted by liquid nitrogen with 3 minutes of rest ... 85
Table A-15. Anova of percentage of rice wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 86
xiv
Table A-16. Percent of rice wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest . 86 Table A-17. Anova of percentage of wheat wax extracted by liquid nitrogen with 1 minute of rest ... 87 Table A-18. Percent of wheat wax extracted by liquid nitrogen with 1 minute of rest ... 87 Table A-19. Anova of percentage of wheat wax extracted by liquid nitrogen with 3 minutes of rest ... 88 Table A-20. Percent of wheat wax extracted by liquid nitrogen with 3 minutes of rest ... 88 Table A-21. Anova of percentage of wheat wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 89 Table A-22. Percent of wheat wax extracted by liquid nitrogen with 1, 3, 5 minutes of rest ... 89
xv
List of figures
Figure 2. 1. Rice plant and rice grain ... 5
Figure 2.2.Structure of rice grain (from Encyclopaedia Britannica, http:// www.britannica.com) ... 7
Figure 2.3.Wheat plant and wheat grain ... 9
Figure 2.4. Struture of wheat grain (from Encyclopaedia Britannica, http://www.britannica.com) ... 11
Figure 2.5. Sorghum plant and sorghum grain ... 12
Figure 2.6.Structure of sorghum grain (Earp.C.F et al., 2003) ... 13
Figure 2.7. Structure of cuticule (lipidlibrary.aocs.org) ... 15
Figure 4.1. Proposed methodology, liquid nitrogen extraction on the left and n-hexane on the right ... 28
Figure 4.2. Description of wax extraction in liquid nitrogen. ... 30
Figure 4.3. Illustration of immersion process in liquid nitrogen ... 30
Figure 4.4.Illustration of reflux system used for wax extraction by n-hexane (www.chem.wisc.edu) ... 32
Figure 5.1. Extraction kinetics of sorghum, rice and wheat wax by reflux extraction using n-hexane. ... 36
Figure 5.2. Extraction kinetics of sorghum, rice and wheat wax by immersion in liquid nitrogen. ... 39
Figure 5.3. Impact of number of immersion cycles on wax yield (%) for sorghum (1 minute rest time) ... 43
Figure 5.4. Impact of rest time on wax yield (%) for sorghum (2 cycles with 1, 3 or 5 minutes rest time) ... 43
Figure 5.5 . Rice wax yield from extraction with liquid nitrogen (1 minute rest time) ... 44
Figure 5.6. Rice wax yield from extraction with liquid nitrogen (3 minutes rest time) ... 44
Figure 5.7. Rice wax yield from extraction with liquid nitrogen (2 cycles with 1, 3 or 5 minutes rest time) ... 46
Figure 5.8. Wheat wax yield from extraction with liquid nitrogen (1 minute rest) ... 48
Figure 5.9. Wheat wax yield from extraction with liquid nitrogen (3 minute rest) ... 48
Figure 5. 10 . Wheat wax yield from extraction with liquid nitrogen (2 cycles with 1, 3 or 5 minutes rest time) ... 49
Figure 5.11. Wax extracted with n-hexane: sorghum (a), wheat (b), rice (c). Sorghum wax extracted by liquid nitrogen (d) ... 51
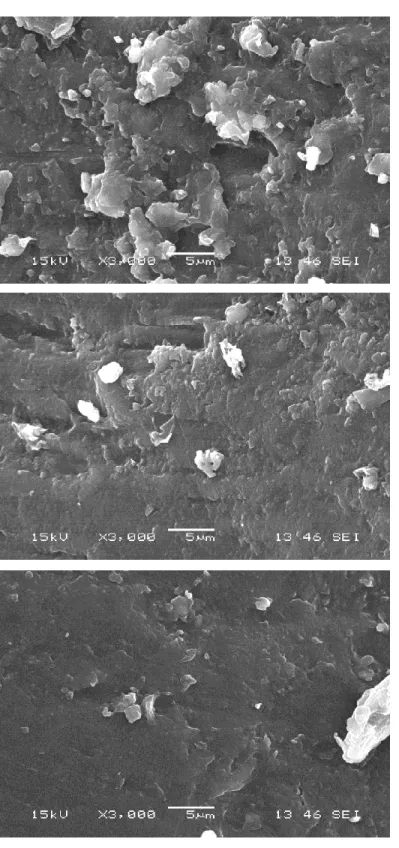
Figure 5.12. Surface visualization by SEM of the sorghum grain: fresh (a), treated by n-hexane (b) and treated by liquid nitrogen (c) ... 53
Figure 5.13. Surface visualization by SEM of the rice grain: fresh (a), treated by n-hexane (b) and treated by liquid nitrogen (c) ... 54
Figure 5.14. Surface visualization by SEM of the wheat grain: fresh (a), treated by n-hexane (b) and treated by liquid nitrogen (c) ... 55
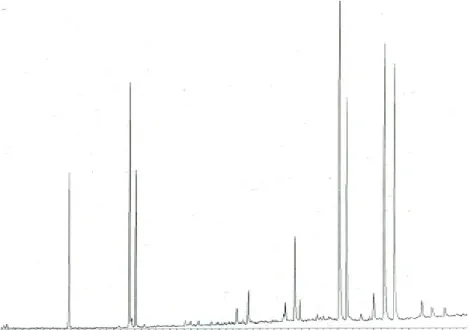
Figure 5.15. GC-MS Chromatogram of rice wax extracted by n-hexane ... 57
Figure 5.16.GC-MS Chromatogram of rice wax extracted by liquid nitrogen ... 57
Figure 5. 17. GC-MS Chromatogram of sorghum wax extracted by n-hexane ... 58
xvi
Figure 5.19. GC-MS Chromatogram of wheat wax extracted by n-hexane ... 59
Figure 5.20. GC-MS Chromatogram of wheat wax extracted by liquid nitrogen ... 59
Figure 5.21. GC-FID Chromatogram of rice wax extracted by n-hexane... 61
Figure 5.22. GC-FID Chromatogram of sorghum wax extracted by liquid nitrogen ... 61
Figure 5.23. GC-FID Chromatogram of sorghum wax extracted by n-hexane ... 62
Figure 5.24. GC-FID Chromatogram of sorghum wax extracted by liquid nitrogen ... 62
Figure 5.25. GC-FID Chromatogram of wheat wax extracted by n-hexane ... 63
Figure 5.26. GC-FID Chromatogram of wheat wax extracted by liquid nitrogen ... 63
xvii
Remerciements
J'aimerais remercier à ma directrice, Madame la professeure Cristina Ratti qui est très gentille et sympathique. Je suis vraiment très chanceuse d'avoir travaillé avec elle. Merci de ses conseils, ses supports, sa patience et ses encouragements tout au long de mes études et aussi dans ma vie. Je me sens de temps en temps coupable quand je n'ai pas pu accomplir bien des taches au laboratoire.
J'aimerais consacrer mes remerciements à Monsieur le professeur Paul Angers, mon co-directeur pour ses supports, ses suggestions, sa disponibilité et ses encouragements précieux durant mes études de la maitrise.
Merci beaucoup à Monsieur Ronan Corcuff de m'avoir permis d'utiliser le système de Gas Chromatography-Mass spectrometry et de m‟aider à faire des analyses des composants de la cire par cette technique.
Merci également à Monsieur Richard Janvier qui m'a aidé à générer des photos par la microscopie électronique.
Merci bien Céline Paquin, Jocelyne Giasson et Diane Gagnon pour leurs supports techniques et leurs conseils au laboratoire.
Merci beaucoup à mon mari qui m'accompagne dans la vie, a supporté et encouragé tout au long de ma maitrise. Je pense toujours à mon fils de son courage pendant le combat avec son cancer. C'était lui qui m'a donné la force afin de surmonter toutes les difficultés dans la vie. Merci beaucoup à ma famille, surtout ma mère et ma belle-mère qui sont venues à Québec m'aider quand ma vie est dans le besoin et dans la difficulté. Merci à tous mes amis à Québec et au Vietnam pour leur encouragement pendant mes études. Finalement, Je remercie le CRSNG pour le soutien financier de ce projet.
xix
1
CHAPTER 1. INTRODUCTION
Cereals are members of the grass family. They are among the important foods for humans and have been used for thousands of years. Production of cereals in the world is more than 2500 million tonnes in the year 2013 (FAO, 2013). Wheat, rice and sorghum are in the group of major cereals of the world and provide high of the world‟s dietary energy supply.
Rice is very popular in Africa and especially in Asia and sorghum is especially well known and accepted in Africa while wheat is mostly planted in North America and Europe. Bread wheat (Triticum aestivum), Asian rice (Oryza sativa L.) and Sorghum (Sorghum bicolor L.) are the mostly widely cultivated in the world among of other varieties. In 2010, world production of wheat, rice and sorghum paddies was 651 million tonnes, 672 million tonnes and 57 million tonnes, respectively (FAO, 2012).
The surface of cereal kernels is covered by a layer containing wax, called bran, a natural lipid biochemically synthesized during growth. Bran is highly hydrophobic so it is used as a natural protector against water and moisture loss, even against microorganisms. The major components of cereal wax are esters of long chain fatty acids and long chain fatty alcohols or sterols, hydrocarbons, acylglycerides, free fatty acids and free alcohols (Ohnishi et al., 1985; Vali et al., 2005; Kim, 2008). These waxes have been considered as a by-product or waste material from oil production and are usually removed by refining processes called winterization before the oils are marketed (Kim, 2008).
Nowadays, cereal waxes get increased attention since they can have many interesting applications. In cosmetic industries, for instance, they play an important role in the production of creams, crayons and lipsticks because of their ability for making skin smooth and soft. Also, waxes can form a protective film to prevent the loss of skin moisture. Moreover, waxes of these grains are used in pharmaceutical industries, thanks to the significant presence of policosanols (Lui et al., 2005; Wang et al., 2007; Irmak et al., 2006;
2
Chen et al., 2008; Dunford et al., 2010). This component, which is a mixture of long chain saturated alcohols, is believed to lower the bad cholesterol, to treat gastric and duodenal ulcers and especially to exhibit substantial anti-inflammatory activity (Vali et al., 2005). Waxes also have other potential applications in the food, polymer and leather industries.
To extract waxes from cereals, many methods using organic solvents have been utilized. Due to their intrinsic hydrophobicity, waxes have mainly been extracted by non-polar solvents or mixtures of solvent like chloroform-methanol (Ohnishi et al., 1986), benzene (Avato et al., 1990) and hexane (Hwang et al., 2002; 2004; 2005). For the further purpose of separating the wax from the extract, two ways have been proposed. The first one consists of evaporation of the solvent from the mixture containing waxes and mixing the wax extract with other polar solvents (acetone or alcohol), followed by precipitation of waxes and filtration. The second one, in crystallizing the waxes by incubation of the extraction mixture at -18oC, and then the crystallized waxes are collected by filtration (Hwang et al., 2002). Although solvent wax extraction methods are simple, and economical require no complex equipment, they are not considered to be environmental-friendly, with potential harm to human health.
Recently, research focused on taking advantage of liquid nitrogen to pre-treat whole fruits prior to drying. Ketata et al. (2012) showed that liquid nitrogen could decrease wax content on the surface of blueberries, resulting in shortening the drying process. Similarly, blueberries, seabuckthorn fruits and green grapes treated with liquid nitrogen showed a decrease in drying times for different drying methods (vacuum, hot air and freeze-drying). The surface of these fruits was observed by scanning electron microscopy before and after liquid nitrogen pre-treatments, pointing out the disappearance of their epicuticular wax, which was mainly responsible for slowing down drying (Thromas et al., 2010; 2011). These studies strongly support the fact that liquid nitrogen could be used to extract waxes from cereal grains like wheat, rice and sorghum. In addition, liquid nitrogen is proved to be non-toxic, colorless, odorless, tasteless, inflammable and inert. Especially, it vaporizes itself at room temperature so there would be no leftover residue in extracted waxes neither in grains. Therefore, the aim of this research is to study the possibility of extracting waxes
3 from wheat (Triticum aestivum), rice (Oryza sativa L.) and sorghum (Sorghum bicolor L.) by immersion in liquid nitrogen as well as to determine composition of the extract.
5
CHAPTER 2. LITERATURE REVIEW
2.1. Rice (Oryza sativa L.)
Rice is one of the world's important cereals, serving as a staple food for a large section of the population, especially in Asia and Africa. It has been under intensive cultivation in Asia for over 4 000 years and has since spread across the world, where almost a third of the population depends on rice for vital nutrition (Grist, 1975). Among the 40 000 different rice varieties, Asian rice (Oryza sativa L.) and African rice (Oryza glaberrima) are the most popular cultivated in the world (Grist, 1972). They belong to genus Oryza and there are only small differences between these species, mainly in grain size and glume pubescence.
Oryza sativa L. is divided into four groups: indica, japonica, brevindica and brevis.
However, the sub-species brevindica and brevis are not generally recognized. Japonica varieties are usually cultivated in dry fields, in temperate East Asia, upland areas of Southeast Asia and high elevations in South Asia, while indica varieties are mainly lowland rice, grown mostly submerged, throughout tropical Asia (Figure 2.1). The grain of japonica is shorter and broader than the grain indica. Japonica is sticky, and it tends to soften rapidly after certain time of cooking while Indica is non-sticky and usually can be overcooked (Grist, 1975).
6
2.1.1. Production of rice
The world production of rice is very large and it has risen from 519 million tonnes of rice paddy in 1990 to 672 million tonnes in 2010 (FAO, 2012; table 2.1). Although the production is large, only 5-6% of produced rice is traded internationally. Asian countries produce most of the rice in the world. In 2010, Thailand, Vietnam and India were the three largest exporters of rice. They accounted for nearly 70% of the world rice exports.
Table 2.1. World production of rice (Source FAO, 2012)
Year
Production (metric tonnes)
World Asian countries African countries American countries North American countries 1990 518 568 263 477 692 981 12 697 109 22 655 617 7 080 000 2000 599 355 455 545 546 464 17 476 517 32 032 396 8 657 820 2010 672 015 587 607 328 408 22 855 318 37 170 221 11 027 000
2.1.2. Structure and composition of rice grain
Rice has different names with different nutrition values depending on where it is positioned in the processing line. To understand better the description of rice and its different names, Figure 2.2 shows a schematic cross section of a grain of rice, with its main components. Rice harvested from the field is called paddy which is enclosed by the hull, or husk. Milling usually removes both the hull and bran layers of the rice. In the rice milling process, first the outermost layer, the hull, is removed to produce brown rice. This process is the least damaging to the nutritional value of the rice and avoids the unnecessary loss of nutrients that occurs with the further processing. Brown rice would be considered a whole grain. It is a source of thiamine, niacin, riboflavin, iron, and calcium. Rice that is milled to remove the bran as well is called white rice. White rice has greatly diminished its nutrient content from brown rice. When white rice forms a major portion of the diet (lacking of important
7 nutrients), there is a risk of „beriberi‟, a disease resulting from the deficiency of thiamine and minerals.
Figure 2.2.Structure of rice grain (from Encyclopaedia Britannica, http:// www.britannica.com)
In parts of India and some countries of West Africa, parboiled rice is popular. Parboiled rice is a term indicating that rice paddy has been subjected to a steaming or parboiling process. This makes nutrients from the outer husk, especially thiamine, move into the grain itself. The rice is then dried, and can be milled as usual or used as brown rice. The milled parboiled rice is nutritionally superior to the standard milled rice. Table 2.2 presents the composition of brown, milled raw and milled parboiled rice (Adair, 1972).
8
Table 2.2. Composition of brown, milled and parboiled rice (source: Adair, 1972) Component Brown rice Milled raw rice Milled parboiled rice Moisture (%) Calories (/100g) Protein (%) Fat (%) N-free extract (%) Fiber (%) Ash (%) Thiamine (mg/100 g) Riboflavin (mg/100 g) Niacin (mg/100 g) 12.0 360.0 7.5 1.9 77.4 0.9 1.2 0.34 0.05 4.7 12.0 363.0 6.7 0.4 80.4 0.3 0.5 0.07 0.03 1.6 10.3 369.0 7.4 0.3 81.3 0.2 0.7 0.4 … 3.5
Rice processing releases many by-products. Firstly, the by-products of milling, including bran and rice polish (finely powdered bran and starch resulting from polishing), are used as livestock feed. Secondly, oil is processed from the bran for both food and industrial uses. Thirdly, broken rice is used in brewing, distilling, and manufacturing starch and rice flour. Fourthly, hulls are used for fuel, packing material, industrial grinding, and fertilizer manufacture. Finally, straw is used for feed, livestock bedding, roof thatching, mats, garments and packing material. Table 2.3 presents an estimation of the percentage of different types of rice and by-products in a paddy milling processing operation (Kahlon, 2009).
Table 2.3. White rice and by-products from paddy milling process (Source: Kahlon, 2009)
Composition White rice Broken rice Bran Hulls
9
2.2. Wheat (Triticum aestivum)
Wheat is one of the oldest and the most extensively grown among grain crops. It emerged as a crop about 10 000 years ago and became a major component of most diets of the world because of its agronomic adaptability, ease of storage, nutritional goodness and the ability of its flour to produce a variety of palatable, interesting and satisfying foods (Feldman, 2001). Wheat origin is in southwest Asia and it is now cultivated worldwide. Wheat belongs to the genus Triticum, and the most widely cultivated in the world is common wheat or bread wheat (Triticum aestivum) (Figure 2.3) (Pomeranz, 1978). Bread wheat is a hexaploid species and constitutes about 90% of the wheat grown worldwide, 93% of the wheat produced in the United States and 95% in Canada (Wrigley C.W., 2009). It has high economic value because of the kernel hardness and high protein content. Common wheat may be of either winter or spring growing habit and may have either red or white kernels (Shellenberger, 1978). Besides of Triticum aestivum, Triticum durum is the second most widely cultivated wheat. It belongs to tetraploid species and is used primarily for pasta production. Triticum monococcum, Triticum dicoccum, and Triticum spelta are species of wheat cultivated in limited quantities in the world (Wrigley C.W., 2009).
10
2.2.1. Wheat production
As shown in Table 2.4, the world production of wheat is very large and has been stable between 1990 and 2010. According to the statistics of FAO, the major wheat production comes from Asian, European and North American countries. More than 80% of the wheat production is consumed within source countries and mainly as human food, while 20% (more than 100 million tonnes) enters into international trade annually. For that reason, wheat becomes the most-traded cereal in the world (Wrigley C.W., 2009).
Table 2.4. The production of wheat (Source FAO, 2012)
Year
Production (metric tonnes)
World Asian countries European countries American countries North American countries 1990 592 310 517 203 062 087 233 143 757 127 159 034 106 392 000 2000 585 690 370 254 524 075 183 599 454 110 863 548 87 174 900 2010 650 881 002 292 441 446 201 149 388 112 690 549 83 269 400
2.2.2. Structure and composition of wheat
Figure 2.4 shows the cross section of a wheat grain with its main structural components. Wheat grains are generally oval shape although different wheat species have grains that range from almost spherical to long, narrow and flattened shapes. The grain is usually between 5 and 9 mm in length and between 35 and 50 mg in weight. When the husk is removed, the brown grain is exposed. It contains 2-3% germ, 13-17% bran and 80-85% endosperm (Šramková et al., 2009).
11 Figure 2.4. Struture of wheat grain (from Encyclopaedia Britannica,
http://www.britannica.com)
Most of wheat constituents have important applications and nutritional values. The bran is made up of several layers and acts as a protective covering. It is an important by-product in milling processing. Wheat bran is rich in vitamins B, antioxidants, minerals and also a source of fibre. It is used in feed-stock and adds fibre to foods, many recipes are available today that use wheat bran. Wheat germ is an especially rich source of α- and β- tocopherols and oil produced from it provides more vitamin E activity than most other oils (Piironen et
al., 2009). Wheat germ is added to baked goods and casseroles and boosts the nutritional
value of the food. In addition to vitamins and minerals, wheat germ provides us with naturally occurring antioxidants. The major component of wheat is flour and starchy endosperm and most bread are made with wheat flour, including many breads named for the other grains they contain like most rye and oat breads (Ponte, 1978).
2.3. Sorghum (Sorghum bicolor L.)
Sorghum (Figure 2.5) is the fifth important crop in the world coming after wheat, rice, maize and barley (FAO, 2012). It is a member of grass family and difficult to classify because of its genetic variability. This cereal has different names like: milo, jowar, kafir corn, guinea corn and cholam (Raiph, 2003).
12
Sorghum is used for several purposes. It provides a major source of food and feed in many countries, especially Africa, the United States, Asia and Latin America. In the United States, sorghum grain is used primarily for animal feed, but also is used in the food industry. In Africa, sorghum is the fourth main source of energy after cassava, maize, yam (Henley et al., 2010). Worldwide, haft of sorghum production is used for human consumption. It is also used to produce biofuels like ethanol and co-productions. Sorghum is a source of alcoholic beverages in many countries (Moench et al., 1970). Nowadays, sorghum is known like a gluten-free food for people who have been the diagnosed with celiac disease (Liu et al., 2012). Sorghum is also high in antioxidants and policosanols, group of long chain alcohols, so it might help lower the risk of cancer, diabetes and heart diseases (Henley, 2010).
Figure 2.5. Sorghum plant and sorghum grain 2.3.1 Sorghum production
Table 2.5 shows the production of sorghum in the world. The world production of sorghum has been stable for 2 decades, about 56 million metric tons in 1990 and 57 million metric tons in 2012. From 1990 to 2012, the production of sorghum in Africa increased from 11 million tons to 23 million tons while in Asia, it decreased a half, from 18 million tons to 9.5 million tons (FAO, 2012). The weather of Europe is not suitable for the growth of sorghum and also the habit of consumers is not familiar with sorghum. The annual production in this
13 area is less than 1 million ton. In the United States, sorghum is widely used for livestock feed and the production has been very stable from the year 1990 until now.
Table 2.5. The production of sorghum (Source FAO, 2012)
Year
Production (metric tons)
World Africa countries American countries Asian countries Europe countries 1990 56 807 007 11 980 273 24 643 325 18 571 513 665 155 2000 55 856 128 18 412 620 23 244 861 11 317 149 762 047 2012 57 004 922 23 312 557 21 170 356 9 502 615 775 888
2.3.2 Structure and composition of sorghum.
Figure 2.6.Structure of sorghum grain (Earp.C.F et al., 2003)
Sorghum grain has different colors from pale yellow through various shades of red and brown to a deep purple brown. Figure 2.6 shows the cross section of a sorghum grain. Like most other cereals, sorghum grain has three major components: the pericarp (outer layer), the endosperm (storage tissue) and the germ (embryo). The pericap, endosperm and germ contribute for about 6, 84 and 10%, respectively, of the grain weight.
14
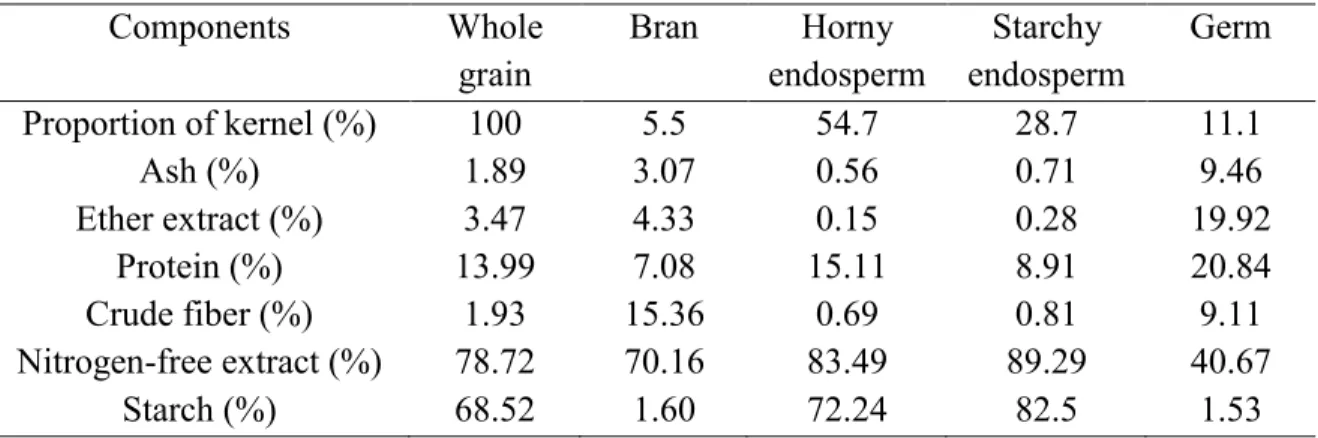
The pericarp is composed of three tissues: epicarp, mesocarp and endocarp (Earp and Rooney, 1982). The epicarp is generally covered with a thin layer of wax. The endosperm is the main part of the grain. It contains starch granules, protein, group B vitamin, and it is also a good source of carbohydrates. The germ contains two parts: embryonic and scutellum. Table 2.6 presents the composition of the different parts of sorghum grain. The composition of sorghum is also different depending on the varieties and growing conditions and it also varies during sorghum plant development.
Table 2.6. Composition of the whole kernel and its part for sorghum (moisture-free basis) (Wall et Charles, 1970) Components Whole grain Bran Horny endosperm Starchy endosperm Germ Proportion of kernel (%) Ash (%) Ether extract (%) Protein (%) Crude fiber (%) Nitrogen-free extract (%) Starch (%) 100 1.89 3.47 13.99 1.93 78.72 68.52 5.5 3.07 4.33 7.08 15.36 70.16 1.60 54.7 0.56 0.15 15.11 0.69 83.49 72.24 28.7 0.71 0.28 8.91 0.81 89.29 82.5 11.1 9.46 19.92 20.84 9.11 40.67 1.53
15
2.4. Rice, wheat and sorghum waxes and their applications
2.4.1. Wax generalities: types and composition
Figure 2.7. Structure of cuticule (lipidlibrary.aocs.org)
Wax is a fat-like material which exists on the surface of plant material as well as on the surface coating of insects or animal skin. According to the origin of wax, it can be classified into four groups: animal and insect waxes (beeswax, spermaceti and lanolin); vegetable wax (bayberry, carnauba, cane sugar, jojoba, sorghum, rice bran and wheat bran); petroleum and mineral wax (paraffin, ceresin, petrolatum and microcrystalline) and synthetic wax (polyethylene, carbowax, polarwax, syncrowax, acrawax and stearone) (Li., 1999).
The compositions of wax vary from one to another based on their sources. They typically consist of several components, including wax esters, wax acids, wax alcohols, and hydrocarbons. For this reason, organic solvents are conventionally used to extract wax and wax-like materiels. Among them, wax esters of long-chain fatty acids and long-chain fatty
16
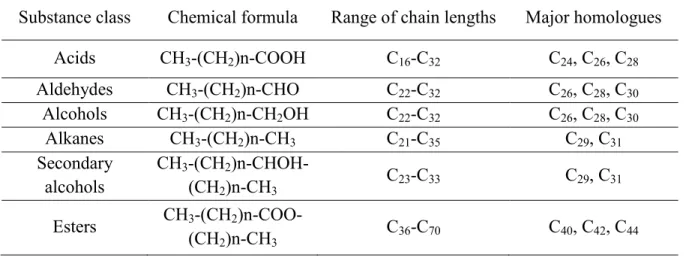
alcohols are the major components which has interesting biological characteristics such as to reduce cholosterols in humains (Christie W., 2011). The Table 2.7 is an example of vegetable wax composition. It shows that long chain hydrophobe substances are present in vegetable waxes. The carbon number varies greatly from 16 carbons for fatty acid and up to 70 carbons for esters (Luka et al., 2009).
Table 2. 7. The most common substance classes of cuticular waxes (Luka et al., 2009) Substance class Chemical formula Range of chain lengths Major homologues
Acids CH3-(CH2)n-COOH C16-C32 C24, C26, C28 Aldehydes CH3-(CH2)n-CHO C22-C32 C26, C28, C30 Alcohols CH3-(CH2)n-CH2OH C22-C32 C26, C28, C30 Alkanes CH3-(CH2)n-CH3 C21-C35 C29, C31 Secondary alcohols CH3-(CH2 )n-CHOH-(CH2)n-CH3 C23-C33 C29, C31 Esters CH3-(CH2 )n-COO-(CH2)n-CH3 C36-C70 C40, C42, C44
Wax in grains is a type of vegetable (plant) wax and exists on the surface of grains. It is believed to help grains to control water evaporation, to maintain moisture and to protect grains from micro-organisms (Hwang et al., 2004). The amount of wax in grains depends both on the type of grains and their variety (Imark and Dunford, 2005). Figure 2.7 shows us that vegetable waxes are from two types of waxes: epicuticular waxes and intracuticular waxes. Epicuticular waxes are present in the outer surface of the grain coating whereas intracuticular waxes are inside the grain coating layer. Epicuticular wax is rich in alkanes while intracuticular wax is rich in primary alcohols and triterpenoids. (Buschhaus et al., 2007). Cereal waxes have not been much studied in the literature despite the large worldwide production of these crops. They are usually obtained as by-products of the rice and wheat bran oil industries or by-products of the milling processing industries. Their properties are similar to carnauba wax and they could be used as a replacement of hard vegetable wax (Huston, 1972; Jones J., 2007).
17 Based on its composition, rice bran wax is classified into soft and hard wax. Both wax types are recovered from rice bran crude oil with melting points of 79.5oC (hard wax) and 74oC (soft wax). The hard wax consists of 64.5% fatty alcohols, 33.5% fatty acids and 2% hydrocarbons. Soft wax includes 51.8% fatty alcohols, 46.2% fatty acids and hydrocarbons (Orthoefer et al., 2005; Kim et al., 2008).
According to the research done by Vali et al. (2005), the major components of rice bran wax are wax esters and they account for 93-94% of total wax esters with even carbon numbers between C44 and C62. Saponification of wax esters will result in the formation of
C24-C40 fatty alcohols and C16-C24 fatty acids. Less than 7% of the total wax esters have odd
carbon numbers of C45-C61 (combination of C16-C24 fatty acids and C25- C39 fatty alcohols).
The compositions of even number fatty alcohols are triacontanol (C30) 24-27%;
dotriacotanol (C32) 17-20%; octacosanol (C28) 12-17%; hexacosanol (C26) 5-7%;
tetracosanol (C24) 2-5%. The major compositions of FA are lignoceric acid (24:0) : 60-67%
and behenic acids (22:0): 17-20%. These results are found to be similar to the studies done by Liu et al. (2008) and Orthoefer et al. (2005).
The average composition of wheat bran wax is similar to that from rice wax. However, up to now there are few reported studies about the composition of wheat bran wax (Ohnishi et
al., 1986). Thin-layer-chromatography (TCL) of waxy esters in wheat showed that the
contents of acylsterols, shorter alkylesters, hydrocarbons and longer alkylesters of the neutral lipids are 7.4, 0.5, 0.4 and <0.1%, respectively. The ratio of alkanes, alkenes and squalene is approximately 79:9:12. The main alcohols of the crude longer alkylesters are tetracosanol (C24) (39.9%), hexacosanol (C26) (20.2%), docosanol (C22) (18.7%) and
octacosanol (C28) (9.1%) whereas fatty acids are palmitic (19.8%), arachidic (17.1%),
behenic (15.0%) and stearic (9.1%) (Ohnishi et al., 1986). Similarly, the research of Dunford et al. (2009) states that the major components of long chain fatty alcohols in wheat bran wax are docosanol (C22), tetracosanol (C24), hexacosanol (C26), and octacosanol (C28).
Hwang et al., 2002 reported that 0.16-0.3% wax could be extracted from sorghum grain depending on the type of solvent used for extraction. The melting point of this extracted
18
wax varied from 77 to 85oC. They are composed of hydrocarbons, wax esters, aldehydes, alcohols and acids with the long hydrocarbon chain. The composition of sorghum wax varied according to the sources of Sorghum and extraction methods. Another research reported that long chain lipids extracted by hot hexane and hot ethanol generated the yield of 0.2-0.3% (w/w) and the extract contained policosanols 37-44%, aldehydes 44-55% and acid 4-5% (Hwang et al., 2004). The main alcohols are octacosanol (C28) (43.7%),
triacontanol (40.9%) and hexacosanol (C26) (8.2%). Cai et al., 2013 also confirm that the
major components of sorghum wax were long chain fatty acid, primary alcohols, aldehydes and alkanes. However, the yields of fatty acids, primary alcohols and aldehydes are 36.39%, 9.3%, 18.38% (w/w), respectively. Among them, compounds with 30 carbon in length were largest part in the sorghum wax.
2.4.2. Cereal wax applications
Among active components in cereal wax, long chain aliphatic primary alcohols (C24-C34),
known as policosanols, are the important components in pharmaceutical industries. Policosanols are popularly used as drugs. Originally, they are extracted from cane sugar, yams and beeswax but today they are also found and extracted from whole grains, especially in sorghum grains (Hwang et al., 2002; 2004; 2005), rice grains (Wang et al., 2007; Cravotto et al., 2004; Vali et al., 2005; Liu et al., 2006) and wheat grains (Irmak et
al., 2005; 2007; Dunford et al., 2009). According to the research of Hargrove et al. (2004),
5-20mg/day of mixed fatty alcohols (C24-C34) can lower LSD-Cholesterol by 21-29% and
raise high-density lipoprotein HDL-Cholesterol by 8-15%. Another research showed that the ingestion of policosanol 5mg/day for 8 weeks reduces serum total cholesterol by 12% and LDL-Cholesterol levels by 17%. It also raises HDL-Cholesterol and improves LDL/HDL ratios in those with elevated blood lipids (Jones J., 2007). Policosanols are believed to be safe in animal studies and even in human studies (Jones J., 2007).
Many waxes such as white wax have been used for cosmetic and pharmaceutical industries. Sabale et al. (2009) showed that ointment base made with rice bran wax had better spreadability (the average spreadability was 16.8±2.4 g.mm-1.s-1) than the standard base
19 (average spreadability 10.13±1.66 g.mm-1.s-1). These authors indicated that ointment prepared with rice bran wax base also showed better diffusibility than the one prepared with standard base. They believed then that rice bran wax could be further used as an oleaginous ointment base as far as their pharmaceutical properties are concerned in contrast to the traditional available costly bases. In addition, the Food and Drug Administration (FDA) allows rice bran wax to be directly added in foods and it is also considered as a safe ingredient in cosmetics.
Cereal bran wax has been extensively applied in cosmetics preparation. They are used to prepare lipsticks, creams and mascaras. They are believed to be able to efficiently remove the anti-wrinkle, restructure the healthy skin and also make the skin smooth and soft. Wax can form a protective film so it prevents the loss of skin moisture (Ito et al., 2003). In addition, the cereal wax is similar with carnauba wax so it can be used in place of or with carnauba wax in car, shoes and floor polishes (Lotchte-Watson et al., 1999)
2.5. Extraction methods of lipid
A large number of lipid extraction methods have been developed and described at laboratory scales. Among them, soxhlet extraction method is one of the most commonly used, in which organic solvents are used to solubilize lipids from solid materials. As the lipids are hydrophobic compounds, they are usually extracted by hexane, benzene, chloroform, light petroleum ether and acetone (Hwang et al., 2002). Up to now, Soxhlet apparatus is widely used in worldwide laboratories and has become the standard and reference method for most of lipid analysis in foods when hexane is used as the solvent. However, the method has several disadvantages such as time-consuming operation, unsafe and flammable solvents and unfriendly aspect of environment and especially hazardous residues remaining in final products. In order to reduce the extraction time, derivative methods of Soxhlet such as microwave-integrated or assisted Soxhlet and Soxtec have been introduced. The main difference is to equip the Sohxlet system with a microwave and/or a heating device as described by (Virot et al., 2007) but the extraction process principle remains similar.
20
Another extraction method, known as supercritical fluid extraction, for food lipids has recently and widely documented for many reasons. The extraction principle replies on the unique ability of a substance to diffuse through solids like a gas, and dissolve materials like a liquid. Carbon dioxide and water are the two commonly used substances to extract hydrophobic and hydrophilic materials, respectively. Supercritical fluid extraction methods using CO2 (SC-CO2) was used to extract many food-based and medicine-based sources
since CO2 can easily become gas at ambient condition (Lang et al., 2001). In addition,
Gomez et al. (1995) reported that SC-CO2 and Soxhlet extraction yielded similar recovery
of grape seed oil. With the distinct properties of low viscosity and high diffusivity of CO2,
it is believed to penetrate more quickly into porous solid food products and render mass transfer more easily, resulting in the extraction process more efficiently (Sahena et al., 2009). Moreover, thanks to the ease to change the properties of the supercritical fluids, via minor changes in temperature or pressure, the extracted compounds may be remarkably selected. For example, Song et al., (1992) claimed that a vidoline component present in the leaves of Cathanranthus roseus was successfully isolated by SC-CO2 from more than 100
alkaloid compounds. Finally, the SC-CO2 is believed to be suitable for extracting thermally
labile compounds as the method is conducted at low temperature. SC-CO2 was used to
extract, for instance, the ginger oils with more protected natural compounds than the distilling method at high temperature (Bartley and Foley, 1994). For those advantages of SC-CO2 over extraction methods using solvent like hexane except for its requirement of
more sophisticated equipment, it is likely that SC-CO2 can be the chosen method to extract
high value compounds present in lipids e.g. compounds with medicinal activity.
Also, liquid CO2 has been used as a solvent to extract essential oils and lipids. This method
is different from the SC-CO2 at the fact that the liquid CO2 method is performed at lower
temperature and pressure than SC-CO2. In the liquid form, CO2 is reported to be a good
solvent to extract lipids (Sahena et al. 2009). For example, it was used by Spricigo et al., (1999) to extract lipids from nutmeg. According to those authors, this method was ideal to fractionate the essential oils since the extraction operation was at mild condition (at ambient temperature). Interestingly, no toxic residues remaining in final products were found, and the process was environmentally friendly. The essential oil of black pepper and
21 clove oil extracted by liquid CO2 were also published by Ferreira et al. (1993) and Guan et
al. (2007), respectively. They reported that liquid CO2 could efficiently extract essential oil
with high yield and up to 96% wt% in the case of black pepper oil. Therefore, liquid CO2 is
promising solvent to extract lipids or more precisely to fractionate essential oil from total lipids of plant-based materials.
As lipids are present in all biological material including foods, many methods attempting to extract more efficiently and intactly these lipids has been described and developed. Each method possesses its own distinct advantages and disadvantages as well as specific purposes (e.g selectivity or yield). Any novel the lipid extraction method would need to be validated and compared to a reference method. In the food industry, Soxhlet extraction method using the solvent n-hexane has been frequently used as the standard and reference method.
2.6. Extraction methods of cereal waxes
Cereal wax is a type of lipid so the method extraction has the same principle with the method extraction of lipid. Rice and wheat bran waxes are mostly obtained during bran oil extraction in the dewaxing step of refining process. Other extraction methods from whole grain cereals have also been reported. Because the surface waxes are very hydrophobic, they may be extracted by non-polar solvents. The wheat grains were then dipped in chloroform-methanol solution (2:1, v/v) for three times and the optimal ratio of grain and solvent is 1:4 (w/v). Then, grains were immersed twice into three volumes of water-saturated butanol. After that, the extracts were washed with water, the solvent was evaporated and the mixture was dried to obtain the wax (Ohnishi et al., 1986). The result shows that the yield of total lipids was 2.7% with the major components were acylsterols, alkylesters, alkanes, alkenes, alcohols and hydrocarbons. Another method was described by Sariava (1995), in which petroleum ether was used to extract wax from the whole sorghum kernel. After filtration to remove the grain from solvent, this solution was stored at low temperature and the precipitated wax was collected. Similarly, the report of Hwang et al. (2002) showed that the sorghum wax could be extracted by refluxing them in organic
22
solvents (hexane, chloroform, light petroleum ether) for an extended time. The wax was then separated from the mixture by two ways. The first one comprises evaporating the solvent from the mixture containing waxes, mixing the wax extract with other polar solvents (acetone or alcohol) followed by filtration to separate the precipitated wax. The second consisted in crystallizing the waxes by incubation of the extraction mixture at -18oC, then the crystallized wax were collected by filtration. Hwang et al. (2004 and 2005) applied the previous described method to extract wax from selected cereals of Korean origin (grain sorghum, brown rice, purple rice, wheat and maize). These grains were refluxed in hexane for 30 minutes, the ratio of hexane and grains are 1:1 (v/w). These mixtures were then filtered at low temperature and precipitated wax was obtained. The wax yield varied from 0.2-0.3% depending on the type of grain and the solvents. The compositions of the wax obtained by this Hwang are acids, aldehydes and alcohols.
Although solvent wax extraction methods are simple and economical, requiring no complex equipment, they are not considered to be environmental-friendly, with potential harm to human health due to solvent residues.
2.7. Liquid nitrogen and its potentials for wax extraction
2.7.1. Properties of liquid nitrogen
Nitrogen is a non-toxic, colorless, odorless and tasteless gas that constitutes 78% of the air atmosphere. It is inert, nonflammable, and it does not support combustion. At -195.8oC (boiling point) and atmospheric pressure, nitrogen becomes a colorless liquid. The liquid to gas expansion ratio of nitrogen is 1:694 at 20 °C so liquid nitrogen boils to fill a volume with nitrogen gas very quickly. The transition between liquid phase and solid phase occurs at -210oC (melting point) and atmospheric pressure. In industry, liquid nitrogen is produced in large quantities by fractional distillation of liquid air (Afsset, 2008). Table 2.8 shows several properties of nitrogen (Afsset, 2008).
23 Table 2.8. Properties of nitrogen (Source Afsset, 2008)
Chemical symbol N2
Molecular Weight (g/mol) 28.01
Boiling point -195.8oC
Melting point -210oC
Gas density 1.2506 kg/m3(P=1.013 bar and T= 0oC) Liquid density 808.607 kg/m3(P=1.013 bar and T= -195.8oC) Solubility in water 0.0234 vol/vol (P=1.013 bar and 0 °C)
Triple point T =-210oC and P=12.5 kPa
Critical point T= -146.9oC; P=3399 kPa
2.7.2. Potential of wax extraction by liquid nitrogen
A few studies revealed the possibility of using liquid nitrogen to extract waxes from fruits. For example, the research done by Ketata et al. (2012) showed that three 8 and 10 seconds immersions of whole highbush and lowbush blueberries in liquid nitrogen increased their coefficients of water loss to 55.39%, 49.23% and the coefficient of sugar gain to 63.46% and 68.81% during further osmotic dehydration, respectively, when compared to non-treated berries. Observed by scanning electron microscopy, their epicutilar wax seemed to be gradually disappearing along with the increase in the number of immersion cycles. After three cycles, the thickness of epidermis decreased more than 80% for highbush blueberries and 55% for lowbush blueberries. Similarly, drying times for different drying methods (vacuum, freeze and convective drying) are markedly shortened for whole blueberries, seabuckthorn fruits and green grapes pretreated with liquid nitrogen, when compared to non-treated samples (Thromas et al., 2010; 2011). The thickness of the epidermis of the fruits (determined by optical microscopy) decreased 38% for seabuckthorn fruits and 22% for green grapes after 5 immersion cycles in liquid nitrogen. Also, dewaxing of the fruit surface was observed by scanning electronic microscopy. These reported findings strongly support the fact that liquid nitrogen could be used to extract waxes from other vegetable surfaces like cereal grains.
25
CHAPTER 3. HYPOTHESIS AND OBJECTIVES
3.1. Hypothesis
Wax from cereal grains (rice, wheat and sorghum) can be extracted by cyclic immersions of different time intervals in liquid nitrogen.
3.2. Objectives
3.2.1 General objective
To assess the potential of a novel „green‟ process, by use of liquid nitrogen, for rice, sorghum and wheat wax extraction as an alternative to traditional extraction methods, by n-hexane.
3.2.2 Specific objectives
- To determine the optimal conditions (duration, number of immersion cycles) for extracting the wax of rice, sorghum and wheat grains with liquid nitrogen.
- To compare the impact of the two extraction methods (liquid nitrogen and n-hexane) on the surface of grains by scanning electron microscope (SEM).
- To analyse the composition of extracted rice, sorghum and wheat waxes.
- To evaluate the effectiveness of liquid nitrogen extraction in comparison to n-hexane extraction in terms of yield of extracted wax and composition.
27
CHAPTER 4. MATERIALS AND METHODS
4.1. Materials
Brown rice (Oryza sativa L.) was bought from Carl Garrich Lone Pine Enterprises, United State. Sorghum (Sorghum bicolor L.) and wheat (Triticum aestivum) were purchased from Farinart, Quebec, Canada. These cereals were dried at 45oC in a ventilated oven, Fisher 338F, Canada for 24 hours to about 10% of moisture (Hwang et al., 2002) and stored in plastic bags under vacuum conditions until used. Liquid nitrogen was obtained from Praxair, Canada. N-hexane and Whatman paper were bought from Fisher Scientific, Canada. (Trimethylsilyl) trifluoroacetamide with 1% trimethylchlorosilane and standard pentadecanoic acids (C15) were purchased from Sigma-Alrich Co., st. Louis, USA.
4.2. Methodology
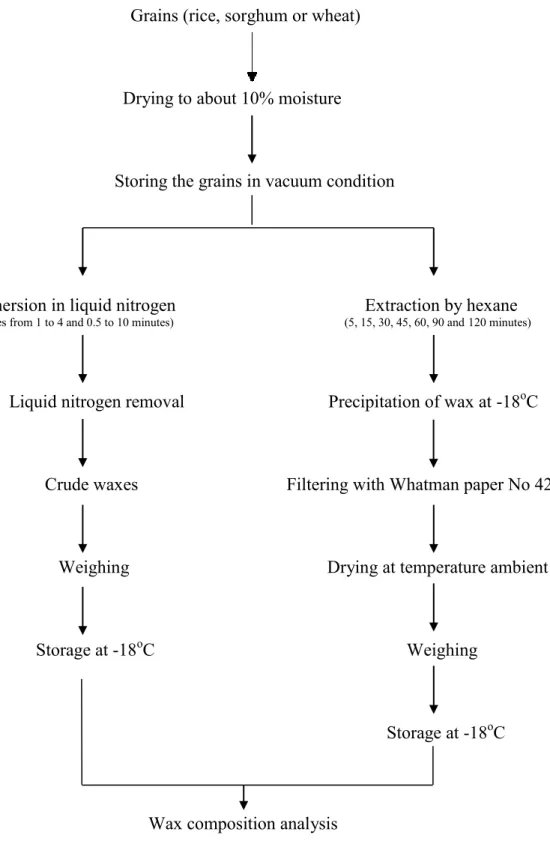
Figure 4.1 shows a schematic diagram of the proposed methodology for this research. Detailed methods and further information on each step follows.
28
Grains (rice, sorghum or wheat)
Drying to about 10% moisture
Storing the grains in vacuum condition
Immersion in liquid nitrogen Extraction by hexane
(Cycles from 1 to 4 and 0.5 to 10 minutes) (5, 15, 30, 45, 60, 90 and 120 minutes)
Liquid nitrogen removal Precipitation of wax at -18oC
Crude waxes Filtering with Whatman paper No 42
Weighing Drying at temperature ambient
Storage at -18oC Weighing
Storage at -18oC
Wax composition analysis
Figure 4.1. Proposed methodology, liquid nitrogen extraction on the left and n-hexane on the right
29 4.2.1. Determination of moisture content
Moisture content of the grains was determined according to the standard method in an oven (AOAC, 1980). The samples were first weighed (W1) with a Mettler Toledo balance
(Model AB104-S, Greinfesee, Switzerland) and then placed in a vacuum oven (Fisher scientific 281A) in the presence of P2O5. The vacuum oven was set at 50oC under gauge
pressure of 25 mmHg. After being dried to constant weight, they were transferred to desiccators for 15 minutes to cool down to the room temperature. Finally, the dried samples were rapidly weighed (W2) again, and their moisture content was calculated by the formula
(4.1).
( ) (4.1) Where: MC (%): Moisture content, wet basis (%)
W1: Initial grain mass (kg)
W2: Dried grain mass (kg)
4.2.2. Wax extraction by liquid nitrogen
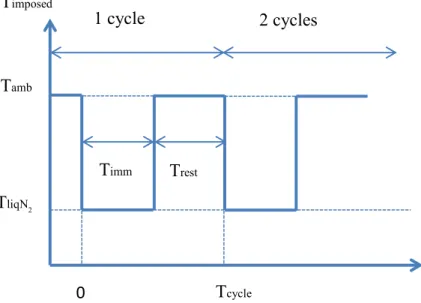
Wax extraction was carried out according to the figures 4.2 and 4.3 which illustrate the immersion process of the grains (rice, sorghum and wheat) in liquid nitrogen. 100 g of grains in a metal strainer was submerged into 500 ml of liquid nitrogen (Figure 4.2) with various durations of immersion (from 0.5 to 10 minnutes) and numbers of cycles (ranging from one to four). One cycle is defined as the period where the samples were in the liquid nitrogen (Timm) and out of liquid nitrogen (Trest) (Figure 4.3). After each immersion in
liquid nitrogen with the temperature -195.8oC (TliqN2), the samples were retained for one
minute at room temperature (Tamb). Once the nitrogen presence in treated samples was
extensively evaporated, the amount of extracted wax was determined by weighing the aluminum foils wrapping the container before and after the extraction (Figure 4.2). The wax were collected and stored at -18oC until analyzed.
30
When the rest time (Trest) was studied, the grains were immersed for 0.5 to 10 minutes in
liquid nitrogen and followed by 1, 3, 5 minutes in ambient temperature (Tamb).
The experiment was triplicated for each cycle and each immersion duration.
Figure 4.2. Description of wax extraction in liquid nitrogen.
Figure 4.3. Illustration of immersion process in liquid nitrogen Tamb TliqN2 0 Tcycle Trest Timm Timposed 1 cycle 2 cycles
31 The extraction time was calculated by the equation (4.2)
( ) (4.2)
Where:
Ncycle: The number of immersion cycle of grains in liquid nitrogen.
Timm: Immersion time (minute).
Trest: The length of time when treated samples remain at ambient temperature until they are
subject to the second treatment (minute). Text: Extraction time (minute)
4.2.3. Wax extraction by n-hexane
A conventional solvent-based wax extraction method was conducted for comparison purposes. We used the method described by Hwang et al. (2005) for wax extraction of Korean cereal grains with some modifications. 50 g of grain was mixed with n-hexane in a round flask, placed in a device with reflux system (Figure 4.4) and extracted for 5, 15, 30, 45, 60, 90 and 120 minutes. The proportion of n-hexane and the sample was 2:1 (v:w), and the temperature in the extraction chamber was fixed at 68-70oC, corresponding to the boiling point of n-hexane at the atmospheric pressure. Once the extraction was finished, the bottom flask containing extracted wax was placed in a freezer at -18oC for about 8 hours. Then, it was filtered through a Whatman No. 42 paper filter under vacuum to collect the wax. The wax was then dried under the hood at room temperature for 24 hours to a constant weight or all the solvent was evaporated. The amount of crude wax was measured and stored at -18oC for the future analysis. Three repetitions for each extraction time were conducted.
32
Figure 4.4.Illustration of reflux system used for wax extraction by n-hexane (www.chem.wisc.edu)
4.2.4. Calculation of wax yield
The amount of wax for each extraction was calculated by the following formula (4.3) (Watson et al., 1999):
( ) ( ) ( ) ( ) (4.3)
MC (%): percent moisture content of the grains, as determined in point 4.2.1.
4.2.5. Examination of wax by scanning electron microscope (SEM)
The fresh and treated grains of cereals (rice, sorghum and wheat) for wax extraction by n-hexane and liquid nitrogen were placed onto the observation support of the scanning electron microscope (JEOL, JSM6360LV, Tokyo, Japan) and metalized with palladium in an argon plasma chamber. The grain surfaces were then captured by the microscope under the accelerating potential of 15kV and the magnification by 3000 times.